Abstract
Objective:
Prophylactic medications can be a source of preventable harm, potentially affecting large numbers of patients. Few data exist about how clinicians change prescribing practices in response to new data and revisions to guidelines about preventable harm from a prophylactic medication. We sought to determine the changes in prescribing practice of seizure medications for patients with intracerebral hemorrhage (ICH) across a metropolitan area before and after new outcomes data and revised prescribing guidelines were published.
Methods:
We conducted an observational study using electronic medical record data from 4 academic medical centers in a large US metropolitan area.
Results:
A total of 3,422 patients with ICH, diagnosed between 2007 and 2012, were included. In 2009, after a publication found an association of phenytoin with higher odds of dependence or death, the use of phenytoin declined from 9.6% in 2009 to 2.2% in 2012 (p < 0.00001). Conversely, the use of levetiracetam more than doubled, from 15.1% in 2007 to 35% in 2012 (p < 0.00001). Use of levetiracetam varied among the 4 institutions from 6.7% to 29.8% (p < 0.00001).
Conclusions:
New data that led to revised prescribing guidelines for prophylactic seizure medications for patients with ICH were temporally associated with a significant decrease in use of the medication, potentially reducing adverse outcomes. However, a corresponding increase in the use of an alternative medication, levetiracetam, occurred despite limited knowledge about its potential effects on outcomes. Future guideline changes should anticipate and address alternatives.
For many conditions without curative treatment, avoiding unintended harm from medications is one of the few potential avenues to maximize outcomes while new curative therapies are in development. However, if a medication has unintended harmful effects, there could be a significant public health benefit to reducing its use. With the advent of large clinical databases, such as registries, and access to more comprehensive outcomes data, new knowledge about previously unrecognized harmful effects of medications can be generated more easily. Whether and how this new knowledge influences prescribing practices is not clear.
Intracerebral hemorrhage (ICH) is the most deadly form of stroke and has no Food and Drug Administration (FDA)–approved treatment. Seizures are a common occurrence in patients with ICH, affecting up to 16% of patients.1 When seizures occur, they increase the risk of midline shift and poor outcome,2 suggesting that the prevention of seizures is a worthwhile goal. While the use of prophylactic seizure medication was supported by guidelines issued in 19993 and 2007,4 subsequent data suggested that early seizures were less common3 and prophylactic treatment might have unintended consequences. In 2009, a single-center registry study found that phenytoin (a sodium blocker approved by the FDA in 1953), but not levetiracetam (a more recently approved medication for partial seizures), was associated with more fever and worse functional outcomes at 3 months in patients with ICH compared with no prophylactic seizure medication.5 A subsequent article described a post hoc analysis of an industry-sponsored clinical trial that associated seizure medication in general with worse outcomes.6 Citing these findings, the American Heart Association guidelines were updated in 2010 to state that “prophylactic seizure medications should not be used” as a new recommendation.7
The greater Chicago area offers an opportunity to assess the potential effect of new data and revised prescribing guidelines for several reasons: (1) presence of several large health care institutions; (2) independent clinical practices at each institution with established teams in neurology, neurosurgery, and neurocritical care; and (3) diverse patient populations (e.g., race/ethnicity, socioeconomic and health insurance status). While prescribing differences within a practice in a single center are difficult to assess because of small sample sizes and potential lack of generalizability, differences across multiple, independent, major health care institution practices can be determined.
We tested the hypothesis that the use of prophylactic seizure medications has changed over time across multiple major health care institutions in a large US metropolitan area following the publication of new data and revised prescribing guidelines. Further, we tested the hypothesis that there are institutional differences in the use of seizure medications.
METHODS
We retrieved data from the Chicago HealthLNK Data Repository (HealthLNK), a health data exchange consisting of merged and de-duplicated patient electronic health records from 7 major health care institutions in the Chicago area. HealthLNK includes demographic and clinical visit data from 5 major academic medical centers (Loyola University Medical Center, Northwestern Medicine, Rush University Medical Center, University of Chicago Medical Center, and University of Illinois Hospital and Health Sciences System), a large county health care system (Cook County Health and Hospital Systems), and a network of outpatient community health centers (Alliance of Chicago). Unique patient IDs are created for patients in HealthLNK from patient demographic data, using a Health Insurance Portability and Accountability Act–compliant Secure Hash Algorithm 512 hashing algorithm, which allows the merging of patient data across sites without sharing protected health information. De-duplication of patient records allows for a more accurate count for patient cohorts. Merging data from multiple sites creates a more complete overall record of patient care and accounts for diagnoses and procedures received at more than one institution.
We identified patients with ICD-9 diagnosis for ICH (431), excluding traumatic brain injury, which might have an intracranial hematoma as a complication rather than the primary diagnosis.
Medical record data from 2007 through 2012 (the most recent data available) for diagnoses and administered medications were available from 4 institutions: Loyola University Medical Center (western suburbs), Rush University Medical Center (west of downtown), University of Chicago Medicine (southern Chicago), and Northwestern Medicine (north of downtown). Demographic data included age, sex, and race/ethnicity; patients cannot be re-identified by end users of the data and presentation of data that could reasonably lead to re-identification of the practices of individual reporting sites is prohibited.
We analyzed use of seizure medications during the calendar month of ICH, since administration in later months would be less likely to be intended for seizure prophylaxis because seizures typically occur within the first few days of ICH onset. The 1999 and 2007 guidelines underscored the use of prophylactic seizure medications within a month of ICH onset.
To determine rates of craniotomy over time, we used the same methodology employed in a previous study using a prospective registry, the Northwestern University Brain Attack Registry (NUBAR).8
Normally distributed data (e.g., age) were compared between groups with analysis of variance, while categorical data (e.g., use of levetiracetam, institution) were compared using χ2. We performed logistic regression for the probability of receiving levetiracetam, controlling for institution, year, and their interaction. Statistical analyses were calculated with standard commercial software (NCSS 9; NCSS Inc., Kaysville, UT).
RESULTS
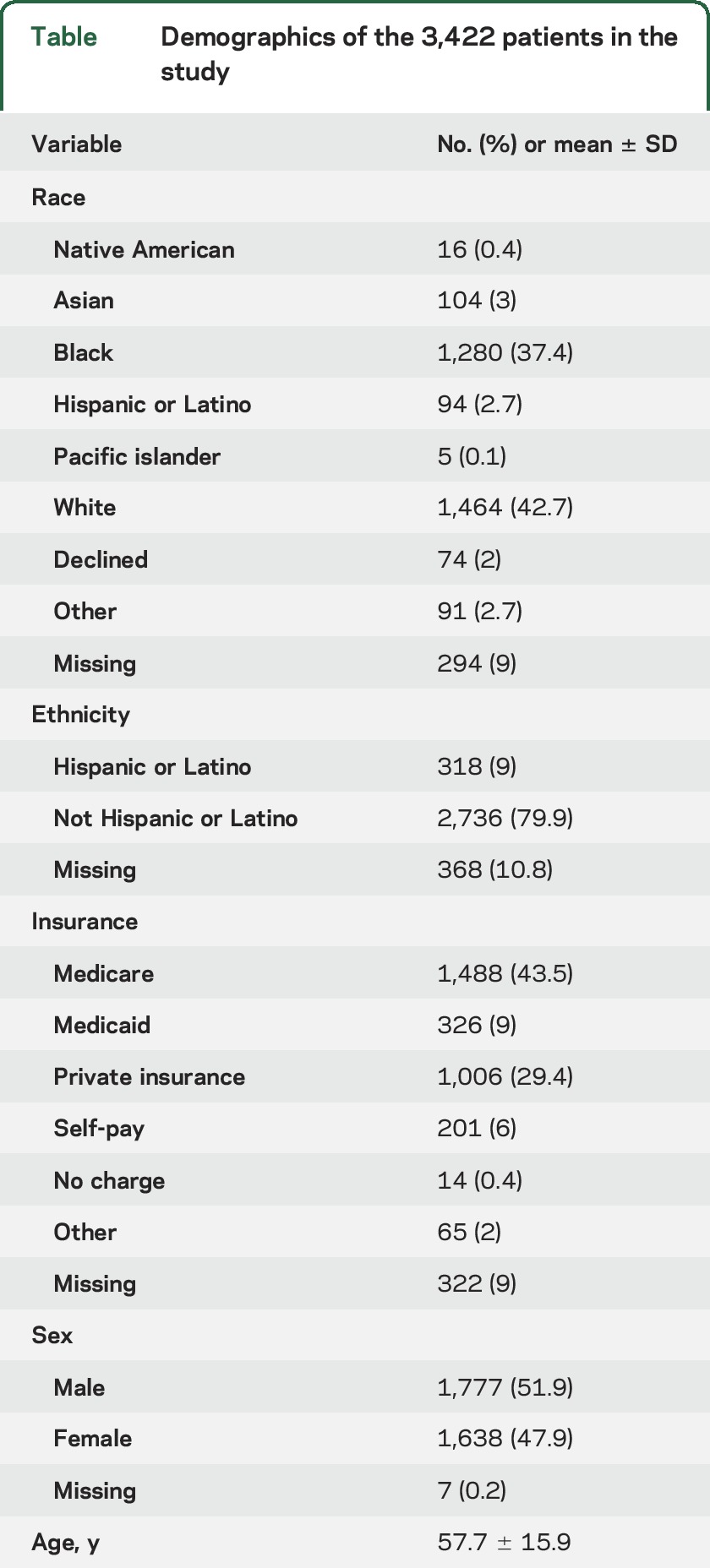
The cohort included 3,422 patients (table). The sample was ethnically diverse (nearly 40% African American, 10% of Hispanic or Latino origin), and patients were covered by a variety of insurance payers. Patients were unevenly distributed among the 4 institutions. Institution 5 admitted 1,202 (35.1%) patients with ICH; institution 7 admitted 1,014 (29.6%); institution 4 admitted 725 (21.2%); and institution 2 admitted 481 (14.1%).
Table.
Demographics of the 3,422 patients in the study
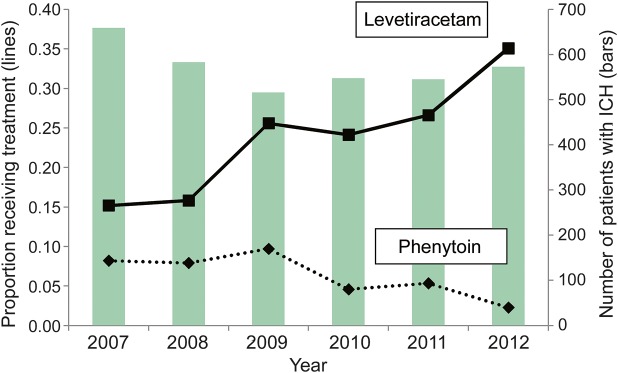
During the study period, the number of patients admitted with ICH remained stable from year to year, but the percentage of patients treated with seizure medications changed (figure 1). Levetiracetam (n = 922, 26.9%) and phenytoin (n = 479, 13.9%) were the most commonly prescribed seizure medications, with few patients receiving valproate (43, 1.2%), carbamazepine (20, 0.5%) or lacosamide (18, 0.5%).The use of phenytoin declined from 9.6% in 2009 to 2.2% in 2012 (p < 0.00001); there was no difference in the rate of phenytoin use between institutions (p = 0.1).
Figure 1. Change in seizure medication use over time.
Percentage of patients who received phenytoin (dashed line) and levetiracetam (solid line) per year over 4 institutions are shown (left-sided axis). While stable in 2007 and 2008, from 2009 onwards the percentage of patients receiving levetiracetam (solid line) more than doubled, while the percentage of patients receiving phenytoin (dashed line) declined. The number of patients with intracerebral hemorrhage (ICH) remained stable (vertical bars, right-sided axis).
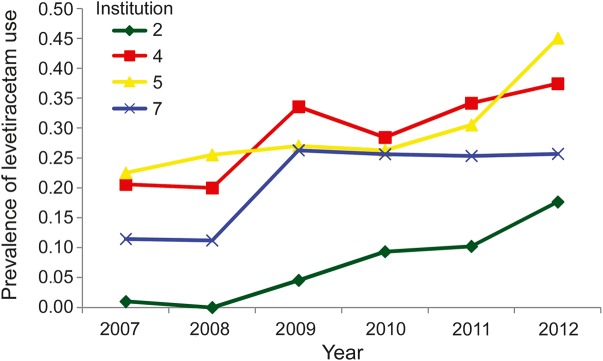
The use of levetiracetam, in contrast to the use of phenytoin, more than doubled from 15.1% in 2007 to 35% of patients in 2012 (p < 0.00001). Levetiracetam use varied considerably between institutions (ranging from 6.7% at institution 2 to 29.8% at institution 5, p < 0.00001), as shown in figure 2. The probability of receiving levetiracetam was different from year to year (p < 0.00001), and there was a significant year by institution interaction (p = 0.006), indicating that the probability of receiving levetiracetam in any given year varied with the institution. All institutions increased their use of levetiracetam during the study period. Overall, the use of either phenytoin or levetiracetam increased from a baseline of 23% in 2007 and 2008 to 35% in 2009, 28% in 2010, 32% in 2011, and 37% in 2012.
Figure 2. Institutional practice variability was prominent.
The prevalence of levetiracetam use increased at each of the 4 institutions in the city during the study period, but varied with both the year and the institution (interaction p = 0.006).
We considered that an increase in the rates of craniotomy might have prompted an increase in the use of levetiracetam. We examined the rates of craniotomy over time in NUBAR because procedure codes are not available in HealthLNK, and found that rates of craniotomy, in fact, progressively decreased over time, from 23% in 2007 to 4% in 2012.
DISCUSSION
We found that the use of phenytoin, a traditional prophylactic seizure medication, was attenuated across multiple institutions in a large metropolitan area shortly after the publication of data from a single center associating it with more fever and greater odds of dependence,5 followed by revised prescribing guidelines. This rapid change in practice suggests that clinicians acknowledged the findings that phenytoin was associated with worse outcomes and largely discontinued its use in patients with ICH at the 4 institutions included in this study. However, use of an alternative seizure medication, which was also not recommended, more than doubled over the same time period, despite revised guidelines stating that prophylactic seizure medications should not be used.7 At this time, there were no data suggesting any association between the alternative medication, levetiracetam, and worse outcomes, although the sample sizes analyzed were small.5,9 Clinicians may respond to new data and revised guidelines in unexpected ways, such as narrowly interpreting the findings, which suggests that revised recommendations should anticipate potential interpretations other than literal adherence.
Clinicians generally respond to new data. For example, the results of the Women's Health Initiative,10 which revealed the cardiovascular risks of hormone replacement therapy in postmenopausal women, led to widespread abandonment of the practice, although there are no data about whether clinicians have switched to prescribing alternative medications perceived to present lower risks. Analyses of other large datasets confirm that clinicians respond to recommendations, such as Get With The Guidelines for cerebrovascular disease,11,12 and have improved compliance with recommended care for patients with stroke including aspirin use and poststroke care. However, seizure medications have not been subjected to randomized controlled trials in patients with ICH, the level of evidence is lower, and there was no widespread outreach to improve guideline adherence.
HealthLNK includes robust patient-level medication use data, but does not include mortality or outcomes such as dependence or health-related quality of life. Although it would have been preferable to limit the cohort to patients surviving at least several days13 or a month, such data would be unlikely to change the overall findings of an increase in levetiracetam and decrease in phenytoin use. Seizure medications have not been independently linked to mortality in patients with ICH, making survival data somewhat less relevant to the results. More detail on the dosing and duration of seizure medication (e.g., a single dose, a few days, or 2 weeks of treatment) would also have been desirable to further specify prescribing practices, although this information would not likely change the main results substantially. We could not examine the use of diagnostic tests to uncover subclinical seizures, although this would be unlikely to explain the change in type of seizure medications during the study period and the overall proportion of patients receiving seizure medications did not change. Indeed, one of the institutions reported a low rate of subclinical seizures in 2009,5 when levetiracetam use was increasing, suggesting that a fear of subclinical seizures is unlikely to account for the increase in levetiracetam use. Heightened concern for subclinical seizures would also not account for a decrease in the use of phenytoin.
HealthLNK identifies patients by ICD-9 codes, while ICD-10 became required in fall 2015. Future ICD-10 data may have greater specificity in terms of the diagnosis and may provide an opportunity to specify whether the hematoma is cortical (ICD-10 code I61.1) rather than deep (I61.0), a subgroup that might be more likely to be benefit from prophylactic seizure medications.
The Ethnic/Racial Variations of ICH (ERICH) study of 744 patients broadly underscores our findings regarding levetiracetam use.14 Between October 2010 and 2012, prophylactic seizure medications were used in 289 (39%) patients with ICH, with 89% of these receiving levetiracetam. Levetiracetam was associated with dependence or death in univariate, but not multivariate, analysis. Data prior to 2010 were not presented, limiting the ability to detect any change in prescribing practice. These data show that phenytoin use had already declined by 2010, in line with the ERICH results.
The potential etiology of variation in practice between institutions is not clear from these data. The ERICH study found that seizure medication use was not associated with race or ethnicity,14 drawing more attention to local practice variation between institutions. Therefore, further research should seek to determine the role of local variation on prescribing practice and outcomes. We found that the rates of craniotomy at one institution decreased, perhaps in response to the lack of benefit in equivocal cases in the Surgical Trial in Intracerebral Haemorrhage trial,15 so an increase in the rate of craniotomy elsewhere is unlikely to account for an increase in the use of levetiracetam. It is possible that referring institutions, not captured in HealthLNK, increased their use of levetiracetam, thus prompting continued use in the study institutions; however, the HealthLNK institutions all had well-established neurocritical care and neurosurgery teams that review all medications and treatment plans on admission throughout the study period, so it seems unlikely that the change would have been predominantly externally driven.
The data are somewhat limited because we could not specify which patients received prophylactic seizure medications exclusively; however, the study only includes seizure medications prescribed during the calendar month of ICH, which are most likely to be prophylactic and were encouraged by previous guidelines. Late seizures occur in approximately 10% of survivors, often presaged by early seizures,16 so most seizure medication use would be considered to be prophylactic. The ease of use of levetiracetam including a rounded number for standard use, IV or oral administration, relatively low side effect profile, and absence of laboratory monitoring are likely to have contributed to its wider use.
Medications that inadvertently increase complications might also increase the costs of care. While these data do not contain costs, other administrative databases, such as the Agency for Healthcare Research and Quality's Healthcare Cost and Utilization Project, might be of interest for future research.
We found that new data and revised prescribing guidelines on the side effects of phenytoin, a widely prescribed medication in patients with ICH, led to a decrease in its use, sparing patients from the potential adverse effects of treatment. However, a corresponding increase was noted in the prescribing of an alternative medication that was also not recommended, although not independently associated with worse outcomes. Clinicians appear to be hesitant to abandon a longstanding clinical practice, such as the use of prophylactic seizure medication in ICH. These findings suggest that new data and revised guidelines may narrowly alter clinical practice, but that literal adherence may not occur for potentially rational reasons. Future guideline revisions should anticipate that clinicians may seek to prescribe an alternative medication and proactively address this type of response, as well any potential effect of levetiracetam on outcomes in patients with ICH.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the centers that contributed medication data to HealthLNK, Loyola University Medical Center, Northwestern Medicine, William Galanter, MD, at University of Illinois Medical Center, Bala Hota, MD, at Rush University Medical Center, and David Meltzer MD, PhD, at the University of Chicago Medical Center.
GLOSSARY
- ERICH
Ethnic/Racial Variations of ICH
- FDA
Food and Drug Administration
- ICD-9
International Classification of Diseases–9
- ICD-10
International Classification of Diseases–10
- ICH
intracerebral hemorrhage
- NUBAR
Northwestern University Brain Attack Registry
Footnotes
Editorial, page 15
AUTHOR CONTRIBUTIONS
Andrew Naidech: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis, obtaining funding. Jennifer Beaumont: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Babak Jahromi: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Shyam Prabhakaran: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval. Abel N. Kho: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Jane L. Holl: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision.
STUDY FUNDING
This project was supported by grant K18HS023437 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.De Herdt V, Dumont F, Henon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology 2011;77:1794–1800. [DOI] [PubMed] [Google Scholar]
- 2.Vespa PM, O'Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003;60:1441–1446. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Adams HP, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1999;30:905–915. [DOI] [PubMed] [Google Scholar]
- 4.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:2001–2023. [DOI] [PubMed] [Google Scholar]
- 5.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke 2009;40:3810–3815. [DOI] [PubMed] [Google Scholar]
- 6.Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care 2009;11:38–44. [DOI] [PubMed] [Google Scholar]
- 7.Morgenstern LB, Hemphill JC III, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology 2013;81:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care 2010;12:165–172. [DOI] [PubMed] [Google Scholar]
- 10.Writing Group for the Women's Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–1640. [DOI] [PubMed] [Google Scholar]
- 12.Ellrodt AG, Fonarow GC, Schwamm LH, et al. Synthesizing lessons learned from get with the guidelines: the value of disease-based registries in improving quality and outcomes. Circulation 2013;128:2447–2460. [DOI] [PubMed] [Google Scholar]
- 13.Battey TW, Falcone GJ, Ayres AM, et al. Confounding by indication in retrospective studies of intracerebral hemorrhage: antiepileptic treatment and mortality. Neurocrit Care 2012;17:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheth KN, Martini SR, Moomaw CJ, et al. Prophylactic antiepileptic drug use and outcome in the ethnic/racial variations of intracerebral hemorrhage study. Stroke 2015;46:3532–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387–397. [DOI] [PubMed] [Google Scholar]
- 16.Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014;45:1971–1976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.