Abstract
Blood-injection-injury (BII) phobia differs from other subtypes of specific phobia in that it is associated with elevated disgust-sensitivity as well as specific autonomic and brain responses during processing of phobia-relevant stimuli. To what extent these features play a role already during threat anticipation is unclear. In the current fMRI experiment, 16 female BII phobics and 16 female healthy controls anticipated the presentation of phobia-specific and neutral pictures. On the behavioral level, anxiety dominated the anticipatory period in BII phobics relative to controls, while both anxiety and disgust were elevated during picture presentation. By applying two different models for the analysis of brain responses to anticipation of phobia-specific versus neutral stimuli, we found initial and sustained increases of activation in anterior cingulate cortex (ACC), insula, lateral and medial prefrontal cortex (PFC), thalamus and visual areas, as well as initial activation in the amygdala for BII phobics as compared to healthy controls. These results suggest that BII phobia is characterized by activation of a typical neural defense network during threat anticipation, with anxiety as the predominant emotion.
Keywords: fMRI, Amygdala, Bed nucleus of the stria terminalis (BNST), Insula, Anterior cingulate cortex
Highlights
-
•
Anxiety, but not disgust, during threat anticipation in blood-injection-injury phobics.
-
•
Distinct temporal characteristics of brain regions involved in threat anticipation.
-
•
Initial and sustained activation in subregions of a neural defense network.
1. Introduction
Specific phobia is characterized by rapid, intense and uncontrollable fear in response to phobia-relevant objects and situations (American Psychiatric Association, 2000). Besides that, most phobics show anticipatory anxiety during expectation of phobia-related situations (Aue and Hoeppli, 2011) that triggers avoidance behavior which in turn prevents fear extinction (Gray and McNaughton, 2000).
A common subtype of specific phobia is blood-injection-injury (BII) phobia with an estimated prevalence of 3–4% in the general population (Wani and Ara, 2014) and a higher prevalence in women (Oosterink et al., 2009). In BII phobia, phobic fears and anxiety emerge in relation to blood withdrawal, medical interventions and the confrontation with a person's own blood or blood of others, especially in the context of injuries. A feature that distinguishes BII phobia from other specific phobias is vasovagal syncope during exposure to phobia-specific objects or situations (Marks, 1988, Page, 1994), which affects approximately 75% of BII phobics (Marks, 1988). This reaction has been attributed to a biphasic autonomic response with a short increase followed by a marked decrease of heart rate (Page, 1994). Furthermore, besides fear and anxiety, BII phobics generally also experience strong disgust during symptom provocation (de Jong and Merckelbach, 1998, Sawchuk et al., 2002, Tolin et al., 1997). BII phobics often avoid medical treatment and even decline necessary treatment (Wani and Ara, 2014). Consequently, BII phobia can have detrimental effects and the investigation of its neural correlates could provide important insights for the development of an effective treatment.
In general, functional magnetic resonance imaging (fMRI) studies in specific phobia point towards involvement of amygdala, insula, anterior cingulate cortex (ACC) as well as prefrontal and orbitofrontal cortex during fear and anxiety, although the neural correlates of specific phobia are still not definitive and most studies were concerned with the animal subtype of specific phobia (for review see Del Casale et al., 2012, Ipser et al., 2013, Linares et al., 2012). To date, BII phobia has received little attention in neuroscientific research (Del Casale et al., 2012). Unfortunately, results are rather inconclusive and seem to critically depend on experimental designs and procedures. Confrontation with phobia-relevant or generally disgusting images has been associated with diminished medial prefrontal cortex (PFC) activity (Hermann et al., 2007) and relatively unspecific activations in thalamus and occipital cortex in BII phobics (Caseras et al., 2010a, Schienle et al., 2003). Direct comparison between BII and animal phobics revealed that only spider phobics showed activations in key areas for emotional processing, i.e. insula and ACC, when confronted with phobia-specific pictures (Caseras et al., 2010a, Lueken et al., 2011). In contrast, another study reported similar activation patterns in BII and spider phobics in the amygdala, insula, ACC, thalamus and orbitofrontal cortex (OFC) (Caseras et al., 2010b).
While confrontation with phobia-related stimuli is associated with strong disgust responses as well as partially different autonomic and brain responses in BII phobics as compared to other subtypes of specific phobia, it is as yet unknown to what extent these responses play a role already during anticipation of phobia-related threat. Since anticipatory anxiety includes negative affect, arousal and hypervigilance and leads to avoidance behavior and maintenance of symptoms (Gray and McNaughton, 2000), understanding its neural basis is of utmost importance. In spider phobia, anticipation of phobia-relevant in contrast to neutral pictures led to enhanced activation of ACC, insula, thalamus and visual cortex (Straube et al., 2007). Moreover, anxiety ratings during anticipation of aversive stimuli correlated with activations in dorsal and rostral ACC as well as medial PFC (Straube et al., 2007). These findings are in line with studies on anticipation of aversive stimuli in healthy subjects (e.g. Alvarez et al., 2011, Carlson et al., 2011, Chua et al., 1999, Drabant et al., 2011, Grupe et al., 2013, Kalisch et al., 2006, Nitschke et al., 2006, Shankman et al., 2014, Simmons et al., 2004, Simmons et al., 2006). Furthermore, anticipatory anxiety has been shown to lead to ACC activation in patients with panic disorder while expecting a panic attack (Boshuisen et al., 2002), and to insula activation in social anxiety when anticipating public speaking (Boehme et al., 2014, Lorberbaum et al., 2004).
Aside from these brain regions, Straube et al. (2007) also reported activation in the bed nucleus of the stria terminalis (BNST) during threat anticipation in spider phobics, suggesting involvement of this part of the so-called extended amygdala in anticipatory anxiety in animal phobia (also see Münsterkötter et al., 2015; but see Lueken et al., 2014). A growing body of research emphasizes a dissociation between amygdala and BNST, with the amygdala being involved in rapid processing of imminent threat and the BNST modulating sustained anxiety states in unpredictable threat contexts (Davis et al., 2010, Walker et al., 2003). In some studies with healthy subjects the anticipatory period was analyzed in such a way that it was possible to detect amygdala and BNST activation in one and the same experiment (Alvarez et al., 2011, Grupe et al., 2013, Somerville et al., 2013), for example by separately modeling phasic and sustained brain responses (Grupe et al., 2013).
The current fMRI study aimed to investigate neural correlates of threat anticipation in BII phobia by comparing anticipation of phobia-specific and neutral pictures. Additionally, anxiety and disgust ratings as well as changes in heart rate were examined to control for characteristic emotional and autonomic responses. Based on previous research, we were interested in brain activations in amygdala, BNST, ACC, insula, PFC, thalamus and visual areas during anticipation of phobia-specific in contrast to neutral pictures. Especially with regard to amygdala and BNST, we used an initial as well as a sustained model for BOLD responses in order to separate phasic and sustained brain activation, respectively. We hypothesized that initial amygdala activation and sustained BNST activation would be evident in phobic participants as compared to healthy controls during anticipation of phobia-specific versus neutral stimuli.
2. Material and methods
2.1. Subjects
Sixteen right-handed female subjects with BII phobia (age: 24.1 ± 3.82 years) and 16 right-handed female healthy control subjects (age: 23.7 ± 4.44 years) participated in the study. Only female participants were included since BII phobia is most common in young women (Miloyan and Eaton, 2016, Wani and Ara, 2014) and the majority of studies in specific phobia investigated female samples (for review see Del Casale et al., 2012, Ipser et al., 2013, Van Houtem et al., 2013), which makes the current study more comparable to other studies, especially to the anticipation study by Straube et al. (2007). Participants were recruited by public advertisement and received monetary reimbursement (10 €) or course credit for participation. BII-phobic subjects were selected by means of a short clinical interview (mini-DIPS, Margraf, 1994) based on DSM-IV (American Psychiatric Association, 2000) and ICD-10 (World Health Organization, 1992). Patients and controls were matched with regard to age and level of education. Phobics scored significantly higher than non-phobic controls on the Mutilation Questionnaire (MQ, Klorman et al., 1974) (phobics: mean = 21.5, S.D. = 3.27; controls: mean = 5.94, S.D. = 2.29; t[30] = 15.6, p < 0.001, d = 5.52) and the Disgust Propensity and Sensitivity Scale (DPSS-R, van Overveld et al., 2006) (phobics: mean = 48.06, S.D. = 7.13; controls: mean = 31.25, S.D. = 7.37; t[30] = 6.56, p < 0.001, d = 2.32). In one of the control subject, the clinical interview indicated diagnostic criteria for spider phobia, but without sufficient psychological strain. For none of the reported effects, this subject was an outlier. Participants with psychopathological disorders other than BII phobia were excluded. Additionally, participants had no history of or current neurological disorder and traumatic brain injury. None of the participants had taken psychotropic drugs or beta-blockers for a period of at least three months prior to the study (also see Del Casale et al., 2012). The study conforms to the Declaration of Helsinki and was approved by the ethics committee of the University of Jena, Germany. All participants gave informed consent prior to the experiment.
2.2. Experimental design
In the scanner, participants anticipated the presentation of phobia-specific or neutral pictures. Pictures were selected from the International Affective Picture System (IAPS, Lang et al., 2008). Phobia-specific pictures showed bloody injuries of people and limbs as well as blood-withdrawal (# 3010, 3030, 3051, 3060, 3071, 3100, 3130, 3150, 9405, 9592). Neutral pictures showed people or objects (# 2200, 2214, 2215, 2270, 2383, 5395, 5520, 7010, 7090, 7503) and were matched to the phobia-specific pictures with regard to features, complexity, and color scheme (Adobe Photoshop, Version 13.0.1; Adobe Systems Software Ireland Limited, Ireland; see Supplementary Table 1). Phobia-specific and neutral pictures were used in previous studies on BII phobia (Buodo et al., 2006, Hamm et al., 1997). During the anticipatory period, one of two white cues (circle or square) was presented on black background, indicating whether the following picture would be phobia-specific or neutral. Assignment of the symbols to the conditions was counterbalanced across subjects, and participants were informed about cue-condition association before the session. The experiment comprised 10 phobia-specific and 10 neutral trials presented in pseudo-random order. The anticipatory period lasted 10, 12, or 14 s to ensure unpredictability of stimulus onset. The subsequent picture presentation lasted 12 s to establish a sufficiently threatening context and to be able to detect alterations in heart rate. Between trials, a fixation cross was shown for 16 s. In total, the experiment lasted 13 min.
After the scanning session, participants rated phobia-specific and neutral pictures as well as the respective anticipatory periods on the dimensions anxiety (1 = “not anxious at all” to 9 = “very anxious”) and disgust (1 = “not disgusting at all” to 9 = “very disgusting”) using a nine-point Likert-scale. Furthermore, as a measure of avoidance behavior, participants were requested to answer the question “How long did you look at the unpleasant pictures on average?” (“very briefly”, “a few seconds”, “almost the entire time”, or “I never looked away”). Behavioral data were analyzed by means of mixed-model analyses of variance (ANOVAs) using IBM SPSS software (Version 22; IBM, Armonk, New York, USA), with group (BII phobics vs. healthy controls) as between-subject factor and condition (phobia-specific vs. neutral) as within-subject factor. Post-hoc t-tests were performed to resolve interactions when appropriate. Generally, a p-value of < 0.05 was considered statistically significant. Data are presented as mean ± standard error.
2.3. Pulse oximetry
A pulse oximeter (Pulse Oximeter 8600FO; Nonin Medical Inc., Amsterdam, the Netherlands) was used to determine the participants' heart rate as an indirect measure of blood oxygen saturation and blood volume (Bowes et al., 1989). The signal was recorded together with the experimental events. Data were analyzed with Brain Vision Analyzer (Version 1.03; BrainProducts, Munich, Germany). Due to signal disturbances, one phobic and one control participant had to be excluded from further analysis. For all other subjects, the peak of each pulse wave was detected, and the time interval between two peaks was considered an index for the heart rate. This analysis was conducted separately for anticipation and presentation periods, covering a time window from 500 ms before trial onset until the end of the respective trial. The 500 ms before trial onset were used as baseline. Finally, the mean changes in heart rate for phobia-specific and neutral anticipatory and presentation periods for each participant were calculated. Resulting means were analyzed with mixed-model ANOVAs using SPSS (Version 22; IBM, Armonk, New York, USA) with group serving as between-subject and condition as within-subject factor. Post-hoc t-tests were used to resolve interactions. A p-value of < 0.05 was considered statistically significant. Additionally, heart rate variability was calculated (root mean square of successive differences [RMSSD]; see Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996) to control for influence of autonomic data on fMRI data.
2.4. FMRI
Data were collected with a 1.5 T magnetic resonance scanner (“Magnetom Vision Plus”; Siemens Medical Systems, Erlangen, Germany). The scanning session started with a high-resolution T1-weighted anatomical scan. Afterwards functional data were acquired with a T2*-weighted echo-planar sequence (TE = 50 ms, flip angle = 90°, matrix = 64 × 64, FOV = 192 mm, TR = 4000 ms) with 40 axial slices (thickness = 3 mm, gap = 1 mm, in plane resolution = 3 × 3 mm) in each volume. One run consisted of 205 volumes. FMRI data were preprocessed and analyzed with BrainVoyager QX (Version 2.8; Brain Innovation, Maastricht, the Netherlands). During preprocessing, the first four volumes of each run were discarded to ensure adequate saturation. Data were corrected for slice time errors as well as movement artifacts. Anatomical and functional data were co-registered and normalized to fit Talairach space (Talairach and Tournoux, 1988). Subsequently, data were smoothed spatially (6 mm full-width half maximum [FWHM] Gaussian kernel) and temporally (high pass filter: 3 cycles per run; low pass filter: 2.8 s; linear trend removal). Statistical analysis comprised multiple linear regression of the signal time course at each voxel. The expected BOLD signal change for each condition was modelled with a canonical hemodynamic response function (HRF). For the anticipatory period, we calculated two different general linear models (GLM) (also see Herrmann et al., 2016). In the first GLM, the HRF was modelled over the whole phobia-specific and neutral anticipatory periods (sustained response). In the second GLM, the response was modelled as the HRF initiated by the first second of the anticipatory periods (initial response), while the rest of the anticipatory period was modelled separately as a predictor of no interest. Due to the majority of the phobic subjects stating to have avoided at least some part of the picture presentation phase, and due to predictability of the experimental conditions based on the cues presented at the beginning of each trial (phobia-specific or neutral), fMRI analysis of the picture presentation phase was not informative. Picture presentations were thus defined as predictors of no interest in both GLMs. As a first analysis step, percent-standardized predictor estimates were calculated for each participant by dividing the signal in a given voxel at each time point by the mean of the signal time course. Next, random effects analysis with adjustment for autocorrelation following a global AR(2) model across the individual predictor estimates for planned contrasts was performed.
The analyses were conducted for defined regions of interest (ROIs) as defined a priori. The amygdala ROI was extracted from the anatomy toolbox (Eickhoff et al., 2005) and consisted of amygdala maximum probability maps as recommended by Eickhoff et al. (2006) (also see Herrmann et al., 2016). ROIs for ACC, insula, PFC (dorsolateral superior frontal gyrus, medial superior frontal gyrus, middle frontal gyrus), OFC (orbital superior frontal gyrus, orbital middle frontal gyrus, orbital inferior frontal gyrus), thalamus and visual cortex (cuneus, fusiform gyrus) were defined on the basis of the Automated Anatomical Labeling (AAL) atlas included in the Wake Forest University (WFU) PickAtlas software (Maldjian et al., 2004, Maldjian et al., 2003, Tzourio-Mazoyer et al., 2002). Obtained MNI-coordinates were converted into Talairach space with ICBM2tal (Lancaster et al., 2007). Coordinates for bilateral BNST were defined according to an anatomical atlas (Mai et al., 1997).
To correct for multiple comparisons, the cluster-level statistical threshold estimator plugin for BrainVoyager (Goebel et al., 2006) was used. After setting the voxel-level threshold to p < 0.005 (uncorrected; Lieberman and Cunningham, 2009), a mask consisting of all ROIs was applied to the thresholded maps with an estimated full width at half maximum for spatial smoothness following the approach by Forman et al. (1995) and an iterative procedure (Monte Carlo simulation) with 1000 iterations. The minimum cluster size threshold (189 mm3) with a cluster-level false positive rate of 5% was applied to the statistical maps. The potential influence of autonomic data was tested by correlating mean heart rate and heart rate variability across trials with parameter estimates (anticipation of phobia-specific vs. neutral pictures) within a mask, created on the basis of significant activation clusters found in the ROI analyses.
To further analyze temporal dynamics of differential activations, we first z-standardized differences in parameter estimates (anticipation of phobia-specific – neutral stimuli) resulting from the initial and sustained model within clusters of activation in our main regions of interest (amygdala, BNST, ACC and insula). Standardized data were then analyzed for within- (phasic vs. sustained) and between-group contrasts (model by group interaction) by means of t-tests using SPSS (Version 22; IBM, Armonk, New York, USA).
3. Results
3.1. Behavioral data
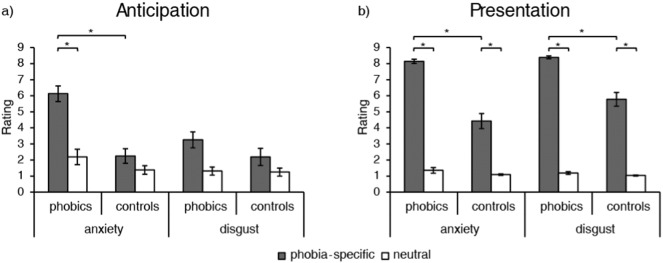
The ANOVA for anxiety ratings during anticipation (Fig. 1) yielded significant main effects for group (F[1, 30] = 25.45, p < 0.001, d = 1.84) and condition (F[1, 30] = 36.89, p < 0.001, d = 2.22) as well as a significant group by condition interaction (F[1, 30] = 14.94, p = 0.001, d = 1.41). Post-hoc analysis revealed significantly higher anxiety ratings for phobia-specific anticipation for phobic as compared to control subjects (t[30] = 5.81, p < 0.001, d = 2.05), while there was no significant group difference for anticipation of neutral pictures. Additionally, phobics, but not control subjects, rated anticipation of phobia-specific stimuli as significantly more anxiety-inducing than anticipation of neutral stimuli (t[15] = 5.96, p < 0.001, d = 2.11). Analysis of disgust ratings for the anticipatory period (Fig. 1) yielded a significant main effect for condition (F[1, 30] = 13.24, p = 0.001, d = 1.33), indicating higher disgust ratings for anticipation of phobia-specific in contrast to neutral pictures. The main effect for group and the interaction effect did not reach significance.
Fig. 1.
Ratings for anxiety (1 = “not anxious at all” to 9 = “very anxious”) and disgust (1 = “not disgusting at all” to 9 = “very disgusting”) in phobic and control subjects for the anticipatory period (a) and picture presentation (b). *p < 0.001.
The ANOVA for anxiety ratings in response to picture presentation (Fig. 1) showed significant main effects for group (F[1, 30] = 58.13, p < 0.001, d = 2.78) and condition (F[1, 30] = 369.97, p < 0.001, d = 7.02), as well as a significant group by condition interaction (F[1, 30] = 43.48, p < 0.001, d = 2.41). Post-hoc analysis revealed that anxiety ratings for phobia-specific pictures were significantly higher in the phobic group than in the control group (t[30] = 7.63, p < 0.001, d = 2.70), while this was not the case for the neutral pictures. Additionally, phobic as well as control participants rated phobia-specific pictures as significantly more anxiety-inducing than neutral pictures (phobics: t[15] = 27.98, p < 0.001, d = 9.89; controls: t[15] = 7.13, p < 0.001, d = 2.52). Disgust ratings for pictures (Fig. 1) also resulted in significant main effects for group (F[1, 30] = 37.14, p < 0.001, d = 2.23) and condition (F[1, 30] = 744.09, p < 0.001, d = 9.96) as well as a significant group by condition interaction (F[1, 30] = 31.28, p < 0.001, d = 2.04). Post-hoc analysis showed significantly higher disgust ratings for phobia-specific pictures in phobic as compared to control subjects (t[30] = 5.98, p < 0.001, d = 2.11), while ratings did not differ between groups for neutral pictures. In both groups, phobia-specific pictures were rated as significantly more disgust-inducing than neutral pictures (phobics: t[15] = 60.49, p < 0.001, d = 21.37; controls: t[15] = 11.27, p < 0.001, d = 3.99).
Answers on avoidance behavior during the experiment (“How long did you look at the unpleasant pictures on average?”) differed between groups. The majority of phobic subjects answered with “almost the entire time” (56.25%), while only few phobics chose “a few seconds” (25%), “I never looked away” (12.5%), or “very briefly” (6.25%). In the control group, most of the subjects answered “I never looked away” (87.5%), while only two subjects answered with “almost the entire time” (12.5%). Statistical analysis showed a significant association between group and chosen answer (Fisher's exact test: p < 0.001).
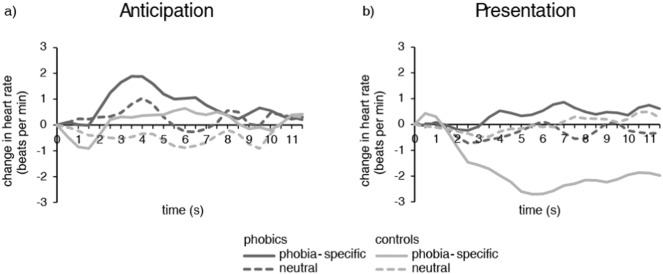
3.2. Heart rate
With regard to the anticipatory period, there were no significant main effects for group or condition, and no significant interaction. For the presentation phase, there was a significant main effect for group (F[1, 28] = 5.99, p = 0.021, d = 0.89) and a significant interaction between group and condition (F[1, 28] = 7.24, p = 0.012, d = 0.98), but no significant main effect for condition. Post-hoc analysis revealed that phobic subjects had a significantly lower change in heart rate during presentation of phobia-specific pictures than healthy controls (0.37 ± 0.59 vs. − 1.78 ± 0.40; t[28] = 3.00, p = 0.006, d = 1.06), while there was no significant difference between groups during presentation of neutral pictures (− 0.28 ± 0.25 vs. − 0.01 ± 0.35). Furthermore, controls had a significantly decreased heart rate in phobia-specific as compared to neutral presentation phases (t[14] = − 3.38, p = 0.005, d = 1.20), while there was no significant difference within the phobic group (Fig. 2).
Fig. 2.
Change in heart rate (beats per minute) for phobic and control subjects over the anticipatory period (a) and picture presentation (b).
3.3. FMRI data
3.3.1. Sustained response
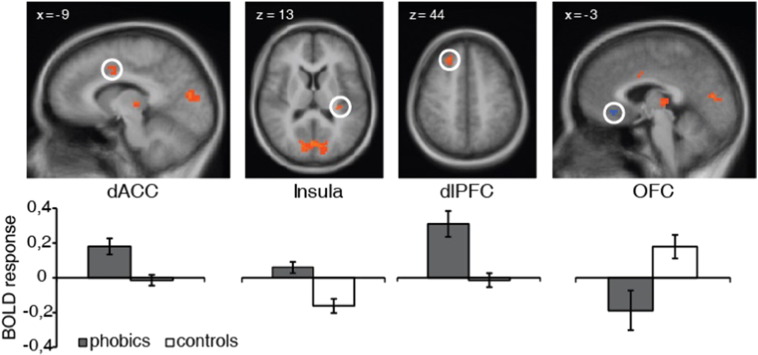
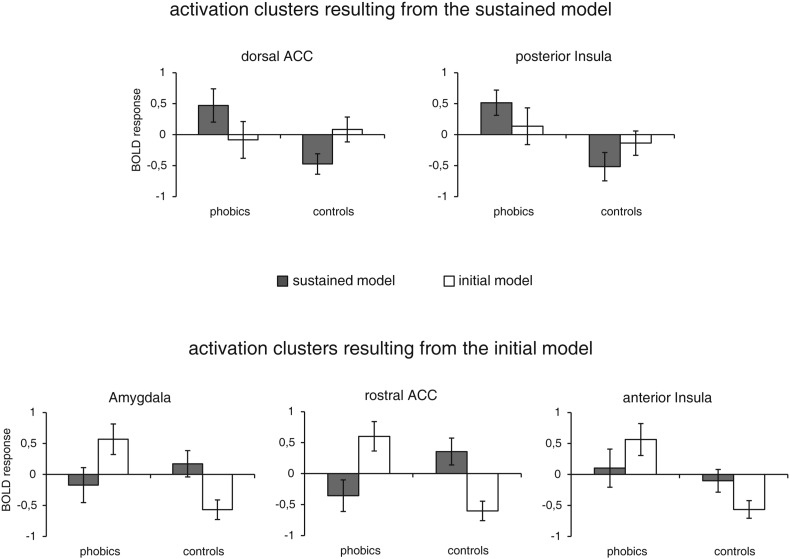
In the first analysis step, the GLM covering the whole anticipatory period was analyzed. ROI analyses for group effects resulted in several activation differences for the contrast phobia-specific > neutral anticipation (Table 1, Fig. 3). Compared to healthy control subjects, phobic subjects showed increased activation in dorsal ACC, posterior insula, dorsolateral prefrontal cortex (dlPFC), dorsomedial prefrontal cortex (dmPFC), thalamus, cuneus and fusiform gyrus as well as decreased activation in medial OFC. There were no significant correlations between these differential activations and heart rate or heart rate variability. The t-tests for standardized parameter estimates for activation clusters in dorsal ACC and posterior insula showed a significant model by group interaction in the dorsal ACC (t[30] = 1.83, p = 0.039, d = 0.65) (Fig. 5).
Table 1.
Significant sustained activations during anticipation of phobia-specific vs. neutral pictures as revealed by ROI analysis.
| Region | Phobics > controls |
Controls > phobics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t-Value | mm3 | x | y | z | t-Value | mm3 | ||
| ACC | L | − 8 | 0 | 37 | 3.20 | 378 | |||||
| Insula | L | − 32 | − 28 | 13 | 3.67 | 189 | |||||
| PFC | |||||||||||
| dlPFC | L | − 25 | 10 | 57 | 3.76 | 297 | |||||
| R | 21 | − 7 | 57 | 3.25 | 567 | ||||||
| R | 22 | 38 | 46 | 3.88 | 351 | ||||||
| dmPFC | R | 12 | 12 | 63 | 3.82 | 432 | |||||
| L/R | 3 | 30 | 60 | 3.29 | 270 | ||||||
| OFC | L/R | − 5 | 32 | − 4 | 3.32 | 243 | |||||
| Thalamus | L | − 4 | − 22 | 5 | 3.59 | 1161 | |||||
| Visual cortex | |||||||||||
| Cuneus | L | − 17 | − 79 | 23 | 3.70 | 324 | |||||
| R | 18 | − 78 | 35 | 4.71 | 999 | ||||||
| L/R | 4 | − 80 | 10 | 4.38 | 4239 | ||||||
| Fusiform gyrus | L | − 26 | − 56 | − 17 | 3.84 | 864 | |||||
| L | − 36 | − 21 | − 17 | 3.75 | 216 | ||||||
ACC, anterior cingulate cortex; PFC, prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; OFC, orbitofrontal cortex; L, left; R, right; (x,y,z), Talairach coordinates of maximally activated voxel (activation threshold: p < 0.05 corrected)
Fig. 3.
Phobic subjects showed increased sustained activation during anticipation of phobia-specific in contrast to neutral pictures in dorsal anterior cingulate cortex (dACC), insula and dorsolateral prefrontal cortex (dlPFC) as well as decreased activation in orbitofrontal cortex (OFC) as compared to healthy control subjects. Statistical parametric maps are overlaid on an averaged T1 scan (radiological convention: left = right). Graphs display contrasts of parameter estimates (phobia-specific vs. neutral picture anticipation; mean ± standard error for activation cluster).
Fig. 5.
Standardized differences in parameter estimates (anticipation of phobia-specific – neutral stimuli; mean ± standard error) resulting from the initial and sustained model within clusters of activation in dorsal anterior cingulate cortex (ACC), posterior insula, amygdala, rostral ACC and anterior insula.
3.3.2. Initial response
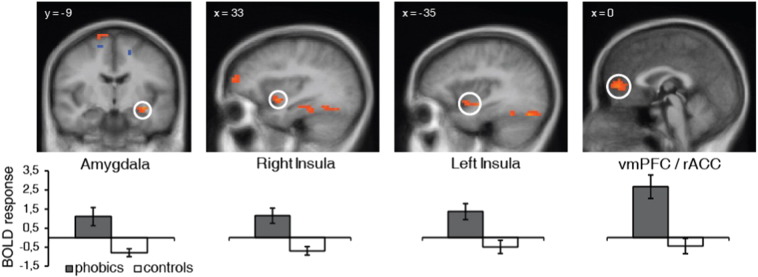
In the ROI analyses for the initial response model of the anticipatory period, phobic as compared to control subjects showed increased activation for the contrast phobia-specific > neutral anticipation in the amygdala, rostral ACC, anterior insula, dmPFC, ventrolateral prefrontal cortex (vlPFC), ventromedial prefrontal cortex (vmPFC), thalamus and fusiform gyrus as well as decreased activation in dmPFC (Table 2, Fig. 4). Again, these differential activations did not correlate with heart rate or heart rate variability. The t-tests for standardized parameter estimates for activation clusters in amygdala, rostral ACC and anterior insula showed significant model by group interactions (amygdala: t[30] = 2.84, p = 0.004, d = 1.00; rostral ACC: t[30] = 3.47, p = 0.001, d = 1.23; anterior insula: t[30] = 1.78, p = 0.043, d = 0.63) (Fig. 5). Furthermore, phobics showed significantly higher parameter estimates in the initial relative to the sustained model in the rostral ACC (t[15] = 2.16, p = 0.024, d = 0.76) and healthy controls showed significantly higher parameter estimates in the sustained relative to the initial model in the amygdala (t[15] = 2.76, p = 0.007, d = 0.98), rostral ACC (t[15] = 2.90, p = 0.006, d = 1.03) and anterior insula (t[15] = 1.81, p = 0.045, d = 0.64).
Table 2.
Significant initial activations during anticipation of phobia-specific vs. neutral pictures as revealed by ROI analysis.
| Region | Phobics > controls |
Controls > phobics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t-Value | mm3 | x | y | z | t-Value | mm3 | ||
| Amygdala | L | − 30 | − 7 | − 10 | 4.68 | 297 | |||||
| ACC | L/R | − 1 | 46 | 7 | 3.79 | 351a | |||||
| Insula | L | − 33 | − 7 | − 9 | 4.08 | 378 | |||||
| R | 31 | 5 | − 7 | 4.15 | 324 | ||||||
| PFC | |||||||||||
| dmPFC | L | − 16 | − 6 | 55 | 3.44 | 189 | |||||
| R | 9 | − 6 | 72 | 3.24 | 270 | 13 | − 4 | 61 | 3.29 | 189 | |
| vlPFC | L | − 29 | 38 | 14 | 3.11 | 189 | |||||
| R | 31 | 57 | 19 | 3.48 | 594 | ||||||
| vmPFC | R | 18 | 38 | 14 | 3.41 | 513 | |||||
| L/R | − 2 | 48 | 8 | 4.29 | 1836a | ||||||
| Thalamus | L | − 9 | − 13 | 11 | 3.29 | 540 | |||||
| Visual cortex | |||||||||||
| Fusiform gyrus | L | − 41 | − 48 | − 17 | 4.39 | 1107 | |||||
| L | − 38 | − 69 | − 20 | 4.79 | 945 | ||||||
| R | 26 | − 44 | − 16 | 4.54 | 3510 | ||||||
ACC, anterior cingulate cortex; PFC, prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; L, left; R, right; (x,y,z), Talairach coordinates of maximally activated voxel (activation threshold: p < 0.05 corrected)
These activation clusters are interconnected.
Fig. 4.
Phobic subjects showed increased initial activation during anticipation of phobia-specific in contrast to neutral pictures in left amygdala, right and left anterior insula and a cluster of increased activation extending from the ventromedial prefrontal cortex (vmPFC) to the rostral anterior cingulate cortex (rACC). Statistical parametric maps are overlaid on an averaged T1 scan (radiological convention: left = right). Graphs display contrasts of parameter estimates (phobia-specific vs. neutral picture anticipation; mean ± standard error for activation cluster).
4. Discussion
The present study demonstrates increased activation of BII phobics in several brain regions during anticipatory anxiety. Specifically, sustained phobia-specific relative to neutral picture anticipation was associated with hyperactivations in ACC, insula, PFC, thalamus and visual areas in phobics as compared to controls. For initial phobia-specific relative to neutral anticipation, phobic subjects as compared to controls showed increased activation in amygdala, ACC, insula, PFC, thalamus and fusiform gyrus. On the behavioral level, the anticipatory period was characterized by increased anxiety but not disgust and measures of heart rate did not indicate biphasic response patterns in phobic subjects.
BII phobia is associated with high disgust-sensitivity (de Jong and Merckelbach, 1998, Sawchuk et al., 2002, Tolin et al., 1997). This is confirmed by the present rating data for picture presentation. However, no such effect was found for the anticipatory period. This stands in contrast to increased anxiety ratings in BII phobia for both anticipation and presentation of phobia-specific pictures. Consequently, anxiety seems to be the dominant emotion during threat anticipation, which makes the emotional experience similar to other specific phobias (Straube et al., 2007) and other anxiety disorders (Andrews et al., 1994, Grillon et al., 2008, Grillon et al., 2009). The current fMRI results support this assumption, as findings replicate activation patterns previously shown for anticipatory anxiety in spider phobia (Münsterkötter et al., 2015, Straube et al., 2007), social anxiety disorder (Boehme et al., 2014, Lorberbaum et al., 2004) and panic disorder (Boshuisen et al., 2002, Wittmann et al., 2014). This is especially interesting in the light of fMRI studies that investigated confrontation with phobia-relevant stimuli in BII phobia which suggest inconclusive and unspecific results (Caseras et al., 2010a, Caseras et al., 2010b, Hermann et al., 2007, Lueken et al., 2011, Schienle et al., 2003). More specifically, direct comparison between BII and animal phobics showed distinct neural substrates for the two subtypes (Caseras et al., 2010a, Caseras et al., 2010b, Lueken et al., 2011).
During fear and anxiety, ACC activation is often associated with insula hyper-reactivity (Critchley et al., 2004, Medford and Critchley, 2010, Shenhav et al., 2013, Straube et al., 2006a, Straube et al., 2006b) and both regions are implicated in specific phobia (Del Casale et al., 2012, Ipser et al., 2013, Linares et al., 2012). This is reflected by the current results, with increased activation of dorsal ACC and posterior insula during sustained anticipation of phobia-specific pictures in BII phobics. ACC has been shown to be active during threat processing in animal phobia (Britton et al., 2009, Carlsson et al., 2004, Goossens et al., 2007, Straube et al., 2006a, Straube et al., 2006b). More specifically, dorsal ACC has been associated with increased drive for action, scanning of the environment and adaptive control during anticipation of aversive stimuli (Grupe et al., 2013, Straube et al., 2007). Insula hyperactivation, on the other hand, has been implicated in interoception and representation of bodily states (Craig, 2002, Craig, 2009, Critchley et al., 2004, Grupe and Nitschke, 2013). Several studies have also shown insula activation during anticipation in patients with specific phobia (Straube et al., 2007), social phobia (Boehme et al., 2014, Lorberbaum et al., 2004), or panic disorder with agoraphobia (Wittmann et al., 2014), and also in healthy controls (e.g. Carlson et al., 2011, Chua et al., 1999, Ploghaus et al., 1999, Simmons et al., 2004, Simmons et al., 2006). The combination of ACC and insula activation in the current study suggests that BII phobics allocate more attention, externally as well as internally, during anticipation of phobia-specific pictures. In line with this notion, increased activation in visual areas (fusiform gyrus and cuneus) was observed in BII phobics. This is consistent with previous symptom provocation studies in specific phobia (Caseras et al., 2010a, Schienle et al., 2003, Straube et al., 2005, Straube et al., 2006b), and has been attributed to increased visual attention in the expectation of behaviorally relevant visual input during anticipatory anxiety (Straube et al., 2007). Furthermore, in accordance with results for other anxiety disorders (Boshuisen et al., 2002, Straube et al., 2007), increased thalamus activation was found during phobia-specific anticipation in BII phobics, which is suggested to reflect general arousal states and stress (Vertes et al., 2015).
Current findings include several clusters of increased activation during sustained anticipation in medial and lateral PFC for BII phobics as compared to healthy controls. This region has generally been suggested to integrate emotional and cognitive processing (Pessoa, 2008), and seems to be important for appraisal and regulation of emotionally relevant situations (Amodio and Frith, 2006, Hermann et al., 2007, Kalisch et al., 2006, Kerr et al., 2012, Ochsner and Gross, 2005), also with regard to specific phobia (Del Casale et al., 2012, Ipser et al., 2013, Linares et al., 2012). Additionally, mPFC is part of the so-called default mode network under baseline and resting state conditions, and is involved in introspection as well as self-referential mental activity (Raichle, 2015, Raichle et al., 2001). In previous studies, medial and lateral PFC have been shown to be involved in anticipatory anxiety in healthy subjects (Drabant et al., 2011, Nitschke et al., 2006, Ploghaus et al., 1999). On the contrary, the current findings show decreased activation of the medial part of the OFC in BII phobics in contrast to healthy controls. Similar deactivation has been reported in BII phobics during symptom provocation, likely reflecting reduced control over emotional responses (Hermann et al., 2007). The simultaneous increase and decrease of activation in PFC subregions in the present study indicates differential functions of the PFC in sustained anticipatory anxiety in BII phobics. Enhanced prefrontal activity could reflect increased appraisal of the anticipatory context as well as increased self-perception in BII phobics. Deactivation of medial OFC, on the other hand, indicates that BII phobics might have difficulties down-regulating negative emotions. Furthermore, it has been suggested that the default mode network is directly competing with other systems and as a consequence focused attention on external stimuli tends to reduce activation in the default mode network (Buckner et al., 2008). An alternative explanation could thus be that the activation increases and decreases in PFC subregions reflect competing systems. Consequently, the medial OFC as part of the default mode network might show decreased activation because the anticipation of phobia-specific stimuli demands more attentional resources in BII phobics as compared to controls.
Beyond that, the current analysis revealed activation of the amygdala with a model for the initial anticipatory period. Such an effect has not been found in studies which only considered sustained anticipatory anxiety (e.g. Boshuisen et al., 2002, Chua et al., 1999, Jensen et al., 2003, Ploghaus et al., 1999, Straube et al., 2007). In line with previous studies in healthy subjects (Alvarez et al., 2011, Grupe et al., 2013, Somerville et al., 2013), analysis of initial brain responses facilitated the detection of amygdala activation, even though a prolonged anticipation design was used. This supports involvement of the amygdala in rapid threat processing (LeDoux, 1998, Öhman and Mineka, 2001) and again demonstrates that it is possible to meet temporal characteristics with specific analysis strategies (Grupe et al., 2013, Somerville et al., 2013).
There was no differential activation of BNST. This stands in contrast to findings reported for spider phobia (Münsterkötter et al., 2015, Straube et al., 2007). Research in animals and humans revealed intensive connections of BNST with other limbic regions (Avery et al., 2014, Davis et al., 2010) and indicates a role of BNST in sustained anxiety states (Alvarez et al., 2011, Grupe et al., 2013, Somerville et al., 2013). The emotional relevance of the stimulus material also in healthy control subjects (Bradley et al., 2001, Codispoti et al., 2003, Grondin et al., 2014) could have made the detection of a differential effect in a small brain region like the BNST difficult.
With regard to different specific phobia subtypes, the present study demonstrates commonalities among these subtypes during anticipatory anxiety. Increased activation was found in brain regions that were previously suggested to play a role in anticipation of phobia-specific stimuli in spider phobia (Münsterkötter et al., 2015, Straube et al., 2007). This stands in contrast to studies on phobia-specific picture processing, which found similar (Caseras et al., 2010b) but also distinct brain activations (Caseras et al., 2010a, Lueken et al., 2011) for animal phobia in comparison to BII phobia. Taken together, these findings suggest that diagnostic classifications for specific phobia are meaningful for emotional processing during confrontation with phobia-specific stimuli, but that anticipatory anxiety could be a common denominator among specific phobia subtypes.
The current results demonstrate different temporal characteristics of brain areas under investigation. All brain regions found to be active during sustained anticipation were also detected in the initial phase of anticipation, with the exception of the amygdala which was only active during onset of the anticipatory period. However, while posterior insula was active during sustained anticipation, initial anticipation led to anterior insula activation. Similarly, dorsal ACC was active in the sustained condition, while rostral ACC activity was found in the initial anticipatory period. These differences might reflect a role of different subregions with distinct functions and temporal characteristics during anticipatory anxiety and should be investigated in more detail in the future.
The results provide no evidence for a change in heart rate, and consequently no evidence for a biphasic response pattern in BII phobics during anticipation or presentation of phobia-specific in contrast to neutral pictures. Even though BII phobia is consistently associated with a biphasic autonomic response pattern, results in previous studies are equivocal (Lumley and Melamed, 1992, Sarlo et al., 2008, Thyer and Curtis, 1985). It seems as if only a minority of BII phobics displays a biphasic autonomic response (Ritz et al., 2013). Additionally, longer anticipation and presentation intervals might have been necessary to detect distinct alterations in heart rate (Graham et al., 1961). The drop in heart rate in the control group during phobia-specific picture presentation has also been observed in other studies (Caseras et al., 2010b) and was suggested to indicate defensive reactivity in response to threatening stimuli (Azevedo et al., 2005). The reason why only healthy controls show a deceleration in heart rate could be that the majority of phobic subjects stated to have avoided at least part of the phobia-specific picture presentation, while healthy controls did not exhibit such avoidance behavior. As a consequence, the pictures might have led to stronger physiological responses in this group.
In the end, some limitations of the current study should be mentioned. The sample size was quite small. However, due to clear a priori hypotheses, the current findings should be considered relevant for a neurobiological model of BII phobia. Furthermore, the current sample included only female BII phobics. Although specific phobias are more prevalent in women than in men (Bienvenu and Eaton, 1998, Ost, 1992), the generalizability of the current results to BII phobics in general is restricted. Regarding the analysis, results are constrained by the choice of ROIs. However, especially with regard to the small sample size, a ROI-based approach is reasonable in order to avoid disregarding important neural activations. Future studies with whole-brain analyses are needed in order to test the specificity of the current findings, also in samples with both male and female participants. Additionally, validity of anticipatory cues could be varied to gain a more detailed understanding of threat anticipation and its neural correlates in specific phobia.
5. Conclusions
The current study demonstrates brain activations related to anticipatory anxiety in BII phobia. While previous studies on symptom provocation in specific phobia reported inconsistent results, neural correlates during anticipatory anxiety could constitute a common denominator for specific phobia subtypes. In the future, it would be interesting to extent the current results by directly comparing different subtypes of specific phobia during anticipation of phobia-specific stimuli. Furthermore, modeling of initial and sustained brain responses revealed distinct temporal dynamics of the brain regions involved in anticipatory anxiety, also within one and the same brain region. These temporal dynamics should be considered during conceptualization of study designs and data analysis in future studies.
The following are the supplementary data related to this article.
Characteristics of phobia-specific and neutral pictures (IAPS)1.
Conflicts of interest
The authors report no financial relationships with commercial interests.
Acknowledgments
This work was supported by the German Research Foundation (DFG: SFB/TRR 58: C06, C07) and the Open Access Publication Fund of the University of Muenster.
References
- Alvarez R.P., Chen G., Bodurka J., Kaplan R., Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (Washington, D.C.) [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews G., Freed S., Teesson M. Proximity and anticipation of a negative outcome in phobias. Behav. Res. Ther. 1994;32:643–645. doi: 10.1016/0005-7967(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Aue T., Hoeppli M.-E. Evidence for an encounter expectancy bias in fear of spiders. Cognit. Emot. 2011;26:727–736. doi: 10.1080/02699931.2011.602241. [DOI] [PubMed] [Google Scholar]
- Avery S.N., Clauss J.A., Winder D.G., Woodward N., Heckers S., Blackford J.U. BNST neurocircuitry in humans. NeuroImage. 2014;91C:311–323. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo T.M., Volchan E., Imbiriba L.A., Rodrigues E.C., Oliveira J.M., Oliveira L.F., Lutterbach L.G., Vargas C.D. A freezing-like posture to pictures of mutilation. Psychophysiology. 2005;42:255–260. doi: 10.1111/j.1469-8986.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Bienvenu O.J., Eaton W.W. The epidemiology of blood-injection-injury phobia. Psychol. Med. 1998;28:1129–1136. doi: 10.1017/s0033291798007144. [DOI] [PubMed] [Google Scholar]
- Boehme S., Ritter V., Tefikow S., Stangier U., Strauss B., Miltner W.H., Straube T. Brain activation during anticipatory anxiety in social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2014;9:1413–1418. doi: 10.1093/scan/nst129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuisen M.L., Ter Horst G.J., Paans A.M., Reinders A.A., den Boer J.A. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol. Psychiatry. 2002;52:126–135. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Bowes W.A., Corke B.C., Hulka J. Pulse oximetry: a review of the theory, accuracy, and clinical applications. Obstet. Gynecol. 1989;74:541–546. [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Cuthbert B.N., Lang P.J. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Britton J.C., Gold A.L., Deckersbach T., Rauch S.L. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depress. Anxiety. 2009;26:796–805. doi: 10.1002/da.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buodo G., Sarlo M., Codispoti M., Palomba D. Event-related potentials and visual avoidance in blood phobics: is there any attentional bias? Depress. Anxiety. 2006;23:304–311. doi: 10.1002/da.20172. [DOI] [PubMed] [Google Scholar]
- Carlson J.M., Greenberg T., Rubin D., Mujica-Parodi L.R. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc. Cogn. Affect. Neurosci. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K., Petersson K.M., Lundqvist D., Karlsson A., Ingvar M., Öhman A. Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 2004;4:340–353. doi: 10.1037/1528-3542.4.4.340. [DOI] [PubMed] [Google Scholar]
- Caseras X., Giampietro V., Lamas A., Brammer M., Vilarroya O., Carmona S., Rovira M., Torrubia R., Mataix-Cols D. The functional neuroanatomy of blood-injection-injury phobia: a comparison with spider phobics and healthy controls. Psychol. Med. 2010;40:125–134. doi: 10.1017/S0033291709005972. [DOI] [PubMed] [Google Scholar]
- Caseras X., Mataix-Cols D., Trasovares M.V., Lopez-Sola M., Ortriz H., Pujol J., Soriano-Mas C., Giampietro V., Brammer M.J., Torrubia R. Dynamics of brain responses to phobic-related stimulation in specific phobia subtypes. Eur. J. Neurosci. 2010;32:1414–1422. doi: 10.1111/j.1460-9568.2010.07424.x. [DOI] [PubMed] [Google Scholar]
- Chua P., Krams M., Toni I., Passingham R., Dolan R. A functional anatomy of anticipatory anxiety. NeuroImage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Codispoti M., Gerra G., Montebarocci O., Zaimovic A., Augusta Raggi M., Baldaro B. Emotional perception and neuroendocrine changes. Psychophysiology. 2003;40:863–868. doi: 10.1111/1469-8986.00104. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel – now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong P.J., Merckelbach H. Blood-injection-injury phobia and fear of spiders: domain specific individual differences in disgust sensitivity. Personal. Individ. Differ. 1998;24:153–158. [Google Scholar]
- Del Casale A., Ferracuti S., Rapinesi C., Serata D., Piccirilli M., Savoja V., Kotzalidis G.D., Manfredi G., Angeletti G., Tatarelli R., Girardi P. Functional neuroimaging in specific phobia. Psychiatry Res. 2012;202:181–197. doi: 10.1016/j.pscychresns.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Drabant E.M., Kuo J.R., Ramel W., Blechert J., Edge M.D., Cooper J.R., Goldin P.R., Hariri A.R., Gross J.J. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. NeuroImage. 2011;55:401–410. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Heim S., Zilles K., Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L., Schruers K., Peeters R., Griez E., Sunaert S. Visual presentation of phobic stimuli: amygdala activation via an extrageniculostriate pathway? Psychiatry Res. Neuroimaging. 2007;155:113–120. doi: 10.1016/j.pscychresns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Graham D.T., Kabler J.D., Lunsford L., Jr. Vasovagal fainting: a diphasic response. Psychosom. Med. 1961;23:493–507. doi: 10.1097/00006842-196111000-00004. [DOI] [PubMed] [Google Scholar]
- Gray L.A., McNaughton N. Oxford University Press; Oxford: 2000. The Neuropsychology of Anxiety. [Google Scholar]
- Grillon C., Lissek S., Rabin S., McDowell D., Dvir S., Pine D.S. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am. J. Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Pine D.S., Lissek S., Rabin S., Bonne O., Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin S., Laflamme V., Gontier É. Effect on perceived duration and sensitivity to time when observing disgusted faces and disgusting mutilation pictures. Atten. Percept. Psychophys. 2014;76:1522–1534. doi: 10.3758/s13414-014-0682-7. [DOI] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D.W., Oathes D.J., Nitschke J.B. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb. Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm A.O., Cuthbert B.N., Globisch J., Vaitl D. Fear and the startle reflex: blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Hermann A., Schäfer A., Walter B., Stark R., Vaitl D., Schienle A. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biol. Psychol. 2007;75:124–130. doi: 10.1016/j.biopsycho.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Boehme S., Becker M.P., Tupak S.V., Guhn A., Schmidt B., Brinkmann L., Straube T. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum. Brain Mapp. 2016;37:1091–1102. doi: 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser J.C., Singh L., Stein D.J. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin. Neurosci. 2013;67:311–322. doi: 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- Jensen J., McIntosh A.R., Crawley A.P., Mikulis D.J., Remington G., Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kalisch R., Wiech K., Critchley H.D., Dolan R.J. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. NeuroImage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kerr D.L., McLaren D.G., Mathy R.M., Nitschke J.B. Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Front. Psychol. 2012;3(557) doi: 10.3389/fpsyg.2012.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klorman R., Hastings J.E., Weerts T.C., Melamed B.G., Lang P.J. Psychometric description of some specific-fear questionnaires. Behav. Ther. 1974;5:401–409. [Google Scholar]
- Lancaster J.L., Tordesillas-Gutierrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. (Technical Report A-8). [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biol. Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares I.M., Trzesniak C., Chagas M.H., Hallak J.E., Nardi A.E., Crippa J.A. Neuroimaging in specific phobia disorder: a systematic review of the literature. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) 2012;34:101–111. [PubMed] [Google Scholar]
- Lorberbaum J.P., Kose S., Johnson M.R., Arana G.W., Sullivan L.K., Hamner M.B., Ballenger J.C., Lydiard R.B., Brodrick P.S., Bohning D.E., George M.S. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Lueken U., Kruschwitz J.D., Muehlhan M., Siegert J., Hoyer J., Wittchen H.-U. How specific is specific phobia? Different neural response patterns in two subtypes of specific phobia. NeuroImage. 2011;56:363–372. doi: 10.1016/j.neuroimage.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Lueken U., Hilbert K., Stolyar V., Maslowski N.I., Beesdo-Baum K., Wittchen H.U. Neural substrates of defensive reactivity in two subtypes of specific phobia. Soc. Cogn. Affect. Neurosci. 2014;9:1668–1675. doi: 10.1093/scan/nst159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley M.A., Melamed B.G. Blood phobics and nonphobics: psychological differences and affect during exposure. Behav. Res. Ther. 1992;30:425–434. doi: 10.1016/0005-7967(92)90026-d. [DOI] [PubMed] [Google Scholar]
- Mai J.K., Assheuer J., Paxinos G. Academic Press; San Diego: 1997. Atlas of the Human Brain. [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Margraf J. Springer; 1994. Diagnostisches Kurz-Interview bei psychischen Störungen. [Google Scholar]
- Marks I. Blood-injury phobia: a review. Am. J. Psychiatry. 1988;145:1207–1213. doi: 10.1176/ajp.145.10.1207. [DOI] [PubMed] [Google Scholar]
- Medford N., Critchley H.D. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloyan B., Eaton W.W. Blood-injection-injury phobia in older adults. Int. Psychogeriatr. 2016:1–6. doi: 10.1017/S1041610215002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterkötter A.L., Notzon S., Redlich R., Grotegerd D., Dohm K., Arolt V., Kugel H., Zwanzger P., Dannlowski U. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depress. Anxiety. 2015;32:656–663. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- Nitschke J.B., Sarinopoulos I., Mackiewicz K.L., Schaefer H.S., Davidson R.J. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Öhman A., Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol. Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Oosterink F.M., de Jongh A., Hoogstraten J. Prevalence of dental fear and phobia relative to other fear and phobia subtypes. Eur. J. Oral Sci. 2009;117:135–143. doi: 10.1111/j.1600-0722.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- Ost L.G. Blood and injection phobia: background and cognitive, physiological, and behavioral variables. J. Abnorm. Psychol. 1992;101:68–74. doi: 10.1037//0021-843x.101.1.68. [DOI] [PubMed] [Google Scholar]
- Page A.C. Blood-injury phobia. Clin. Psychol. Rev. 1994;14:443–461. [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Ploghaus A., Tracey I., Gati J.S., Clare S., Menon R.S., Matthews P.M., Rawlins J.N. Dissociating pain from its anticipation in the human brain. Sci. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Meuret A.E., Simon E. Cardiovascular activity in blood-injection-injury phobia during exposure: evidence for diphasic response patterns? Behav. Res. Ther. 2013;51:460–468. doi: 10.1016/j.brat.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Sarlo M., Buodo G., Munafo M., Stegagno L., Palomba D. Cardiovascular dynamics in blood phobia: evidence for a key role of sympathetic activity in vulnerability to syncope. Psychophysiology. 2008;45:1038–1045. doi: 10.1111/j.1469-8986.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- Sawchuk C.N., Lohr J.M., Westendorf D.H., Meunier S.A., Tolin D.F. Emotional responding to fearful and disgusting stimuli in specific phobics. Behav. Res. Ther. 2002;40:1031–1046. doi: 10.1016/s0005-7967(01)00093-6. [DOI] [PubMed] [Google Scholar]
- Schienle A., Schäfer A., Stark R., Walter B., Kirsch P., Vaitl D. Disgust processing in phobia of blood-injection-injury: an fMRI study. J. Psychophysiol. 2003;17:87–93. [Google Scholar]
- Shankman S.A., Gorka S.M., Nelson B.D., Fitzgerald D.A., Phan K.L., O'Daly O. Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport. 2014;25:596–600. doi: 10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Matthews S.C., Stein M.B., Paulus M.P. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- Simmons A., Strigo I., Matthews S.C., Paulus M.P., Stein M.B. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol. Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Wagner D.D., Wig G.S., Moran J.M., Whalen P.J., Kelley W.M. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb. Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Straube T., Glauer M., Dilger S., Mentzel H.-J., Miltner W.H.R. Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol. Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. NeuroImage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; New York: 1988. Co-planar Stereotactic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thyer B.A., Curtis G.C. On the diphasic nature of vasovagal fainting associated with blood-injury-illness phobia. The Pavlovian Journal of Biological Science. 1985;20:84–87. doi: 10.1007/BF03003257. [DOI] [PubMed] [Google Scholar]
- Tolin D.F., Lohr J.M., Sawchuk C.N., Lee T.C. Disgust and disgust sensitivity in blood-injection-injury and spider phobia. Behav. Res. Ther. 1997;35:949–953. doi: 10.1016/s0005-7967(97)00048-x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Houtem C.M., Laine M.L., Boomsma D.I., Ligthart L., van Wijk A.J., De Jongh A. A review and meta-analysis of the heritability of specific phobia subtypes and corresponding fears. J. Anxiety Disord. 2013;27:379–388. doi: 10.1016/j.janxdis.2013.04.007. [DOI] [PubMed] [Google Scholar]
- van Overveld W.J.M., de Jong P.J., Peters M.L., Cavanagh K., Davey G.C.L. Disgust propensity and disgust sensitivity: separate constructs that are differentially related to specific fears. Personal. Individ. Differ. 2006;41:1241–1252. [Google Scholar]
- Vertes R.P., Linley S.B., Hoover W.B. Limbic circuitry of the midline thalamus. Neurosci. Biobehav. Rev. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Toufexis D.J., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wani A.L., Ara A. Blood injury and injection phobia: the neglected one. Behav. Neurol. 2014;2014:471340. doi: 10.1155/2014/471340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann A., Schlagenhauf F., Guhn A., Lueken U., Gaehlsdorf C., Stoy M., Bermpohl F., Fydrich T., Pfleiderer B., Bruhn H., Gerlach A.L., Kircher T., Straube B., Wittchen H.U., Arolt V., Heinz A., Strohle A. Anticipating agoraphobic situations: the neural correlates of panic disorder with agoraphobia. Psychol. Med. 2014;44:2385–2396. doi: 10.1017/S0033291713003085. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of phobia-specific and neutral pictures (IAPS)1.