Abstract
Purpose
The increasing demand for esthetically pleasing results has contributed to the use of ceramics for dental implant abutments. The aim of this study was to compare the biological response of epithelial tissue cultivated on lithium disilicate (LS2) and zirconium oxide (ZrO2) ceramics. Understanding the relevant physicochemical and mechanical properties of these ceramics will help identify the optimal material for facilitating gingival wound closure.
Methods
Both biomaterials were prepared with 2 different surface treatments: raw and polished. Their physicochemical characteristics were analyzed by contact angle measurements, scanning white-light interferometry, and scanning electron microscopy. An organotypic culture was then performed using a chicken epithelium model to simulate peri-implant soft tissue. We measured the contact angle, hydrophobicity, and roughness of the materials as well as the tissue behavior at their surfaces (cell migration and cell adhesion).
Results
The best cell migration was observed on ZrO2 ceramic. Cell adhesion was also drastically lower on the polished ZrO2 ceramic than on both the raw and polished LS2. Evaluating various surface topographies of LS2 showed that increasing surface roughness improved cell adhesion, leading to an increase of up to 13%.
Conclusions
Our results demonstrate that a biomaterial, here LS2, can be modified using simple surface changes in order to finely modulate soft tissue adhesion. Strong adhesion at the abutment associated with weak migration assists in gingival wound healing. On the same material, polishing can reduce cell adhesion without drastically modifying cell migration. A comparison of LS2 and ZrO2 ceramic showed that LS2 was more conducive to creating varying tissue reactions. Our results can help dental surgeons to choose, especially for esthetic implant abutments, the most appropriate biomaterial as well as the most appropriate surface treatment to use in accordance with specific clinical dental applications.
Keywords: Ceramics, Dental abutments, Dental esthetics, Embryo culture techniques
Graphical Abstract
INTRODUCTION
Ceramics used in medical fields have evolved rapidly over the last 20 years. In particular, dental glass ceramics have been developed because they have advantageous physicochemical and cosmetic properties [1]. Ceramics therefore may replace metallic materials in the oral cavity, maintaining the same high quality level in dental rehabilitation [2].
Introduced to dental markets over a decade ago, all-ceramic systems covered a wide range of indications, from thin veneers used in single-tooth restorations to crowns and wide-span bridges [3]. Titanium abutments were a standard option for dental implant-supported restorations. Due to potential recession of the soft tissue margin in oral implants [4] and coloration of the gingiva [5], the all-ceramic system helps maintain the esthetics of the implant, as demonstrated by the widespread current use of this system [6]. Ceramic abutments, fabricated from yttrium stabilized-zirconium oxide (ZrO2), have been developed for their color, which is similar to that of teeth, high loading strength, tissue tolerability, and intrasulcular design enhancement [7]. Transformation toughening of ZrO2 results in extremely high component stability and extraordinary bending and tensile strength, as well as fracture and chemical resistance [8,9]. These properties allow ZrO2 to self-repair micro-crack initiation by stopping crack propagation. ZrO2 is considered to be a highly biocompatible material, and has already been used in medical applications such as artificial hip joints.
As a result of patient demand, veneers and crowns are currently available in ZrO2 or, recently, in lithium disilicate (LS2) ceramic [1,2]; they can be used with the press technique as well as computer-assisted design and manufacturing technology [10]. In this way, dental zirconia has been used daily by dental practitioners (e.g., IPS e.max® ZirCAD, Ivoclar Vivadent SAS, Saint-Jorioz, France) which is an yttrium-stabilized ZrO2. Its biological properties are very satisfactory and clinical results have proven its suitability for dental applications. Featuring a flexural strength of 900 MPa, more than double that of glass ceramic (e.g., IPS e.max® Press or CAD, Ivoclar Vivadent SAS), this biomaterial can be used for almost all applications for which metal has exclusively been used up to now, especially for the posterior teeth, long bridges, and implant armatures [8]. Nevertheless, dental zirconia ceramics are considered less esthetic than glass ceramics due to their white shade and high opacity. This is the fundamental problem for dental implant abutments; however, LS2 ceramic has a similar appearance to natural teeth and could therefore improve the esthetic outcomes. The esthetic characterization of implant abutments and its ceramic restorations involves the reflection and transmission of light. IPS e.max® LS2 is a high-strength ceramic material with 360–400 MPa of flexural strength. Moreover, this biomaterial was recently reported to be one of the most robust and durable all-ceramic systems tested to date [8].
In order to be a viable and superior treatment choice, ceramic restorations must be cosmetically and functionally appropriate. The surface and biological requirements in dental implantology are as follow: (1) no cell adhesion or proliferation on the esthetic crown for easy cleaning; (2) cell adhesion and proliferation around the esthetic abutment for a tight junction; and (3) strong cell adhesion under the epithelia-conjunctive junction to preserve the dental implant from buccal bacteria for an extended period.
The aim of this in vitro study was to compare 2 polished biomaterials for cytocompatibility and biological response. We used a combination of contact angle assessments and interferometry measurements to determine surface wettability. An organotypic culture of chicken epithelium was then performed.
MATERIALS AND METHODS
Two types of biomaterials were used (IPS e.max® Press, IPS e.max® ZirCAD, Ivoclar Vivadent SAS). The first was an LS2 ceramic (IPS e.max® Press, Ivoclar Vivadent SAS) composed of silicon dioxide (SiO2) (57%–80%), lithium oxide (Li2O) (11%–19%), potassium superoxide (KO2) (0%–13%), phosphorus pentoxide (P2O5) (0%–11%), ZrO2 (0%–8%), zinc oxide (ZnO) (0%–8%), and other oxides and pigments (0%–10%). The second one was a dental yttrium-stabilized ZrO2 (IPS e.max® ZirCAD, Ivoclar Vivadent SAS) composed of ZrO2 (87%–95%), hafnium oxide (HfO2) (1%–5%), aluminium oxide (Al2O3) (0%–1%), yttrium oxide (Y2O3) (4%–6%), and other oxides and pigments (0%–8%). The control samples used Thermanox® (Thx), a cell culture-treated plastic (Nunc® batch #628934) showing excellent cell migration properties associated with low cell adhesion. Thx is a very smooth material that is traditionally used for cell culture control samples.
Sample preparation
LS2 ceramic samples
Square samples (1.4×1.4×0.1 cm) were separately obtained by the loose-wax technique and completely fritted. All samples were cleaned by an acid (Invex®, Ivoclar Vivadent SAS) and sanded in house with glass-bead blasting before use (raw-LD). Then, a surface treatment was performed using manual polishing. The mechanical polishing was done by the same operator with the Optrafine® pack (batch #NL1757, Ivoclar Vivadent SAS). A manual dental piece was used without water spray: 15 seconds with a large drill (DC 83103040, Komet, Paris, France) at 30,000 rpm, 15 seconds with a dark-blue diamond polisher in cup shape at 7,000 rpm (polisher P), 15 seconds with a light-blue diamond finisher in cup shape at 7,000 rpm (finisher F), and 15 seconds with a small brush accompanied by polishing paste (Optrafine® HP, lot JL1606, Ivoclar Vivadent SAS) at 7,000 rpm (pol-LD).
Eighty square samples were set up: 40 samples without a surface treatment (raw-LD) and 40 samples with polishing (pol-LD).
ZrO2 ceramic samples
The zirconia blocks (size MO0 B85 L-22; batch #P01949; enlargement factor, 1.228) were cut into plates (1.5×1.5×0.2 cm) using a rotating diamond saw before sintering. The plates were then ground on the surface using silicon carbure particles and water. The samples were dried afterward at 80°C–120°C for 2 hours using a drying furnace. They were then fired in a Programat S1 at 1,500°C (IPS e.max® ZirCAD program, Ivoclar Vivadent SAS) with a holding time of 30 minutes at the maximum temperature. The samples were completely sintered before use (raw-Zr). In order to achieve a reasonable polishing for flat surfaces using dental instruments, polishing was carried out after the final densification in the S1 using a Struers TegraPol-35 grinding/polishing unit (300 rpm). After sintering, apex diamond grinding discs were applied (20-, 6-, and 0.5-µm diamond wheels). Eighty square samples were also prepared, including 40 raw samples (raw-Zr) and 40 polished samples (pol-Zr).
Physicochemical surface characterizations
Contact angle measurements
Contact angles of water droplets were determined at ambient temperature (21°C–24°C) with a drop shape analysis apparatus (DSA-10, Krüss GmbH, Hamburg, Germany). The measuring system recorded the drop shape by a charge-coupled device (CCD) camera and determined the contact angles using image analysis software, taking into account the entire drop shape [11].
Six water droplets were deposited on each surface, and the values of the contact angle (°) represent the averages of 12 contact angle measurements (6 on the right side and 6 on the left side).
Scanning white-light interferometry
A Zygo® NewView 200 (Zygo Corporation, Middlefield, CT, USA) apparatus was used with frequency domain analysis to generate quantitative 3-dimensional images of surfaces [12]; measurements were made using a white-light filter based on a center wavelength of 600 nm, with a bandwidth of 125 nm. The interference patterns were recorded by a CCD camera, and each measurement contained 320×240 data points. Three objectives with magnifications of ×2.5, ×10, and ×50 were used, and 3 images were recorded for each magnification. The scan size and sampling interval were fixed by the magnification of the optical system. For the magnification of ×2.5, the sampling interval was 8.8 µm in both directions. The vertical resolution was lower than 1 nm, while lateral resolution of the microscope was limited by the aperture of the objective. This technique was used to determine the biomaterial surface roughness.
Scanning electron microscopy
Samples of tissues cultivated on the different materials were rinsed in phosphate-buffered saline (PBS), fixed in 3% glutaraldehyde in Rembaum buffer (pH 7.4) for 1 hour [13], dehydrated in a series of graded alcohols, critical-point dried using CO2 (Polaron Instrument Inc., Nottingham, UK), sputter-coated with gold (Polaron Instrument Inc.) and examined with a Philips scanning electron microscope (ESEM FEG XL 30, Philips, Amsterdam, The Netherlands).
Organotypic culture method
Before culture, the ceramic samples were ultrasonically cleaned in acetone for 10 minutes, then rinsed in distilled water (10 minutes), and autoclaved at 120°C for 20 minutes.
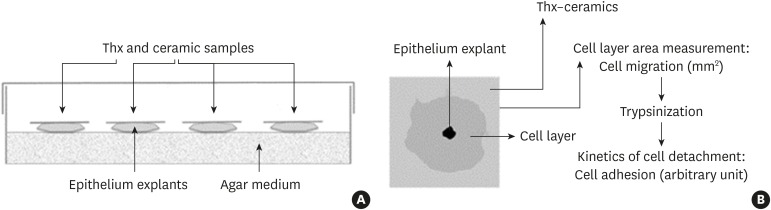
An organotypic culture method [14] was used to examine the cellular response to ceramic samples (Figure 1A). Culture dishes were pretreated with a nutrient medium consisting of 50% Bacto agar 1% (Difco™, Fisher, Illkirch-Graffenstaden, France) in Gey's solution [15], 38.5% DMEM (Gibco™, Invitrogen, Paris, France), 10% fetal calf serum (Gibco™, Invitrogen), 1% L-glutamine (Gibco™, Invitrogen), and 0.05% penicillin/streptomycin solution (Gibco™, Invitrogen). Skin was isolated from 7-day-old white leghorn chicken eggs and placed in sterile PBS. The samples were cut into 1-mm2 pieces and layered onto the bottom of a 70-cm2 petri dish (Dominique Dutscher, Issy-les-Moulineaux, France). Nine fragments were placed in each petri dish and a 2-cm2 piece of material was deposited on each of those fragments.
Figure 1.
(A) Organotypic culture description. (B) Method of measurement of cell migration and adhesion.
Thx, Thermanox®.
Each dish was used for only 1 material, and 4 dishes were prepared for each material. The cultures were incubated at 37°C with 5% CO2 for 7 days, after which the materials were removed and stained with neutral red. Next, the total surface area covered by tissue was measured using a stereomicroscope equipped with a camera and Image J software (National Institutes of Health, Bethesda, MD, USA) [16]. This area corresponded to the total surface of the cell layer minus the initial explant surface.
Additionally, the cells were detached from the materials using 0.025% trypsin-EDTA (Gibco™, Invitrogen) in Isoton® II electrolyte solution (Beckman Coulter, Villepinte, France).
The rate of cell detachment was determined by counting the detached cells with a Multisizer® (Beckman Coulter) after 5, 10, 20, 30, and 60 minutes. The rate of detachment as a function of time was used as a measure of adhesion strength, and we defined an arbitrary index (Figure 1B).
Statistical analysis
All data were represented as means±standard error of the mean. The data were compared using 1-way analysis of variance followed by the Tukey-Kramer multiple comparisons test (InStat, GraphPad software, La Jolla, CA, USA). P values <0.05 were considered to indicate statistical significance.
RESULTS
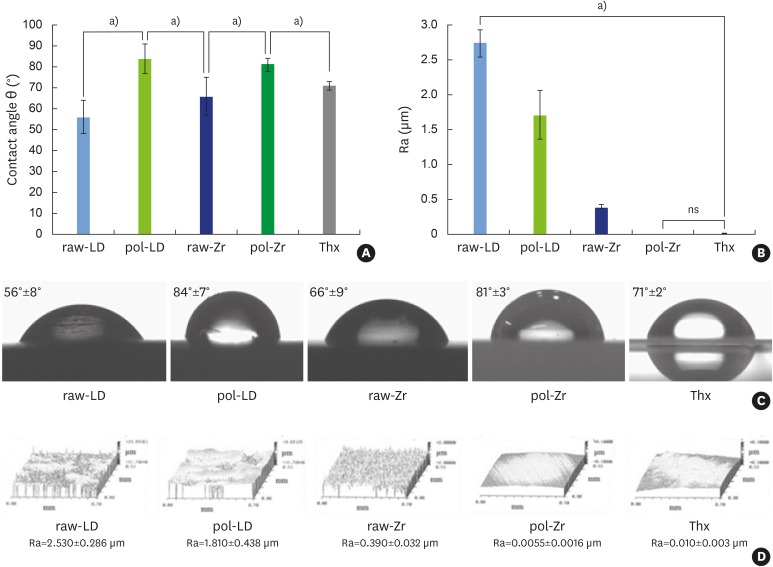
The contact angle measurements showed significantly different results between the controls and the ceramics (Figure 2A and 2C). The polishing treatment of the ceramics reduced their hydrophilicity, as illustrated by the contact angles of the water droplets, which increased from 56° to 84° for LS2 ceramic sample (LD) and from 66° to 81° for ZrO2 ceramic sample (Zr) following the treatment. This property is expected to influence protein adsorption and cell attachment.
Figure 2.
Physicochemical analyses of raw-LD, pol-LD, raw-Zr, and pol-Zr compared to Thx. (A) Contact angle θ (°); (B) Ra (μm); (C) images of water droplets; (D) interferometry microscopy images. Error bars indicate significant differences.
raw-Zr, raw ZrO2 ceramic sample; pol-Zr, polished ZrO2 ceramic sample; raw-LD, raw LS2 ceramic sample; pol-LD, polished LS2 ceramic sample; Thx, Thermanox®; ns, not significant; Ra, roughness.
a)Statistically significant differences (P<0.001).
The roughness of the ceramics compared to controls, as measured by scanning white-light interferometry, showed significantly different values between Thx and ceramics and between LD and Zr (Figure 2B and 2D). Pol-Zr had the same roughness as the control Thx. These results were correlated with the cell adhesion assessment. The decreasing values of roughness in raw-LD, pol-LD, raw-Zr, and pol-Zr were correlated with reduced cell adhesion.
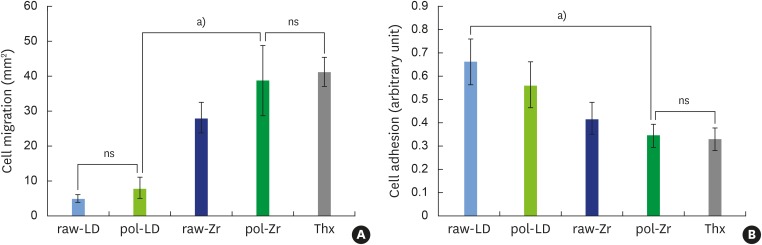
After 7 days in organotypic culture, the epithelial layers grown on the materials (Figure 3A) showed significant differences between the control and the ceramics, and between the ceramics themselves. LD did not enhance cell migration compared to Thx, while Zr significantly increased cell migration compared to LD.
Figure 3.
Organotypic culture on raw-LD, pol-LD, raw-Zr, and pol-Zr compared to Thx. (A) Cell migration (mm2); (B) cell adhesion (arbitrary unit). Error bars indicate significant differences.
raw-Zr, raw ZrO2 ceramic sample; pol-Zr, polished ZrO2 ceramic sample; raw-LD, raw LS2 ceramic sample; pol-LD, polished LS2 ceramic sample; Thx, Thermanox®; ns, not significant.
a)Statistically significant differences (P<0.001).
Interestingly, the cell adhesion assessment (Figure 3B) showed strong cell adhesion on the raw-LD but very weak cell adhesion on the pol-Zr and Thx (P<0.001). Zr (both polished and raw) showed significantly weaker cell adhesion than LD.
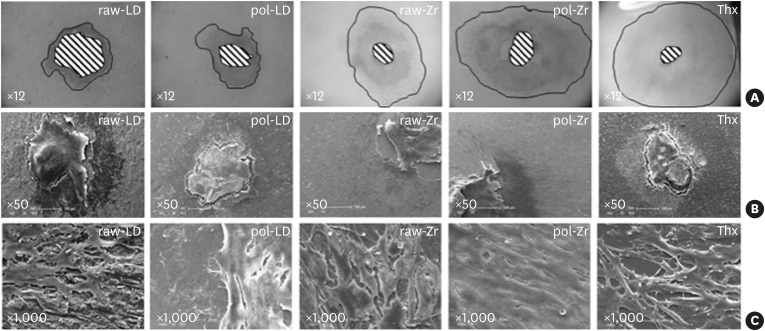
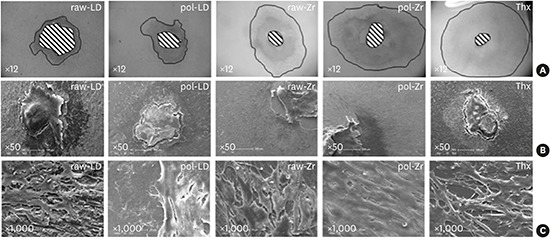
Samples observed by scanning electron microscopy (Figure 4) showed drastic differences in cell shape on the ceramics compared to Thx. A proportional increase of the cell layer area was observed in the raw-LD compared to Thx. On Thx and polished ceramics, which favored cell migration, the cells were elongated. Cells on the raw ceramics were cuboidal in shape, forming a squamous epithelium.
Figure 4.
Scanning results of raw-LD, pol-LD, raw-Zr, and pol-Zr compared to Thx. (A) White-light interferometry measuring the area with neutral red staining using imaging software (×12); and (B, C) scanning electron microscopy showing shapes and layers of cells (B, ×50; C, ×1,000).
raw-Zr, raw ZrO2 ceramic sample; pol-Zr, polished ZrO2 ceramic sample; raw-LD, raw LS2 ceramic sample; pol-LD, polished LS2 ceramic sample; Thx, Thermanox®.
DISCUSSION
Biocompatibility and chemical durability are highly important properties in dental materials. Zirconia ceramics have been reported not to have potential toxic or genotoxic effects [17,18,19] and to present satisfactory soft tissue responses [20]. Esthetic implant abutments must minimize or reduce plaque adhesion and possess light dynamic qualities comparable to natural teeth. The first objective is to allow healing around the crown and the gingiva, which requires significant roughness of the dental crown, leading to strong cell adhesion. The second objective is to avoid biofilm formation on the dental prosthesis, which involves creating surfaces that prevent both cell adhesion and protein adsorption. Dental ceramics must offer maximal mechanical stability, outstanding biocompatibility, and accelerated and dense peri-implant soft tissue attachment [21]. They are generally required to be low-adhesive materials to avoid biofilm formation on the teeth [22].
In accordance with the periodontal environment, our organotypic culture method was used in this study instead of classic cell culture because it approaches the complexity of living tissue. With this technique, the organ in contact with the biomaterial develops a tissue comparable to that of the human gingival epithelium [23].
It is well known that migration and adhesion are biological parameters that are not necessarily directly linked. Cells can migrate rapidly with very low adhesion, which is the case for the epithelial tongue during the new-epithelialization phase of wound healing. Our approach allowed us to study the behavior of cells in direct contact with biomaterials according to these 2 parameters [23,26].
It is now widely known that cell adhesion depends on the physicochemical properties of a biomaterial through protein adsorption and extracellular matrix constitution. First, the wettability properties, assessed by the contact angle measurements, influence protein adsorption onto a biomaterial [24]. A hydrophobic surface generally induces protein adsorption and then cell adhesion, while a hydrophilic surface often prevents any adsorption and subsequent cell adhesion. Second, the roughness of a biomaterial also drastically influences protein adsorption and the behavior of the cells [25]. In this study, the cell adhesion on the different ceramics was controlled by their roughness. The biological parameters assessed using our culture model have already been demonstrated to have relationships with cell migration, cell adhesion, and physicochemical properties in other biomaterials [26].
Finally, we confirmed in vitro that the LD dental ceramic (in this case IPS e.max® Press, Ivoclar Vivadent SAS) was not cytotoxic [27,28] and is a promising material for improving the esthetic outcomes of dental implants and for tightening the peri-implant junction [23] due to simple surface modifications making it possible to create 2 different types of interfaces on a single material. The abrasion provides micro-rough surfaces with strong wettability, which allows strong adhesion of epithelial tissues. This type of surface will be optimal for gingival adhesion around the dental abutment. In contrast, applying the glazing technique to the same material will provide a smooth surface with strong hydrophilicity [23]; which gives restricted adhesion properties to the material, as appropriate for a dental surface designed to prevent biofilm formation in the septic environment of the mouth [29].
The key to an esthetically pleasing appearance lies in the clinician's ability to properly manage the soft tissue profile around dental implants [30]. An all-ceramic system with LS2 ceramic could improve the final esthetic results. Our study clearly demonstrates that it is easily possible, by simple surface treatments that can be performed by a practitioner, to modify the properties of the ceramics used in dentistry. These results demonstrate how simple surface modifications (i.e., polished vs. raw) can finely modulate tissue adhesion.
Dental surgeons need to choose the best surface treatment for each clinical application, especially in dental implantology. Soft tissue management for the visible dental zone could be improved by choosing LD press ceramics as an esthetic and biocompatible material for a tailor-made, manually modifiable abutment.
ACKNOWLEDGEMENTS
The authors are very thankful to Ivoclar Vivadent SAS company (Saint-Jorioz, France) for providing samples.
Footnotes
Funding: This study was partly supported by the administration of the Picardie Region and the Feder fund.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Valenti M, Valenti A. Retrospective survival analysis of 261 lithium disilicate crowns in a private general practice. Quintessence Int. 2009;40:573–579. [PubMed] [Google Scholar]
- 2.Gehrt M, Wolfart S, Rafai N, Reich S, Edelhoff D. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin Oral Investig. 2013;17:275–284. doi: 10.1007/s00784-012-0700-x. [DOI] [PubMed] [Google Scholar]
- 3.Herrguth M, Wichmann M, Reich S. The aesthetics of all-ceramic veneered and monolithic CAD/CAM crowns. J Oral Rehabil. 2005;32:747–752. doi: 10.1111/j.1365-2842.2005.01498.x. [DOI] [PubMed] [Google Scholar]
- 4.Bengazi F, Wennström JL, Lekholm U. Recession of the soft tissue margin at oral implants. A 2-year longitudinal prospective study. Clin Oral Implants Res. 1996;7:303–310. doi: 10.1034/j.1600-0501.1996.070401.x. [DOI] [PubMed] [Google Scholar]
- 5.Bressan E, Paniz G, Lops D, Corazza B, Romeo E, Favero G. Influence of abutment material on the gingival color of implant-supported all-ceramic restorations: a prospective multicenter study. Clin Oral Implants Res. 2011;22:631–637. doi: 10.1111/j.1600-0501.2010.02008.x. [DOI] [PubMed] [Google Scholar]
- 6.Ekfeldt A, Fürst B, Carlsson GE. Zirconia abutments for single-tooth implant restorations: a retrospective and clinical follow-up study. Clin Oral Implants Res. 2011;22:1308–1314. doi: 10.1111/j.1600-0501.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 7.Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005;25:113–119. [PubMed] [Google Scholar]
- 8.Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010;23:434–442. [PubMed] [Google Scholar]
- 9.Van Dooren E, Calamita M, Calgaro M, Coachman C, Ferencz JL, Pinho C, et al. Mechanical, biological and clinical aspects of zirconia implants. Eur J Esthet Dent. 2012;7:396–417. [PubMed] [Google Scholar]
- 10.Wittneben JG, Wright RF, Weber HP, Gallucci GO. A systematic review of the clinical performance of CAD/CAM single-tooth restorations. Int J Prosthodont. 2009;22:466–471. [PubMed] [Google Scholar]
- 11.Grundke K, Bogumil T, Werner C, Janke A, Pöschel K, Jacobasch HJ. Liquid-fluid contact angle measurements on hydrophilic cellulosic materials. Colloids Surf A Physicochem Eng Asp. 1996;116:79–91. [Google Scholar]
- 12.de Groot P, Deck L. Surface profiling by frequency-domain analysis of white light interferograms. Proc SPIE. 1994;2248:101–104. [Google Scholar]
- 13.Rajaraman R, Rounds DE, Yen SP, Rembaum A. A scanning electron microscope study of cell adhesion and spreading in vitro . Exp Cell Res. 1974;88:327–339. doi: 10.1016/0014-4827(74)90248-1. [DOI] [PubMed] [Google Scholar]
- 14.Duval JL, Letort M, Sigot-Luizard MF. Comparative assessment of cell/substratum static adhesion using an in vitro organ culture method and computerized analysis system. Biomaterials. 1988;9:155–161. doi: 10.1016/0142-9612(88)90115-9. [DOI] [PubMed] [Google Scholar]
- 15.Sigot-Luizard MF, Lanfranchi M, Duval JL, Benslimane S, Sigot M, Guidoin RG, et al. The cytocompatibility of compound polyester-protein surfaces using an in vitro technique. In Vitro Cell Dev Biol. 1986;22:234–240. doi: 10.1007/BF02621224. [DOI] [PubMed] [Google Scholar]
- 16.Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 17.Josset Y. Oum’Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ, Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999;47:481–493. doi: 10.1002/(sici)1097-4636(19991215)47:4<481::aid-jbm4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Covacci V, Bruzzese N, Maccauro G, Andreassi C, Ricci GA, Piconi C, et al. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials. 1999;20:371–376. doi: 10.1016/s0142-9612(98)00182-3. [DOI] [PubMed] [Google Scholar]
- 19.Warashina H, Sakano S, Kitamura S, Yamauchi KI, Yamaguchi J, Ishiguro N, et al. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials. 2003;24:3655–3661. doi: 10.1016/s0142-9612(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 20.van Brakel R, Meijer GJ, Verhoeven JW, Jansen J, de Putter C, Cune MS. Soft tissue response to zirconia and titanium implant abutments: an in vivo within-subject comparison. J Clin Periodontol. 2012;39:995–1001. doi: 10.1111/j.1600-051X.2012.01931.x. [DOI] [PubMed] [Google Scholar]
- 21.De Sanctis M, Baldini N, Vignoletti F. Soft tissue management around teeth and implants. J Parodontol Implantol Oral. 2010;29:245–269. [Google Scholar]
- 22.Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002;17:793–798. [PubMed] [Google Scholar]
- 23.Brunot-Gohin C, Duval JL, Azogui EE, Jannetta R, Pezron I, Laurent-Maquin D, et al. Soft tissue adhesion of polished versus glazed lithium disilicate ceramic for dental applications. Dent Mater. 2013;29:e205–12. doi: 10.1016/j.dental.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Müller C, Lüders A, Hoth-Hannig W, Hannig M, Ziegler C. Initial bioadhesion on dental materials as a function of contact time, pH, surface wettability, and isoelectric point. Langmuir. 2010;26:4136–4141. doi: 10.1021/la903299y. [DOI] [PubMed] [Google Scholar]
- 25.Bolortuya G, Ebihara A, Ichinose S, Watanabe S, Anjo T, Kokuzawa C, et al. Effects of dentin surface modifications treated with Er:YAG and Nd:YAG laser irradiation on fibroblast cell adhesion. Photomed Laser Surg. 2012;30:63–70. doi: 10.1089/pho.2011.3132. [DOI] [PubMed] [Google Scholar]
- 26.Duval JL, Dinis T, Vidal G, Vigneron P, Kaplan DL, Egles C. Organotypic culture to assess cell adhesion, growth and alignment of different organs on silk fibroin. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1916. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 27.Messer RL, Lockwood PE, Wataha JC, Lewis JB, Norris S, Bouillaguet S. In vitro cytotoxicity of traditional versus contemporary dental ceramics. J Prosthet Dent. 2003;90:452–458. doi: 10.1016/s0022-3913(03)00533-x. [DOI] [PubMed] [Google Scholar]
- 28.Brackett MG, Lockwood PE, Messer RL, Lewis JB, Bouillaguet S, Wataha JC. In vitro cytotoxic response to lithium disilicate dental ceramics. Dent Mater. 2008;24:450–456. doi: 10.1016/j.dental.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004;75:292–296. doi: 10.1902/jop.2004.75.2.292. [DOI] [PubMed] [Google Scholar]
- 30.Rompen E, Raepsaet N, Domken O, Touati B, Van Dooren E. Soft tissue stability at the facial aspect of gingivally converging abutments in the esthetic zone: a pilot clinical study. J Prosthet Dent. 2007;97:S119–25. doi: 10.1016/S0022-3913(07)60015-8. [DOI] [PubMed] [Google Scholar]