Abstract
Objective:
To establish reference values for isometric strength of 12 muscle groups and flexibility of 13 joint movements in 1,000 children and adults and investigate the influence of demographic and anthropometric factors.
Methods:
A standardized reliable protocol of hand-held and fixed dynamometry for isometric strength of ankle, knee, hip, elbow, and shoulder musculature as well as goniometry for flexibility of the ankle, knee, hip, elbow, shoulder, and cervical spine was performed in an observational study investigating 1,000 healthy male and female participants aged 3–101 years. Correlation and multiple regression analyses were performed to identify factors independently associated with strength and flexibility of children, adolescents, adults, and older adults.
Results:
Normative reference values of 25 strength and flexibility measures were generated. Strong linear correlations between age and strength were identified in the first 2 decades of life. Muscle strength significantly decreased with age in older adults. Regression modeling identified increasing height as the most significant predictor of strength in children, higher body mass in adolescents, and male sex in adults and older adults. Joint flexibility gradually decreased with age, with little sex difference. Waist circumference was a significant predictor of variability in joint flexibility in adolescents, adults, and older adults.
Conclusions:
Reference values and associated age- and sex-stratified z scores generated from this study can be used to determine the presence and extent of impairments associated with neuromuscular and other neurologic disorders, monitor disease progression over time in natural history studies, and evaluate the effect of new treatments in clinical trials.
Meaningful, reliable, and sensitive outcome measures are required to monitor treatment and progression of neuromuscular and other neurologic disorders. While there have been substantial advances in the understanding of the pathogenesis and natural history of many neuromuscular disorders, the identification and development of new outcome measures that best reflect the efficacy of specific treatments have not advanced at the same rate.1 Establishing valid and responsive outcome measures is a priority for the field.2 To assist in the development of new outcome measures, normative reference values generated from large populations across the lifespan using standardized methods are required. Normative reference values can be utilized to generate z scores, which can be used in multicenter studies to improve outcome measure precision and responsiveness.
Muscle weakness and joint contractures predispose to numerous pathologies requiring intervention. Reference data play an important role in identifying and quantifying these impairments and evaluating the effectiveness of interventions. Currently, few comprehensive datasets detail the normal variation of active range of motion in healthy individuals and are limited by the number of joints assessed,3 the age range of participants,4,5 or insufficient sex representation.6,7 Similar limitations exist in strength reference datasets, relevant only to children8–11 or adult populations,12–16 or strength measured using equipment not readily available in clinic.17–19
The purpose of this study was to generate a reference dataset of normative values across the lifespan for an extensive set of isometric muscle strength and joint flexibility items, stratified for age and sex, and to investigate the influence of demographic and anthropometric factors.
METHODS
Study design and participants.
Data were collected as part of the 1,000 Norms Project, an observational study investigating physical function and self-reported health in 1,000 people across the lifespan (see full protocol20). One thousand people aged between 3 and 101 years from the Greater Sydney metropolitan area in Australia participated in the project. Participants were recruited from January 2014 to September 2015 using highly structured convenience and snowball sampling techniques, including advertising via social media, e-newsletters, and community flyers. Presentations were held at social and volunteer groups, aged care organizations, playgroups, and schools. Eligible participants were aged ≥3 years, considered themselves healthy for their age, and could participate in age-appropriate activities of daily living. People with significant health conditions affecting physical performance or an inability to follow age-appropriate instructions were excluded. Potential participants with the following conditions were also excluded: diagnosed diabetes mellitus; malignant cancers; demyelinating, inflammatory, or degenerative neurologic conditions; pregnancy; class 3 obesity; severe cardiac or pulmonary disease; joint replacement; infectious or inflammatory arthropathies; or severe mobility impairment necessitating dependence on mobility aids for all ambulation. Equal numbers of male and female participants were recruited and were stratified into 9 age categories. One hundred people per decade were recruited in the age groups of 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80+ years. In order to represent the rapid periods of growth and maturation and to distinguish between young children and adolescents, 20 children per year from 3 to 9 years of age and 16 per year from 10 to 19 years of age were recruited.
Standard protocol approvals, registrations, and patient consents.
Ethical approval was granted by the institutional Human Research Ethics Committee (HREC 2013/640) and written informed consent was obtained from all participants or parents/guardians of children.
Procedure.
Participants attended the University of Sydney Performance Laboratory once for a 2-hour assessment. Participants had their height, body mass, waist circumference, and lower limb alignment measured. Foot structure was assessed using the Foot Posture Index, a 6-item summed scale from −12 (supinated) to +12 (pronated).21 Age, sex, current work status, and self-reported ethnicity were collected from all participants or parents/guardians. Work status was classified as working (full-time, part-time, or unpaid) or not currently working (unemployed, student, or retired). Ethnicity was classified into 5 categories: British/European, American, Asian, African, and Aboriginal/Torres Strait Islander.
Two experienced clinical evaluators (physiotherapists) assessed isometric strength and joint flexibility using standardized methodology, including instructions, positioning, and scoring.20 The dominant limb was assessed and determined as the hand used to write with and the foot used to kick a ball. The strength of 12 muscle groups—hand grip, ankle dorsiflexors and plantarflexors, knee flexors and extensors, hip abductors, internal and external rotators, elbow flexors and extensors, and shoulder internal and external rotators—were assessed by maximal voluntary isometric contraction using a portable hand-held dynamometer (Citec dynamometer CT 3001; CIT Technics, Groningen, Netherlands). The dynamometer was calibrated 0–500 N with certified weights monthly throughout data collection. The strength of knee musculature in participants ≥12 years of age was assessed by fixed dynamometry (CSMi; HUMAC NORM, Stoughton, MA). For unit of measure consistency, knee flexor and extensor strength in children aged 3–11 years were converted to Newton-meters (Nm) using anthropometric tables.22 Rather than using the fixation device outlined in the protocol, ankle plantarflexion strength was assessed using hand-held dynamometry in long sitting, heel over plinth edge.
Joint flexibility was assessed using a universal goniometer, digital inclinometer, or bubble inclinometer (Baseline; Fabrication Enterprises Inc., White Plains, NY) depending on the joint assessed. Thirteen active joint range movements were assessed: ankle dorsiflexion and plantarflexion, knee flexion and extension, hip flexion, internal and external rotation, elbow flexion and extension, shoulder internal and external rotation, and cervical flexion and extension. Interrater reliability of the clinical evaluators demonstrated satisfactory repeatability of all strength and flexibility measures (intraclass correlation coefficient2,1 0.80–0.99) in a pilot study of 10 participants aged 6–67 years.
Data analysis.
Data were collected and managed using REDCap electronic data capture and manually checked for transcription errors. Reference values were generated for each age group and sex in SPSS v22 Statistics for Windows (IBM SPSS; Armonk, NY). Normality of the data was assessed using Kolmogorov-Smirnov test. For analysis, age categories were children (3–9 years), adolescents (10–19 years), adults (20–59 years), and older adults (60+ years). To determine if strength and flexibility differed between male and female participants, independent t tests were conducted. A series of multiple regression models was constructed to determine the extent to which muscle strength and joint flexibility were influenced by participant demographic (age and sex) and anthropometric factors (height, body mass, waist circumference, foot posture, and lower limb alignment). First, Pearson product-moment correlation coefficients (r) were generated to explore the bivariate relationships between strength and anthropometric and demographic factors. The same correlations were explored for each joint flexibility measure. Second, factors identified to have an association (r ≥ 0.3, p < 0.05) with strength or joint flexibility were entered simultaneously into a stepwise multiple regression model, which was reduced to a set of factors that best predicted and could be regarded as independent determinants of each strength and joint flexibility measure. To avoid multicollinearity, only one variable from highly correlated (r ≥ 0.7) variables was included. Standardized β weights were calculated to provide an indication of the relative importance of the contribution of the various factors entered into the model to explain the variance in joint flexibility or muscle strength. Variables were retained in the multiple regression model if p < 0.05.
RESULTS
To recruit 1,000 participants, 2,972 e-mails and 240 phone calls were logged. Ninety-one potential participants were excluded in accordance with the inclusion and exclusion criteria. Among adults aged over 18 years, 56% were currently working and 44% were not (31% retired and 13% students or unemployed). Participants were of diverse geographic ancestry, although the majority of participants were British/European ethnicity (74.4%), followed by Asian (16.6%), North or South American (5.1%), African (2.4%), and Aboriginal or Torres Strait Islander (1.5%). The sample mean (SD) age was 40.9 years (26.1), body mass 62.9 kg (21.1), height 1.61 m (0.02), waist circumference 78.6 cm (15.4), Foot Posture Index 3.5 (2.4), and lower limb alignment 1.8° (2.7). Ninety-three percent were right-footed and 91% were right-handed.
All missing data were accounted for. Four children declined to perform ankle dorsiflexion strength and 12 children were unable to perform cervical flexion and extension joint movements in accordance with the protocol. Ankle plantarflexors for 7 male adults and ankle dorsiflexors for 1 male adult were not assessed with hand-held dynamometry during periods of offsite calibration and servicing; 6 adults and 8 older adults were not assessed using fixed dynamometry due to safety concerns.
Normative reference values for the strength of 13 muscle groups per age category (children, adolescents, adults, and older adults) and sex are presented in table 1 and per decade in table e-1 at Neurology.org. From adolescence, male participants were significantly stronger in all muscle groups across all ages. There were no significant (p < 0.05) differences between the strength measures of boys or girls aged 3–9 years, except for shoulder internal rotators (p = 0.031), where boys were stronger. Correlations between strength and participant demographics and anthropometrics for children, adolescents, adults, and older adults are presented in table e-2. In children and adolescents, strength and age were highly correlated (p < 0.05), confirming that children become significantly stronger as they age from childhood and through adolescence. From 20 years of age, the relationship between strength and age changed. In adults aged 20–59 years, reduced strength with age was evidenced by significant, although weak, correlations with hand grip, ankle dorsiflexors, knee flexors and extensors, and shoulder external rotators. In older adults, decreased strength with increasing age was evidenced in all muscle groups. All muscle groups across all age categories demonstrated that greater height, body mass, and waist circumference were significantly associated with greater strength. The changes in strength measures with advancing age are shown in figure 1. Table e-3 shows the results of the multiple analyses. In children, height, followed by waist circumference, was the most significant predictor of strength. In adolescents, a combination of body mass, sex, and age were shown to be the strongest independent predictors of strength. Sex (male) was the most significant predictor of strength in adults, followed by height and body mass with lower predictive values. In older adults, sex (male) was the most significant predictor, with body mass, height, and age demonstrating lower predictive values.
Table 1.
Isometric strength reference values of children (3–9 years), adolescents (10–19 years), adults (20–59 years), and older adults (60+ years)a
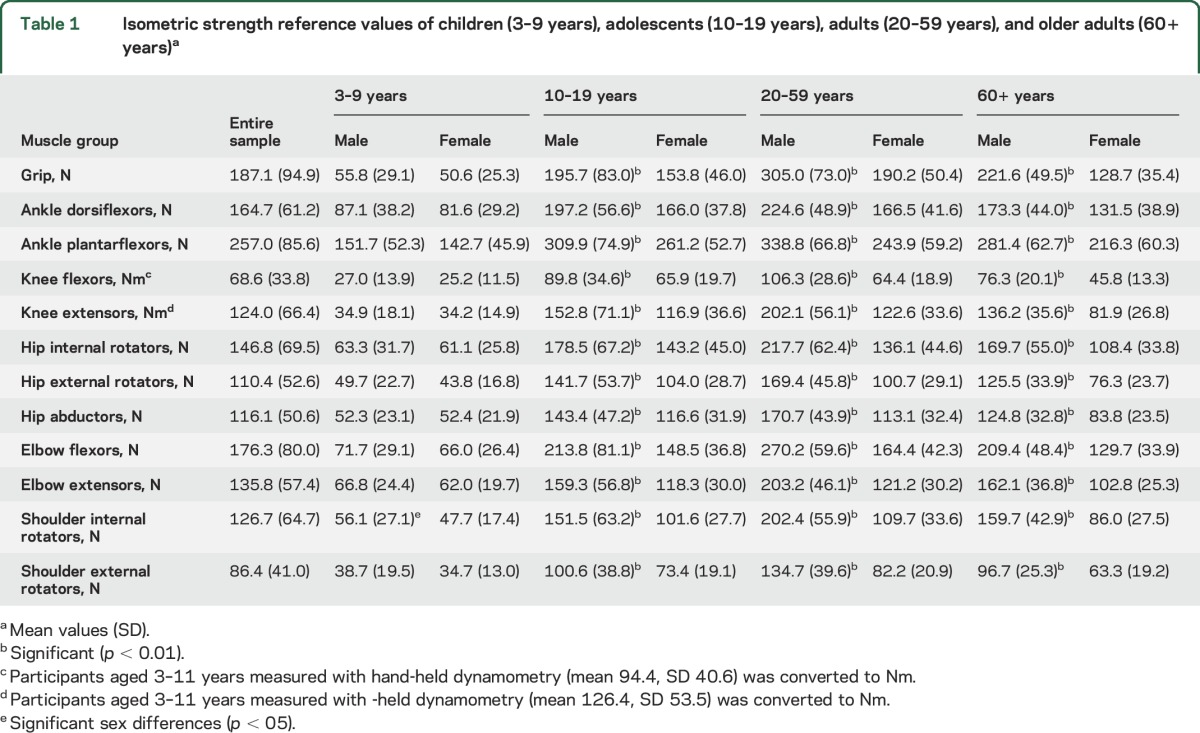
Figure 1. Scatterplots of muscle strength vs age for 1,000 children and adults.
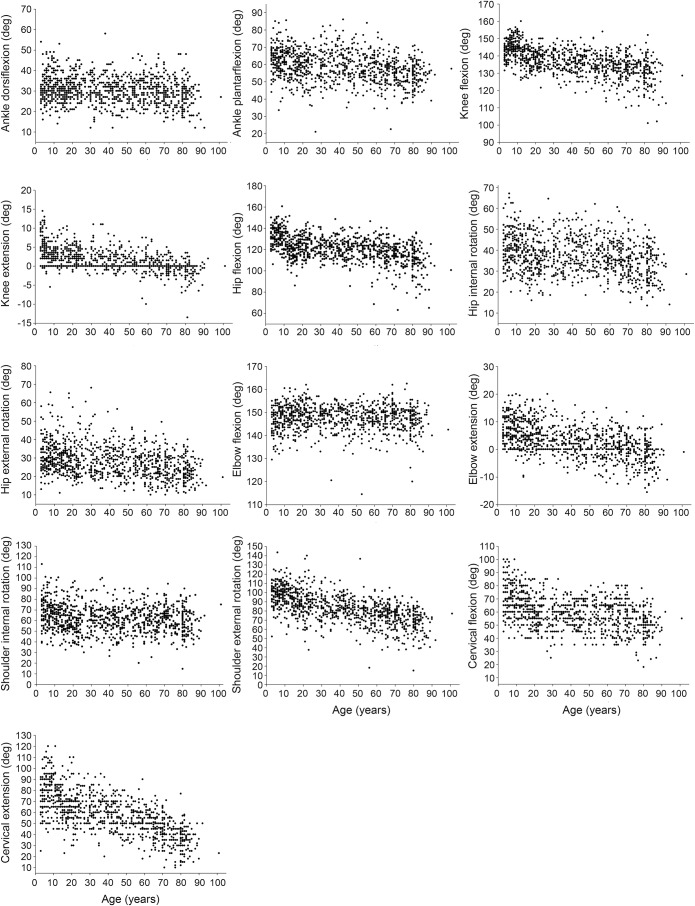
Normative reference values for active range of motion per age category (children, adolescents, adults, and older adults) and sex are presented in table 2 and per decade in table e-4. There was no significant difference (p < 0.05) in joint flexibility between boys and girls aged 3–9 years, except for hip internal rotation (p = 0.017), where girls had greater flexibility. Active range of motion was greatest in children compared to older adults. Figure 2 illustrates the inverse relationship between joint flexibility in all joints with age. Pearson correlations (table e-2) demonstrate that a decrease in flexibility with aging occurred in 8 of 13 joints of both adolescents and adults and in 12 joints of older adults (p < 0.05). Greater body mass and waist circumference were associated with a decrease in joint flexibility from 10 years of age. The correlation between height and joint flexibility was strongest in adolescents, where taller individuals demonstrated less joint range of motion. From adolescence to older adulthood, a more pronated foot posture was associated with a greater range of ankle dorsiflexion. Lower limb alignment did not demonstrate any significant correlations, beyond very weak associations, with measures of flexibility in any age category. In children, 2 multiple regression models (see table e-4) reached significance (knee extension and neck extension) and revealed height as the most significant predictor. Age, waist circumference, and height were the strongest independent predictors of flexibility in adolescents. For all adults older than 20 years, age, sex, and waist circumference were the strongest predictors of joint flexibility.
Table 2.
Joint flexibility reference values of children (3–9 years), adolescents (10–19 years), adults (20–59 years), and older adults (60+ years)a

Figure 2. Scatterplots of joint flexibility vs age for 1,000 children and adults.
DISCUSSION
This study has established a comprehensive reference dataset of isometric muscle strength and joint flexibility in 1,000 healthy people aged 3–101 years. The associations between strength and flexibility measures with demographic and anthropometric variables within different age categories identified some important relationships. As expected, there is a highly significant increase in strength of all muscle groups as children rapidly develop through to early adulthood. From adulthood, this relationship changes and a decrease in muscle strength with aging starts to occur; by older adulthood, all muscle groups demonstrate loss of strength with aging. From 10 years of age, a time of life coinciding with rapid growth and maturation, males are significantly stronger in all measures. In contrast, joint flexibility demonstrates a steady decline with age and no meaningful difference between males and females.
Our results are consistent with previous studies investigating isometric muscle strength in children8,9 and adults.12–14 However, direct comparison is limited by differences in age range, sample size, and muscle groups evaluated. Some studies report body mass as the strongest correlate with muscle strength in children,8–11 while others demonstrate as we did that height showed the strongest relationship and was the most significant predictor of strength.23,24 In adults, a decline in strength was most strongly associated with aging in 5 muscle groups (hand grip, ankle dorsiflexors, shoulder external rotators, knee flexors, and extensors), while in older adulthood all muscle groups demonstrated a significant decline in strength associated with aging. These results suggest a muscle-specific response to aging during adulthood and that generalized weakness does not occur until older adulthood. This highlights the importance of using age- and sex-matched reference data for specific muscle groups to avoid overrepresentation or underrepresentation of the force capabilities of a particular muscle group. Similar relationships between aging and muscle weakness have been reported in a limited number of adult studies.12–14
The association between waist circumference and strength and flexibility has not been reported previously. Waist circumference was identified as a significant predictor of flexibility in adolescents, adults, and older adults and of strength in children. Epidemiologic studies have identified an association between waist circumference and tendon pathology,25 with preliminary evidence supporting either a mechanical effect (due to increased load) or systemic effect (due to circulating lipids).26 The influence of adiposity on localized musculo-tendinous tissues in neuromuscular disorders will be an important factor to evaluate with the increasing rates of obesity in society.
Few studies report normative reference values for flexibility in children. Our normative reference values for adults are consistent with the literature.3,4,7 We identified only one sex difference in the flexibility of children (namely hip internal rotation), and only small differences (2°–6°) between men and women from adolescence through to older adulthood. As such, sex does not seem to have a clinically important effect on the joint flexibility of healthy adolescents and adults. There is no consensus in the literature regarding sex differences and flexibility; some studies report, as we have, that there is no clinically relevant difference,4 while others report sex differences.5 We identified a linear decrease in joint flexibility associated with advancing age, consistent with the adult literature.3 It is likely that in healthy individuals, joint flexibility declines gradually and steadily with age and a substantial or sudden decline should be considered indicative of an underlying pathology.
Studies characterizing the functional decline and rate of progression of neuromuscular disorders such as amyotrophic lateral sclerosis,27 Duchenne muscular dystrophy,28 and Charcot-Marie-Tooth disease29 depend on hand-held dynamometry to capture and track relevant changes in muscle strength. Access to reliable and expansive normative reference values and associated age- and sex-matched z scores are necessary to accurately and precisely quantify response to new interventions and to establish minimum clinically important differences.
This study is not without limitation. Participants were recruited through convenience sampling methods and with the exclusion criteria of conditions affecting physical performance may have resulted in a population that were particularly healthy and physically capable for their age. While the mixed ethnicity of our sample is reflective of the Australian population, the ethno-geographic variation in strength and flexibility measures could not be established. The cross-sectional study design was effective in achieving our study aim of generating a reference dataset of strength and flexibility across the lifespan; however, the direction of some of the cause and effect relationships can only be identified in longitudinal studies that track the changes in these measures over time. Ankle plantarflexion strength in healthy adolescents and adults can only ever be estimated with hand-held dynamometry due to the very high force capability (often exceeding 1,000 N).30 The reported reference values for ankle plantarflexors are likely to underestimate the force capabilities of this muscle group, and values should be used as a lower threshold for weakness in patients with neuromuscular and other neurologic disorders. Finally, the strength and flexibility reference values are specific to the Citec hand-held dynamometer and Baseline goniometer and inclinometer and may not be interchangeable with data obtained from other devices.
The normative reference data generated from this study can be used to determine the presence and extent of impairments associated with neuromuscular disorders and to monitor disease progression over time. The reference values and associated age- and sex-matched z scores can be used to develop outcome measures with enhanced precision and responsiveness to be used in clinical trials for neuromuscular and other neurologic disorders.
Supplementary Material
ACKNOWLEDGMENT
The authors thank John Eisenhuth for technical assistance, Ray Patton for calibration of the hand-held dynamometer, and the 1,000 volunteers.
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: 1000 Norms Project Consortium, Jennifer Baldwin, Marnee McKay, Paulo Ferreira, Elizabeth Nightingale, Fereshteh Pourkazemi, Amy Sman, Claire Hiller, Seyed Mousavi, Leslie Nicholson, Kristy Rose, Angus Chard, Martin Mackey, Niamh Moloney, Jacqueline Raymond, Joshua Burns, Alycia Fong Yan, Markus Hübscher, Milena Simic, Kathryn Refshauge, Natalie Vanicek, Caleb Wegener, Fiona Lee, Kathryn North, and Kate Quinlan
AUTHOR CONTRIBUTIONS
Marnee J. McKay: study design, data collection, analysis and interpretation, drafting and revising the manuscript. Jennifer N. Baldwin: study design and data collection. Paulo Ferreira: study design and revising the manuscript. Milena Simic: study design and revising the manuscript. Natalie Vanicek: study design and revising the manuscript. Joshua Burns: study conceptualization and design, data interpretation, drafting and revising the manuscript.
STUDY FUNDING
Study funded by National Health and Medical Research Council of Australia Centre for Research Excellence in Neuromuscular Disorders (NHMRC 1031893) and the Australian Podiatry Education and Research Foundation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lunn MP, Van den Bergh PY. Outcome measures in neuromuscular disease: is the world still flat? J Peripher Nerv Syst 2015;20:255–259. [DOI] [PubMed] [Google Scholar]
- 2.Mercuri E, Messina S, Pane M, Bertini E. Current methodological issues in the study of children with inherited neuromuscular disorders. Dev Med Child Neurol 2008;50:417–421. [DOI] [PubMed] [Google Scholar]
- 3.Roach KE, Miles TP. Normal hip and knee active range of motion: the relationship to age. Phys Ther 1991;71:656–665. [DOI] [PubMed] [Google Scholar]
- 4.Hallaceli H, Uruc V, Uysal HH, et al. Normal hip, knee and ankle range of motion in the Turkish population. Acta Orthop Traumatol Turc 2014;48:37–42. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta P, De S, Pal A, Maity P, Banerjee M, Dhara PC. Variation of range of joint motion in Bengalee (Indian) healthy adult subjects. J Life Sci 2012;4:123–133. [Google Scholar]
- 6.Boone DC, Azen SP. Normal range of motion of joints in male subjects. J Bone Joint Surg Am 1979;61:756–759. [PubMed] [Google Scholar]
- 7.Gunal I, Kose N, Erdogan O, Gokturk E, Seber S. Normal range of motion of the joints of the upper extremity in male subjects, with special reference to side. J Bone Joint Surg Am 1996;78:1401–1404. [DOI] [PubMed] [Google Scholar]
- 8.Backman E, Odenrick P, Hendriksson KG, Ledin T. Isometric muscle force and anthropometric values in normal children aged between 3.5 and 15 years. Scand J Rehab Med 1989;21:105–114. [PubMed] [Google Scholar]
- 9.Beenakker E, Van der Hoeven J, Fock J, Maurits N. Reference values of maximum isometric muscle force obtained in 270 children aged 4–16 years by hand-held dynamometry. Neuromuscul Disord 2001;11:441–446. [DOI] [PubMed] [Google Scholar]
- 10.Eek MN, Kroksmark A-K, Beckung E. Isometric muscle torque in children 5 to 15 years of age: normative data. Arch Phys Med Rehabil 2006;87:1091–1099. [DOI] [PubMed] [Google Scholar]
- 11.Hébert LJ, Maltais DB, Lepage C, Saulnier J, Crête M. Hand-held dynamometry isometric torque reference values for children and adolescents. Pediatr Phys Ther 2015;27:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther 1996;76:248–259. [DOI] [PubMed] [Google Scholar]
- 13.Bäckman E, Johansson V, Häger B, Sjöblom P, Henriksson K. Isometric muscle strength and muscular endurance in normal persons aged between 17 and 70 years. Scand J Rehab Med 1995;27:109–117. [PubMed] [Google Scholar]
- 14.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil 1997;78:26–32. [DOI] [PubMed] [Google Scholar]
- 15.Meldrum D, Cahalane E, Conroy R, Fitzgerald D, Hardiman O. Maximum voluntary isometric contraction: reference values and clinical application. Amyotroph Lateral Scler 2007;8:47–55. [DOI] [PubMed] [Google Scholar]
- 16.Van der Ploeg R, Fidler V, Oosterhuis H. Hand-held myometry: reference values. J Neurol Neurosurg Psychiatry 1991;54:244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danneskiold-Samsøe B, Bartels E, Bülow P, et al. Isokinetic and isometric muscle strength in a healthy population with special reference to age and gender. Acta Physiol 2009;197:1–68. [DOI] [PubMed] [Google Scholar]
- 18.Hogrel J-Y, Payan CA, Ollivier G, et al. Development of a French isometric strength normative database for adults using quantitative muscle testing. Arch Phys Med Rehabil 2007;88:1289–1297. [DOI] [PubMed] [Google Scholar]
- 19.The National Isometric Muscle Strength (NIMS) Database Consortium. Muscular weakness assessment: use of normal isometric strength data. Arch Phys Med Rehabil 1996;77:1251–1255. [DOI] [PubMed] [Google Scholar]
- 20.McKay MJ, Baldwin JN, Ferreira P, et al. 1000 Norms Project: protocol of a cross-sectional study cataloguing human variation. Physiotherapy 2016;102:50–56. [DOI] [PubMed] [Google Scholar]
- 21.Redmond AC, Crosbie J, Ouvrier RA. Development and validation of a novel rating system for scoring standing foot posture: the Foot Posture Index. Clin Biomech 2006;21:89–98. [DOI] [PubMed] [Google Scholar]
- 22.Winter DA. Biomechanics and Motor Control of Human Movement. New York: John Wiley & Sons; 2009. [Google Scholar]
- 23.Hogrel JY, Decostre V, Alberti C, et al. Stature is an essential predictor of muscle strength in children. BMC Musculoskel Disord 2012;13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macfarlane TS, Larson CA, Stiller C. Lower extremity muscle strength in 6-to 8-year-old children using hand-held dynamometry. Pediatr Phys Ther 2008;20:128–136. [DOI] [PubMed] [Google Scholar]
- 25.Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Care Res 2009;61:840–849. [DOI] [PubMed] [Google Scholar]
- 26.Scott A, Zwerver J, Grewal N, et al. Lipids, adiposity and tendinopathy: is there a mechanistic link? Crit Rev Br J Sports Med 2015;49:984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shefner JM, Liu D, Leitner ML, et al. Quantitative strength testing in ALS clinical trials. Neurology 2016:87:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirschner J, Schessl J, Schara U, et al. Treatment of Duchenne muscular dystrophy with cyclosporine A: a randomised, double-blind, placebo-controlled multicentre trial. Lancet Neurol 2010;9:1053–1059. [DOI] [PubMed] [Google Scholar]
- 29.Cornett KM, Menezes MP, Bray P, et al. Phenotypic variability of childhood Charcot-Marie-Tooth disease. JAMA Neurol 2016;73:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelln BM, McKeon PO, Gontkof LM, Hertel J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil 2008;17:160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.