Abstract
Quantitative EEG measurement of the scalp vertex theta/beta ratio (TBR) is marketed as a tool for use in the evaluation of patients who may have attention-deficit/hyperactivity disorder (ADHD). The American Academy of Neurology (AAN) recently assessed the literature about this tool. The assessment urged caution, considering that the TBR remains an investigational research tool at this time. This perspective comments further on that assessment and its rationale, and recommends a perspective for the clinician and payer.
The use of quantitative EEG (QEEG) continues to receive attention as a controversial diagnostic aid for attention-deficit/hyperactivity disorder (ADHD). For the purposes of this perspective piece, attention-deficit disorder is included in the definition of ADHD.
The QEEG generally considered in the United States is the theta/beta ratio (TBR). TBR is the ratio of the amount of theta activity divided by the amount of beta activity, where both are measured at the scalp vertex site Cz. The ears are linked to use as reference electrode sites for the measurements, so these measurements include the EEG activity from both the scalp vertex and the lower temporal regions. In many studies, the TBR measurement is a single channel EEG based on 1–3 minutes of EEG.
TBR measurement is available in commercial equipment. Commercial units display results as low, moderate, or high TBR without giving specific numeric values or raw EEG displays. The norms are age-adjusted.
American Academy of Neurology practice and advisory findings
The American Academy of Neurology (AAN) assessment1 found a relative lack of support for the use of TBR tests for the diagnosis of ADHD. The 3 conclusions from that assessment are as follows:
Clinicians should inform patients with suspected ADHD and their families that the combination of TBR and frontal EEG beta power should not replace a standard clinical evaluation.
There is a risk for substantial harm to patients misdiagnosed with ADHD, given the high false-positive diagnostic rate of TBR and frontal EEG beta power.
Clinicians should inform patients with suspected ADHD and their families that the TBR should be used neither to confirm an ADHD diagnosis nor to support further testing after a clinical evaluation, unless such diagnostic assessments take place within the limits of a research study.
Although some studies showed that TBR has a relatively high sensitivity and specificity for ADHD, the published literature was inconclusive.
For example, one large recent study2 based TBR measurements on 60 seconds of EEG. The gold standard for ADHD was unusual or unclear because patients with the most ADHD symptoms constituted the “ADHD-negative” control group. All patients were children referred to a specialized center for assessment of ADHD. Those considered to have a primary diagnosis of an anxiety, anger, or another disorder along with ADHD as a secondary diagnosis were considered ADHD-negative for this study, a classification that seemed peculiar because otherwise these patients would have been considered ADHD-positive patients. The classification decision ultimately compromised the gold standard in the study. There was no comparison to other childhood psychiatric or neurologic disorders as controls, and there were no normal controls. The diagnoses for the study were established upon initial referral, not upon follow-up, and the eventual diagnosis was unknown. The cutoff for “abnormal” was set at 1.5 SDs above the mean for age, so that 15% of the normal population would be expected by chance alone to be falsely positive. The authors were involved with commercialization of this product, which could be viewed as a conflict of interest. It seems wise to wait for further studies from investigators who do not have a commercial conflict of interest, who could run a study with a better gold standard, and in which other disorders and normal controls could be assessed.
Uncertainties abound. Increased theta is a well-known, nonspecific EEG finding common to a wide variety of conditions. Many pathologic conditions cause excess central theta, and even simple drowsiness does so. EEG is subject to normal variant rhythms that are well-known to have no pathologic implications, including benign temporal theta or its central analog the Ciganek rhythm. Temporal theta could show up in these Cz channels because the ear reference electrodes are active and will detect temporal rhythms. For all these reasons, increased central theta or TBR is a suspect sign on which to base a diagnosis of ADHD.
Background about ADHD and the TBR
Increased central theta in children with behavioral disorders was reported in the 1930s.3 Studies in the 1960s and 1970s confirmed excess theta activity in some childhood behavioral disorders.4 The push to use a TBR as a diagnostic test began more than 15 years ago. Initial studies5 described good sensitivity, specificity, and reliability for TBR as a diagnostic tool. TBR in children correlated with behavioral measures of impulsivity and inhibition both in ADHD and normal children.6 TBR findings in adults correlated with response times that were used as a marker for attention.7
However, problems began to appear with the accuracy of TBR when attempted as a diagnostic test. Attempts to reproduce earlier studies found only a 63% sensitivity and 58% specificity and even worse diagnostic accuracies for TBR in diagnosis of ADHD.8-10 The TBR has a dramatic age effect, decreasing markedly as a child ages from childhood toward adulthood.10 TBR results differed depending on whether the child had hyperactive or inattentive subtype.
One study evaluated TBR correlation with ADHD symptoms in 562 patients and 309 control subjects.11 Results showed a correlation of r = 0.10 for children and r = −0.14 for adults, specifically a low correlation for younger children, poor correlation for adolescents, and the inverse correlation for adults.
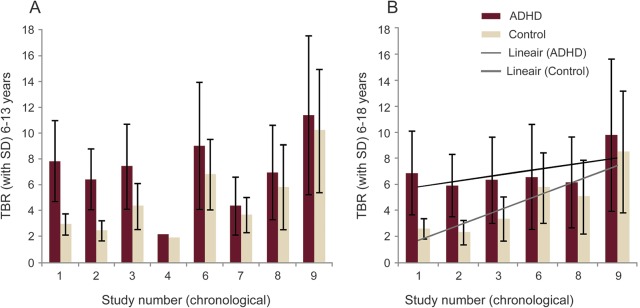
TBR diagnostic effect size decreased corresponding to the year of a study's publication.12 The report showed that the enthusiasm of a notable TBR effect by initial investigators was tempered by a lack of confirmation in follow-up studies, so that the effect size decreased in proportion to the calendar year in which the study was published. Original studies showed an effect, and more recent studies failed to confirm such a distinct TBR diagnostic effect (figure).
Figure. Meta-analysis of 9 studies of patients with attention-deficit/hyperactivity disorder (ADHD) vs controls.
Two age groups are presented: younger children (age 6–13; A) and all children (age 6–18; B). Theta/beta ratio (TBR) means and SDs are presented. The 9 studies are shown chronologically as published. Note how the differences between ADHD and controls narrowed over time, and how much scatter is seen in TBR measurements among studies of younger children. From Arns et al.12
Overall, these results suggest several conclusions: TBR may be increased in some children with ADHD. This effect may be seen only in one or another subtype (e.g., inattentive). The effect may disappear in older children and adolescents. The effect may actually be reversed in adults. TBR may not differentiate well between ADHD and normal controls, depending on which study is consulted. The effect in other disorders is unknown. The effect with drowsiness and medications is uncertain. False-positive and false-negative results may be common.
Clinical lessons
The AAN Guideline recommends that the TBR test is not yet ready for routine clinical use. Rather, it is best used in formal structured research investigations that may better define its role, if any. A similar recommendation was reached by a group of psychology QEEG experts.13 That group considered that TBR cannot be used to diagnose or rule out ADHD, and it does not replace a regular clinical examination.
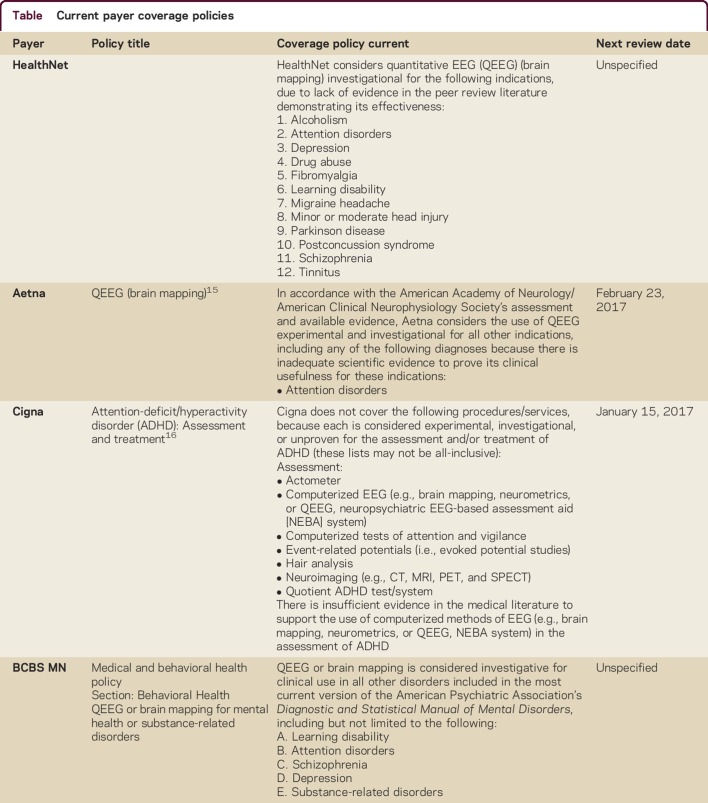
Payers have taken a similar position (table). They consider the use of any QEEG for ADHD investigational or experimental.
Table.
Current payer coverage policies
The Food and Drug Administration,14 while giving its approval for commercial marketing, cautioned that TBR must be used only upon a physician's order, by a medical professional qualified and experienced to diagnose ADHD, only after a traditional evaluation, and not as a stand-alone test. It cannot be used if a routine EEG has shown any abnormalities, or if the patient has a history of seizures, or is on any seizure medication. Then a low TBR value would suggest that the clinician should check for other disorders that might have produced the patient's symptoms.
The clinician should exercise caution in use and interpretation of TBR results. Meanwhile, we await further research by investigators who are not affiliated with companies marketing these services and devices.
ACKNOWLEDGMENT
The authors thank members of the American Academy of Neurology Payment Policy Subcommittee as well as the authors of the AAN's guideline document for their support of concept and review of early versions of the manuscript.
AUTHOR CONTRIBUTIONS
All authors contributed to drafting/revising the manuscript, information and data acquisition, and critical revision of the manuscript for intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
M.R. Nuwer has received travel reimbursement to serve as a representative at the AMA, CPT, and RUC meetings on behalf of the American Academy of Neurology and American Clinical Neurophysiology Society; serves on the editorial boards of Neurology® Clinical Practice, Journal of Clinical Neurophysiology, and Practical Neurology and as Honorary Consulting Editor for Clinical Neurophysiology; receives publishing royalties for Intraoperative Neurophysiologic Monitoring (Cambridge University Press, 2008); serves as laboratory medical director for SleepMed, where he advises on and reviews laboratory procedures; receives research support from NIH/National Institute of Neurological Disorders and Stroke and CDMRP; receives stock, stock options, and/or Board of Directors compensation from Corticare, which outsources ICU EEG monitoring; and has served as an expert in legal proceedings related to QEEG. J. Buchhalter serves on scientific advisory boards for NIH, NINDS, Charlie Foundation, and IDIC 15; has received funding for travel and/or speaker honoraria from AAN, Eisai Ltd, Lundbeck, and Upsher-Smith; served on the editorial advisory board for Clinical Neurology News; has served as a consultant to Lundbeck, Inc., Eisai Ltd., UCB, and Upsher-Smith; and receives research support from Alberta Health Services. K. Shepard serves as Director of Medical Economics for the American Academy of Neurology. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Gloss D, Varma JK, Nuwer MR. Practice advisory: the utility of EEG theta/beta power ratio in ADHD diagnosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2016;87:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder SM, Rugino TA, Hornig M, Stein MA. Integration of an EEG biomarker with a clinician's ADHD evaluation. Brain Behav 2015;5:e00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jasper H, Solomon P, Bradley C. Electroencephalographic analyses of behavior problem children. Am J Psychiatry 1938;95:641. [Google Scholar]
- 4.Satterfield JH, Lesser LI, Saul RE, Cantwell DP. EEG aspects in the diagnosis and treatment of minimal brain dysfunction. Ann NY Acad Sci 1973;205:274–282. [DOI] [PubMed] [Google Scholar]
- 5.Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology 2001;15:136–144. [DOI] [PubMed] [Google Scholar]
- 6.Williams LM, Hermens DF, Thein T, et al. Using brain-based cognitive measures to support clinical decisions in ADHD. Pediatr Neurol 2010;42:118–126. [DOI] [PubMed] [Google Scholar]
- 7.Van Dongen-Boomsma M, Lansbergen MM, Bekker EM, et al. Relation between resting EEG to cognitive performance and clinical symptoms in adults with attention-deficit/hyperactivity disorder. Neurosci Lett 2010;469:102–106. [DOI] [PubMed] [Google Scholar]
- 8.Ogrim G, Kropotov J, Hestad K. The quantitative EEG theta/beta ration in attention deficit/hyperactivity disorder and normal controls: sensitivity, specificity, and behavioral correlates. Psychiatry Res 2012;198:482–488. [DOI] [PubMed] [Google Scholar]
- 9.Liechti MD, Valko L, Müller UC, et al. Diagnostic value of resting electroencephalogram in attention-deficit/hyperactivity disorder across the lifespan. Brain Topogr 2013;26:135–151. [DOI] [PubMed] [Google Scholar]
- 10.Buyck I, Wieserma JR. Resting electroencephalogram in attention deficit hyperactivity disorder: developmental course and diagnostic value. Psychiatry Res 2014;216:391–397. [DOI] [PubMed] [Google Scholar]
- 11.Loo SK, Cho A, Hale TS, McGough J, McCracken J, Smalley SL. Characterization of the theta to beta ratio in ADHD: identifying potential sources of heterogeneity. J Atten Disord 2013;17:384–392. [DOI] [PubMed] [Google Scholar]
- 12.Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord 2013;17:374–383. [DOI] [PubMed] [Google Scholar]
- 13.Arns M, Loo SK, Sterman MB, et al. Editorial Perspective: how should child psychologists and psychiatrists interpret FDA device approval? Caveat emptor. J Child Psychol Psychiatr 2016;57:656–658. [DOI] [PubMed] [Google Scholar]
- 14.The Department of Health & Human Services, Food and Drug Administration. Letter to NEBA Health, LLC. Re: K112711 Neuropsychiatric EEG-based assessment aid for ADHD (NEBA) System. 2013. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf11/K112711.pdf. Accessed July 29, 2016.
- 15.Aetna Quantitative EEG (Brain Mapping) Policy Number 0221. Available at: aetna.com/cpb/medical/data/200_299/0221.html. Accessed July 29, 2016. [Google Scholar]
- 16.Cigna Attention-Deficit/Hyperactivity Disorder (ADHD): Assessment and Treatment Policy Number 0231. Available at: cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0231_coveragepositioncriteria_adhd_assessment_and_treatment.pdf. Accessed July 29, 2016. [Google Scholar]