Abstract
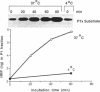
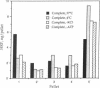
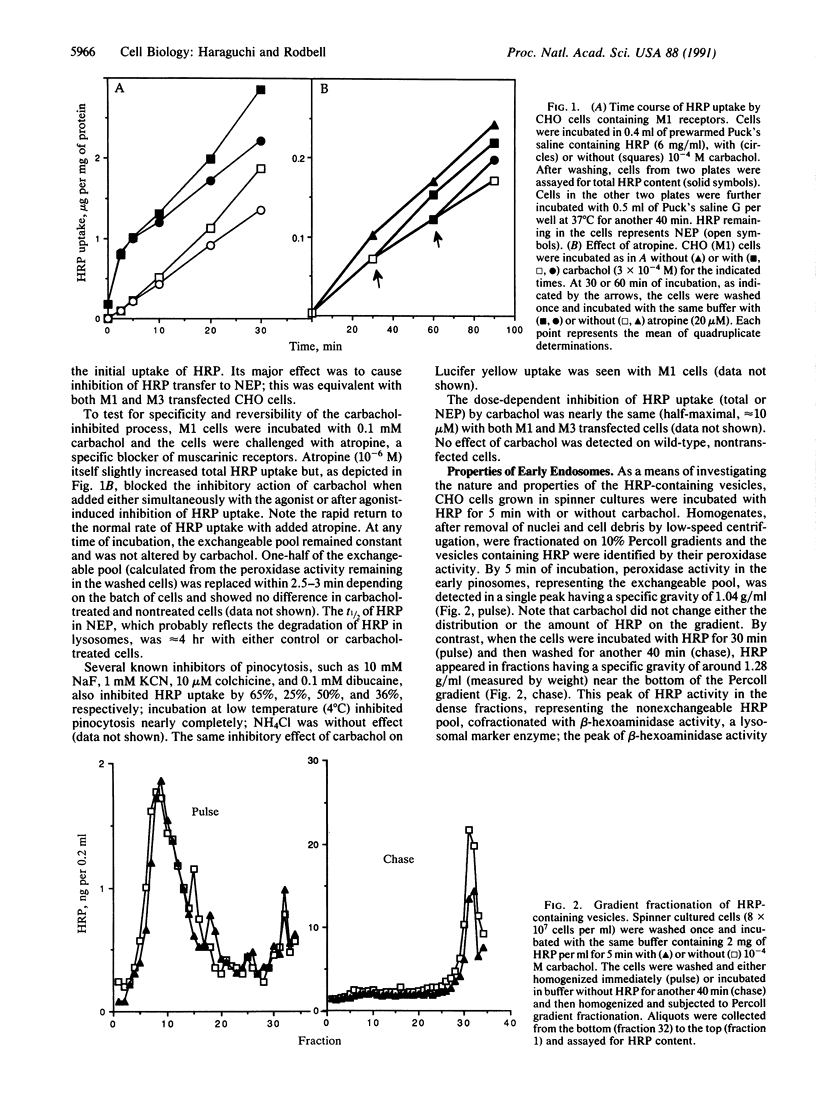
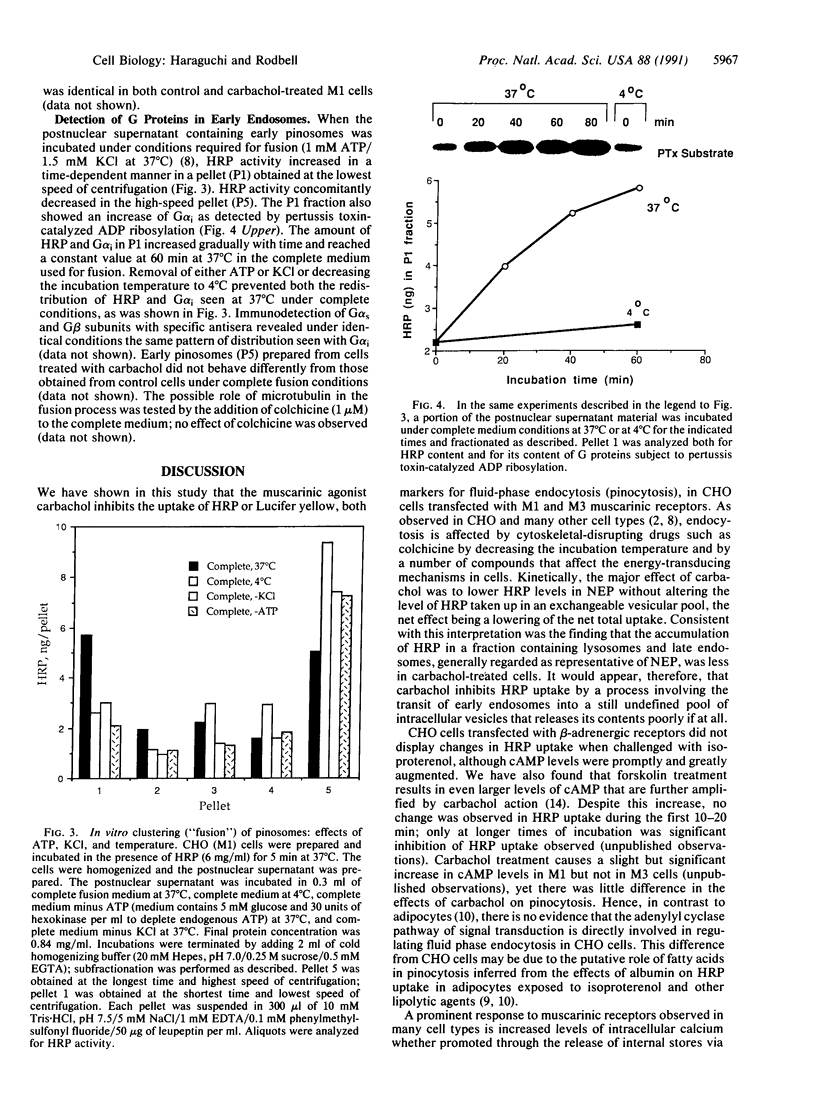
We examined the effects of isoproterenol and carbachol on fluid-phase endocytosis by Chinese hamster ovary (CHO) cells transfected with beta-adrenergic, M1, or M3 cholinergic receptors. Isoproterenol increased cAMP production and carbachol increased intracellular Ca, indicating successful expression of the receptor genes and coupling to typical signal transduction pathways. Carbachol inhibited the uptake of horseradish peroxidase (HRP) or Lucifer yellow (markers of fluid-phase endocytosis) in both M1- and M3-containing cells but not in wild-type cells, whereas isoproterenol did not affect pinocytosis in cells transfected with beta-adrenergic receptors. Carbachol inhibited the transit of HRP from an exchangeable pool to a nonexchangeable pool by a latent process requiring minimally 5 min of incubation. During the latent period, only one peak of low-density HRP-containing vesicles was found on Percoll gradients; after 5 min, HRP appeared in both high- and low-density vesicles. Carbachol-treated cells contained less HRP in the high-density fraction enriched in lysosomal markers. Early endosomes from CHO cells labeled for 5 min with HRP underwent fusion to make a more dense population of vesicles in the presence of ATP and KCl at 37 degrees C but not at 4 degrees C. The fused material contained increased levels of G proteins as detected either by ADP ribosylation with appropriate toxins or by immunoblotting with specific antibodies. These findings suggest that GTP binding proteins are internalized in endocytic vesicles and enter into a complex trafficking process involving fusion with other vesicular compartments. Trafficking of endosomes to these compartments is inhibited by activated M1 and M3 muscarinic receptors in CHO cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Ramachandran J., Capon D. J. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989 Jul 13;340(6229):146–150. doi: 10.1038/340146a0. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Biochemistry of interorganelle transport. A new frontier in enzymology emerges from versatile in vitro model systems. J Biol Chem. 1989 Oct 15;264(29):16965–16968. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck M. A., Fraser C. M. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties. Biochem Biophys Res Commun. 1990 Dec 14;173(2):666–672. doi: 10.1016/s0006-291x(05)80087-7. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Bird G. S., Obie J. F., Putney J. W., Jr The mechanism for synergism between phospholipase C- and adenylylcyclase-linked hormones in liver. Cyclic AMP-dependent kinase augments inositol trisphosphate-mediated Ca2+ mobilization without increasing the cellular levels of inositol polyphosphates. J Biol Chem. 1991 Mar 15;266(8):4772–4781. [PubMed] [Google Scholar]
- Carpentier J. L. The cell biology of the insulin receptor. Diabetologia. 1989 Sep;32(9):627–635. doi: 10.1007/BF00274248. [DOI] [PubMed] [Google Scholar]
- Casey K. A., Maurey K. M., Storrie B. Characterization of early compartments in fluid phase pinocytosis: a cell fractionation study. J Cell Sci. 1986 Jul;83:119–133. doi: 10.1242/jcs.83.1.119. [DOI] [PubMed] [Google Scholar]
- Cushman S. W. Structure-function relationships in the adipose cell. II. Pinocytosis and factors influencing its activity in the isolated adipose cell. J Cell Biol. 1970 Aug;46(2):342–353. doi: 10.1083/jcb.46.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Mayorga L. S., Mayorga L. E., Stahl P. In vitro clustering and multiple fusion among macrophage endosomes. J Biol Chem. 1989 Aug 5;264(22):13171–13180. [PubMed] [Google Scholar]
- Felder C. C., Dieter P., Kinsella J., Tamura K., Kanterman R. Y., Axelrod J. A transfected m5 muscarinic acetylcholine receptor stimulates phospholipase A2 by inducing both calcium influx and activation of protein kinase C. J Pharmacol Exp Ther. 1990 Dec;255(3):1140–1147. [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J Biol Chem. 1989 Dec 5;264(34):20356–20362. [PubMed] [Google Scholar]
- Fraser C. M., Wang C. D., Robinson D. A., Gocayne J. D., Venter J. C. Site-directed mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Mol Pharmacol. 1989 Dec;36(6):840–847. [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Cell-free systems to study vesicular transport along the secretory and endocytic pathways. FASEB J. 1989 Nov;3(13):2488–2495. doi: 10.1096/fasebj.3.13.2680705. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. E., Howell K. E. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO J. 1986 Dec 1;5(12):3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot F. L., Audus K. L. Angiotensin peptide regulation of fluid-phase endocytosis in brain microvessel endothelial cell monolayers. J Cereb Blood Flow Metab. 1990 Nov;10(6):827–834. doi: 10.1038/jcbfm.1990.139. [DOI] [PubMed] [Google Scholar]
- Haraguchi K., Rodbell M. Isoproterenol stimulates shift of G proteins from plasma membrane to pinocytotic vesicles in rat adipocytes: a possible means of signal dissemination. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1208–1212. doi: 10.1073/pnas.87.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Hellmiss R., Duerson K., Ennulat D., David N., Clapham D., Peralta E. Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J. 1990 Dec;9(13):4381–4390. doi: 10.1002/j.1460-2075.1990.tb07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Colombo M. I., Stahl P. D. GTP gamma S stimulation of endosome fusion suggests a role for a GTP-binding protein in the priming of vesicles before fusion. Cell Regul. 1989 Nov;1(1):113–124. doi: 10.1091/mbc.1.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Plasma membrane-derived vesicles containing receptor-ligand complexes are fusogenic with early endosomes in a cell-free system. J Biol Chem. 1988 Nov 25;263(33):17213–17216. [PubMed] [Google Scholar]
- Mullock B. M., Branch W. J., van Schaik M., Gilbert L. K., Luzio J. P. Reconstitution of an endosome-lysosome interaction in a cell-free system. J Cell Biol. 1989 Jun;108(6):2093–2099. doi: 10.1083/jcb.108.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Baker D., van Tuinen E., Schekman R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol. 1988 May;106(5):1453–1461. doi: 10.1083/jcb.106.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Ramachandran J., Capon D. J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988 Aug 4;334(6181):434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. Muscarinic acetylcholine receptor regulates phosphatidylcholine phospholipase D in canine brain. J Biol Chem. 1989 Dec 25;264(36):21720–21724. [PubMed] [Google Scholar]
- Racoosin E. L., Swanson J. A. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989 Nov 1;170(5):1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Dunia I., Delavier-Klutchko C., Kaveri S., Strosberg A. D., Benedetti E. L. Internalization of beta-adrenergic receptor in A431 cells involves non-coated vesicles. Eur J Cell Biol. 1989 Dec;50(2):340–352. [PubMed] [Google Scholar]
- Raposo G., Dunia I., Marullo S., André C., Guillet J. G., Strosberg A. D., Benedetti E. L., Hoebeke J. Redistribution of muscarinic acetylcholine receptors on human fibroblasts induced by regulatory ligands. Biol Cell. 1987;60(2):117–123. doi: 10.1111/j.1768-322x.1987.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Ray P., Middleton W., Berman J. D. Mechanism of agonist-induced down-regulation and subsequent recovery of muscarinic acetylcholine receptors in a clonal neuroblastoma x glioma hybrid cell line. J Neurochem. 1989 Feb;52(2):402–409. doi: 10.1111/j.1471-4159.1989.tb09135.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Rodbell M. Pertussis toxin induces structural changes in G alpha proteins independently of ADP-ribosylation. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2577–2581. doi: 10.1073/pnas.86.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L. Cell biology of intracellular protein trafficking. Annu Rev Immunol. 1990;8:195–229. doi: 10.1146/annurev.iy.08.040190.001211. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L. Intracellular pathways and mechanisms of sorting in receptor-mediated endocytosis. Trends Pharmacol Sci. 1989 Nov;10(11):458–462. doi: 10.1016/S0165-6147(89)80011-2. [DOI] [PubMed] [Google Scholar]
- Storrie B., Pool R. R., Jr, Sachdeva M., Maurey K. M., Oliver C. Evidence for both prelysosomal and lysosomal intermediates in endocytic pathways. J Cell Biol. 1984 Jan;98(1):108–115. doi: 10.1083/jcb.98.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udrisar D., Rodbell M. Microsomal and cytosolic fractions of guinea pig hepatocytes contain 100-kilodalton GTP-binding proteins reactive with antisera against alpha subunits of stimulatory and inhibitory heterotrimeric GTP-binding proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6321–6325. doi: 10.1073/pnas.87.16.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. S., Chin G. J., Schwartz J. H., Reese T. S. Pertussis toxin-sensitive G proteins are transported toward synaptic terminals by fast axonal transport. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1775–1778. doi: 10.1073/pnas.88.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B. Role of membrane fusion in hormonal regulation of epithelial transport. Annu Rev Physiol. 1986;48:213–223. doi: 10.1146/annurev.ph.48.030186.001241. [DOI] [PubMed] [Google Scholar]
- Wollman S. H. Turnover of plasma membrane in thyroid epithelium and review of evidence for the role of micropinocytosis. Eur J Cell Biol. 1989 Dec;50(2):247–256. [PubMed] [Google Scholar]
- Zimmerman D. L., Walter P. Reconstitution of protein translocation activity from partially solubilized microsomal vesicles. J Biol Chem. 1990 Mar 5;265(7):4048–4053. [PubMed] [Google Scholar]