Abstract
Fibroblast growth factor 21 (FGF21) is an endocrine-member of the FGF family. It is synthesized mainly in the liver, but it is also expressed in adipose tissue, skeletal muscle, and many other organs. It has a key role in glucose and lipid metabolism, as well as in energy balance. FGF21 concentration in plasma is increased in patients with obesity, insulin resistance, and metabolic syndrome. Recent findings suggest that such increment protects tissue from an increased oxidative stress environment. Different types of physical stress, such as strenuous exercising, lactation, diabetic nephropathy, cardiovascular disease, and critical illnesses, also increase FGF21 circulating concentration. FGF21 is now considered a stress-responsive hormone in humans. The discovery of an essential response element in the FGF21 gene, for the activating transcription factor 4 (ATF4), involved in the regulation of oxidative stress, and its relation with genes such as NRF2, TBP-2, UCP3, SOD2, ERK, and p38, places FGF21 as a key regulator of the oxidative stress cell response. Its role in chronic diseases and its involvement in the treatment and follow-up of these diseases has been recently the target of new studies. The diminished oxidative stress through FGF21 pathways observed with anti-diabetic therapy is another clue of the new insights of this hormone.
Keywords: FGF21, Oxidative stress, Insulin resistance, Metabolic syndrome, Diabetes
1. Introduction
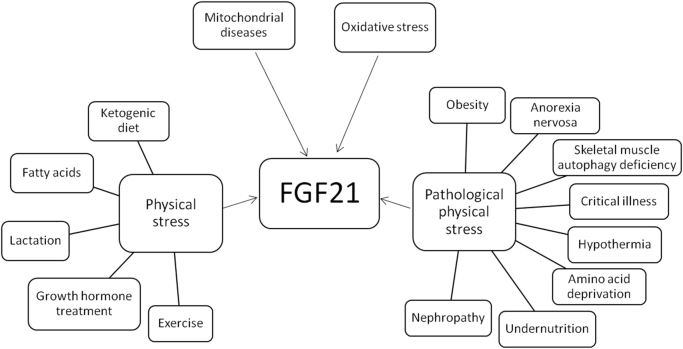
Fibroblast growth factor 21 (FGF21) is a 209 amino acid protein in humans [1]. Its main actions are to regulate glucose and lipid metabolism, and energy balance [1]. It is synthesized mainly in the liver [2], [3], but it is also expressed in white adipose tissue (WAT), brown adipose tissue (BAT) [4], pancreas [5], skeletal muscle [6], cardiac endothelial cells [7], and hypothalamus [8]. The main actions of circulating FGF21 are to increase glucose uptake in adipose tissue [5], augment lipolysis, enhance production of ketone bodies in the liver [9], [10], and to regulate energy balance and physical stress responsiveness in humans [11]. FGF21 plasma concentration may increase with intense physical activity [12], after growth hormone treatment [13], during lactation [14], and after cold exposure [15]. Pathological physical stress conditions like obesity [16], anorexia nervosa [17], skeletal muscle autophagy deficiency [18], critical illness [19], hypothermia [20], amino acid deprivation or undernutrition [21], and nephropathy [22], also induce FGF21 expression (Fig. 1).
Fig. 1.
Conditions associated with an increased FGF21 expression. FGF21 increases in four main circumstances: a) Mitochondrial diseases; b) oxidative stress, c) physical stress situations, such as ketogenic diets, free fatty acids release, lactation, treatment with exogenous growth hormone, and moderate to vigorous exercising; d) pathological physical stress such as obesity, anorexia nervosa, skeletal muscle autophagy deficiency, critical illness, hypothermia, amino acid deprivation, undernutrition, and diabetic nephropathy.
Many intracellular disturbances are associated with an increased expression of FGF21. Mitochondrial disorders that impair the oxidative phosphorylation (OXPHOS) and cause a diminished production of ATP [23], induce elevation of FGF21 serum concentration [24], thus it has been proposed as a serological marker in mitochondrial diseases [24]. Other kinds of intracellular stressors such as autophagy deficiency [18], disruption of the endoplasmic reticulum (ER) calcium homeostasis, and alteration of the ER redox balance [25], [26] could induce FGF21 expression. Although the mechanisms by which FGF21 responds to oxidative stress are still subject of research, it is currently considered an important stress response hormone [9]. This review aims to summarize the role of FGF21 in the regulation of oxidative cell damage and the action of proteins and transcription factors involved in these pathways.
2. FGF21 and cellular oxidative stress
Oxidative stress is defined as an imbalance between pro-oxidant and anti-oxidant factors in favor of the former [27], [28]. Pro-oxidants, such as reactive oxygen species (ROS), are chemically reactive molecules containing oxygen, hydroxyl radicals, hydroperoxyl, hydrogen peroxide, ketoaldehydes, and hydroxynonenal. ROS exert damage to DNA, proteins, and enzymes [29].
Human cells have defense mechanisms to protect against these harmful metabolites, for example, enzymes such as catalase and superoxide dismutase reduce oxygen radicals to H2O2 in the mitochondria [30], [31]. In addition, dietary anti-oxidant molecules, like tocopherol or ascorbate, can donate hydrogen atoms to fatty acid radicals, stabilize cell membranes or change the function of enzymes like occurs with xanthine oxidase, alleviating oxidative stress [32].
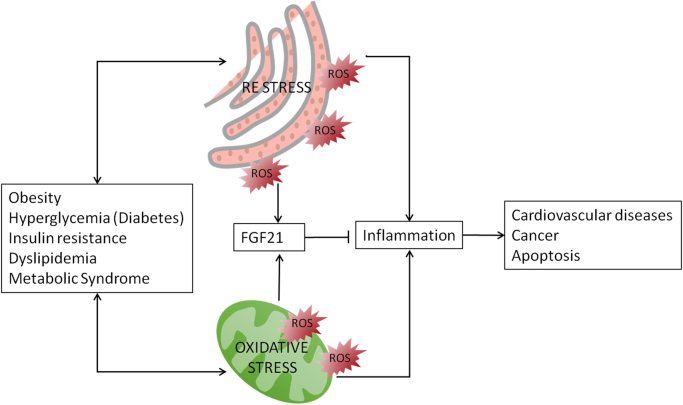
Many chronic diseases are associated with an increased intracellular oxidative stress [33] (Fig. 2).
Fig. 2.
FGF21 and its association with oxidative stress. Metabolic diseases, such as obesity, hyperglycemia, insulin resistance, dyslipidemia, and metabolic syndrome are in both-ways involved with the presence of endoplasmic reticulum stress and oxidative stress. Oxidative stress leads to inflammation responses that result in apoptosis and other pathologies like cardiovascular diseases and cancer. FGF21 inhibits inflammation in response to oxidative stress.
Recently, FGF21 has been considered a novel regulator of oxidative stress in humans. In cultured endothelial cells treated with oxidized low-density lipoproteins (oxLDL), an increased FGF21 mRNA expression and protein concentration was observed [34]. The FGF21 gene promoter has specific response elements (amino acid-responsive element [AARE1 and AARE2]) that are activated by the activating transcription factor 4 (ATF4), which is in turn, stimulated by ER stress produced by aminoacid deprivation or oxidative stress [25].
ER stress and oxidative stress is associated with the pathophysiology of metabolic disorders, contributing to insulin resistance, obesity, and type 2 diabetes mellitus (T2DM) [35], [36], [37], [38]. ERS can be prompted by an increased unfolded protein load, altered calcium homeostasis or perturbed redox balance. If the homeostasis of the ER is altered, the unfolded protein response (UPR) is activated and in consequence FGF21 expression increases. (Fig. 2).
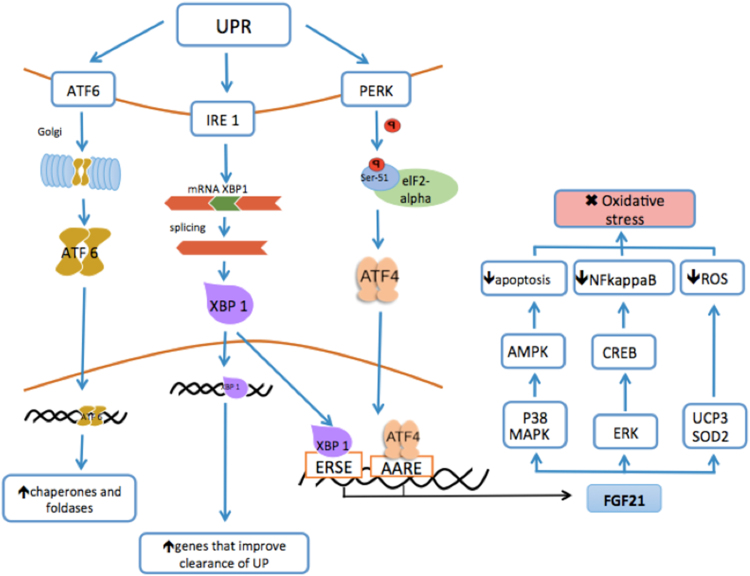
In consequence of ER stress many pathways are activated. Firstly, a transient protein synthesis arrest is observed; then, the ER increases its capacity to handle unfolded proteins, and the UPR target genes are activated [39]. This step restores the translational pathway. When the UPR is activated, three pathways are switched on: 1) the activating transcription factor 6 (ATF6), 2) the inositol-requiring enzyme 1 (IRE1), and 3) the protein kinase-like endoplasmic reticulum kinase (PERK). These ER membrane proteins are sensors of the ER that bind to the luminal chaperone and then, the immunoglobulin protein (BiP) GRP78 binds too [39], [40] (Fig. 3).
Fig. 3.
Activation of FGF21 by the endoplasmic reticulum stress. Three pathways are induced by ER (Endoplasmic reticulum) stress: 1) the activating transcription factor 6 (ATF6), 2) the inositol-requiring enzyme 1 (IRE1), and 3) the protein kinase-like endoplasmic reticulum kinase (PERK). ATF6 increases the expression of chaperones and foldases promoting the degradation of unfolded proteins. IRE1 increases ER folding capacity by detecting misfolded ER proteins and inducing the site-specific splicing of X-box-binding protein 1(XBP1). XBP1 activation up-regulates genes that improve clearance of unfolded proteins and enhance cell survival and binds the endoplasmic reticulum stress element (ERSE) to enhance the expression of FGF21. The PERK activation leads to the phosphorylation of serine-51 (Ser-51) of eukaryotic initiation factor 2 alpha (EIF2 alpha), a transcription factor that catalyzes the first step in the beginning of protein synthesis, in order to decrease the ER load. Furthermore, EIF2 alpha phosphorylation prompts simultaneous induction of ATF4 (activating transcription factor 4), which initiates the expression of its target gene, transcription factor C/EBP homologous protein (CHOP). Three antioxidant mechanisms are activated when FGF21 is expressed due to ER stress: 1) UCP3 (uncoupling protein 3) and the SOD2 (superoxide dismutase-2), decreasing the action of ROS (reactive oxygen species). 2) ERK (extracellular signal-regulated kinase) induces the activation of CREB (cAMP responsive element binding protein) repressing NFkappaB that works as a pro-inflammatory factor, and 3) MAPK (mitogen-activated protein kinase) and p38 that activate AMPK (adenosine monophosphate kinase), decreasing the apoptosis. Finally, oxidative stress is diminished.
However, when cells are exposed to ER stress, BiP separates from these sensors leading to their activation [41]. ATF6 increases chaperones and foldases expression as well as unfolded proteins degradation [42]. As part of the UPR, IRE1 increases ER folding capacity by detecting misfolded ER proteins and activates the transcription factor, X-box-binding protein 1 (XBP1). The activation of IRE1 induces site-specific splicing of XBP1 mRNA. The genes upregulated by XBP1 mRNA improve clearance of unfolded proteins and are associated with the increase of pro-survival functions, [43] besides, XBP1 binds to the endoplasmic reticulum stress elements (ERSE), that promote the expression of FGF21 [44], [45]. When all these protective steps are unable to control the injuring stimulus, intracellular death pathways are activated [46], [47].
PERK works as a protein sensor that mediates translational inhibition. During ER stress, PERK is activated and promotes the phosphorylation of serine 51 (Ser 51) of the eukaryotic initiation factor 2 alpha (eIF2 alpha). The eIF2 alpha inactivates protein synthesis in order to decrease the ER stress load [37], [48], [49]. Furthermore, eIF2 alpha phosphorylation prompts simultaneous induction of ATF4 [50], initiating the expression of its target gene, transcription factor C/EBP homologous protein (CHOP) [51], [52].
This association has been demonstrated in CHOP−/− mouse primary hepatocytes. When exposed to TG-induced ER stress, FGF21 transcriptional activation was impaired. On the other hand, over-expression of ATF4 and CHOP are related to FGF21 promoter activation, in a time and dose-dependent manner [26].
ATF4 is a transcription factor that promotes the expression of FGF21 when ER stress is present [25]. It acts as a regulator of genes involved in redox homeostasis and amino acid metabolism [53]. ATF4 also up-regulates the expression of beta-Klotho, the co-receptor of FGF21 [54]. The common endpoint of these pathways is the inhibition of protein synthesis, increasing the translation of full-length ATF4, which in turn, regulates expression of DNA damage gene 34 (GADD34).
GADD34 is a subunit of the protein phosphatase complex that dephosphorylates eIF2 alpha, allowing the resumption of protein synthesis and translation of the UPR reprogrammed mRNA pool [55]. This gene is also involved in gene expression and amino acid metabolism related to antioxidant defense.
These pathways aim to restore protein synthesis, activate kinases and transcription factors to diminish ROS, NFkappaB action, apoptosis, and subsequently oxidative stress. FGF21 helps to diminish importantly the oxidative stress inducing three antioxidant mechanisms:1) activation of the uncoupling protein 3 (UCP3), and superoxide dismutase-2 (SOD2) that decrease ROS [7], 2) ERK (extracellular signal-regulated kinase), which induces activation of CREB (cAMP responsive element binding protein), repressing NFkappaB, that acts as a pro-inflammatory factor [56], and 3) activation of MAPK and p38, activates AMPK and decrease the apoptosis [4], [57]. This evidence strongly indicates that ER stress increases FGF21 synthesis as a protective event.
3. FGF21 and transcription factors related to oxidative stress (Table 1)
Table 1.
Relationship between FGF21 and key transcription factors associated with oxidative stress.
| Transcription factor | Association with FGF21 | Bibliography |
|---|---|---|
| Nrf2 | When Nrf2 is induced, FGF21 gene expression and FGF21 plasma concentration increases in db/db diabetic mice | [64] |
| ATF4 | It has been linked to the adaptive response to oxidative stress and identified as a clear FGF21 expression inductor | [25], [26], [54] |
| The promoter region of FGF21 has specific binding sites for ATF4 | ||
| TBP-2 | Mice with liver deletion of Tbp-2 show enhanced insulin sensitivity as well as an increased expression of FGF21 | [72] |
Nrf2: nuclear factor erythroid-derived 2; ATF4: activating transcription factor 4; TBP2: thioredoxin binding protein-2.
The hepatic expression of FGF21, induced by protein restriction, may act as an endocrine signal of low-protein intake. This augmented expression correlates with a phosphorylation of eIF2 alpha in the liver [58], which stimulates ATF4 [50]. FGF21 KO mice are fully resistant to low protein-induced changes in food intake, energy expenditure (EE), body weight gain, and metabolic gene expression. This has been confirmed in an experimental study performed for 6 months [59].
Nrf2 (nuclear factor E2-related factor 2) is another transcription factor related to oxidative stress that promotes diverse antioxidant genes. Nrf2 is a key redox regulator in many organs and also it has been involved in cardiovascular diseases [60]. In the pancreatic beta cells it induces the expression of glutathione-related genes in order to reduce apoptosis mediated by nitric oxide [61]. Its functions have been described under basal and stress conditions [62]. Nrf2 is negatively regulated by an adaptor protein Keap1 (Kelch-like ECH-associated protein 1) [63].
Nrf2 increases hepatic FGF21 expression and plasma FGF21 concentration in diabetic db/db and high-calorie-diet-induced obesity mice models [64]. When Keap1 is exposed to oxidative stimuli, Nrf2 is protected against the proteasome-mediated degradation [65], translocates and accumulates in the nucleus and forms an heterodimer with small Maf proteins, then it binds to the antioxidat/electrophile responsive element (ARE/EpRE). This oxidative stress-response system is called the Keap1-Nrf2 system [66]. Besides the important functions of antioxidance and detoxification, the Keap1–Nrf2 system is involved in the regulation of metabolically stressed conditions [67], [68], [69]. Keap1 knock-out mice show an increase in FGF21 plasma concentration and FGF21 hepatic expression, by Nrf2 induction. Also FGF21 increases when Nrf2 is induced by oleanolic triterpenoid 1-[2-cyano-3,12-dioxooleane-1, 9(11)-dien-28-oyl] imidazole in diabetic db/db and high-calorie-diet-induced obesity mice models [64]. Thus, FGF21 is a biomarker of the activation of the Keap1-Nrf2 system [64].
Thioredoxin binding protein-2 (TBP-2), also known as thioredoxin-interacting protein, is an alpha arrestin protein that binds to thioredoxin, an antioxidant protein involved in redox signaling, essential for cell growth and survival [70]. The over-expression of TBP-2 causes impairment of insulin sensitivity and insulin secretion, leading to beta cell apoptosis [71]. It has also been involved in the regulation of transcription factors associated with G protein-coupled receptors involved in metabolic homeostasis and cancer suppression [70].
Mice with liver deletion of TBP-2 showed an enhanced insulin sensitivity with improvement in glucose-induced insulin secretion related with higher expression of PPAR alpha target genes such as FGF21 [72].
Autophagy is a cellular process that transports cytoplasmic constituents to lysosomes for degradation of proteins and recycling of organelles or nutrients [73]. Autophagy defects have been associated with altered insulin secretion [74] and insulin resistance [75]. Since skeletal muscle accounts for 80% of whole-body insulin-mediated glucose utilization [76], a mice model with skeletal muscle autophagy-deficiency with a deletion of autophagy related 7 (Atg7) transcription factor showed altered mitochondrial function. Interestingly, induction of FGF21 by the ATF4 pathway was reported, exerting a decrease in fat mass, improving insulin sensitivity, and showing resistance to diet-induced obesity [18].
4. FGF21 and diseases with an increased oxidative stress
Mitochondrial DNA mutations cause elevation of FGF21 [24]. Recently, it was shown that preprogeroid polymerase gamma mutator (POLG) mouse that accumulates point mutations and deletions in their mitochondrial genome, produced an increment in FGF21. When challenged with a high fat diet, these mice were resistant to diet-induced obesity, highlighting a metabolically favorable synergy between mitochondrial stress and FGF21 [77].
Critical illnesses are also characterized by mitochondrial damage and FGF21 elevation [24], [78]. In a cross-sectional study of 405 critically ill subjects, serum FGF21 concentration was 8-fold higher than in control subjects (P<0.0001). In a rabbit model of critical illness, hepatic FGF21 expression was correlated with mitochondrial dysfunction and an integrated stress response (ISR) markers (r2=0.48, p<0.0006; and r2=0.73, p<0.0001 respectively) [19]. Also, the correction of hyperglycemia decreased FGF21 concentrations. Noteworthy, elevated serum FGF21 concentration was higher in the sickest patients who did not survive (p<0.006), suggesting that FGF21 is a stress or cell damage-induced response [19]. As described above, the ISR activation in critical illness phosphorylates eIF2 alpha which blocks the activation of protein translation, and promotes the translation of transcription factor ATF4, regulating FGF21 expression [79].
Diabetic nephropathy is an oxidative-stress related condition [80]. In previous studies with patients with T2DM and diabetic nephropathy, there was proof of higher serum concentration of FGF21, demonstrating a negative relationship between FGF21 and glomerular filtration rate [22]. In addition, FGF21 have shown a positive correlation with albuminuria [81]. The intraperitoneal administration of FGF21 in mice, exerted an improvement in albuminuria, reversing mesangial expansion, and reducing pro-fibrotic molecules such as inhibitor-1 plasminogen activator (PAI-1) and transforming growth factor beta 1 (TGF beta1). Moreover, FGF21 reduced the oxidative stress in the kidneys inhibiting the pro-inflammatory pathway of nuclear factor kappa beta (NF-kB) [82]. The association between diabetic nephropathy pathophysiology and FGF21 concentration plays an important role in the inhibition of oxidative stress and subsequent fibrosis [83], as well as its action in decreasing lipotoxicity damage and apoptosis.
5. Effect of FGF21 on anti-diabetic drugs and its relationship with oxidative stress
FGF21 appears to be a mediator of the therapeutic effects of drugs involved in the treatment of some metabolic diseases [84]. Metformin reduces the plasma glucose concentration through the inhibition of glucose absorption in the intestine, suppression of gluconeogenesis in the liver and the improvement of the insulin action in the periphery [85]. To suppress liver gluconeogenesis, metformin induces adenosine monophosphate kinase (AMPK) activation, which in turn inhibits transcription of hepatic gluconeogenic enzymes [86]. Some studies have shown increased FGF21 serum concentration after metformin treatment in hepatocytes in an AMPK activation-dependent manner [87]. Also, it has been suggested that the FGF21 upregulation by metformin depends on the elF2 alpha-ATF4 axis [88], which is involved in the oxidative stress response. Moreover, the increased expression of FGF21 in the liver may be associated with the gluconeogenic gene glucose 6-phosphatase (G6Pase) suppression, and the increased glucose uptake by GLUT1 [89], [90]. Taking together, the increment of FGF21 serum concentration contributes to the beneficial metabolic effects of metformin [91].
Other drugs to treat diabetes and also related with increment of FGF21, are the glucagon like peptide-1 (GLP1) analogs. GLP1 is an incretin hormone released by L-cells at small intestine that enhances beta cells insulin release under hyperglycemia, and suppresses glucagon secretion by pancreatic alpha cells. In addition, GLP1 inhibits gastric emptying contributing to satiety sensation and reduction in food intake [92]. GLP1 analogs protect cardiomyocytes against apoptosis via inhibition of endoplasmic reticulum stress [93]. GLP1-derived nonapeptide GLP1(28-36) protected pancreatic β-cells from glucolipotoxicity in increased oxidative stress conditions, independently of the GLP1 receptor [94].
GLP1 analogs are also able to promote FGF21 expression. Especially, Liraglutide induce FGF21 gene expression in the liver [95]. The administration of another GLP1 analog, Exendine-4, for 10 weeks augmented hepatic FGF21 gene expression in mice fed with high fat diet compared to control [96]. However, opposite results were recently reported, where hepatic expression and FGF21 serum concentration were decreased with exedin-4 treatment in mice also fed with high fat diet for 4 weeks [84]. Therefore, more studies are needed to clarify FGF21 role using such drugs in patients with diabetes.
Also, the administration of FGF21 analogs in humans have demonstrated favorable effects on body weight, fasting insulin, and adiponectin when administered for 28 days in obese T2DM subjects [97]. Recently, in a phase I study, a long acting FGF21 analog produced a decrease in triglyceride concentration, as well as a reduction in total cholesterol and low-density lipoprotein cholesterol, and an increase in high-density lipoprotein cholesterol observed in the high-dose groups [98].
6. Conclusions
FGF21 is considered a new novel metabolic hormone related with glucose and lipid metabolism, insulin resistance, and obesity. Its role as an important regulator of mitochondrial and oxidative stress has been consistently demonstrated in experimental studies. Also the multiple beneficial effects on human disorders and its therapeutic potential by attenuating apoptosis, ER stress, inflammation, and its consequences have been studied recently. Therefore, FGF21 is a human stress-response hormone, synthesized and released in order to decrease cell damage. Prospective studies are required to address the questions if supra-physiological concentrations of FGF21 might improve the conditions associated with an increased oxidative stress, and to assess the effects of an increased oxidative stress in FGF21 knock-out mice.
Declaration of interest
The authors have no multiplicity of interest to disclose.
Funding
This research was funded by the Department of Endocrinology and Metabolism. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Vasco de Quiroga # 15, Sección XVI Tlalpan 14000, Mexico City, Mexico.
Author contributions
All authors contributed equally to this work.
Acknowledgements
MAGS would like to acknowledge Luz del Carmen Abascal Olascoaga for her support.
Contributor Information
Miguel Ángel Gómez-Sámano, Email: miguelangelgomezsamano@gmail.com.
Mariana Grajales-Gómez, Email: marianagrago@gmail.com.
Julia María Zuarth-Vázquez, Email: juliazuarth@gmail.com.
Ma. Fernanda Navarro-Flores, Email: fer.navarroflores@gmail.com.
Mayela Martínez-Saavedra, Email: mayela.saa@gmail.com.
Óscar Alfredo Juárez-León, Email: oajl09@gmail.com.
Mariana G. Morales-García, Email: marianaguadalupe.morales@upaep.edu.mx.
Víctor Manuel Enríquez-Estrada, Email: vicmanuel280@gmail.com.
Francisco J. Gómez-Pérez, Email: gomezperezfco@gmail.com.
Daniel Cuevas-Ramos, Email: ceptamim@gmail.com.
References
- 1.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., Sandusky G.E., Hammond L.J., Moyers J.S., Owens R.A., Gromada J., Brozinick J.T., Hawkins E.D., Wroblewski V.J., Li D.S., Mehrbod F., Jaskunas S.R., Shanafelt A.B. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura T., Nakatake Y., Konishi M., Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys. Acta. 2000;1492:203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 3.Kong L.J., Feng W., Wright M., Chen Y., Dallas-Yang Q., Zhou Y.P., Berger J.P. FGF21 suppresses hepatic glucose production through the activation of atypical protein kinase Ci/λ. Eur. J. Pharmacol. 2013;702:302–308. doi: 10.1016/j.ejphar.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Hondares E., Iglesias R., Giralt A., Gonzalez F.J., Giralt M., Mampel T., Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wente W., Efanov A.M., Brenner M., Kharitonenkov A., Köster A., Sandusky G.E., Sewing S., Treinies I., Zitzer H., Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 6.Yoon J.H., Kim J., Song P., Lee T.G., Suh P.-G., Ryu S.H. Secretomics for skeletal muscle cells: a discovery of novel regulators? Adv. Biol. Regul. 2012;52:340–350. doi: 10.1016/j.jbior.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Planavila A., Redondo-Angulo I., Ribas F., Garrabou G., Casademont J., Giralt M., Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc. Res. 2014;106:19–31. doi: 10.1093/cvr/cvu263. [DOI] [PubMed] [Google Scholar]
- 8.Bookout A.L., de Groot M.H.M., Owen B.M., Lee S., Gautron L., Lawrence H.L., Ding X., Elmquist J.K., Takahashi J.S., Mangelsdorf D.J., Kliewer S.A. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.K.H. Kim, M. Lee, FGF21 as a Stress Hormone : The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases, 2014, pp. 245–251. [DOI] [PMC free article] [PubMed]
- 10.Domouzoglou E.M., Maratos-Flier E. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am. J. Clin. Nutr. 2011;93:901S–905S. doi: 10.3945/ajcn.110.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuevas-Ramos D., Aguilar-Salinas C.A. Modulation of energy balance by fibroblast growth factor 21. Horm. Mol. Biol. Clin. Investig. 2016 doi: 10.1515/hmbci-2016-0023. [DOI] [PubMed] [Google Scholar]
- 12.Cuevas-Ramos D., Almeda-Valdés P., Meza-Arana C.E., Brito-Córdova G., Gómez-Pérez F.J., Mehta R., Oseguera-Moguel J., Aguilar-Salinas C. a. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J., Zhao L., Wang A., Eleswarapu S., Ge X., Chen D., Jiang H. Growth hormone stimulates transcription of the fibroblast growth factor 21 gene in the liver through the signal transducer and activator of transcription 5. Endocrinology. 2012;153:750–758. doi: 10.1210/en.2011-1591. [DOI] [PubMed] [Google Scholar]
- 14.Schoenberg K.M., Giesy S.L., Harvatine K.J., Waldron M.R., Cheng C., Kharitonenkov A., Boisclair Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology. 2011;152:4652–4661. doi: 10.1210/en.2011-1425. [DOI] [PubMed] [Google Scholar]
- 15.Lee P., Linderman J.D., Smith S., Brychta R.J., Wang J., Idelson C., Perron R.M., Werner C.D., Phan G.Q., Kammula U.S., Kebebew E., Pacak K., Chen K.Y., Celi F.S. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Yeung D.C.Y., Karpisek M., Stejskal D., Zhou Z.-G., Liu F., Wong R.L.C., Chow W.-S., Tso A.W.K., Lam K.S.L., Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 17.Fazeli P.K., Misra M., Goldstein M., Miller K.K., Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J. Clin. Endocrinol. Metab. 2010;95:369–374. doi: 10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K.H., Jeong Y.T., Oh H., Kim S.H., Cho J.M., Kim Y.-N., Kim S.S., Kim D.H., Hur K.Y., Kim H.K., Ko T., Han J., Kim H.L., Kim J., Back S.H., Komatsu M., Chen H., Chan D.C., Konishi M., Itoh N., Choi C.S., Lee M.-S. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 19.Thiessen S.E., Vanhorebeek I., Derese I., Gunst J., Van den Berghe G. FGF21 Response to Critical Illness: effect of Blood Glucose Control and Relation With Cellular Stress and Survival. J. Clin. Endocrinol. Metab. 2015;100:E1319–E1327. doi: 10.1210/jc.2015-2700. [DOI] [PubMed] [Google Scholar]
- 20.Hanssen M.J.W., Broeders E., Samms R.J., Vosselman M.J., van der Lans A.A.J.J., Cheng C.C., Adams A.C., van Marken Lichtenbelt W.D., Schrauwen P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015;5:10275. doi: 10.1038/srep10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sousa-Coelho A.L., Relat J., Hondares E., Pérez-Martí A., Ribas F., Villarroya F., Marrero P.F., Haro D. FGF21 mediates the lipid metabolism response to amino acid starvation. J. Lipid Res. 2013;54:1786–1797. doi: 10.1194/jlr.M033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Z., Zhou Z., Liu Y., Gong Q., Yan X., Xiao J., Wang X., Lin S., Feng W., Li X. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.E. Ylikallio, A. Suomalainen, Mechanisms of mitochondrial diseases, Ann. Med. 2012. http://www.tandfonline.com/doi/full/10.3109/07853890.2011.598547. (accessed 10.09.15) [DOI] [PubMed]
- 24.Suomalainen A. Fibroblast growth factor 21: a novel biomarker for human muscle-manifesting mitochondrial disorders. Expert Opin. Med. Diagn. 2013;7:313–317. doi: 10.1517/17530059.2013.812070. [DOI] [PubMed] [Google Scholar]
- 25.Schaap F.G., Kremer A.E., Lamers W.H., Jansen P.L.M., Gaemers I.C. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–699. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Wan X., Lu X., Xiao Y., Lin Y., Zhu H., Ding T., Yang Y., Huang Y., Zhang Y., Liu Y.-L., Xu Z., Xiao J., Li X. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed. Res. Int. 2014;2014:807874. doi: 10.1155/2014/807874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebert M.A., Jones D.P. Clinical Measures of the Balance, ANTIOXIDANTS REDOX Signal. 2006;8(8):1–16. [Google Scholar]
- 28.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gracy R., Talent J., Kong Y., Conrad C. Reactive oxygen species: the unavoidable environmental insult? Mutat. Res. Mol. Mech. Mutagen. 1999;428:17–22. doi: 10.1016/s1383-5742(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 30.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–166. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohen R., Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. 〈http://www.ncbi.nlm.nih.gov/pubmed/12512863〉 (accessed 24.05.15) [DOI] [PubMed] [Google Scholar]
- 32.Dizdaroglu M., Jaruga P., Birincioglu M., Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 32. 2002:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. 〈http://www.ncbi.nlm.nih.gov/pubmed/12031895〉 (accessed 15.10.15) [DOI] [PubMed] [Google Scholar]
- 33.Rahman T., Hosen I., Islam M.M.T., Shekhar H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012;3:997–1019. [Google Scholar]
- 34.Lü Y., Liu J.H., Zhang L.K., Du J., Zeng X.J., Hao G., Huang J., Zhao D.H., Wang G.Z., Zhang Y.C. Fibroblast growth factor 21 as a possible endogenous factor inhibits apoptosis in cardiac endothelial cells. Chin. Med. J. (Engl.). 2010;123:3417–3421. [PubMed] [Google Scholar]
- 35.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozcan U., Cao Q., Yilmaz E., Lee A.-H., Iwakoshi N.N., Ozdelen E., Tuncman G., Görgün C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 37.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozcan L., Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu. Rev. Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 40.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 41.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarti A., Chen A.W., Varner J.D. A review of the mammalian unfoded protein response. Biotechnol. Bioeng. 2012;108:2777–2793. doi: 10.1002/bit.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee A.-H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. 〈http://www.ncbi.nlm.nih.gov/pubmed/14559994〉 (accessed 06.01.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asada R., Kanemoto S., Matsuhisa K., Hino K., Cui M., Cui X., Kaneko M., Imaizumi K. IRE1α-XBP1 is a novel branch in the transcriptional regulation of Ucp1 in brown adipocytes. Sci. Rep. 2015;5:16580. doi: 10.1038/srep16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonikas M.C., Collins S.R., Denic V., Oh E., Quan E.M., Schmid V., Weibezahn J., Schwappach B., Walter P., Weissman J.S., Schuldiner M. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter F., Schmid J., Düssmann H., Concannon C.G., Prehn J.H.M. Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival TL - 22. Cell Death Differ. 2015;22 doi: 10.1038/cdd.2014.241. (VN-r) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y., Vattem K.M., Sood R., An J., Liang J., Stramm L., Wek R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. 〈http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=109330&tool=pmcentrez&rendertype=abstract〉 (accessed 09.12.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R.D. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Regulated translation initiation controls stress-induced gene expression in mammalian cells. - PubMed - NCBI, (n.d.). http://www.ncbi.nlm.nih.gov/pubmed/11106749. (accessed January 6, 2016). [DOI] [PubMed]
- 51.Tsaytler P., Harding H.P., Ron D., Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 52.Harding H.P., Zhang Y., Scheuner D., Chen J.-J., Kaufman R.J., Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc. Natl. Acad. Sci. USA. 2009;106:1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rössler O.G., Thiel G. Specificity of stress-responsive transcription factors Nrf2, ATF4, and AP-1. J. Cell. Biochem. 2017;118:127–140. doi: 10.1002/jcb.25619. [DOI] [PubMed] [Google Scholar]
- 54.Dong K., Li H., Zhang M., Jiang S., Chen S., Zhou J., Dai Z., Fang Q., Jia W. Endoplasmic reticulum stress induces up-regulation of hepatic β-Klotho expression through ATF4 signaling pathway. Biochem. Biophys. Res. Commun. 2015;459:300–305. doi: 10.1016/j.bbrc.2015.02.104. [DOI] [PubMed] [Google Scholar]
- 55.Novoa I., Zeng H., Harding H.P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. 〈http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2174339&tool=pmcentrez&rendertype=abstract〉 (accessed 11.09.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Planavila A., Redondo I., Hondares E., Vinciguerra M., Munts C., Iglesias R., Gabrielli L.A., Sitges M., Giralt M., van Bilsen M., Villarroya F., van Bilsen M., van der Vusse G.J., Reneman R.S., Smeets P.J., Planavila A., Iglesias R., Giralt M., Villarroya F., Fredj S., Bescond J., Louault C., Potreau D., Gnecchi M., Doroudgar S., Glembotski C.C., Frost R.J., Engelhardt S., Stastna M., Chimenti I., Marban E., Van Eyk J.E., Badman M.K., Galman C., Inagaki T., Muise E.S., Izumiya Y., Hondares E., Hondares E., Kharitonenkov A., Dutchak P.A., Kharitonenkov A., Kurosu H., Jin Y., Ogawa Y., Uebanso T., Karliner J.S., Xu J., Xu J., Liu S.Q., Booysen H.L., Norton G.R., Opie L.H., Woodiwiss A.J., Lehman J.J., Kelly D.P., Schilling J., Alvarez-Guardia D., Fisher F.M., Stachowiak E.K., Fang X., Myers J., Dunham S., Stachowiak M.K., Ventura-Clapier R., Garnier A., Veksler V., Eisele P.S., Salatino S., Sobek J., Hottiger M.O., Handschin C., Purushotham A., Amat R. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 2013;4:2–14. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 57.Jeanson Y., Ribas F., Galinier A., Arnaud E., Ducos M., Andre M., Chenouard V., Villarroya F., Casteilla L., Carriere A. Lactate induces FGF21 expression in adipocytes through a p38-MAPK pathway. Biochem. J. 2016;473:685–692. doi: 10.1042/BJ20150808. [DOI] [PubMed] [Google Scholar]
- 58.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C., Münzberg H., Hutson S.M., Gettys T.W., Schwartz M.W., Morrison C.D. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laeger T., Albarado D.C., Burke S.J., Trosclair L., Hedgepeth J.W., Berthoud H.-R., Gettys T.W., Collier J.J., Münzberg H., Morrison C.D. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16:707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barančík M., Grešová L., Barteková M., Dovinová I., Ík M.B., Grešová L., Barteková M., Dovinová I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 2016;65:S1–S10. doi: 10.33549/physiolres.933403. 〈http://www.ncbi.nlm.nih.gov/pubmed/27643930〉 (accessed 16.12.16) [DOI] [PubMed] [Google Scholar]
- 61.Yagishita Y., Fukutomi T., Sugawara A., Kawamura H., Takahashi T., Pi J., Uruno A., Yamamoto M. Nrf2 protects pancreatic β-Cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 2013;63:1–48. doi: 10.2337/db13-0909. [DOI] [PubMed] [Google Scholar]
- 62.Chartoumpekis D.V., Ziros P.G., Psyrogiannis A.I., Papavassiliou A.G., Kyriazopoulou V.E., Sykiotis G.P., Habeos I.G. Nrf2 represses FGF21 during long-term high-fat diet - Induced obesity in mice. Diabetes. 2011;60:2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furusawa Y., Uruno A., Yagishita Y., Higashi C., Yamamoto M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells. 2014 doi: 10.1111/gtc.12186. [DOI] [PubMed] [Google Scholar]
- 65.Takaya K., Suzuki T., Motohashi H., Onodera K., Satomi S., Kensler T.W., Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uruno A., Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide Biol. Chem. 2011;25:153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/Small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 68.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uruno A., Furusawa Y., Yagishita Y., Fukutomi T., Muramatsu H., Negishi T., Sugawara A., Kensler T.W., Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 2013;33:2996–3010. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masutani H., Yoshihara E., Masaki S., Chen Z., Yodoi J. Thioredoxin binding protein (TBP)-2/Txnip and α-arrestin proteins in cancer and diabetes mellitus. J. Clin. Biochem. Nutr. 2012;50:23–34. doi: 10.3164/jcbn.11-36SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 72.Oka S., Yoshihara E., Bizen-Abe A., Liu W., Watanabe M., Yodoi J., Masutani H. Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxisome proliferator-activated receptor function. Endocrinology. 2009;150:1225–1234. doi: 10.1210/en.2008-0646. [DOI] [PubMed] [Google Scholar]
- 73.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung H.S., Chung K.W., Won Kim J., Kim J., Komatsu M., Tanaka K., Nguyen Y.H., Kang T.M., Yoon K.-H., Kim J.-W., Jeong Y.T., Han M.S., Lee M.-K., Kim K.-W., Shin J., Lee M.-S. Loss of autophagy diminishes pancreatic β cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Yang L., Li P., Fu S., Calay E.S., Hotamisligil G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baron A.D., Brechtel G., Wallace P., Edelman S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 255. 1988 doi: 10.1152/ajpendo.1988.255.6.E769. 〈http://www.ncbi.nlm.nih.gov/pubmed/3059816〉 (accessed 18.01.16) [DOI] [PubMed] [Google Scholar]
- 77.Wall C.E., Whyte J., Suh J.M., Fan W., Collins B., Liddle C., Yu R.T., Atkins A.R., Naviaux J.C., Li K., Bright A.T., Alaynick W.A., Downes M., Naviaux R.K., Evans R.M. High-fat diet and FGF21 cooperatively promote aerobic thermogenesis in mtDNA mutator mice. Proc. Natl. Acad. Sci. Usa. 2015;112:8714–8719. doi: 10.1073/pnas.1509930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanhorebeek I., Ellger B., De Vos R., Boussemaere M., Debaveye Y., Vander Perre S., Rabbani N., Thornalley P.J., Van den Berghe G. Tissue-specific glucose toxicity induces mitochondrial damage in a burn injury model of critical illness. Crit. Care Med. 2009;37:1355–1364. doi: 10.1097/CCM.0b013e31819cec17. [DOI] [PubMed] [Google Scholar]
- 79.De Sousa-Coelho A.L., Marrero P.F., Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem. J. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 80.Miranda-Díaz A.G., Pazarín-Villaseñor L., Yanowsky-Escatell F.G., Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res. 2016;2016:1–7. doi: 10.1155/2016/7047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jian W.-X., Peng W.-H., Jin J., Chen X.-R., Fang W.-J., Wang W.-X., Qin L., Dong Y., Su Q. Association between serum fibroblast growth factor 21 and diabetic nephropathy. Metabolism. 2012;61:853–859. doi: 10.1016/j.metabol.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Kim H.W., Lee J.E., Cha J.J., Hyun Y.Y., Kim J.E., Lee M.H., Song H.K., Nam D.H., Han J.Y., Han S.Y., Han K.H., Kang Y.S., Cha D.R. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology. 2013;154:3366–3376. doi: 10.1210/en.2012-2276. [DOI] [PubMed] [Google Scholar]
- 83.Zhang C., Shao M., Yang H., Chen L., Yu L., Cong W., Tian H., Zhang F., Cheng P., Jin L., Tan Y., Li X., Cai L., Lu X. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal Inflammation. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0082275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim K.H., Lee M.S. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs. J. Endocrinol. 2015;226:R1–R16. doi: 10.1530/JOE-15-0160. [DOI] [PubMed] [Google Scholar]
- 85.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A., Radovick S., Wondisford F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim M.-H., Jee J.-H., Park S., Lee M.-S., Kim K.-W., Lee M.-K. Metformin enhances glucagon-like peptide 1 via cooperation between insulin and Wnt signaling. J. Endocrinol. 2014;220:117–128. doi: 10.1530/JOE-13-0381. [DOI] [PubMed] [Google Scholar]
- 88.Kim K.H., Jeong Y.T., Kim S.H., Jung H.S., Park K.S., Lee H.-Y., Lee M.-S. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem. Biophys. Res. Commun. 2013;440:76–81. doi: 10.1016/j.bbrc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 89.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teillon S., Calderon G. a., Rios M. Diminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of PPARa?? And FGF21 in mice with hepatic ablation of brain-derived neurotropic factor. J. Endocrinol. 2010;205:37–47. doi: 10.1677/JOE-09-0405. [DOI] [PubMed] [Google Scholar]
- 91.Kim K.H., Jeong Y.T., Kim S.H., Jung H.S., Park K.S., Lee H.-Y., Lee M.-S. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem. Biophys. Res. Commun. 2013;440:76–81. doi: 10.1016/j.bbrc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 92.Meier J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 93.Younce C.W., Burmeister M.A., Ayala J.E. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a, Am. J. Physiol. - Cell Physiol. 2013;304:C508–C518. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- 94.Liu Z., Stanojevic V., Brindamour L.J., Habener J.F. GLP1-derived nonapeptide GLP1(28-36)amide protects pancreatic -cells from glucolipotoxicity. J. Endocrinol. 2012;213:143–154. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nonogaki K., Hazama M., Satoh N. Liraglutide suppresses obesity and hyperglycemia associated with increases in hepatic fibroblast growth factor 21 production in KKAy Mice. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/751930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J., Hong S.-W., Park S.E., Rhee E.-J., Park C.-Y., Oh K.-W., Park S.-W., Lee W.-Y. Exendin-4 regulates lipid metabolism and fibroblast growth factor 21 in hepatic steatosis. Metabolism. 2014;63:1041–1048. doi: 10.1016/j.metabol.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L., Kharitonenkov A., Bumol T., Schilske H.K., Moller D.E. The effects of LY2405319, an FGF21 Analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 98.Dong J.Q., Rossulek M., Somayaji V.R., Baltrukonis D., Liang Y., Hudson K., Hernandez-Illas M., Calle R.A. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br. J. Clin. Pharmacol. 2015;80:1051–1063. doi: 10.1111/bcp.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]