Abstract
Background
Approximately 20% to 40% of patients with gastroesophageal reflux disease (GERD) are refractory to standard-dose proton-pump inhibitor (PPI) treatment.
Objective
We compared the efficacy and quality-of-life effects of 20 mg once daily (QD) versus 10 mg twice daily (BID) rabeprazole (RPZ) in patients with refractory GERD-related symptoms and sleep disturbances.
Methods
This multicenter, prospective, randomized, open-label study included patients in whom PPI treatment >4 weeks was ineffective. According to the Global Overall Symptom (GOS) scale, PPI-refractory GERD was defined as ≥1 category with >3 points among 10 specific upper gastrointestinal symptoms. Seventy-eight patients were randomly assigned to 20 mg QD and 10 mg BID RPZ groups for 8 weeks. Efficacy was evaluated using self-reported questionnaires, including the GOS scale and Pittsburg Sleep Quality Index (PSQI), whereas quality of life was assessed using the Short-Form 8 Health Survey (SF-8), at 4 and 8 weeks. Patients showing improvement at 8 weeks received follow-up every 4 to 8 weeks.
Results
GOS scale scores were significantly improved at 8 weeks in both groups, with no significant intergroup differences. Although SF-8 scores showed an increasing trend over 8 weeks in both groups, the physical component summaries in the 10 mg BID group significantly improved. The mental component summaries clearly improved in the 10 mg BID group. Of the 74 cases (4 missing), 51 (68.9%) had PSQI scores ≥5.5. PSQI scores remained unchanged during follow-up in both groups. The recurrence rate was not significantly different (46.1% vs 47.1% in the 20 mg QD and 10 mg BID groups, respectively) during the follow-up period at median (interquartile range) 24.0 (30.5) months.
Conclusions
In patients with refractory GERD, there was no significant difference in GOS scale score, PSQI, or recurrence rate between the groups. With regard to subscores of the SF-8, the 10 mg BID group might be potentially effective.
Key words: proton-pump inhibitor, quality of life, refractory gastroesophageal reflux disease, sleep disturbances
Highlights
-
•
This multicenter prospective randomized comparative study compares the efficacy on symptoms, QOL and sleep disturbance between two ways of double dose PPI; once-daily 20mg RPZ or twice-daily 10mg RPZ.
-
•
The patients with PPI refractory GERD were suffered from severe acid reflux symptoms, sleep disturbance and general distress. For these reasons, to establish the treatments to refractory GERD was important to improve their QOL. In patients with refractory GERD, there was no significant difference in GOS, PSQI, or recurrence rate between the groups.
Introduction
Gastroesophageal reflux disease (GERD) is widespread in the increasingly aging Japanese society because of increased gastric acid secretion associated with aging, the Westernization of eating habits, and decreased Helicobacter pylori infection rate.1
Gastric acid secretion gradually increases in patients with proton-pump inhibitor (PPI)-refractory GERD. Approximately 10% of erosive reflux disease and approximately 50% of nonerosive reflux disease are refractory to PPIs.2 The causes of refractory GERD are nonacid regurgitation of bile acid; esophageal hypersensitivity to gastric acid; delayed gastric emptying; and patient comorbidities, such as mental disease, functional disturbances, early metabolism of PPI (CYP2C19 homeEM), and gastric acid regurgitation at midnight.3 Some reports showed that excess acid secretion in the duodenum led to hypersensitivity of the esophagus to gastric acid and delayed gastric emptying.4, 5 Because of this, we speculated that stronger inhibition of gastric acid secretion with double-dose PPI might improve the symptoms of refractory GERD. However, there are 2 approaches for administering double-dose PPI: rabeprazole (RPZ) 20 mg once daily (QD) or 10 mg twice daily (BID). There are no reports to determine which strategy is more effective and reliable for reducing the symptoms of PPI-refractory GERD. Refractory GERD causes typical symptoms, such as heartburn, and leads to a decreased quality of life (QOL)6, 7 such as sleep disturbances.8 Therefore, the establishment of treatments for refractory GERD is important for improving QOL. Thus, the present study aimed to compare the efficacy and QOL effects of 20 mg QD RPZ versus 10 mg BID RPZ in patients with symptoms of refractory GERD.
Subjects and Methods
Study design
This multicenter prospective, randomized, open-label comparative study was approved by the review board of Keiyu Hospital and was performed in accordance with the tenets of Declaration of Helsinki.
Subjects
Patients from Keiyu Hospital and 6 other clinics were enrolled between November 2011 and September 2015. Inclusion criteria included patients in whom a standard dose of PPI (RPZ 10 mg/d, lansoprazole 15 mg/d, omeprazole 10 mg/d, or esomeprazole 10 mg/d) over 4 weeks had not been effective. Patients were diagnosed using the Global Overall Symptom (GOS) scale.9 Those who scored >3 points out of 10 specific upper gastrointestinal symptoms were diagnosed with PPI-refractory GERD. Exclusion criteria were as follows: patients who had been treated with double-dose PPI during the past 4 weeks, pregnant patients, nursing mothers, and night shift workers. Disease-related exclusion criteria were as follows: age <20 or >90 years; patients with mental disorders undergoing treatment; patients with allergic reactions to RPZ; patients with HIV treated with atazanavir; severe diseases such as malignancies, active peptic ulcer, or a past history of upper gastrointestinal surgery; patients who had undergone H pylori eradication therapy within 6 months; and patients unable to undergo esophagogastroduodenoscopy.
Patients were not permitted to take prescription medications, such as histamine-H2 blockers, prokinetic agents, or gastroprotective, drugs for 48 days after starting the study protocol.
Study protocol and evaluation criteria
We obtained written informed consent from each patient before participation in this study. Eligible patients with PPI-refractory GERD were randomly assigned into 2 groups: those who received 20 mg RPZ once daily (20 mg QD group) after breakfast and those who received 10 mg RPZ twice daily (10 mg BID group) after breakfast and dinner. The randomization was by means of randomly permuted and sealed scratch cards each having a 6-digit number. Patients were instructed to take RPZ after breakfast and after dinner. Patients completed the GOS scale questionnaire every week for 8 weeks, and the Pittsburgh Sleep Quality Index (PSQI)10, 11 and Short-Form 8 Health Survey12, 13 (SF-8) questionnaires at 4 and 8 weeks. The sequence of this survey was not ordered.
All patients underwent esophagogastroduodenoscopy before treatment. On esophagogastroduodenoscopy, the GERD grade was defined as the presence of a mucosal break in the esophageal mucosa according to the Los Angeles classification grade A through D.
GOS scale score
The GOS scale9 is the optimal method for measuring the severity of dyspepsia symptoms. It grades 10 specific symptoms—epigastric pain, heartburn, regurgitation, upper abdominal bloating, nausea, excessive belching, postprandial fullness, and early satiety—using a 7-point Likert severity scale that ranges from 1 = no problem to 7 = a very severe problem. The total GOS scale score was divided into the scores for the 1 question concerning abdominal pain, for 2 questions concerning acid reflex-related symptoms, and for the 5 questions concerning dyspeptic (dysmotility) symptoms. Improvement of symptoms was defined as a GOS score of 1 (no problem) or 2 (minimal problem) for each item.14
PSQI
PSQI was used to measure the patient’s recent sleep quality during the previous month. The Japanese version of PSQI11 consists of 17 questions, which are organized into 7 components, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, and daytime disturbances. The score of each component score ranged from 0 to 3. The total score of these 7 component scores provides a global PSQI score, which ranges from 0 to 21. Higher scores indicate poorer sleep.10, 11 A cutoff score >5.5 has a sensitivity of 80.0% to 85.7% for various patient groups and a specificity of 86.6% for control subjects in the Japanese version of PSQI.11
SF-8
Health-related QOL was assessed using the validated 8-item SF-8 questionnaire (with a 4-week recall period).12, 13 SF-8 is a generic questionnaire derived from the 36-item Short-Form Health Survey and was developed to estimate QOL according to the scores from 8 domains. The Japanese version of the SF-8 was developed using a cross-cultural adaptation method that requires translation of the original English version into Japanese followed by back-translation into English, and its validity was confirmed using the Japanese general population.14 Scores for the 8 domains and the physical component summary (PCS) and mental component summary (MCS) were calculated according to the manual for the Japanese version of SF-8.15 A score of 50 is the mean for the Japanese general population across the 8 domains and 2 summary scores; higher scores indicate a better QOL.
Adverse events and tolerability
Adverse events and tolerability were assessed by recording all adverse events, and changes in clinical variables were measured at the 4- and 8-week follow-up visit after treatment. An adverse event was defined as any unfavorable or unintended sign, whether it was considered to be causally related to the drugs used in this study. The type and severity of adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 4.0.
Follow-up
In this study protocol, double-dose RPZ was stopped at 8 weeks and then standard dose (10 mg RPZ QD) was started in all patients. Patients were monitored for GERD symptoms after the 8-week study period using the GOS scale self-administered questionnaire every 4 to 8 weeks.
Statistical analysis
The study’s sample size was calculated based on 10 for SD, 6 to 7 for difference of 2 groups in the summed GOS scale score.16 A 1-point reduction of all terms resulted in an 8-point reduction of the summed GOS scale score. With a power of 0.8 at the 0.05 2-sided significance level, 33 to 45 patients per group were required to detect statistical significance using the unpaired t test. A χ2 test or Student t test was used to assess the significance of the differences in categorized data or numerical data, respectively. The significance of time-course changes at 4 or 8 weeks, compared with baseline data, was determined by repeated measurement of a generalized linear model and Bonferroni’s method. The significance of the difference in time-course change between the 2 groups was determined by repeated measurement of a generalized linear model. P values < 0.05 were considered to indicate statistical significance. All statistical analysis was performed using IBM-SPSS version 22.0 (IBM-SPSS Japan Inc, Tokyo, Japan).
Literature was reviewed from Medline (accessed through PubMed Central). We used the search terms GERD, PPI, PPI refractory, GOS score, sleep disturbance, QOL, and recurrence, plus the logical operators AND and OR for combinations and tracking. The last search was performed in June 2016.
Results
Patient characteristics
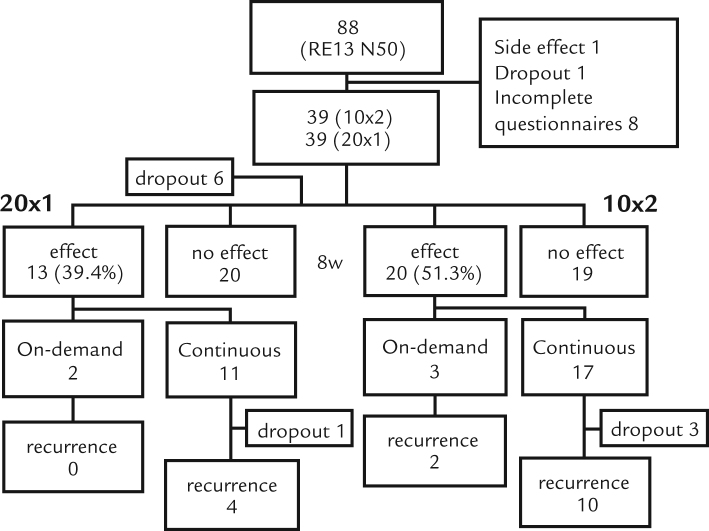
A total of 88 patients were enrolled in this study. One patient discontinued treatment because of edema of extremities, which was suspected to be a side effect of RPZ. One patient dropped out, and 8 submitted incomplete questionnaires. Therefore, the final number of patients included in the efficacy analysis was 78 (n = 39 in each group) (Figure 1). Treatment groups did not differ in age, gender, or other characteristics (Table I).
Figure 1.
Flow chart of patients. 20 × 1 = 20 mg RPZ QD group; 10 × 2 = 10 mg RPZ BID group.
Table I.
Clinical characteristics of patients with gastroesophageal reflux disease (GERD).
| 20 mg QD rabeprazole |
10 mg BID rabeprazole |
P value |
|
|---|---|---|---|
| n = 39 | n = 39 | ||
| Age*, y | 60.5 (14.8) | 64.5 (13.4) | 0.224 |
| Male sex | 17 | 26 | 0.243 |
| Female sex | 22 | 26 | 0.243 |
| Body mass index* | 23.0 (4.1) | 22.8 (4.0) | 0.888 |
| Smoking habits† | 5 (12.8) | 8 (20.5) | 0.441 |
| Drinking habits† | 12 (30.8) | 14 (35.9) | 0.734 |
| Helicobacter pylori† | 2/34 (5.9) | 6/33 (18.2) | 0.168 |
| GERD subtypes | |||
| Nonerosive reflux disease | 28 | 30 | |
| Erosive reflux disease | 7 | 9 | |
| Grade A‡ | 5 | 5 | |
| Grade B‡ | 0 | 2 | |
| Grade C‡ | 1 | 0 | |
| Grade D‡ | 1 | 1 | |
| Missing | 4 | 1 | |
| Global Overall Symptom* | 23.1 (9.1) | 22.8 (7.1) | 0.824 |
| Pittsburg Sleep Quality Index* | 7.8 (3.8) | 7.5 (4.1) | 0.742 |
| Short Form 8 | |||
| Physical Component* *Summaries | 44.7 (7.9) | 43.1 (5.7) | 0.328 |
| Mental Component* Summaries | 42.7 (7.4) | 46.5 (7.4) | 0.051 |
Values are given as mean (SD).
Values are given as n (%).
Grade was determined based on Los Angeles classification.
GOS scale score
In the evaluation of the patients’ GOS scale scores, the proportion of patients who showed effective improvement at 8 weeks was 42.4% (14 out of 33) in the RPZ 20 mg QD group and 48.7% (19 out of 39) in the 10 mg BID group; there was no significant difference between the 2 groups (P = 0.384).
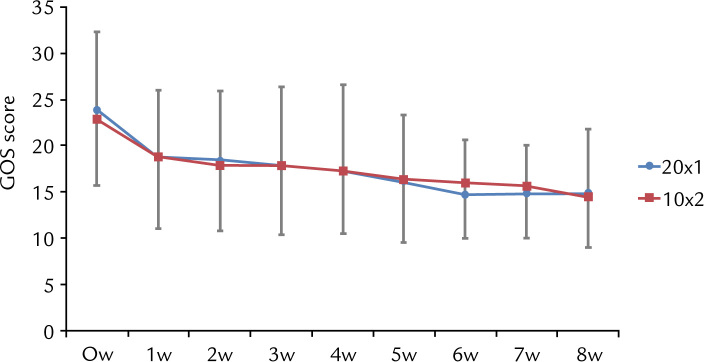
The mean (SD) sum of GOS scores (sGOS) before treatment was 23.1 (9.1) and 22.8 (7.1) in the 20 mg QD group and 10 mg BID group, respectively. sGOS scores in both groups significantly decreased after 1 week of treatment, at 18.6 (7.4) and 18.8 (7.8) for the 20 mg QD group and the 10 mg BID group, respectively (P < 0.05) and gradually decreased during the course of treatment (Figure 2). There was no significant difference between the two groups in sGOS scores throughout the 8-week treatment period (Table II).
Figure 2.
Time-course changes in the total Global Overall Symptom (GOS) score. The red line corresponds to the 20 mg RPZ QD (20×1) group and the blue line corresponds to the 10 mg RPZ BID (10×2) group over the course of treatment.
Table II.
Time course changes of gastroesophageal reflux disease symptoms, sleep dysfunction, and quality-of-life score.
| Measurement | 20 mg QD rabeprazole |
10 mg BID rabeprazole |
||||
|---|---|---|---|---|---|---|
| Mean | SD | P value* | Mean | SD | P value* | |
| Global Overall Symptom | ||||||
| Baseline | 23.1 | 9.2 | 22.8 | 7.1 | ||
| 4 wk | 16.8 | 7.8 | 0.002 | 17.2 | 6.7 | < 0.001 |
| 8 wk | 14.9 | 6.9 | < 0.001 | 14.5 | 5.5 | < 0.001 |
| **P = 0.921 | ||||||
| Pittsburg Sleep Quality Index | ||||||
| Baseline | 7.8 | 3.8 | 7.4 | 4.1 | ||
| 4 wk | 7.5 | 3.9 | 0.538 | 7.2 | 4.2 | 0.593 |
| 8 wk | 7.5 | 3.8 | 0.507 | 6.8 | 4.0 | 0.138 |
| **P = 0.615 | ||||||
| Short Form 8 | ||||||
| Physical component summaries | ||||||
| Baseline | 44.9 | 7.8 | 43.1 | 5.7 | ||
| 4 wk | 44.8 | 8.1 | 0.989 | 45.0 | 5.6 | 0.049 |
| 8 wk | 46.5 | 6.5 | 0.181 | 46.1 | 5.9 | 0.005 |
| **P = 0.635 | ||||||
| Mental component summaries | 42.9 | 9.1 | 46.5 | 7.4 | ||
| Baseline | 44.6 | 7.5 | 0.084 | 47.9 | 7.6 | 0.227 |
| 4 wk | 44.5 | 8.5 | 0.219 | 48.4 | 6.6 | 0.106 |
| 8 wk | **P = 0.028 | |||||
P value indicates comparison to the value of baseline by repeated measurement general linear model and Bonferroni׳s method.
P value indicates differences in time course changes between 2 groups by repeated measurement general linear model.
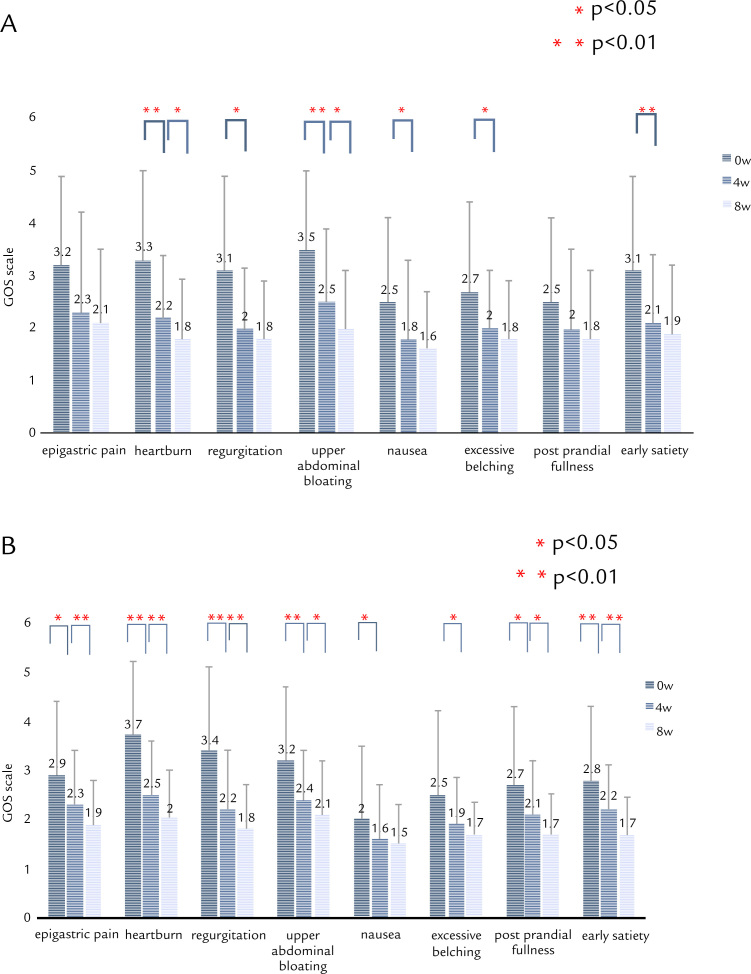
Time-course changes in symptoms on the GOS scale are shown in Figure 3A (20 mg QD group) and Figure 3B (10 mg BID group). In the 20 mg QD group, epigastric pain or postprandial fullness was not significantly improved. On the other hand, all symptoms were significantly improved in the 10 mg BID group (Figure 2B).
Figure 3.
Time-course changes in each symptom on the Global Overall Symptom (GOS) scale after treatment with double-dose RPZ. (A) 20 mg RPZ QD. (B) 10 mg RPZ BID. 4w = 4 weeks; 8w = 8 weeks; 0w = baseline.
PSQI score
Sleep disturbance, defined as a global PSQI score >5.5, was found in 24 out of 34 (70.6%) and 26 out of 39 (66.7%) patients from the 20 mg QD group and 10 mg BID group, respectively. The mean PSQI scores were not different between the 2 groups, and no significant improvement was observed in either group during the 8-week study (Table II). The proportion of patients with sleep disturbances at 8 weeks was not different from baseline in either group (22 out of 32 [68.8%] vs 24 out of 38 [63.2%] in the 20 mg QD and 10 mg BID groups, respectively.
The mean (SD) GOS scale score in patients with sleep disturbances was not significantly different compared with patients without sleep disturbances (24.4 [8.3] vs 20.7 [7.7], respectively; P = 0.074). There was no significant correlation between GOS scale score and PSQI score (r = 0.201; P = 0.089). These data suggest that the severity of GERD symptoms is not associated with a reduction in sleep quality or quantity.
SF-8 score
With regard to the SF-8 results, the mean PCS scores were not different between the 2 groups, but mean (SD) MCS scores in the 10 mg BID group (46.5 [7.4]) were slightly higher than those in 20 mg QD group (42.7 [7.4]); however, the differences were not statistically significant (P = 0.051). Significant improvement in PCS scores from baseline were observed in the 10 mg BID group, but not in the 20 mg QD group (P < 0.05). The trend of improvement in MCS scores from baseline was significantly different between the 2 groups (P < 0.05), and a clearer trend of improvement was observed in the 10 mg BID group (Table II). Domains of general health, physical functioning, body pain, vitality, and mental health were significantly improved at 4 and 8 weeks in the 20 mg QD group (P < 0.05). Body pain, vitality, and mental health were significantly improved in the 10 mg BID group (P < 0.05) (Supplement Table 1).
Follow-up
The median follow-up period (interquartile range) was 24.0 months (30.5 months; range = 1.8–50.3 months). Four patients (1 from the 20 mg QD group and 3 from the 10 mg BID group) were excluded from final evaluation because they were lost to follow-up (no visits and no responses to 2 telephone calls). Twenty-nine patients were available for the final assessment. Of the 14 patients who showed effective improvement at 8 weeks in the 20 mg QD group, 13 received continuous RPZ therapy at 10 mg/d and 3 dropped out. Of the 10 patients on continuous therapy, 4 experienced recurrent symptoms of GERD, and 1 dropped out. One patient was treated on demand, and no patients developed recurrence. The recurrence rate of patients on continuous RPZ therapy in the 20 mg QD group was 4 out of 10 (40.0%). Of the 19 patients who experienced improvement at 8 weeks in the 10 mg BID group, 13 were given continuous therapy, and 3 were given RPZ on demand. Of 13 patients on continuous therapy, 9 had recurrent symptoms and 3 dropped out. Of the 3 patients who received RPZ on demand, none experienced recurrent symptoms. The recurrence rate for patients on continuous therapy in the 10 mg BID group was 9 out of 16 (56.3%). There was no significant (P = 0.420) difference between the 2 methods of administration.
Adverse effect and tolerability
The overall incidence of adverse events and adverse drug reactions was very small (mild lower extremity edema and nausea in 1 patient each).
Discussion
The present study was a randomized, parallel comparative study that examined the efficacy and QOL effects of 20 mg RPZ QD versus 10 mg RPZ BID in patients with GERD-related symptoms and sleep disturbances refractory to PPIs.
The pathophysiology of GERD involves the development of mucosal damage as a result of reflux of the gastric contents into the esophagus. Therefore, the use of acid secretion inhibitory agents like PPIs has been established as the therapeutic strategy for GERD. However, approximately 20% to 40% of patients with GERD are refractory to standard-dose PPI treatment.17, 18 Susceptibility to acid reflux, bile reflux, hypersensitivity to gastric acid, delayed gastric emptying, comorbid psychiatric disease and functional GI disorders, PPI metabolism, and nighttime acid reflux are proposed mechanisms associated with PPI failure.18, 19
Several therapeutic options for PPI-refractory GERD have been reported. Fujiwara et al20 performed a questionnaire-based survey completed in 6 Asian countries, including Japan. A total of 876 physicians participated in the study. Physicians preferred an increase in PPI dosage (53%) or combination of a PPI and other drugs (48%), including prokinetics, histamine H2 blockers, and mucosal protective drugs, for the treatment of PPI-refractory GERD. In addition, Becker et al21 reported that 90.9% patients in the group with pathologic findings in pH/multichannel intraluminal impedance monitoring showed significant symptom relief after increasing PPI dosage.
Appropriate acid suppression for the management of GERD can be measured as the percentage of time that gastric pH is ≥4.0.22, 23 Adachi et al24 reported 24-hour gastric median pH trend grams in 2 healthy volunteer groups who received either a 14-day course of 20 RPZ mg given in the morning or a 14-day course of 10 mg RPZ given in the morning and evening. Dosage of 10 mg RPZ BID was more effective for the inhibition of nocturnal gastric acid secretion compared with 20 mg RPZ given once in the morning, although the total daily doses were equivalent. In addition, the percentage of time gastric pH was ≥4.0 was significantly longer in the group that received 10 mg RPZ BID. On the basis of this, we surmised that 10 mg RPZ BID might be more effective for the relief of symptoms of PPI-refractory GERD. Shimatani et al25 showed that the percentage of time with intragastric pH >4 for 24 hours on Day 7 was greater with 10 mg RPZ BID than at 20 mg RPZ QD. Their data support our findings that the efficacy of RPZ to improve QOL was higher in the twice-daily 10 mg PPI than that in once-daily 20 mg PPI dosage, although actual efficacy on refractory GERD symptoms was not different between the 2 groups. Approximately half of patients achieved symptom relief, defined as a GOS scale score of 1 (no problem) or 2 (minimal problem); however, there was no significant difference between the 2 groups. In addition, the changes in sGOS scores in double-dose PPI treatment for 8 weeks were similar between the 2 groups. In both groups, the symptoms as measured by sGOS score after 1 week of double-dose treatment were significantly decreased. Fujiwara et al8 reported that more than half of all Japanese patients with GERD experience sleep disturbances for which PPI treatment was effective. This study demonstrates that many patients with PPI-refractory GERD experience sleep disturbances. The same researchers reported a significant positive correlation between frequency scale for symptoms of GERD and PSQI scores8; however, the present study showed no correlation between GOS scores and PSQI scores. Another scale; for example, the Epworth Sleepiness Scale, would be needed to evaluate the sleep disturbances in patients with PPI-refractory GERD in future studies.
Assessment of QOL facilitates the translation and interpretation of clinical improvements into outcomes that are important and meaningful to patients and physicians. For the purpose of monitoring QOL during treatment as well as in the context of comparing the effectiveness of different therapies, health outcomes, and QOL represent useful tools for evaluating the efficacy of the care provided.
Hongo et al13 reported that QOL in Japanese patients with GERD is significantly decreased, compared with those of healthy individuals in Western countries. In the study, patients with GERD showed low PCS and MCS scores of 44.6 and 46.8, respectively.1 In the present study, mean (SD) PCS scores in patients with refractory GERD were worse than their MCS scores (42.9 [7.8] vs 45.5 [8.1]), but the difference was not statistically significant. These data suggest that patients with PPI-refractory GERD were more distressed than patients with GERD. After 8 weeks of treatment with double-dose RPZ, symptom relief was observed in approximately 50% of patients, but SF-8 scores were not improved to the level of the general Japanese population (ie, >50). Only PCS scores among patients in the 10 mg BID group were significantly increased compared with baseline. Consequently, we concluded that 10 mg RPZ taken twice daily might be more effective for the improvement of QOL in patients with PPI-refractory GERD.
We also investigated whether double-dose RPZ for 8 weeks can prevent recurrence of severe acid reflux symptoms. In the present study, the recurrence rate was 14 out of 30 (46.7%). Approximately half of patients with PPI-refractory GERD experienced recurrent symptoms after treatment. Although double-dose PPI was effective against symptoms of PPI-refractory GERD, the treatment could not prevent any recurrences. For patients who experience recurrent symptoms, we might have to continue double-dose PPI or stronger acid-suppressive agents for better symptom control. Hsu et al26 reported that 8 weeks of PPI therapy decreases the relapse of symptoms, compared with 8 weeks of therapy, among patients with Los Angeles grade A or B erosive esophagitis. The cumulative 12-week incidence of symptom relapse was higher for the 4-week group than for the 8-week group (62.5% vs 47.8%).
Several limitations to the present study warrant mention. A definitive conclusion could not be drawn because of the limited number of patients. In addition, we did not perform a pH-monitoring study or endoscopy to confirm healing of reflux esophagitis. To the best of our knowledge, the present study is the first randomized controlled trial to comprehensively evaluate the acid reflux symptoms, sleep disturbances, and general distress of Japanese patients with PPI-refractory GERD.
Conclusions
In patients with refractory GERD, there was no significant difference in GOS scale score, PSQI, or recurrence rate between 10 mg RPZ twice-daily and 20 mg RPZ once-daily groups. With regard to subscores of SF-8, the 10 mg RPZ twice-daily group might have the potential to be effective.
Acknowledgments
Akira Mizuki, Masayuki Tatemichi and Hiroshi Nagata designed the trial. Akira Mizuki, Terue Sakakibara, Yukihiko Miura, Shigeyuki Zeki, Mitsuru Ohata, Kanji Matsuo, Fumio Kawamura and Hiroshi Nagata did the trial. Masayuki Tatemichi performed the analysis of the clinical data. Akira Mizuki, Masayuki Tatemichi and Hiroshi Nagata interpreted the data and erote the manuscript. All authors critically revised and approved the final manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.curtheres.2016.11.002.
Appendix A. Supplementary material
Supplementary material
References
- 1.Hiyama T., Yoshihara M., Tanaka S., Haruma K., Chayama K. Strategy for treatment of nonerosive reflux disease in Asia. World J Gastroenterol. 2008;1:3123–3128. doi: 10.3748/wjg.14.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fass R., Shapiro M., Dekel R., Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease--where next? Aliment Pharmacol Ther. 2005;22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 3.Fass R. Proton pump inhibitor failure--what are the therapeutic options? Am J Gastroenterol. 2009;104:S33–S38. doi: 10.1038/ajg.2009.50. [DOI] [PubMed] [Google Scholar]
- 4.Hobson A.R., Khan R.W., Sarkar S., Furlong P.L., Aziz Q. Development of esophageal hypersensitivity following experimental duodenal acidification. Am J Gastroenterol. 2004;99:813–820. doi: 10.1111/j.1572-0241.2004.04167.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.J., Vos R., Janssens J., Tack J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am J Physiol Gastrointest Liver Physiol. 2004;286:G278–G284. doi: 10.1152/ajpgi.00086.2003. [DOI] [PubMed] [Google Scholar]
- 6.Dimenäs E. Methodological aspects of evaluation of Quality of Life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl. 1993;199:18–21. [PubMed] [Google Scholar]
- 7.Vakil N., van Zanten S.V., Kahrilas P., Dent J., Jones R., Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara Y., Kohata Y., Kaji M., Nebiki H., Yamasaki T., Sasaki E., Hayakawa T., Machida H., Tanigawa T., Watanabe K., Watanabe T., Tominaga K., Arakawa T. Sleep disturbances in Japanese patients with gastroesophageal reflux disease: prevalence, risk factors, and efficacy of rabeprazole. Digestion. 2010;81:135–141. doi: 10.1159/000253849. [DOI] [PubMed] [Google Scholar]
- 9.Veldhuyzen van Zanten S.J., Chiba N., Armstrong D., Barkun A.N., Thomson A.B., Mann V., Escobedo S., Chakraborty B., Nevin K. Validation of a 7-point Global Overall Symptom scale to measure the severity of dyspepsia symptoms in clinical trials. Aliment Pharmacol Ther. 2006;23:521–529. doi: 10.1111/j.1365-2036.2006.02774.x. [DOI] [PubMed] [Google Scholar]
- 10.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Doi Y., Minowa M., Okawa M. Development of the Japanese version of the Pittsburgh Sleep Quality Index (in Japanese) Jpn J Pshychiatr Treat. 1998;13:755–763. [Google Scholar]
- 12.Wiklund I. Quality of life in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:S46–S53. doi: 10.1016/s0002-9270(01)02591-6. [DOI] [PubMed] [Google Scholar]
- 13.Hongo M., Kinoshita Y., Miwa H., Ashida K., Hongo M., Kinoshita Y., Miwa H., Ashida K. The demographic characteristics and health-related quality of life in a large cohort of reflux esophagitis patients in Japan with reference to the effect of lansoprazole: the REQUEST study. J Gastroenterol. 2008;43:920–927. doi: 10.1007/s00535-008-2257-7. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S., Suzukamo Y. Institute for Health Outcomes & Process Evaluation Research; Kyoto: 2004. Manual of the SF-8 Japanese version. (in Japanese) [Google Scholar]
- 15.Stanghellini V., Armstrong D., Mönnikes H., Bardhan K.D. Systematic review: do we need a new gastro-oesophageal reflux disease questionnaire? Aliment Pharmacol Ther. 2004;19:463–479. doi: 10.1046/j.1365-2036.2004.01861.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagahara A., Hojo M., Asaoka D., Sasaki H., Watanabe S. A randomized prospective study comparing the efficacy of on-demand therapy versus continuous therapy for 6 months for long-term maintenance with omeprazole 20 mg in patients with gastroesophageal reflux disease in Japan. Scand J Gastroenterol. 2014;49:409–417. doi: 10.3109/00365521.2013.878380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klinkenberg-Knol E.C., Nelis F., Dent J., Snel P., Mitchell B., Prichard P., Lloyd D., Havu N., Frame M.H., Romàn J., Walan A., Long-Term Study Group Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661–669. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 18.Fass R. Epidemiology and pathophysiology of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98:S2–S7. doi: 10.1016/s0002-9270(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 19.Kohata Y., Fujiwara Y., Machida H., Okazaki H., Yamagami H., Tanigawa T., Watanabe K., Watanabe T., Tominaga K., Arakawa T. Pathogenesis of proton-pump inhibitor-refractory non-erosive reflux disease according to multichannel intraluminal impedance-pH monitoring. J Gastroenterol Hepatol. 2012;27:58–62. doi: 10.1111/j.1440-1746.2012.07074.x. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara Y., Takahashi S., Arakawa T., Sollano J.D., Zhu Q., Kachintorn U., Rani A.A., Hahm K.B., Joh T., Kinoshita Y., Matsumoto T., Naito Y., Takeuchi K., Furuta K., Terano A., IGICS Study Group A 2008 questionnaire-based survey of gastroesophageal reflux disease and related diseases by physicians in East Asian countries. Digestion. 2009;80:119–128. doi: 10.1159/000226088. [DOI] [PubMed] [Google Scholar]
- 21.Becker V., Bajbouj M., Waller K., Schmid R.M., Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther. 2007;26:1355–1360. doi: 10.1111/j.1365-2036.2007.03529.x. [DOI] [PubMed] [Google Scholar]
- 22.Bell N.J., Burget D., Howden C.W., Wilkinson J., Hunt R.H. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51:59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 23.Hunt R.H. Importance of pH control in the management of GERD. Arch Intern Med. 1999;159:649–657. doi: 10.1001/archinte.159.7.649. [DOI] [PubMed] [Google Scholar]
- 24.Adachi K., Komazawa Y., Fujishiro H., Mihara T., Ono M., Yuki M., Kawamura A., Karim Rumi M.A., Amano Y., Kinoshita Y. Nocturnal gastric acid breakthrough during the administration of rabeprazole and ranitidine in Helicobacter pylori-negative subjects: effects of different regimens. J Gastroenterol. 2003;38:830–835. doi: 10.1007/s00535-003-1157-0. [DOI] [PubMed] [Google Scholar]
- 25.Shimatani T., Inoue M., Kuroiwa T., Horikawa Y. Rabeprazole 10 mg twice daily is superior to 20 mg once daily for night-time gastric acid suppression. Aliment Pharmacol Ther. 2004;19:113–122. doi: 10.1046/j.1365-2036.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 26.Hsu P.I., Lu C.L., Wu D.C., Kuo C.H., Kao S.S., Chang C.C., Tai W.C., Lai K.H., Chen W.C., Wang H.M., Cheng J.S., Tsai T.J., Chuah S.K. Eight weeks of esomeprazole therapy reduces symptom relapse, compared with 4 weeks, in patients with Los Angeles grade A or B erosive esophagitis. Clin Gastroenterol Hepatol. 2015;13:859–866. doi: 10.1016/j.cgh.2014.09.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material