Abstract
This work investigated the capacity of (−)-epicatechin to prevent the renal damage induced by LPS administration in rats. Male Sprague Dawley rats were fed for 4 days a diet without or with supplementation with (−)-epicatechin (80 mg/kg BW/d), and subsequently i.p. injected with lipopolysaccharide (LPS). Six hours after injection, LPS-treated rats exhibited increased plasma creatinine and urea levels as indicators of impaired renal function. The renal cortex of the LPS-treated rats showed: i) increased expression of inflammatory molecules (TNF-α, iNOS and IL-6); ii) activation of several steps of NF-κB pathway; iii) overexpression of TLR4, and iv) higher superoxide anion production and lipid peroxidation index in association with increased levels of gp91phox and p47phox (NOX2) and NOX4. Pretreatment with dietary (−)-epicatechin prevented the adverse effects of LPS challenge essentially by inhibiting TLR4 upregulation and NOX activation and the consequent downstream events, e.g. NF-kB activation.
Abbreviations: IκBα, inhibitor of κB α; IKKα/β, IκB kinase α/β; IL-6, interleukin-6; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MDA, malondialdehyde; NOX, NADPH oxidase; SOD, superoxide dismutase; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor α
Keywords: Endotoxemia, Flavonoids, Renopathies, Toll-like receptors, Reactive oxygen species
1. Introduction
Chronic inflammation is a common feature on many cardiometabolic diseases such as obesity, insulin-resistance and dyslipidemia [1], [2]. All these conditions contribute to the development of chronic kidney disease [3]. In addition, inflammatory processes are deeply involved in acute kidney failure, a frequent clinical problem in hospitalized patients, characterized by a sudden loss of kidney ability to excrete wastes, maintain fluid balance, etc. [4].
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) comprises a family of pleiotropic transcription factors that play an important role in regulating the expression of genes involved in different cell processes, including inflammation [5], [6]. NF-κB activation is considered a hallmark in chronic as well as in acute inflammatory processes [5]. Lipopolysaccharide (LPS) is a component of the outer membrane of Gram-negative bacteria used in experimental models of inflammation. LPS is a potent ligand for toll-like receptor 4 (TLR4), leading to the canonical activation of NF-κB, and the associated expression of proinflammatory mediators, such as tumor necrosis factor α (TNFα), and interleukin-6 (IL-6) [5], [7].
Additionally, it was reported that LPS triggers a NADPH oxidase (NOX)-dependent oxidant generation. LPS administration has been proposed to activate NOX via TLR4 [8], [9]. The molecular associations between TLR4 and NOX2 are not elucidated, but for NOX4 a direct interaction of the NOX4 protein with the COOH-terminal region of TLR4 has been proposed as the activating mechanism [10].
The beneficial effects of fruits and vegetables on human health can be in part attributed to their high content of flavonoids [11], [12]. Epidemiological and experimental data demonstrate that dietary flavonoids and their metabolites contribute to the prevention and/or amelioration of the risk factors for cardiometabolic diseases [13], [14], [15]. Many in vitro and in vivo studies in various tissues support the anti-inflammatory effects of the flavanol (−)-epicatechin by attenuating the activation of the NF-κB signaling pathway [16], [17], [18], [19].
The aim of this work was to evaluate the capacity of (−)‐epicatechin to mitigate the kidney dysfunction induced by LPS administration in rats. Our results show that (‒)-epicatechin supplementation prevented kidney functional alterations induced by LPS administration by inhibiting the activation TLR4-NF-κB pathway and the NOX-dependent oxidant production.
2. Materials and methods
2.1. Materials
Primary antibodies for inducible nitric oxide synthase (iNOS) (#7271), TNFα (#1350), heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) (#32301), IL-6 (#1265), TLR4 (#30002), p47phox (#7660), gp91phox (#5827), NOX4 (#21860) and β-actin (#47778), and secondary antibodies rabbit anti-goat IgG-HRP (#2768), mouse anti-rabbit IgG-HRP (#2357), and goat anti-mouse IgG-HRP (#2005) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The primary and secondary antibodies for IκB kinase α/β (IKKα/β) (#11930), p-IKKα/β (Ser176/180) (#2697), inhibitor of κBα (IκBα) (#4814), p-IkBα (Ser32) (#2859), NF-κB p65 (#8242) and p-NF-κB p65 (Ser536) (#3033) were from Cell Signaling Technology (Boston, MA, USA). The primary antibody for rat CD 68, clone ED1 (MCA341R) was from BioRad Laboratories Inc. (Hercules, CA, USA). (−)-Epicatechin, LPS from Escherichia coli serotype 0127: B8, NADPH, 2-thiobarbituric acid, N,N′-dimethyl-9,9′-biacridinium dinitrate (lucigenin), and superoxide dismutase (SOD) were from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Animals, diets and experimental design
All procedures were in agreement with standards for the care of laboratory animals as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and were approved by the Secretary of Science and Technology of the School of Pharmacy and Biochemistry, University of Buenos Aires (EXP-FYB n° 0067147/2015). Male Sprague Dawley rats were housed under conditions of controlled temperature (21–25 °C) and humidity, with a 12 h light/dark cycle. Rats were randomly divided in 2 groups: one group receiving tap water ad libitum and a standard rat chow diet and the other receiving tap water ad libitum and the standard rat chow diet supplemented with (−)-epicatechin (80 mg/kg BW/d). After 4 days on the respective diet, half of the animals that received standard rat chow were administered LPS from Escherichia coli serotype 0127: B8 (4 mg/kg BW) i.p. (L group) and the other half were administered saline solution i.p. (C group). All the animals that received the diet supplemented with (−)-epicatechin were administered LPS i.p. (4 mg/kg BW) (LE group) (n=8 for all groups). After 6 h, the animals were weighed and euthanized with CO2. A group with rats receiving only (−)-epicatechin was not included because the objective of the model used was to establish if (−)-epicatechin can prevent LPS-mediated damage through a mechanism that is only operative under inflammatory conditions. Blood was collected from the abdominal aorta into heparinized tubes, plasma was obtained after centrifugation (600×g, 15 min, 4 °C), and frozen at −80 °C. Kidneys were excised and renal cortex was immediately isolated and processed for immunohistochemistry, Western blot analysis, and biochemical determinations.
2.3. Plasma determinations
Creatinine and urea levels in plasma were measured using commercially available kits from Wiener Lab. (Rosario, Argentina). All the determinations were performed following the manufacturers’ protocols.
2.4. Immunohistochemistry of renal cortex
Renal cortex portions were fixed in 10% formaldehyde in PBS (7.6 mM KH2PO4, 42.4 mM K2HPO4, 150 mM NaCl, pH: 7.4), and embedded in paraffin. Three-micron sections were cut and subjected to immunochemistry. Briefly, the sections were deparaffinized with xylene, rehydrated through graded series of ethanol to water, and then incubated in blocking solution (PBS plus 1% bovine serum) at room temperature for 1 h. Then the sections were incubated overnight at 4 °C with primary antibodies against the monocyte and macrophage marker ED1 (1:400 in PBS in blocking solution), or against IL-6 (1:100 in PBS in blocking solution). Immunostaining was carried out with an avidin-biotin-peroxidase complex kit and hematoxylin counterstaining as described [20], and quantified as the percentage of positive staining per area from 20 random images viewed at x400 magnification. Section analysis was done using a light microscope Nikon E400 (Nikon Instrument Group, Melville, NY, USA) equipped with a digital camera. All measurements were carried out using an image analyzer, Image-Pro Plus version 4.5 for Windows (Media Cybernetics, LP, Silver Spring, MD, USA).
2.5. Western blot analysis
A portion of the renal cortex was homogenized in lysis buffer (150 mM NaCl, 50 mM Trizma-HCl, 1% (v/v) NP-40, pH: 8.0) in the presence of protease and phosphatase inhibitors, and centrifuged at 600×g for 20 min. The supernatant was collected and used as total homogenate. Cytosolic and nuclear fractions were isolated as described previously [21]. Protein content was measured by the Lowry method [22]. Total homogenates, and cytosolic and nuclear fractions were added with a 2X solution of sample buffer (62.5 mM Tris-HCl, pH: 6.8 containing 2% (w/v) SDS, 25% (w/v) glycerol, 5% (v/v) β-mercaptoethanol, and 0.01% (w/v) bromophenol blue) and heated at 95 °C for 2 min. Sample aliquots containing 30 μg of protein were subjected to reducing 10% (w/v) polyacrylamide gel electrophoresis, and then transferred to polyvinylidenedifluoride membranes. Colored molecular weight standards (GE Healthcare, Piscataway, NJ, USA) were run simultaneously. Membranes were blotted for 2 h in 5% (w/v) nonfat milk, and incubated overnight in the presence of the corresponding primary antibody (1:1000 dilution in PBS). After a subsequent incubation for 90 min at room temperature in the presence of the corresponding HRP-conjugated secondary antibody (1:5000 dilution in PBS), complexes were visualized by chemiluminescence. Films were scanned and a densitometric analysis was performed using Image J (National Institute of Health, Bethesda, Maryland, USA). Total homogenate and cytosolic protein bands were normalized to the β-actin content. Bands from the nuclear fractions were normalized to the hnRNP A1 content.
2.6. Determination of NADPH-dependent superoxide anion production
A portion of the renal cortex was homogenized in PBS, and centrifuged at 600×g for 20 min at 4 °C. Supernatants were centrifuged at 10,000×g for 20 min at 4 °C to obtain mitochondria-free homogenates that were stored at −80 °C until analysis. Protein content was measured by the Lowry method [22]. Lucigenin-enhanced chemiluminescence was measured according to Li et al. [23]. Briefly, aliquots of mitochondria-free homogenates were added to vials containing 1 ml of 50 mM potassium phosphate at 37 °C, pH: 7.4 containing lucigenin and NADPH (5 μM and 40 μM final concentrations, respectively) in the absence or the presence of 400 U of SOD. Light emission was measured every 10 s for 10 min using a LKB Wallac 1209 Rackbeta Liquid Scintillation Counter (Turku, Finland) in the chemiluminescence mode. Superoxide anion produced by NADPH-dependent enzymes was calculated as the difference between the areas under the curve in the absence and in the presence of SOD.
2.7. Determination of malondialdehyde (MDA) content
A portion of the renal cortex was homogenized in PBS and centrifuged at 600×g for 20 min at 4 °C. Supernatants were used for determination of MDA content by HPLC-analysis after derivatization with thiobarbituric acid as previously described [24]. Briefly, samples were mixed with 0.2% (w/v) BHT in ethanol and 6% (v/v) perchloric acid and the resulting suspension was centrifugated at 4000×g for 10 min for 4 °C. The obtained supernatants were added 0.6% (p/v) 2-thiobarbituric acid, heated at 95 °C for 45 min, cooled and filtered through 0.22 µm filters. Derivatized MDA was resolved by HPLC with an Agilent 1100 Series equipment from Agilent Technologies (Santa Clara, CA, USA) in reverse phase with a column Luna C-18 (length: 100 mm, diameter: 4.6 mm, particle size: 3 µm) from Phenomenex (Torrance, CA, USA) using a mobile phase consisting of 65% (v/v) 50 mM KH2PO4, pH: 7.0 and 35% (v/v) methanol. Detection was done fluorometrically (λex: 515 nm; λem: 555 nm), and characterized by the retention time of the adduct MDA-TBA. 1, 1, 3, 3-Tetramethoxypropane was used to prepare MDA.
2.8. Statistical analysis
Results are shown as mean±SEM. Except for immunohistochemistry, data were analyzed by one-way analysis of variance (ANOVA) using StatView 5.0 (SAS Institute, Cary, NC, USA), and Fisher's significance difference test was used to examine differences between group means. Immunhistochemical data were analyzed by nonparametric Kruskal-Wallis test and Dunn's multiple comparisons test using absolute values processed through GraphPad Prism 5.01 (GraphPad Software, Inc. San Diego, CA, USA), and Dunn´s multiple comparison test. A value of p<0.05 was considered statistically significant.
3. Results
3.1. (−)-Epicatechin mitigated LPS-induced kidney damage and inflammation
Body weight and the renal weight/body weight ratio were similar among the different experimental groups. Plasma creatinine and urea contents were measured as markers of kidney functional damage. In the L group, creatinine and urea plasma levels were significantly higher than in both C group (30% and 102%, respectively) and LE group (27% and 59%, respectively) (Table 1).
Table 1.
Effects of (‒)-epicatechin on metabolic and renal function parameters from LPS-treatad rats.
| Parameter | C | L | LE |
|---|---|---|---|
| Body weight (g) | 255±15 | 241±6 | 230±8 |
| Kidney weight/body weight (mg/g) | 9.2±0.2 | 9.3±0.2 | 9.9±0.3 |
| Plasma creatinine (mg/dl) | 0.45±0.04 | 0.58±0.03* | 0.46±0.01# |
| Plasma urea (mg/dl) | 28±3 | 56±7* | 34±2# |
C: control, L: LPS, and LE: LPS‐(‒)-epicatechin groups. Values are expressed as means`±SEM; n=8 rats/group.
p<0.05 respect to C.
p<0.05 respect to L.
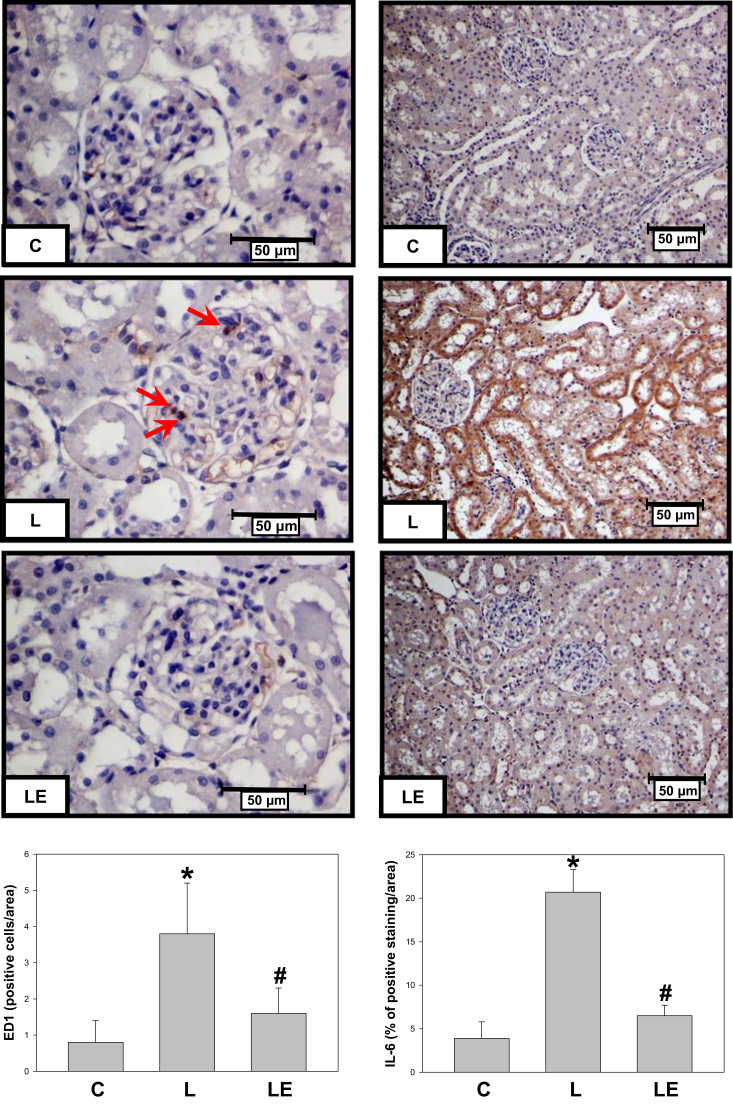
ED1 positive cells and IL-6 were evaluated as inflammation markers in renal cortex by immunohistochemistry (Fig. 1). The number of ED1 positive cells was 4.3-fold higher in the L group respect to the C group, while the LE group did not show this increment. In the case of IL-6, a stronger staining was observed in the renal cortex from L group animals respect to the C (5.0-fold) and to LE (2.2-fold) groups.
Fig. 1.
Effect of dietary (−)-epicatechin on monocyte and macrophage infiltration and IL-6 expression in renal cortex from LPS-treated rats. Representative images and quantification of ED1 (left panels) and IL-6 (right panels) immunostaining in renal cortex from C: control; L: LPS, and LE: LPS-(−)-epicatechin groups. Values are expressed as means±SEM; n=8 rats/group. *p<0.05 respect to C, #p<0.05 respect to L.
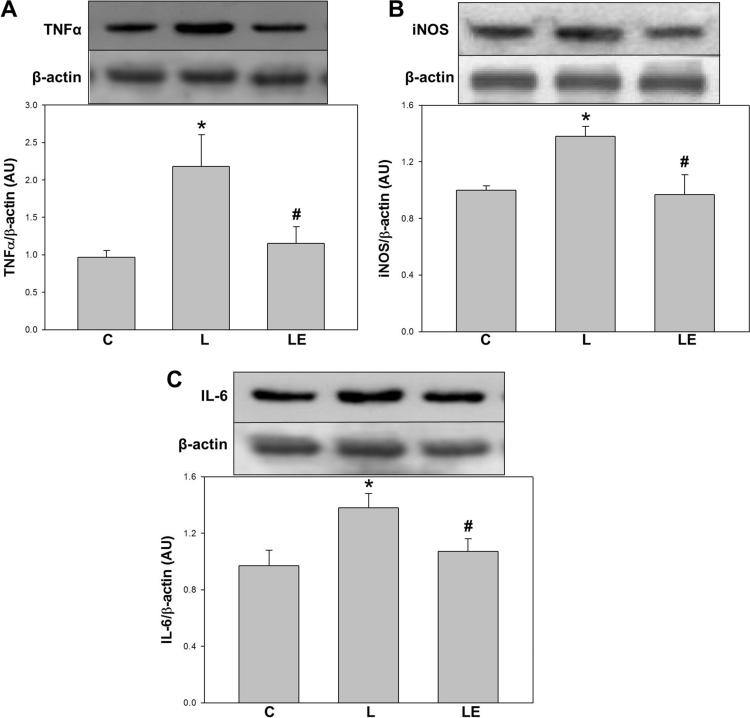
The expression of pro-inflammatory markers was evaluated by Western Blot in kidney cortex. Significantly higher levels of TNF-α (92%), iNOS (38%), and IL-6 (34%), were observed in the L group respect to C and LE groups (Fig. 2).
Fig. 2.
Effects of dietary (−)-epicatechin on TNFα, iNOS and IL-6 expression in renal cortex from LPS-treated rats. TNFα (A), iNOS (B), and IL-6 (C) expressions were determined by Western blot in renal cortex homogenates from C: control, L: LPS, and LE: LPS-(−)-epicatechin groups. Values are expressed as means±SEM; n=8 rats/group. *p<0.05 respect to C, #p<0.05 respect to L.
3.2. (−)-Epicatechin prevented LPS-induced NF-κB activation and TLR4 expression
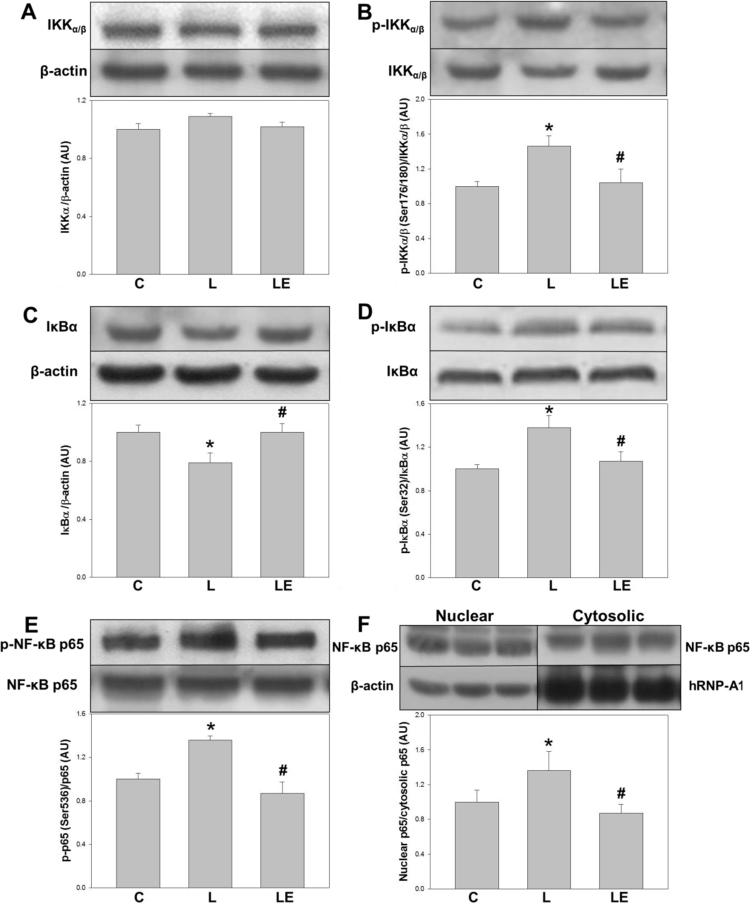
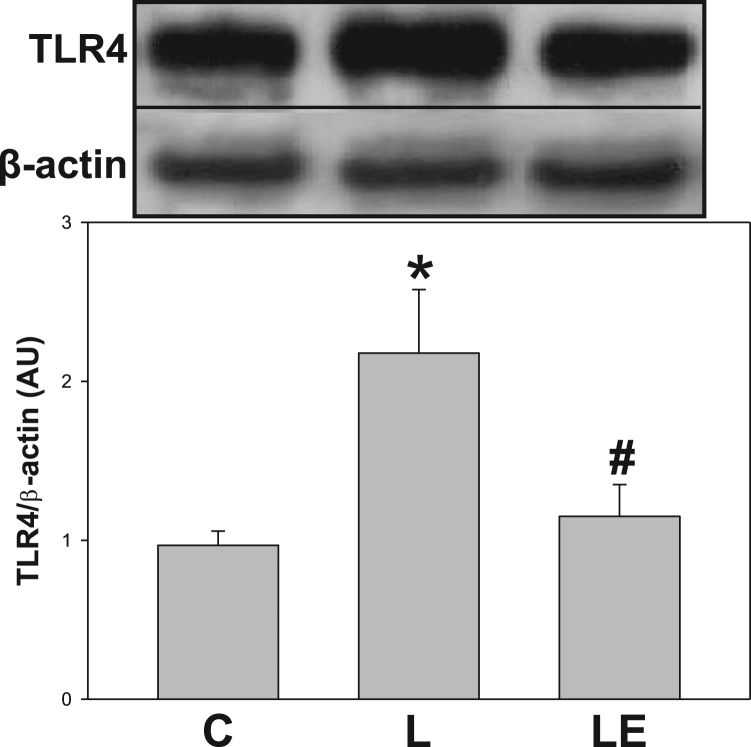
To explore a possible activation of the NF-κB pathway in the renal cortex, several components of this via were evaluated (Fig. 3). IKKα/β phosphorylation at Ser(176/180) (p-IKKα/β) was higher in the L group respect to the C and LE groups (46% and 40% respectively, p<0.05) (Fig. 3B). Total content of IκBα, which is degraded upon NF-κB activation, was lower in the L group respect to C and LE groups (approximately 21% for both) (Fig. 3C). Levels of IkBα phosphorylation at Ser32 (p-IκBα), were higher in the L group respect to C and LE groups (38% and 30%, respectively) (Fig. 3D). NF-κB p65 phosphorylation at Ser(536), as well as the nuclear/cytosolic NF-κB p65 ratio, both parameters of nuclear translocation, were higher in the L group compared to C and LE groups (Figs. 3E and 3F). Fig. 4 shows the expression of TLR4 in renal cortex total homogenates, which was 48% higher in the L group compared to C and LE groups.
Fig. 3.
Effects of dietary (−)-epicatechin on different effectors of NFkB activation in renal cortex from LPS-treated rats. IKKα/β content (A), IKKα/β phosphorylation in Ser(176/180) (B), IkB content (C), IkBα phosphorylation in Ser(32) (D), NF-κB p65 phosphorylation at Ser536 (E) and nuclear/cytosolic NFkB p65 content (F) determined by Western blot in homogenates of renal cortex from C: control, L: LPS, and LE: LPS-(‒)-epicatechin groups. Values are expressed as means±SEM; n=8 rats/group. *p<0.05 respect to C, #p<0.05 respect to L.
Fig. 4.
Effects of dietary (−)-epicatechin on TLR4 expression in renal cortex from LPS-treated rats. TLR4 determined by Western blot in homogenates of renal cortex from C: control, L: LPS, and LE: LPS-(‒)-epicatechin groups. Values are expressed as means±SEM; n=8 rats/group. *p<0.05 respect to C, #p<0.05 respect to L.
3.3. (−)-Epicatechin prevented LPS-induced NOX expression and activation
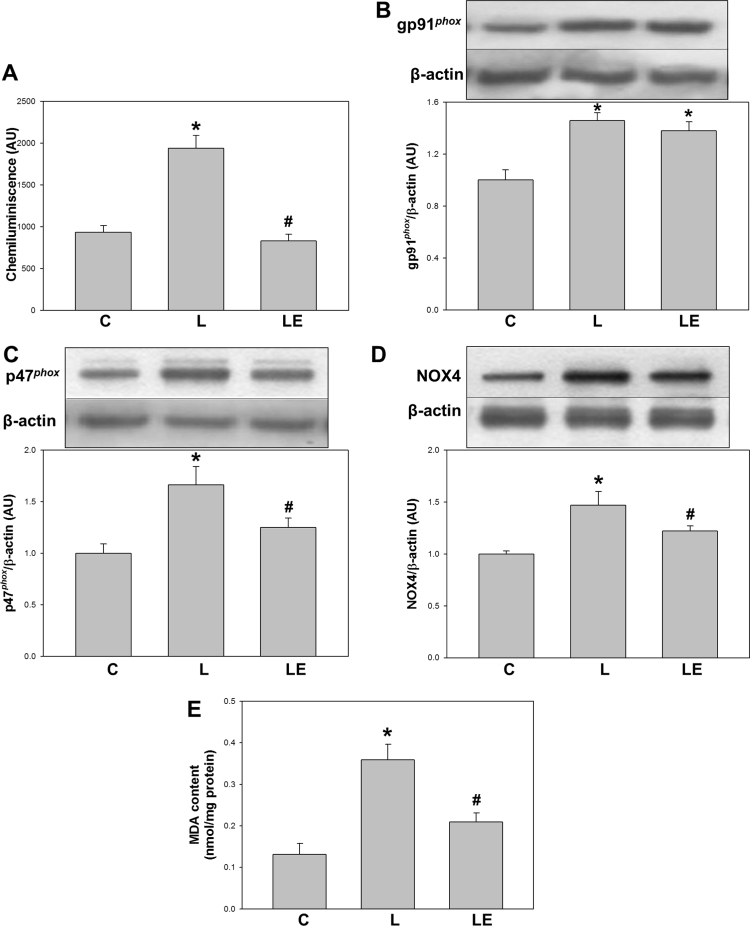
NADPH-dependent superoxide anion production was evaluated ex vivo in mitochondria-free homogenates from renal cortex through a chemiluminescence assay in the absence and presence of SOD. The production of superoxide anion was significantly higher in the L group than in the C group (107%) and the LE group (92%) (Fig. 5A).
Fig. 5.
Effects of dietary (−)-epicatechin on NADPH-dependent oxidant species, NOX2 and NOX4 subunits expressions and MDA content in renal cortex from LPS-treated rats. NADPH-dependent chemiluminescence measured in mitochondria-free homogenates of renal cortex (A), expression levels of gp91phox (B), p47phox (C) and NOX4 (D) determined by Western blot and MDA content determined by HPLC in renal cortex homogenates (E) from C: control, L: LPS, and LE: LPS-(‒)-epicatechin groups. Values are expressed as means±SEM; n=8 rats/group. *p<0.05 respect to C, #p<0.05 respect to L.
Expression of NOX2 and NOX4 subunits were measured in total homogenates. The expression of gp91phox, the constitutive and catalytic subunit of NOX2, resulted higher in both L and LE groups respect to C group (46% and 38%, respectively). The expression of p47phox, one of the activating subunits of NOX2, was higher in the L group respect to C and LE groups (66% and 25%, respectively) (Figs. 5B and 5C). The expression of NOX4. catalytic subunit of NOX4, was higher in the L group respect to C and LE groups (47% and 22%, respectively) (Fig. 5D). Consistently, a higher content of lipid peroxidation products in kidney cortex was observed in the L group as compared to C group (174%) and LE group (72%) (Fig. 5E).
4. Discussion
In the present study, we demonstrated that in rats subjected to LPS-mediated inflammation, dietary administration of (−)-epicatechin prevented the associated renal dysfunction, inflammation, activation of TLR4-NF-κB pathway, and NOX-dependent oxidant production.
Firstly, we observed that the pretreatment of the rats with (−)-epicatechin protected them from LPS-mediated kidney dysfunction and inflammation. Comparable anti-inflammatory actions have been previously reported in several in vivo models of endotoxemia when using flavonoid-rich plant extracts, as well as for isolated flavonoids or their metabolites [25], [26], [27], [28], [29]. Regarding the actions of (−)-epicatechin, we have previously reported that in fructose-fed and in high fat-fed rats inflammatory markers were mostly normalized by the presence of (−)-epicatechin in the diet [30], [31]. Then, the present results are confirmatory of an anti-inflammatory action of (−)-epicatechin, in this case associated with improved kidney function.
In terms of mechanisms of action and in view of the relevance of the TLR4-NF-κB pathway in the damage induced by LPS, we first determined the expression of TLR4 in renal cortex. The high levels of TLR4-expression induced by LPS were not present in the animals receiving (−)-epicatechin. The augmentation in TLR4 levels following LPS challenge can be related to an inflammatory cytokine-mediated increase in the receptor expression [29], [32], [33], [34]. Then, the observed suppression of the increase in TLR4 expression in the kidneys of rats fed with (−)-epicatechin could be due to: i) a prevention of the LPS-induced upregulation of TLR4, as was suggested by dietary administration of a flavonoid-rich supplement [29], and/or ii) a stimulation of TLR4 degradation, as was shown to occur with the addition of epigallocatechin gallate to LPS-treated adipocytes [35]. In addition, the observed positive effects associated with (−)-epicatechin supplementation can also be related to the regulation of the LPS-mediated TLR4 activation by: i) blocking the access to TLR4, as was shown for oligomerized (−)-epicatechin inhibiting the binding of LPS in HEK293 cells [36]; ii) inhibiting the capping of lipids rafts, as was shown for quercetin and luteolin in macrophages stimulated with LPS [37]; and iii) inhibiting TLR4 dimerization, as was shown for the flavonoid isoliquiritigenin, in Ba/F3 cells [38].
The LPS-dependent activation of the NF-κB canonical pathway downstream of TLR4 was prevented by (−)-epicatechin as evidenced by the inhibition of the different steps in the NF-κB cascade. The levels of the intermediates of the NF-κB activation were consistent with the levels of TLR4 expression and also with the expression of cytokines related with this transcription factor, i.e. TNFα, iNOS and IL-6. This suggests that the effect of (−)-epicatechin on TLR4 expression could be a major upstream event cancelling the LPS-mediated NF-κB activation in the kidney.
In terms of oxidant production, rats receiving (−)-epicatechin previous to LPS showed that the capacity of their kidney cortex to produce superoxide anion was similar to control values. Such regulation of oxidant production corresponded with the inhibition of the in vivo lipid peroxidation determined in the kidney of these rats. (−)-Epicatechin has been suggested to be able to modulate oxidative stress following NOX-dependent oxidant production by: i) scavenging superoxide anion and related oxidants [39], [40], and/or ii) inhibiting the activity of the enzymatic complex through a reaction rather specific for (−)-epicatechin [39]. Taking into account the submicromolar concentration that (−)-epicatechin reaches in tissues [41], [42], the possibility for this compound to act as a free radical scavenger in vivo in the kidney is unlikely [11], [43]. Then, indirect antioxidant mechanisms, as the inhibition of NOX activity and/or assembly are expected to be operative in the kidney. Our results are compatible with the hypothesis that (−)-epicatechin downregulates LPS-induced NOX activity by translational and post-translational modifications. For NOX2, (−)-epicatechin prevented the increase in the expression of the modulating protein p47phox; agreeing with previous reports [31], [44], but did not affect the increased expression in the catalytic subunit (gp91phox). For NOX4, the effect of (−)-epicatechin was on the expression of the catalytic subunit. A similar pattern for the expression of NOX subunits was observed in the kidney cortex of fructose-fed rats treated with (−)-epicatechin [31]. Then, it can be concluded that the actions of (−)-epicatechin on oxidant metabolism in the kidney are more related to limiting oxidant production than to scavenging already formed radicals. With regard to NF-κB activation, even when the observed effects of (−)-epicatechin in this model appear to be associated with the inhibition of TLR4 overexpression, a regulation of NF-κB activation triggered by NOX-derived oxidants cannot be ruled out [45], [46].
In summary, (−)-epicatechin dietary administration protected against the renal dysfunction induced by LPS, by preventing both the activation of TLR4-NF-κB pathway and the NOX activation and the consequent inflammatory damage. Although more studies are necessary to understand the mechanisms finally mediating (−)-epicatechin actions, the presented results reinforce the idea that appropriate dietary components could benefit health, both when an acute inflammation is triggered, and in the cases of systemic low-grade inflammation.
Acknowledgements
This work was supported by grants from the Universidad de Buenos Aires (UBACyT 20020120100177 and 20020130100760BA); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP0612); and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2012-0765).
References
- 1.Basciano H., Federico L., Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Lastra G., Manrique C., McFarlane S.I., Sowers J.R. Cardiometabolic syndrome and chronic kidney disease. Curr. Diabetes Rep. 2006;6:207–212. doi: 10.1007/s11892-006-0036-5. [DOI] [PubMed] [Google Scholar]
- 4.Schrier R.W., Wang W., Poole B., Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J. Clin. Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence T. The nuclear factor NF-κB pathway in. Inflamm. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden M.S., West A.P., Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 8.Fan J., Frey R.S., Malik A.B. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J. Clin. Invest. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin L., Li G., Qian X., Liu Y., Wu X., Liu B., Hong J.-S., Block M.L. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- 10.Park H.S., Jung H.Y., Park E.Y., Kim J., Lee W.J., Bae Y.S. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. Baltim. Md 1950. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 11.Fraga C.G. Vol. 59. 2007. Plant polyphenols: how totranslate their in vitro antioxidant actions to in vivo conditions; pp. 308–315. (IUBMB Life). [DOI] [PubMed] [Google Scholar]
- 12.Fraga C.G., Litterio M.C., Prince P.D., Calabró V., Piotrkowski B., Galleano M. Cocoa flavanols: effects on vascular nitric oxide and blood pressure. J. Clin. Biochem. Nutr. 2011;48:63–67. doi: 10.3164/jcbn.11-010FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.-P., Nettleton J.A., Jacobs D.R. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 14.Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., Ryder J.J., Hall W.L., Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Galleano M., Calabro V., Prince P.D., Litterio M.C., Piotrkowski B., Vazquez-Prieto M.A., Miatello R.M., Oteiza P.I., Fraga C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012;1259:87–94. doi: 10.1111/j.1749-6632.2012.06511.x. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie G.G., Oteiza P.I. Modulation of transcription factor NF-kappaB in Hodgkin's lymphoma cell lines: effect of (-)-epicatechin. Free Radic. Res. 2006;40:1086–1094. doi: 10.1080/10715760600788396. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Prieto M.A., Bettaieb A., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2012;527:113–118. doi: 10.1016/j.abb.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettaieb A., Vazquez Prieto M.A., Rodriguez Lanzi C., Miatello R.M., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014;72:247–256. doi: 10.1016/j.freeradbiomed.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison M., van der Heijden R., Heeringa P., Kaijzel E., Verschuren L., Blomhoff R., Kooistra T., Kleemann R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis. 2014;233:149–156. doi: 10.1016/j.atherosclerosis.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Giani J.F., Muñoz M.C., Pons R.A., Cao G., Toblli J.E., Turyn D., Dominici F.P. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am. J. Physiol. Ren. Physiol. 2011;300:F272–F282. doi: 10.1152/ajprenal.00278.2010. [DOI] [PubMed] [Google Scholar]
- 21.Aimo L., Mackenzie G.G., Keenan A.H., Oteiza P.I. Gestational zinc deficiency affects the regulation of transcription factors AP-1, NF-κB and NFAT in fetal brain. J. Nutr. Biochem. 2010;21:1069–1075. doi: 10.1016/j.jnutbio.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Li J.-M., Wheatcroft S., Fan L.M., Kearney M.T., Shah A.M. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation. Circulation. 2004;109:1307–1313. doi: 10.1161/01.CIR.0000118463.23388.B9. [DOI] [PubMed] [Google Scholar]
- 24.Litterio M.C., Jaggers G., Celep G. Sagdicoglu, Adamo A.M., Costa M.A., Oteiza P.I., Fraga C.G., Galleano M. Blood pressure-lowering effect of dietary (-)-epicatechin administration in L-NAME-treated rats is associated with restored nitric oxide levels. Free Radic. Biol. Med. 2012;53:1894–1902. doi: 10.1016/j.freeradbiomed.2012.08.585. [DOI] [PubMed] [Google Scholar]
- 25.Yao J., Pan D., Zhao Y., Zhao L., Sun J., Wang Y., You Q.-D., Xi T., Guo Q.-L., Lu N. Wogonin prevents lipopolysaccharide-induced acute lung injury and inflammation in mice via peroxisome proliferator-activated receptor gamma-mediated attenuation of the nuclear factor-kappaB pathway. Immunology. 2014;143:241–257. doi: 10.1111/imm.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng P.-Y., Lee Y.-M., Wu Y.-S., Chang T.-W., Jin J.-S., Yen M.-H. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem. Pharmacol. 2007;73:793–804. doi: 10.1016/j.bcp.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Radnai B., Tucsek Z., Bognar Z., Antus C., Mark L., Berente Z., Gallyas F., Sumegi B., Veres B. Ferulaldehyde, a water-soluble degradation product of polyphenols, inhibits the lipopolysaccharide-induced inflammatory response in mice. J. Nutr. 2009;139:291–297. doi: 10.3945/jn.108.097386. [DOI] [PubMed] [Google Scholar]
- 28.Pallarès V., Fernández-Iglesias A., Cedó L., Castell-Auví A., Pinent M., Ardévol A., Salvadó M.J., Garcia-Vallvé S., Blay M. Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radic. Biol. Med. 2013;60:107–114. doi: 10.1016/j.freeradbiomed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Nair A.R., Masson G.S., Ebenezer P.J., Piero F. Del, Francis J. Role of TLR4 in lipopolysaccharide-induced acute kidney injury: protection by blueberry. Free Radic. Biol. Med. 2014;71:16–25. doi: 10.1016/j.freeradbiomed.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Bettaieb A., Cremonini E., Kang H., Kang J., Haj F.G., Oteiza P.I. Anti-inflammatory actions of (-)-epicatechin in the adipose tissue of obese mice. Int. J. Biochem. Cell Biol. 2016 doi: 10.1016/j.biocel.2016.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince P.D., Lanzi C.R., Toblli J.E., Elesgaray R., Oteiza P.I., Fraga C.G., Galleano M. Dietary (-)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016;90:35–46. doi: 10.1016/j.freeradbiomed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 32.El-Achkar T.M., Huang X., Plotkin Z., Sandoval R.M., Rhodes G.J., Dagher P.C. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am. J. Physiol. Ren. Physiol. 2006;290:F1034–F1043. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wang W., Deng M., Liu X., Ai W., Tang Q., Hu J. TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation. 2011;34:509–518. doi: 10.1007/s10753-010-9258-4. [DOI] [PubMed] [Google Scholar]
- 34.Janardhan K.S., McIsaac M., Fowlie J., Shrivastav A., Caldwell S., Sharma R.K., Singh B. Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation. Histol. Histopathol. 2006;21:687–696. doi: 10.14670/HH-21.687. [DOI] [PubMed] [Google Scholar]
- 35.Bao S., Cao Y., Zhou H., Sun X., Shan Z., Teng W. Epigallocatechin gallate (EGCG) suppresses lipopolysaccharide-induced Toll-like receptor 4 (TLR4) activity via 67 kDa laminin receptor (67LR) in 3T3-L1 adipocytes. J. Agric. Food Chem. 2015;63:2811–2819. doi: 10.1021/jf505531w. [DOI] [PubMed] [Google Scholar]
- 36.Delehanty J.B., Johnson B.J., Hickey T.E., Pons T., Ligler F.S. Binding and neutralization of lipopolysaccharides by plant proanthocyanidins. J. Nat. Prod. 2007;70:1718–1724. doi: 10.1021/np0703601. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko M., Takimoto H., Sugiyama T., Seki Y., Kawaguchi K., Kumazawa Y. Suppressive effects of the flavonoids quercetin and luteolin on the accumulation of lipid rafts after signal transduction via receptors. Immunopharmacol. Immunotoxicol. 2008;30:867–882. doi: 10.1080/08923970802135690. [DOI] [PubMed] [Google Scholar]
- 38.Park S.-J., Youn H.-S. Suppression of homodimerization of toll-like receptor 4 by isoliquiritigenin. Phytochemistry. 2010;71:1736–1740. doi: 10.1016/j.phytochem.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Steffen Y., Schewe T., Sies H. (–)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 40.Steffen Y., Gruber C., Schewe T., Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Ottaviani J.I., Momma T.Y., Kuhnle G.K., Keen C.L., Schroeter H. Structurally related (-)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards. Free Radic. Biol. Med. 2012;52:1403–1412. doi: 10.1016/j.freeradbiomed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Actis-Goretta L., Lévèques A., Giuffrida F., Romanov-Michailidis F., Viton F., Barron D., Duenas-Paton M., Gonzalez-Manzano S., Santos-Buelga C., Williamson G., Dionisi F. Elucidation of (-)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free Radic. Biol. Med. 2012;53:787–795. doi: 10.1016/j.freeradbiomed.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Galleano M., Verstraeten S.V., Oteiza P.I., Fraga C.G. Antioxidant actions of flavonoids: thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010;501:23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Guzmán M., Jiménez R., Sánchez M., Zarzuelo M.J., Galindo P., Quintela A.M., López-Sepúlveda R., Romero M., Tamargo J., Vargas F., Pérez-Vizcaíno F., Duarte J. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 2012;52:70–79. doi: 10.1016/j.freeradbiomed.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Gloire G., Legrand-Poels S., Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Fraga C.G., Oteiza P.I. Dietary flavonoids: role of (-)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011;51:813–823. doi: 10.1016/j.freeradbiomed.2011.06.002. [DOI] [PubMed] [Google Scholar]