Abstract

Octopolar D2-symmetric chromophores, based on the MPZnM supermolecular motif in which (porphinato)zinc(II) (PZn) and ruthenium(II) polypyridyl (M) structural units are connected via ethyne linkages, were synthesized. These structures take advantage of electron-rich meso-arylporphyrin or electron-poor meso-(perfluoroalkyl)porphyrin macrocycles, unsubstituted terpyridyl and 4′-pyrrolidinyl-2,2′;6′,2″-terpyridyl ligands, and modulation of metal(II) polypyridyl-to-(porphinato)zinc connectivity, to probe how electronic and geometric factors impact the measured hyperpolarizability. Transient absorption spectra obtained at early time delays (tdelay < 400 fs) demonstrate fast excited-state relaxation, and formation of a highly polarized T1 excited state; the T1 states of these chromophores display expansive, intense T1 → Tn absorption manifolds that dominate the 800–1200 nm region of the NIR, long (μs) triplet-state lifetimes, and unusually large NIR excited absorptive extinction coefficients [ε(T1 → Tn) ∼ 105 M–1 cm–1]. Dynamic hyperpolarizability (βλ) values were determined from hyper-Rayleigh light scattering (HRS) measurements, carried out at multiple incident irradiation wavelengths spanning the 800–1500 nm spectral domain. The measured βHRS value (4600 ± 1200 × 10–30 esu) for one of these complexes, RuPZnRu, is the largest yet reported for any chromophore at a 1500 nm irradiation wavelength, highlighting that appropriate engineering of strong electronic coupling between multiple charge-transfer oscillators provides a critical design strategy to realize octopolar NLO chromophores exhibiting large βHRS values at telecom-relevant wavelengths. Generalized Thomas–Kuhn sum (TKS) rules were utilized to compute the effective excited-state-to-excited-state transition dipole moments from experimental linear-absorption spectra; these data were then utilized to compute hyperpolarizabilities as a function of frequency, that include two- and three-state contributions for both βzzz and βxzx tensor components to the RuPZnRu hyperpolarizability spectrum. This analysis predicts that the βzzz and βxzx tensor contributions to the RuPZnRu hyperpolarizability spectrum maximize near 1550 nm, in agreement with experimental data. The TKS analysis suggests that relative to analogous dipolar chromophores, octopolar supermolecules will be likely characterized by more intricate dependences of the measured hyperpolarizability upon irradiation wavelength due to the interactions among multiple different β tensor components.
Short abstract
Coupling of multiple charge-transfer oscillators generates D2-symmetric octopolar NLO chromophores that exhibit large βHRS values at telecom-relevant wavelengths.
Introduction
Applications in telecommunications, data storage, sensor protection, and imaging drive interest in nonlinear optical (NLO) materials.1−4 Organic NLO materials have been the focus of much attention due to their ease of processing, and the fact that molecular design can tailor the NLO responses for targeted applications.2
Dipolar chromophores based on a donor–bridge–acceptor (D-Br-A) framework define the most widely studied NLO motif.5−20 The highly polarizable porphyrin unit figures prominently in many D-Br-A chromophores that exhibit large dynamic hyperpolarizabilities.9,10,15,16,18−30 Earlier studies demonstrate that ethynyl linkages between multiple porphyrinic components, or between porphyrin moieties and other strong oscillators, give rise to unusually polarizable and hyperpolarizable chromophores31−40 that manifest exciting NLO properties.41−50
An interesting class of porphyrin-based chromophores are those in which (porphinato)zinc(II) (PZn) and metal(II) polypyridyl (M) units are linked via an ethyne bridge; many of these compositions feature extraordinarily large frequency-dependent first hyperpolarizabilities (i.e., high βλ values). In these MPZn supermolecules, PZn π–π* and (polypyridyl)-metal based charge-resonance absorption oscillator strength are extensively mixed, and the respective charge transfer (CT) transition dipoles of these chromophoric units are aligned along the highly conjugated molecular axis. These chromophores evince significant PZn–(polypyridyl)metal electronic interactions, display unusual dependences of the sign and magnitude of the hyperpolarizability upon the incident irradiation frequency, and exhibit substantial βλ values at telecommunications-relevant wavelengths.16,21−23,26,41,42,47
Extensive research over the last several decades has led to the development of dipolar, D-Br-A chromophores having large hyperpolarizabilities across the 1300–1500 nm spectral domain.6,12,16,51,52 Translation of large dipolar molecular hyperpolarizabilities into corresponding bulk-phase macroscopic NLO materials requires ordering of the hyperpolarizable chromophores in a noncentrosymmetric fashion. Dipolar molecules tend to organize in centrosymmetric, antiparallel orientations as their respective ground states possess nonzero dipole moments; such ordering drastically diminishes bulk NLO properties relative to that which could be realized through uniform noncentrosymmetric chromophoric organization. Experimental strategies, such as dispersion of dipolar chromophores in a polymeric host material followed by electric field poling, are known to increase the degree of noncentrosymmetric chromophore orientation in bulk phase materials; the net dipolar order achieved within such macroscopic materials using such approaches rarely exceeds that of a few percent of the total weight fraction of the chromophore present.53−59
Octopolar molecules, which have no permanent dipole moments and are noncentrosymmetric, may provide an approach to achieving macroscopic NLO active assemblies.60−72 Most examples of octopolar NLO chromophores have either D3h or Td symmetry. While D2 and D2d symmetries are also appropriate for octopolar NLO chromophore designs, D2- and D2d-symmetric NLO octopoles that manifest exceptional hyperpolarizabilities are unusual.73,74 It was shown recently that strongly coupled, D2-symmetric oscillators designed from (porphinato)zinc(II) (PZn) and metal(II) polypyridyl (M) structural units connected via ethyne bridges define an exceptional class of octopolar NLO chromophores.21 HRS depolarization experiments demonstrated that the measured hyperpolarizabilities (βHRS values, where βHRS2 = [⟨βzzz2⟩ + ⟨βxzz2⟩] = 120/35 βxyz2 for octopolar (Td, D2, or D2d) compounds) of these MPZnM structures arise predominantly from conformers in which torsional angles between the terpyridyl units and the PZn plane are approximately equal in magnitude and opposite in sign,21 suggesting that modest solution-phase structural subpopulations of these MPZnM chromophores possess exceptional hyperpolarizabilities.
Here, we report a series of octopolar D2-symmetric chromophores, based on the MPZnM motif, that demonstrate that strong electronic coupling between multiple charge-transfer oscillators can provide octopolar NLO chromophores that exhibit impressive β values at 1500 nm. While wavelengths near 1500 nm define a critically important telecommunications spectral region, no molecular chromophore has thus far been delineated that exhibits a dynamic hyperpolarizability measured at a 1.5 μm incident irradiation wavelength that exceeds 700 × 10–30 esu;27,61,65,74−81 this work provides new insights into the design of octopolar NLO chromophores, and demonstrates the utility of MPZnM compositions for realizing substantial βHRS values at long wavelengths.
Results and Discussion
Design and Synthesis
RuPZnRu (1), P1RuPZnP1Ru (2), P1RuRfPZnP1Ru (3), and 5,10-P1RuRfPZnP1Ru (4) (Scheme 1) were synthesized by metal-catalyzed cross-coupling16,32,82−89 of an appropriate 4′-brominated ruthenium bis(2,2′;6′,2″-terpyridine) synthon (RuBr or P1RuBr) and a (di-meso-ethynylporphinato)zinc(II) (EPZnE) complex (Scheme 1). These chromophores are based on the MPZnM motif,21,89 in which two bis(terpyridyl)metal(II) units are connected to a PZn meso carbon position via ethynyl connectivity. RuPZnRu, P1RuPZnP1Ru, P1RuRfPZnP1Ru, and 5,10-P1RuRfPZnP1Ru were isolated as described in the Supporting Information; tetrakis(hexafluorophosphate) salts of these chromophores were used in all the spectroscopic experiments described below.
Scheme 1. General Synthetic Scheme for Compounds 1–4,
(A) Synthetic route to compounds 1–4. (B) Molecular structure of RuPZn.
(a) Pd2(dba)3, AsPh3, THF, CH3CN, Et3N, 60 °C.
Previous work establishes that (i) strongly coupled D2 symmetric oscillators can provide impressive octopolar NLO chromophores, (ii) MPZnM measured hyperpolarizabilities derive largely from conformers in which the torsional angles between the terpyridyl and porphyryl units are approximately equivalent in magnitude and opposite in sign, and (iii) the observed octopolar NLO response derives from the collective response of the component dipolar RuPZn supermolecular units.21 Compounds 1–4 provide a compact set of chromophores that probe how electronic and geometric factors impact the measured hyperpolarizability at long irradiation wavelengths. These structures take advantage of electron-rich 10,20-diarylporphyrin (1, 2) or electron-deficient 10,20-bis(perfluoroalkyl)porphyrin frameworks (3, 4), unsubstituted terpyridyl (1) and 4′-pyrrolidinyl-2,2′;6′,2″-terpyridyl ligands (2, 3, 4), and modulation of metal(II) polypyridyl-to-(porphinato)zinc connectivity (3, 4). The bis[meso-(perfluoroalkyl)porphinato]zinc(II) unit provides a PZn chromophoric building block that features HOMO and LUMO levels stabilized by ∼0.3 eV relative to the corresponding orbitals of [5,15-diarylporphinato]zinc(II);40,90−92 this electronic structural modification of the PZn chromophore provides a convenient means to modulate the supermolecular charge resonance character that originates from M and PZn electronic and excitonic interactions that derive from the porphyrin meso-carbon-to-terpyridyl-carbon ethynyl linkage.16,42 Likewise, relative to an unsubstituted terpyridyl ligand, the 4′-pyrrolidinyl substituent modulates terpyridyl ligand π*energy levels and diminishes the E1/2(M3+/2+) value of the bis(terpyridyl)ruthenium(II) structure by 0.3 eV, regulating the respective energies of the PZnn-derived π → π* and bis(terpyridyl)metal(II) charge-transfer states.41 Further, as both the effective chromophore optical symmetry and the nature of the dipolar coupling between independent RuPZn units in larger MPZn-based supermolecules dramatically modulate NLO response,89 chromophores 3 and 4 provide insight on how transition dipole moment orientation influences electronic structure and the measured hyperpolarizability.94
Electronic Absorption and Potentiometric Data
The electronic absorption spectra (EAS) of these species (Figure 1, Table 1) indicate strong mixing of the PZn-based oscillator strength with ruthenium terpyridyl charge resonance bands. Note that EAS of 1–4 differ markedly from the EAS acquired for their respective ethynyl-PZn and (terpyridyl)ruthenium building blocks (see Figure S1). It is important to note in this regard that supermolecular chromophores 1–4 retain spectroscopic qualities that trace their origin to those of well-studied PZn and [Ru(tpy)2]2+ oscillators. For instance, 1–4 show (i) strong, porphyrin B-state derived (ε > 100,000 M–1 cm–1) absorptions near 460 nm; (ii) visible bands centered at ∼525 nm that feature (terpyridyl)metal-to-ligand singlet charge transfer (1MLCT) character and porphyrin ligand oscillator strength contributions; and (iii) bands localized between 600 and 700 nm that exhibit both porphyrinic 1π–π* Q-state and charge resonance character that derive from conjugation expansion10,15,95 and the strong electronic coupling between the (porphinato)metal and (terpyridyl)metal units.16,42 In order to simplify comparison of the spectral properties of these conjugated supermolecules with those of common porphyrin and (terpyridyl)metal(II) benchmarks, we preserve MLCT (d−π*), Soret (B)-band, and Q-band (π–π*) transition labels. When these terms are used in reference to the absorptions of MPZnM chromophores, they denote only the dominant contributor to the oscillator strength of the specific transition, as MLCT, ligand, Soret, and Q electronic states are extensively mixed in these supermolecules.
Figure 1.
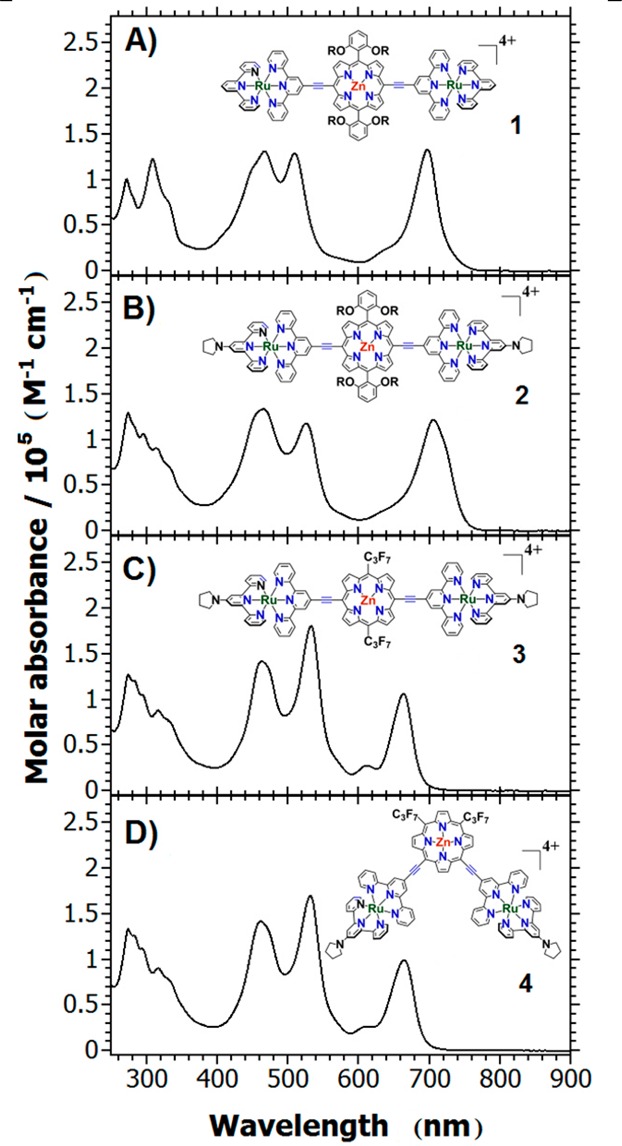
Electronic absorption spectra of (A) RuPZnRu, (B) P1RuPZnP1Ru, (C) P1RuRfPZnP1Ru, and (D) 5,10-P1RuRfPZnP1Ru recorded in CH3CN solvent at 20 °C.
Table 1. Electronic Absorptiona and Potentiometric Datab for Compounds 1–4.
| abs
band maxima [nm] (ε/105 [M–1 cm–1]) |
potentiometric
data (mV) |
|||||||
|---|---|---|---|---|---|---|---|---|
| B band | 1MLCT | Q bands | ZnP0/+ | ZnP+/2+ | M2+/3+ | tpy–/0 | ZnP–/0 | |
| RuPZnRu (1)c,d | 467 (1.31) | 510 (1.29) | 697 (1.33) | 920 | 1155 | 1450 | –895 | –1330 |
| P1RuPZnP1Ru (2)e | 466 (1.33) | 525 (1.18) | 706 (1.22) | 855 | 1195 | 980 | –925 | –1410 |
| P1RuRfPZnP1Ru (3)e | 463 (1.42) | 533 (1.80) | 612 (0.28) | 1380 | 975 | –755 | –965 | |
| 663 (1.06) | ||||||||
| 5,10-P1RuRfPZnP1Ru (4)e | 462 (1.42) | 532 (1.70) | 612 (0.26) | 1405 | 970 | –690 | –1030 | |
| 664 (0.99) | ||||||||
All absorption spectra were recorded in CH3CN; [chromophore] = 1 μM.
Experimental conditions: [chromophore] = 1–4 mM; scan rate = 0.5 V/s; reference electrode = Ag/AgCl; working electrode = Pt disk. E1/2 values are reported relative to SCE; the ferrocene/ferrocenium couple (0.43 V vs SCE) was used as an internal standard.
Potentiometric data recorded in 0.1 M TBAPF6/DMF electrolyte/solvent system.
Potentiometric data recorded in 0.1 M TBAPF6/CH3CN electrolyte/solvent system.
Figure 1 shows that, for all of these compounds, dramatic oscillator strength transfer from the B band to the Q band arises from the coupling between two chromophoric units. Despite significant modulation of the ruthenium oxidation potential (Table 1), incorporation of a pyrrolidine donor on the metal complex has little effect on the ground-state electronic absorption spectrum, because the moderate intensity 1MLCT transition is buried underneath the strong porphyrin-derived S0 → S2 absorption manifold, as is evident from spectra recorded for compounds 1 and 2. Changing PZn ancillary 10,20-meso substitution from an electron rich 3,5-bis(3,3-dimethyl-1-butyloxy)phenyl to an electron deficient heptafluoropropyl group (compare structures of compounds 2 and 3; Figure 1B,C) substantially blue shifts (∼850 cm–1) the low energy Q-state-derived absorption band maximum. Note also that substitution of the PZn ancillary meso aryl moiety with a perfluoroalkyl substituent (Figure 1C,D) drives increased porphyrin ligand oscillator strength contributions to the transition manifold centered near 525 nm, which features augmented metal-to-ligand charge transfer character relative to that observed for compounds 1 and 2 (Figure 1A,B). Potentiometric data for supermolecular chromophores 1–4 are compiled in Table 1.
Excited-State Dynamics
Pump–probe transient absorption spectroscopic studies of 1–4 demonstrate excited-state dynamical characteristics and transient spectral features closely related to those reported for the archetypal dipolar supermolecular donor–acceptor ruthenium(II) [5-(4′-ethynyl-(2,2′;6′2″-terpyridinyl))-10,20-bis(2′,6′-bis(3,3-dimethyl-1-butyloxy)phenyl)porphinato]zinc(II)-(2,2′;6′,2″-terpyridine)2+ bis(hexafluorophosphate) chromophore, RuPZn (Scheme 1).41,42,89 In this respect, it is important to appreciate that the excited-state relaxation dynamics of RuPZn and related chromophores41,42 exhibit significant departures from those characteristic of simple (porphinato)metal and (terpyridyl)metal species. Monomeric (porphinato)zinc(II) species relax typically through both the singlet and triplet manifolds, leading to nanosecond (singlet) and millisecond (triplet) deactivation time scales;31 at room temperature, the initially prepared [Ru(tpy)]2+ electronically excited state relaxes rapidly to the low-lying metal-to-ligand charge transfer (triplet) surface,97−99 where fast intersystem crossing dynamics are driven by thermal population of a low-lying 3MC state, resulting in a 3MLCT lifetime of only a few hundred picoseconds at 298 K.100 In RuPZn,41,42 transient absorption spectra obtained at early time delays (tdelay < 400 fs) demonstrate fast excited-state relaxation and formation of a highly polarized T1 excited state; the combined effects of rapid intersystem crossing and strong coupling to low-lying MLCT states gives rise to excited-state lifetimes >10 μs, and a negligible excite-state population loss to ground-state on the intersystem crossing time scale (τisc ∼ 4 ps) following S0 → S1 excitation. Pump–probe transient optical spectroscopic and dynamical investigations of RuPZn electronically excited states reveal prominent spectral characteristics that include (i) visible spectral domain bleaching due to ground-state depletion, (ii) a broad, weak transient absorption in the spectral region between the B- and Q-state ground-state bleaches, and (iii) an unusually broad, intense T1 → Tn absorption manifold that spans the 800–1200 nm spectral range.41,42
Figure 2 highlights transient absorption spectral data for chromophores 1–4 obtained over a 200 fs-to-1 ns time domain. The transient absorption signals observed at the earliest time delays over the 700–900 nm spectral region suggest that initially prepared excited states for compounds 1–4 are less conjugated than their respective corresponding relaxed excited states. Supermolecules 1 and 2 manifest an ∼15 ps time constant component that leads to an increase in the intensity of the T1 → Tn NIR transient absorption manifold; these dynamics are associated with torsional motion about the ethynyl bridge.33,41,42,101 Global analysis of the excited-state transient dynamical data acquired for 3 and 4 indicates an ultrafast relaxation process (τrelaxation < 1 ps) that leads to an increase in the intensity of the T1 → Tn NIR transient absorption band. This effect likely derives from the electronic structure of the bis[meso-(perfluoroalkyl)porphinato]zinc(II) units of 3 and 4: as the potentiometrically determined HOMO levels of these electron deficient PZn chromophoric building blocks are stabilized by ∼0.3 eV relative to the corresponding orbitals of [meso-arylporphinato]zinc(II),40,90−92 enhanced ground-state charge-resonance interactions between the metal terpyridyl and PZn units drive reduced torsional angle conformational heterogeneity between the porphyryl and terpyridyl least-squares planes relative to that exhibited by 1 and 2. The more planar, conformationally uniform nature of the chromophore 3 and 4 ground states relative to those of 1 and 2 is thus reflected in their order-of-magnitude diminished τrelaxation values, as their mean terpyridyl-(porphinato)metal torsional angles more closely resemble those of their respective relaxed electronically excited states.41,42
Figure 2.
Representative magic angle femtosecond transient absorption spectra recorded for (A) RuPZnRu, (B) P1RuPZnP1Ru, (C) P1RuRfPZnP1Ru, and (D) 5,10-P1RuRfPZnP1Ru at time delays noted. Experimental conditions: λex = (A) 695 nm, (B–D) 672 nm; pump energy = ∼0.5 μJ/pulse; solvent = acetonitrile; T = 20 °C.
Figures 3 and S2 highlight transient absorption spectral data acquired for chromophores 1–4 over ns-to-μs time domains; Table 2 tabulates excited-state lifetimes, T1 → Tn absorption maxima, and T1 → Tn absorption extinction coefficients for these compounds. Note that these estimated T1 → Tn extinction coefficients (ε∼ 105 M–1 cm–1) are calculated from fs transient absorption spectra acquired at 1 ns following excitation, and were estimated by comparing the excited-state absorption to the Qx-band derived ground-state bleach (having a known extinction coefficient), following a method detailed previously.42 Note that λmax(T1 → Tn) increases modestly with incorporation of the pyrrolidine group on the terpyridyl ligand (1, λmax(T1 → Tn) = 980 nm; 2, λmax(T1 → Tn) = 1006 nm), congruent with enhanced electronic delocalization within the compound 2 T1 state. Note also that relative to 1, the enhanced ground-state charge-resonance interactions between the metal terpyridyl and PZn units of chromophores 3 and 4 that derive from the electron-deficient perfluoroalkyl-substituted porphyrin macrocycle result also in red-shifted λmax(T1 → Tn) values (3, λmax(T1 → Tn) = 996 nm; 4, λmax(T1 → Tn) = 988 nm). In sum, Figures 2 and 3 and Table 2 data underscore that the combination of fast intersystem crossing, long triplet-state lifetimes, and unusually large ε(T1 → Tn) values make chromophores 1–4 exceptional excited-state absorbers for this region of the NIR.
Figure 3.

Nanosecond pump–probe transient absorption spectra of (A) P1RuPZnP1Ru (2), (B) P1RuRfPZnP1Ru (3), and (C) 5,10-P1RuRfPZnP1Ru (4) recorded at time delays noted. Experimental conditions: solvent = deaerated acetonitrile; λex = 532 nm; pump energy =1–3 mJ/pulse; ambient temperature.
Table 2. Electronically Excited Triplet State Spectral Dataa for Compounds 1–4.
| chromophore | λmax (T1 → Tn)b (nm) | ε/105 M–1 cm–1 (T1 → Tn)c | τT (μs)d |
|---|---|---|---|
| RuPZnRu (1) | 980e | 1.48 | 10.4e |
| P1RuPZnP1Ru (2) | 1006 | 1.32 | 9.0 |
| P1RuRfPZnP1Ru (3) | 996 | 1.22 | 3.3 |
| 5,10-P1RuRfPZnP1Ru (4) | 988 | 1.14 | 3.3 |
All spectral data were acquired in acetonitrile solvent.
λmax (T1 → Tn) values were determined from transient absorption spectra recorded at a 1 ns time delay.
The excited-state extinction coefficients were estimated using a method described previously;42 reported values were determined at the T1 → Tn λmax.
The triplet-state lifetimes were determined using μs time domain transient absorption measurements carried out in dry dearated acetonitrile solvent.
λmax (T1 → Tn) and triplet lifetime were reported previously.42
Nonlinear Optical Properties
For chromophores with charge transfer (CT) transitions that dominate the NLO response, the dynamic hyperpolarizability can be approximated using a two-state description of β (eq 1).
| 1 |
Here, Pge is the oscillator strength for the CT transition, Δμge the dipole moment difference between ground and CT excited states, Eop the energy gap between the two states, and Einc the incident irradiation energy.102,103 Within the limited context of a single two-level model for charge transfer (along the molecular z-axis) dominated dipolar molecules, βn′ is an excellent approximation for βzzz. Dipolar RuPZn-based chromophores are characterized by large CT transition oscillator strengths (Pges), and significant dipole-moment differences between the ground and excited electronic states (Δμges). These species exhibit complicated nonlinear responses because of two- and three-level contributions arising from mixing of transitions that possess (porphinato)zinc B-, (porphinato)zinc Q-, and ruthenium-to-ligand (MLCT)-state character, and the fact that these transitions have Δμges with both positive and negative sign.16,22,41
A two-state model to describe the electronic hyperpolarizability is appealing for its simplicity and qualitative description of the underlying physical principles. The direct linkage to experimental data, namely, transition dipole moments, optical transition energies, and energy detunings from resonance, is particularly appealing. While of qualitative use for exploring structure–hyperpolarizability relationships, the two-level model does not describe the frequency dispersion of the hyperpolarizability with quantitative accuracy. We recently developed and used generalized Thomas–Kuhn sum (TKS) rules to describe the frequency-dependent hypolarizabilities in a manner that retains the appealing simplicity and direct linkage to linear absorption spectra, while incorporating the influence of multiple excited states that contribute to the hyperpolarizability. Within this framework, a satisfactory description was found for the frequency dispersion of the hyperpolarizabilities for dipolar chromophores.22 Here, we use this approach to describe qualitatively octopolar chromophores with more than two excited states that contribute to the irradiation-wavelength dependent hyperpolarizability. Since multiple absorption manifolds contribute to the nonlinear response in RuPZn-based chromophores, and the transitions that dominate these manifolds possess distinct transition dipole directions, octopolar chromophores 1–4 are anticipated to exhibit complex hyperpolarizability spectra.
Table 3 lists dynamic hyperpolarizability (βλ) values for compounds 1–3, determined from hyper-Rayleigh light scattering (HRS) measurements carried out at different incident irradiation wavelengths (λincs) in CH3CN solvent. The total HRS intensities listed in Table 3 (i.e., the total magnitude of the HRS signal with polarization parallel and perpendicular to the Z-polarized fundamental laser beam, βHRS2 = [⟨βzzz2⟩ + ⟨βxzz2⟩]) provide an average hyperpolarizability value that has not been separated into specific hyperpolarizability tensor components for a specific molecular symmetry.104,105 For chromophores 1–3, hyperpolarizabilities were measured at multiple incident wavelengths, producing a “hyperpolarizability spectrum”; such data are important, as these may be used in combination with appropriate theoretical frameworks to identify electronic states that contribute to the NLO response at a specific λinc, and thereby provide chromophore design insights necessary to optimize the hyperpolarizability at the frequency of interest. Table 3 reports irradiation wavelength-dependent measured hyperpolarizabilities for RuPZnRu (1), P1RuPZnP1Ru (2), P1RuRfPZnP1Ru (3), and the dipolar RuPZn benchmark.
Table 3. Dynamic Hyperpolarizabilities [βHRS, (× 10–30 esu)]a and Depolarization Ratios (ρ) Determined from Hyper-Rayleigh Experiments.
| 800 nm | 840 nm | 1064 nm | 1300 nm | 1320 nm | 1340 nm | 1500 nm | ρb | |
|---|---|---|---|---|---|---|---|---|
| RuPZnc | 200 ± 30 | 700 ± 100 | 1300 ± 100 | 1320 ± 70 | 90 ± 40 | 110 ± 4 | 3.8 | |
| RuPZnRu (1) | 190 ± 30 | 450 ± 70 | 1000 ± 100 | 260 ± 80 | 660 ± 40 | 710 ± 40 | 4600 ± 1200 | 1.7 |
| P1RuPZnP1Ru (2) | 220 ± 10 | 510 ± 80 | 300 ± 40 | 180 ± 70 | 600 ± 100 | 1.9 | ||
| P1RuRfPZnP1Ru (3) | 180 ± 10 | 390 ± 60 | 30 ± 4 | 1.8 |
As a case in point, note that RuPZnRu (Table 3) displays a complex hyperpolarizabilty dispersion: at λinc = 800 nm, the measured hyperpolarizability (β800 = 190 ± 30 × 10–30 esu) is modest. The magnitude of βλ increases as the irradiation wavelength is tuned to 840 and 1064 nm (β840 = 450 ± 70 × 10–30 esu; β1064 = 1000 ± 100 × 10–30 esu), decreases as λinc moves further to the red (β1300 = 260 ± 80 × 10–30 esu), increases steadily at slightly longer irradiation wavelengths (β1320 = 660 ± 40 × 10–30 esu; β1340 = 710 ± 40 × 10–30 esu), but increases dramatically at λinc = 1500 nm (β1500 = 4600 ± 1200 × 10–30 esu). This βHRS value is the largest yet reported for any chromophore at λinc = 1500 nm.
Table 3 further tabulates depolarization ratio measurements determined at λinc = 800 nm. The depolarization ratio, ρ, is the ratio of the HRS signal intensity (I) parallel to (I∥) and perpendicular to (I⊥) the incident polarization (ρ = I∥/I⊥ = ⟨βzzz2⟩/⟨βxzz2⟩). HRS depolarization ratio measurements provide information regarding the optical symmetry of states that give rise to the NLO response. For a classic dipolar NLO chromophore such as disperse red 1, ρ = 3.4; for an octopolar NLO chromophores such as 1,3,5-trihydroxy-2,4,6-trinitrobenzene and crystal violet, ρ = 1.5. Measured depolarization ratios for chromophores 1–3 are ∼1.8; these data, in combination with the chemical topology of the structures, are congruent with the D2 octopolar nature of these structures.
Thomas–Kuhn Sum-over-States (TKS) Analysis
A central goal in nonlinear optics is to establish conceptual frameworks to understand and to tune the hyperpolarizability and frequency dependant nonlinear response. Addressing these design challenges in the frequency domain is especially significant since NLO devices function at finite wavelengths, making it difficult to generalize design-response principles that may be established at either shorter or longer wavelengths. Nonetheless, quantum chemical analysis15,106−114 can be of great value in the regime of nonzero frequency. Developing predictive methods to describe βλ remains an open challenge.115 One bottleneck to predicting βλ is the inclusion of multiple excited states that impact the electronic hyperpolarizability. Excitation energies, the transition moments, basis set, treatment of solvation, and level of electronic structure theory are all well-known to influence the computed nonlinear electronic response. Prior studies9,16,116 have interpreted βλ qualitatively in the context of the two electronic state model (eq 1). This model includes resonant enhancement from one and two photon resonant terms. The excitation energies and the oscillator strengths are provided by the linear absorption spectra. However, the data derived from linear spectra are not adequate to make predictions of hyperpolarizabilities. The reason is that the linear absorption spectra lack transition dipole moment information linking multiple excited states, and this information is needed for three-state (or higher) βλ computation.
We recently showed that the generalized Thomas–Kuhn sum (TKS) rules may be used to compute the effective excited-state-to-excited-state transition dipole moments (within a few-state model) using the experimental linear-absorption spectra. These data can then be used to estimate the hyperpolarizabilities as a function of frequency, including two- and three-state contributions for RuPZn and related dipolar chromophores.22 TKS analysis uses linear-absorption data to extract the transition dipoles and energies of a small number of “effective” excited electronic states, thus providing key input data required to compute βλ (Supporting Information). Our earlier studies found that while structures may possess similar absorption spectra, the frequency-dependent hyperpolarizabilities may be very different because of differences in the influence of excited-state-to-excited-state transition moments.
The TKS analysis enables the use of linear optical absorption spectra and a few hyperpolarizability measurements to estimate the full frequency-dependent NLO response for RuPZn and related dipolar chromophores. Nonetheless, it is critical to recognize that the octopolar D2 symmetric structure for chromophores 1–3 requires that both βzzz and βxzx tensors be computed, making a corresponding TKS analysis of the NLO octopole structures of current interest more complex than those modeled earlier. The fact that the present analysis includes only a limited number of effective excited states makes the octopolar NLO analysis described here more qualitative than that of our earlier studies.
With these caveats in mind, we performed a basic TKS analysis for the RuPZnRu chromophore using its linear-absorption spectrum and the frequency-dependent hyperpolarizability data compiled in Table 3. As βzzz and βxzx tensor components play key roles in determining the hyperpolarizability spectrum for RuPZnRu, Figure 4 compares the computed wavelength-dependent contributions of these tensor components relative to the computed βzzz tensor contribution for RuPZn (Figure 4A),22 which largely determines the βλ spectrum for the dipolar benchmark RuPZn. Figure 4B,C shows the frequency-dependent two- and three-state computed contributions to βzzz and βxzx values for RuPZnRu. These data indicate that, for RuPZnRu, the two- and three-state contributions to βzzz are of smaller magnitude over the entire hyperpolarizability spectrum compared to that computed for RuPZn. This finding underscores the fact that off-diagonal beta tensor components are of critical importance in determining the hyperpolarizability spectrum of octopolar molecules. Congruent with experiment, the TKS computed βzzz value for RuPZn is large at ∼1300 nm. Similarly, the corresponding calculated βzzz and βxzx contributions for RuPZnRu at λinc = 1300 nm are modest, in line with experimental data (Table 3). Note, however, that both the βzzz and βxzx frequency dependent tensor elements for RuPZnRu reach maxima near 1550 nm, in agreement with the observed large βHRS value (4600 ± 1200 × 10–30 esu; Table 3) measured for this chromophore at 1500 nm. At this level of analysis, the TKS rules indicate that relative to analogous dipolar chromophores, octopolar supermolecules will be likely characterized by more intricate dependences of the measured hyperpolarizability upon irradiation wavelength due to the interactions among multiple different β tensor components.
Figure 4.
Comparison of the computed hyperpolarizability tensors βzzz for (A) RuPZn and (B) RuPZnRu, and (C) βxzx for RuPZnRu. The two-level contribution from the Qx-derived transition is β11; the two-level contribution from the 1MLCT-derived transition is β22; the two-level contribution from the B-derived transition is β33; β12, β21, β13, β31, β23, and β32 are the three-level contributions from the coupling of 1Qx-, 1MLCT-, and B-derived excited states. See the Supporting Information for further details.
Experimental Section
Materials and Instrumentation
Inert atmosphere manipulations were carried out under argon prepurified by passage through an O2 scrubbing tower (Schweizerhall R3-11 catalyst) and a drying tower (Linde 3 Å molecular sieves). Air-sensitive solids were handled in a Braun 150-M glovebox. Standard Schlenk techniques were employed to manipulate oxygen and moisture sensitive chemicals. ACS grade CHCl3, CH2Cl2, CH3CN, and hexanes solvents were purchased from Fisher Scientific. Tetrahydrofuran (Fisher Scientific, HPLC grade) was either distilled from potassium/benzophenone or purified using an Innovative Technology Puresolv solvent purification system. Triethylamine was distilled from CaH2; acetonitrile was purified by distillation from CaH2. Pd(PPh3)4, PdCl2(PPh3)2, Pd2(dba)3, and CuI were obtained from either Aldrich or Strem Chemicals. All other commercially available reagents were used as received. An inert atmosphere was maintained in all reactions unless otherwise stated. Chromatographic purification (silica gel, 60 Å pore size, 230–400 mesh, EM Scientific or Silicycle) of all newly synthesized compounds was accomplished on the benchtop. All NMR solvents were used as received. Chemical shifts for 1H NMR spectra are relative to the internal reference (tetramethylsilane) in CDCl3, or solvent residual protium (acetonitrile-d3, δ = 1.93 ppm, THF-d8, δ = 1.85, 3.76 ppm). The number of attached protons and coupling constants are found in parentheses following the chemical shift values. Laboratory instrumentation has been described previously.89
Ultrafast Transient Absorption Experiments
Instrumentation used to acquire ultrafast transient absorption spectra has been described previously.41,89,117 Samples for these studies were prepared, manipulated, and handled as described earlier.41,89 Following acquisition of pump–probe spectroscopic data, linear absorption spectroscopy confirmed that all samples investigated were robust.
Nanosecond Transient Absorption Experiments
Nanosecond transient absorption spectra were acquired utilizing an Edinburgh Instruments LP920 laser flash photolysis spectrometer and Edinburgh L900 Software. Pump pulses were generated from a Q-switched Nd:YAG laser (Quantel, Brillant) and a dual-crystal OPO (OPOTEK, Vibrant LDII). The temporal width of the pump pulses was ∼5 ns; the energy of the pulses exiting the OPO was controlled using neutral density filters. A Xe flash lamp was used as a white light probe source, and a CCD array detector enabled acquisition of transient data over the 185–875 nm wavelength domain. Both the LP920 and Opotek OPO are computer interfaced and controlled by the L900 software. Transient spectra reported derive from data acquired over ∼20–50 scans. Samples were prepared in 1 cm quartz cells and deaerated by 3 freeze–pump–thaw cycles prior to excitation. Excited-state lifetimes were calculated via monoexponential fitting using Origin 9.1 software.
Hyper-Rayleigh Light Scattering (HRS) Measurements
Femtosecond HRS experiments were performed according the method published earlier.27,118,119 The main difference with respect to previous studies involves the spectral domain of these measurements, which included a large number of measurement wavelengths extending to 1500 nm.16,21,89 For this extended set of wavelengths, a variety of reference compounds [crystal violet (CV; β800 (methanol) = 208.6 × 10–30 esu),120p-nitroaniline (pNA; β1064 (acetonitrile) = 8.7 × 10–30 esu),121 and disperse red 1 (DR1; β1300 (chloroform) = 22.4 × 10–30 esu)]119 were used. Reference values at nearby wavelengths were derived from the two-level model. These values have been shown to provide an excellent set of self-consistent points of reference to quantitatively study the frequency dispersion of the hyperpolarizability for porphyrin-derived chromophores.16,21,27,89,122
The
chromophores were dissolved in acetonitrile, and passed through 0.2
μm filters. For the external references in different solvents,
standard local field correction factors were applied 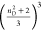 , where n is the refractive
index of the solvent at the sodium D line (1.330 for methanol; 1.344
for acetonitrile; and 1.446 for chloroform). HRS data were acquired
using procedures described previously to ensure linearity of the HRS
signal and that self-absorption of the second harmonic signal was
insignificant;89 depolarization ratios
were likewise determined using approaches detailed in earlier work.89
, where n is the refractive
index of the solvent at the sodium D line (1.330 for methanol; 1.344
for acetonitrile; and 1.446 for chloroform). HRS data were acquired
using procedures described previously to ensure linearity of the HRS
signal and that self-absorption of the second harmonic signal was
insignificant;89 depolarization ratios
were likewise determined using approaches detailed in earlier work.89
Chromophore Synthesis
An exemplary synthesis of bisruthenium(II) 5,15-bis[4′-ethynyl-(2,2′;6′,2″-terpyridinyl)]bis[10,20-bis(2′,6′-bis(3,3-dimethyl-1-butyloxy)phenyl)porphinato]zinc(II)-bis(4′-pyrrolidin-1-yl-2,2′;6′2″-terpyridine) tetrakis(hexafluorophosphate) (P1RuPZnP1Ru, 2) follows below. Synthetic procedures and characterization data for all other compounds may be found in the Supporting Information.
Bisruthenium(II) 5,15-Bis[4′-ethynyl-(2,2′;6′,2″-terpyridinyl)]bis[10,20-bis(2′,6′-bis(3,3-dimethyl-1-butyloxy)phenyl)porphinato]zinc(II)-bis(4′-pyrrolidin-1-yl-2,2′;6′2″-terpyridine) Tetrakis(hexafluorophosphate) (P1RuPZnP1Ru, 2)
[5,15-Diethynyl-10,20-bis[2′,6′-bis(3,3-dimethyl-1-butyloxy)phenyl]porphinato]zinc (50 mg, 0.0513 mmol) and ruthenium(II) (4′-bromo-2,2′;6′,2″-terpyridine)(4′-pyrrolidin-1-yl-2,2′;6′,2″-terpyridine)bis(hexafluorophosphate) (P1RuBr) (Scheme 1, 115 mg, 0.1143 mmol) were placed into a 100 mL Schlenk tube with a stir bar. Trisdibenzylideneacetone dipalladium(0) (7 mg, 7.7 μmol) and triphenylarsine (31 mg, 0.1013 mmol) were added. A solvent mixture of dry THF (12 mL), CH3CN (24 mL), and Et3N (4 mL) was completely degassed via three freeze–pump–thaw cycles and added to the Schlenk tube. The reaction mixture was then stirred under argon atmosphere at 55 °C for 16 h, following which it was cooled to room temperature and the solvent evaporated. The residue was purified by column chromatography on silica gel, eluted with a mixed solvent of CH3CN:H2O:saturated aqueous KNO3 = 90:9:1. The major red-brown band was collected and the solvent evaporated. The residual dark brown solid was dissolved in a minimum volume of CH3CN, and excess ammonium hexafluorophosphate and H2O were added. A dark brown precipitate (2, 110 mg, 0.0389 mmol, 76% yield based on initial bis-ethynyl porphyrin) was collected by filtration. 1H NMR (CD3CN): δ 9.77 (d, J = 4.5 Hz, 4H), 9.14 (s, 4H), 8.75 (d, J = 4.5 Hz, 4H), 8.58 (d, J = 8.0 Hz, 4H), 8.30 (d, J = 8.0 Hz, 4H), 7.84 (t, J = 7.0 Hz, 4H), 7.70–7.63 (m, 10H), 7.39 (d, J = 5 Hz, 4H), 7.21 (d, J = 5 Hz, 4H), 7.12 (t, J = 6 Hz, 4H), 6.99 (d, J = 8.5 Hz, 4H), 6.92 (t, J = 6 Hz, 4H), 3.83 (t, J = 7 Hz, 8H), 3.63 (s, 8H), 2.07 (s, 8H), 0.66 (t, J = 7 Hz, 8H), 0.03 (s, 36 H). Vis (CH3CN): λmax [nm] (ε [× 10–5 M–1 cm–1]) 465 (1.33), 525 (1.18), 705 (1.22). MS (MALDI-TOF) m/z: 2385 (calcd for C128H122F6N18O4PRu2Zn (M – 3PF6)+ 2387), 2528 (calcd for C128H122F12N18O4P2Ru2Zn (M – 2PF6)+ 2532), and 2674 (calcd for C128H122F18N18O4P3Ru2Zn (M – PF6)+ 2678).
Conclusion
Octopolar D2-symmetric chromophores, based on the MPZnM motif in which (porphinato)zinc(II) (PZn) and ruthenium(II) polypyridyl (M) structural units are connected via ethyne linkages, were probed using linear and nonlinear optical spectroscopic methods to determine how electronic and geometric factors impact measured hyperpolarizabilities. Linear electronic spectra of these RuPZnRu, P1RuPZnP1Ru, P1RuRfPZnP1Ru, and 5,10-P1RuRfPZnP1Ru chromophores (Figure 1) display (i) strong, porphyrin B-state derived (ε > 100,000 M–1 cm–1) absorptions near 460 nm; (ii) visible bands centered at ∼525 nm, that feature (terpyridyl)metal-to-ligand singlet charge transfer (1MLCT) and porphyrin ligand oscillator strength contributions; and (iii) high oscillator strength absorption manifolds in the 600–700 nm range that exhibit both porphyrinic 1π–π* Q-state and charge resonance character that derive from conjugation expansion10,15,95 and the strong electronic coupling between the (porphinato)metal and (terpyridyl)metal units. Transient absorption spectra obtained at early time delays (tdelay < 400 fs) demonstrate fast excited-state relaxation, and formation of a highly polarized T1 excited state; the T1 states of these chromophores exhibit a broad, intense T1 → Tn absorption manifold over the 800–1200 nm spectral domain, long (μs) triplet-state lifetimes, and unusually large NIR absorptive extinction coefficients [ε(T1 → Tn) ∼ 105 M–1 cm–1].
Dynamic hyperpolarizability (βλ) values were determined from hyper-Rayleigh light scattering (HRS) measurements carried out at multiple incident irradiation wavelengths spanning the 800–1500 nm spectral domain. These RuPZn-based octopolar chromophores feature complex hyperpolarizability spectra, as (i) multiple transitions contribute to the nonlinear response and (ii) the dipole directions of these directions may be of identical or opposite sign. The measured βHRS value for one of these complexes, RuPZnRu, is the largest yet reported for any chromophore at λinc = 1500 nm (4600 ± 1200 × 10–30 esu), highlighting that engineering of strong electronic coupling between multiple charge-transfer oscillators provides a critical design strategy to realize octopolar NLO chromophores that exhibit impressive βHRS values at long, telecom-relevant wavelengths.
Generalized Thomas–Kuhn sum (TKS) rules were used to compute the effective excited-state-to-excited-state transition dipole moments from experimental linear-absorption spectra; these data were then utilized to compute hyperpolarizabilities as a function of frequency, that include two- and three-state contributions for both the βzzz and βxzx tensor components to the RuPZnRu hyperpolarizability spectrum. This qualitative analysis shows that the βzzz and βxzx tensor contributions to the RuPZnRu hyperpolarizability spectrum reach maxima near 1550 nm, in agreement with experimental data. This qualitative TKS analysis finds that octopolar molecules will be likely characterized by more intricate dependences of the measured hyperpolarizability upon irradiation wavelength due to the interactions among multiple different β tensor components.
Acknowledgments
A.N., J.P., T.V.D., and M.J.T. acknowledge research support from the National Science Foundation through Grant CHE-1413333; K.D.M. and K.C. acknowledge the Fund for Scientific Research-Flanders (Research Grant No. 1510712N) and the University of Leuven (GOA/2011/03); X.H. and D.N.B. thank the National Science Foundation through Grant CHE-1565812 for research support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.6b00291.
Characterization, electrochemical, and computational data, experimental details, and electronic absorption spectra (PDF)
Author Contributions
All authors contributed to writing this manuscript and have given their approval to the final version.
The authors declare no competing financial interest.
Supplementary Material
References
- Verbiest T.; Clays K.; Rodriguez V.. Second-order Nonlinear Optical Characterization Techniques: An Introduction; CRC Press: New York, 2009. [Google Scholar]
- Prasad P. N.; Williams D. J.. Introduction to Nonlinear Optical Effects in Molecules and Polymers; Wiley: New York, 1991. [Google Scholar]
- Beratan D. N. In New Materials for Nonlinear Optics; Hann R. A., Bloor D., Eds.; ACS Symposium Series 455; American Chemical Society: Washington, DC, 1991. [Google Scholar]
- Williams D. J. Organic polymeric and non-polymeric materials with large optical nonlinearities. Angew. Chem., Int. Ed. Engl. 1984, 23, 690–703. 10.1002/anie.198406901. [DOI] [Google Scholar]
- Chauchard E.; Combellas C.; Hendrickx E.; Mathey G.; Suba C.; Persoons A.; Thiebault A. Hyperpolarization properties of pyridinium and quinolinium salts determined by hyper-rayleigh scattering. Chem. Phys. Lett. 1995, 238, 47–53. 10.1016/0009-2614(95)00387-8. [DOI] [Google Scholar]
- Kang H.; Facchetti A.; Zhu P.; Jiang H.; Yang Y.; Cariati E.; Righetto S.; Ugo R.; Zuccaccia C.; Macchioni A.; Stern C. L.; Liu Z.; Ho S.-T.; Marks T. J. Exceptional molecular hyperpolarizabilities in twisted π-electron system chromophores. Angew. Chem., Int. Ed. 2005, 44, 7922–7925. 10.1002/anie.200501581. [DOI] [PubMed] [Google Scholar]
- Kang H.; Facchetti A.; Jiang H.; Cariati E.; Righetto S.; Ugo R.; Zuccaccia C.; Macchioni A.; Stern C. L.; Liu Z.; Ho S.-T.; Brown E. C.; Ratner M. A.; Marks T. J. Ultralarge hyperpolarizability twisted π-electron system electro-optic chromophores: synthesis, solid-state and solution-phase structural characteristics, electronic structures, linear and nonlinear optical properties, and computational studies. J. Am. Chem. Soc. 2007, 129, 3267–3286. 10.1021/ja0674690. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Lou A. J.-T.; He G. S.; Baev A.; Swihart M. T.; Prasad P. N.; Marks T. J. Cooperative coupling of cyanine and tictoid twisted π-systems to amplify organic chromophore cubic nonlinearities. J. Am. Chem. Soc. 2015, 137, 4622–4625. 10.1021/jacs.5b01042. [DOI] [PubMed] [Google Scholar]
- Karki L.; Vance F. W.; Hupp J. T.; LeCours S. M.; Therien M. J. Electronic stark effect studies of a porphyrin-based push-pull chromophore displaying a large first hyperpolarizability: State-specific contributions to beta. J. Am. Chem. Soc. 1998, 120, 2606–2611. 10.1021/ja973593v. [DOI] [Google Scholar]
- LeCours S. M.; Guan H.-W.; DiMagno S. G.; Wang C. H.; Therien M. J. Push-pull arylethynyl porphyrins: new chromophores that exhibit large molecular first-order hyperpolarizabilities. J. Am. Chem. Soc. 1996, 118, 1497–1503. 10.1021/ja953610l. [DOI] [Google Scholar]
- Ledoux I.; Zyss J.; Jutand A.; Amatore C. Nonlinear optical-properties of asymmetric polyphenyls - efficiency versus transparency trade-off. Chem. Phys. 1991, 150, 117–123. 10.1016/0301-0104(91)90061-W. [DOI] [Google Scholar]
- Marder S. R.; Cheng L.-T.; Tiemann B. G.; Friedli A. C.; Blanchard-Desce M.; Perry J. W.; Skindhøj J. Large first hyperpolarizabilities in push-pull polyenes by tuning of the bond length alternation and aromaticity. Science 1994, 263, 511–514. 10.1126/science.263.5146.511. [DOI] [PubMed] [Google Scholar]
- Marder S. R.; Kippelen B.; Jen A. K.-Y.; Peyghambarian N. Design and synthesis of chromophores and polymers for electro-optic and photorefractive applications. Nature 1997, 388, 845–851. 10.1038/42190. [DOI] [Google Scholar]
- Meyers F.; Marder S. R.; Pierce B. M.; Brédas J. L. Electric field modulated nonlinear optical properties of donor-acceptor polyenes: sum-over-states investigation of the relationship between molecular polarizabilities (a, b and g) and bond length alternation. J. Am. Chem. Soc. 1994, 116, 10703–10714. 10.1021/ja00102a040. [DOI] [Google Scholar]
- Priyadarshy S.; Therien M. J.; Beratan D. N. Acetylenyl-linked, porphyrin-bridged, donor-acceptor molecules: A theoretical analysis of the molecular first hyperpolarizability in highly conjugated push-pull chromophore structures. J. Am. Chem. Soc. 1996, 118, 1504–1510. 10.1021/ja952690q. [DOI] [Google Scholar]
- Uyeda H. T.; Zhao Y.; Wostyn K.; Asselberghs I.; Clays K.; Persoons A.; Therien M. J. Unusual frequency dispersion effects of the nonlinear optical response in highly conjugated (polypyridyl)metal-(porphinato)zinc(II) chromophores. J. Am. Chem. Soc. 2002, 124, 13806–13813. 10.1021/ja020651q. [DOI] [PubMed] [Google Scholar]
- Verbiest T.; Houbrechts S.; Kauranen M.; Clays K.; Persoons A. Second-order nonlinear optical materials: recent advances in chromophore design. J. Mater. Chem. 1997, 7, 2175–2189. 10.1039/a703434b. [DOI] [Google Scholar]
- Zhang T.-G.; Zhao Y.; Asselberghs I.; Persoons A.; Clays K.; Therien M. J. Design, synthesis, linear, and nonlinear optical properties of conjugated (porphinato)zinc(ii)-based donor-acceptor chromophores featuring nitrothiophenyl and nitrooligothiophenyl electron-accepting moieties. J. Am. Chem. Soc. 2005, 127, 9710–9720. 10.1021/ja0402553. [DOI] [PubMed] [Google Scholar]
- Zhang T.-G.; Zhao Y.; Song K.; Asselberghs I.; Persoons A.; Clays K.; Therien M. J. Electronic modulation of hyperpolarizable (porphinato)zinc(ii) chromophores featuring ethynylphenyl-, ethynylthiophenyl-, ethynylthiazolyl-, and ethynylbenzothiazolyl-based electron-donating and -accepting moieties. Inorg. Chem. 2006, 45, 9703–9712. 10.1021/ic060898e. [DOI] [PubMed] [Google Scholar]
- LeCours S. M.; DiMagno S. G.; Therien M. J. Exceptional electronic modulation of porphyrins through meso-arylethynyl groups. Electronic spectroscopy, electronic structure, and electrochemistry of [5,15-bis[(aryl)ethynyl]-10,20-diphenylporphinato]zinc(II) complexes. X-ray crystal structures of [5,15-bis[(4′-fluorophenyl)ethynyl]-10,20-diphenylporphinato]zinc(II) and 5,15-bis[(4′-methoxyphenyl)ethynyl]-10,20-diphenylporphyrin. J. Am. Chem. Soc. 1996, 118, 11854–11864. 10.1021/ja962403y. [DOI] [Google Scholar]
- Duncan T. V.; Song K.; Hung S.-T.; Miloradovic I.; Nayak A.; Persoons A.; Verbiest T.; Therien M. J.; Clays K. Molecular symmetry and solution-phase structure interrogated by hyper-Rayleigh depolarization measurements: Elaborating highly hyperpolarizable D2-symmetric chromophores. Angew. Chem., Int. Ed. 2008, 47, 2978–2981. 10.1002/anie.200703187. [DOI] [PubMed] [Google Scholar]
- Hu X.; Xiao D.; Keinan S.; Asselberghs I.; Therien M. J.; Clays K.; Yang W.; Beratan D. N. Predicting the frequency dispersion of electronic hyperpolarizabilities based on absorption data and Thomas-Kuhn sum rules. J. Phys. Chem. C 2010, 114, 2349–2359. 10.1021/jp911556x. [DOI] [Google Scholar]
- Keinan S.; Therien M. J.; Beratan D. N.; Yang W. Molecular design of porphyrin-based nonlinear optical materials. J. Phys. Chem. A 2008, 112, 12203–12207. 10.1021/jp806351d. [DOI] [PubMed] [Google Scholar]
- Reeve J. E.; Collins H. A.; De Mey K.; Kohl M. M.; Thorley K. J.; Paulsen O.; Clays K.; Anderson H. L. Amphiphilic porphyrins for second harmonic generation imaging. J. Am. Chem. Soc. 2009, 131, 2758–2759. 10.1021/ja8061369. [DOI] [PubMed] [Google Scholar]
- Therien M. J. How to improve your image. Nature 2009, 458, 716–717. 10.1038/458716b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T.; Wu S. P.; Miloradovic I.; Therien M. J.; Blasie J. K. Incorporation of designed extended chromophores into amphiphilic 4-helix bundle peptides for nonlinear optical biomolecular materials. Nano Lett. 2006, 6, 2387–2394. 10.1021/nl062091p. [DOI] [PubMed] [Google Scholar]
- De Mey K.; Perez-Moreno J.; Reeve J. E.; Lopez-Duarte I.; Boczarow I.; Anderson H. L.; Clays K. Strong wavelength dependence of hyperpolarizability in the near-infrared biological window for second-harmonic generation by amphiphilic porphyrins. J. Phys. Chem. C 2012, 116, 13781–13787. 10.1021/jp302050j. [DOI] [Google Scholar]
- López-Duarte I.; Reeve J. E.; Pérez-Moreno J.; Boczarow I.; Depotter G.; Fleischhauer J.; Clays K.; Anderson H. L. ″Push-no-pull″ porphyrins for second harmonic generation imaging. Chem. Sci. 2013, 4, 2024–2027. 10.1039/c3sc22306j. [DOI] [Google Scholar]
- Reeve J. E.; Corbett A. D.; Boczarow I.; Kaluza W.; Barford W.; Bayley H.; Wilson T.; Anderson H. L. Porphyrins for probing electrical potential across lipid bilayer membranes by second harmonic generation. Angew. Chem., Int. Ed. 2013, 52, 9044–9048. 10.1002/anie.201304515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senge M. O.; Fazekas M.; Notaras E. G. A.; Blau W. J.; Zawadzka M.; Locos O. B.; Ni Mhuircheartaigh E. M. Nonlinear optical properties of porphyrins. Adv. Mater. 2007, 19, 2737–2774. 10.1002/adma.200601850. [DOI] [Google Scholar]
- Duncan T. V.; Susumu K.; Sinks L. E.; Therien M. J. Exceptional near-infrared fluorescence quantum yields and excited-state absorptivity of highly conjugated porphyrin arrays. J. Am. Chem. Soc. 2006, 128, 9000–9001. 10.1021/ja061897o. [DOI] [PubMed] [Google Scholar]
- Lin V. S.-Y.; DiMagno S. G.; Therien M. J. Highly conjugated, acetylenyl bridged porphyrins: new models for light-harvesting antenna systems. Science 1994, 264, 1105–1111. 10.1126/science.8178169. [DOI] [PubMed] [Google Scholar]
- Rubtsov I. V.; Susumu K.; Rubtsov G. I.; Therien M. J. Ultrafast singlet excited-state polarization in electronically asymmetric ethyne-bridged bis[(porphinato)zinc(II)] complexes. J. Am. Chem. Soc. 2003, 125, 2687–2696. 10.1021/ja021157p. [DOI] [PubMed] [Google Scholar]
- Angiolillo P. J.; Susumu K.; Uyeda H. T.; Lin V. S.-Y.; Shediac R.; Therien M. J. Trends in triplet excitation delocalization in highly conjugated (porphinato)zinc(II) arrays probed by EPR spectroscopy. Synth. Met. 2001, 116, 247–253. 10.1016/S0379-6779(00)00461-6. [DOI] [Google Scholar]
- Duncan T. V.; Wu S. P.; Therien M. J. Ethyne-bridged (porphinato)zinc(ii)-(porphinato)iron(iii) complexes: phenomenological dependence of excited-state dynamics upon (porphinato) iron electronic structure. J. Am. Chem. Soc. 2006, 128, 10423–10435. 10.1021/ja061388m. [DOI] [PubMed] [Google Scholar]
- Fletcher J. T.; Therien M. J. Strongly coupled porphyrin arrays featuring both, π-cofacial and linear-π-conjugative interactions. Inorg. Chem. 2002, 41, 331–341. 10.1021/ic010871p. [DOI] [PubMed] [Google Scholar]
- Lin V. S.-Y.; Therien M. J. The role of porphyrin-to-porphyrin linkage topology in the extensive modulation of the absorptive and emissive properties of a series of ethynyl- and butadiynyl-bridged bis- and tris(porphinato)zinc chromophores. Chem. - Eur. J. 1995, 1, 645–651. 10.1002/chem.19950010913. [DOI] [Google Scholar]
- Shediac R.; Gray M. H. B.; Uyeda H. T.; Johnson R. C.; Hupp J. T.; Angiolillo P. J.; Therien M. J. Singlet and triplet excited states of emissive, conjugated bis(porphyrin) compounds probed by optical and EPR spectroscopic methods. J. Am. Chem. Soc. 2000, 122, 7017–7033. 10.1021/ja9939587. [DOI] [Google Scholar]
- Susumu K.; Duncan T. V.; Therien M. J. Potentiometric, electronic structural, and ground- and excited-state optical properties of conjugated bis[(porphinato)zinc(II)] compounds featuring proquinoidal spacer units. J. Am. Chem. Soc. 2005, 127, 5186–5195. 10.1021/ja040243h. [DOI] [PubMed] [Google Scholar]
- Susumu K.; Therien M. J. Decoupling optical and potentiometric band gaps in π-conjugated materials. J. Am. Chem. Soc. 2002, 124, 8550–8552. 10.1021/ja0203925. [DOI] [PubMed] [Google Scholar]
- Duncan T. V.; Ishizuka T.; Therien M. J. Molecular engineering of intensely near-infrared absorbing excited states in highly conjugated oligo(porphinato)zinc-(polypyridyl)metal(II) supermolecules. J. Am. Chem. Soc. 2007, 129, 9691–9703. 10.1021/ja0707512. [DOI] [PubMed] [Google Scholar]
- Duncan T. V.; Rubtsov I. V.; Uyeda H. T.; Therien M. J. Highly conjugated (polypyridyl)metal-(porphinato)zinc(ii) compounds: long-lived, high oscillator strength, excited-state absorbers having exceptional spectral coverage of the near-infrared. J. Am. Chem. Soc. 2004, 126, 9474–9475. 10.1021/ja0400638. [DOI] [PubMed] [Google Scholar]
- Drobizhev M.; Stepanenko Y.; Dzenis Y.; Karotki A.; Rebane A.; Taylor P. N.; Anderson H. L. Extremely strong near-IR two-photon absorption in conjugated porphyrin dimers: quantitative description with three-essential-states model. J. Phys. Chem. B 2005, 109, 7223–7236. 10.1021/jp044261x. [DOI] [PubMed] [Google Scholar]
- Fisher J. A. N.; Susumu K.; Therien M. J.; Yodh A. G. One- and two-photon absorption of highly conjugated multiporphyrin systems in the two-photon Soret transition region. J. Chem. Phys. 2009, 130, 134506. 10.1063/1.3089795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisaki I.; Hiroto S.; Kim K. S.; Noh S. B.; Kim D.; Shinokubo H.; Osuka A. Synthesis of doubly beta-to-beta 1,3-butadiyne-bridged diporphyrins: Enforced planar structures and large two-photon absorption cross sections. Angew. Chem., Int. Ed. 2007, 46, 5125–5128. 10.1002/anie.200700550. [DOI] [PubMed] [Google Scholar]
- Screen T. E. O.; Thorne J. R. G.; Denning R. G.; Bucknall D. G.; Anderson H. L. Amplified optical nonlinearity in a self-assembled double-strand conjugated porphyrin polymer ladder. J. Am. Chem. Soc. 2002, 124, 9712–9713. 10.1021/ja026205k. [DOI] [PubMed] [Google Scholar]
- Strzalka J.; Xu T.; Tronin A.; Wu S. P.; Miloradovic I.; Kuzmenko I.; Gog T.; Therien M. J.; Blasie J. K. Structural studies of amphiphilic 4-helix bundle peptides incorporating designed extended chromophores for nonlinear optical biomolecular materials. Nano Lett. 2006, 6, 2395–2405. 10.1021/nl062092h. [DOI] [PubMed] [Google Scholar]
- Thorley K. J.; Hales J. M.; Anderson H. L.; Perry J. W. Porphyrin dimer carbocations with strong near infrared absorption and third-order optical nonlinearity. Angew. Chem., Int. Ed. 2008, 47, 7095–7098. 10.1002/anie.200802687. [DOI] [PubMed] [Google Scholar]
- Zou H.; Therien M. J.; Blasie J. K. Structure and dynamics of an extended conjugated NLO chromophore within an amphiphilic 4-helix bundle peptide by molecular dynamics simulation. J. Phys. Chem. B 2008, 112, 1350–1357. 10.1021/jp076643j. [DOI] [PubMed] [Google Scholar]
- Raymond J. E.; Bhaskar A.; Goodson T.; Makiuchi N.; Ogawa K.; Kobuke Y. Synthesis and two-photon absorption enhancement of porphyrin macrocycles. J. Am. Chem. Soc. 2008, 130, 17212–17213. 10.1021/ja804415b. [DOI] [PubMed] [Google Scholar]
- Clays K.; Wostyn K.; Olbrechts G.; Persoons A.; Watanabe A.; Nogi K.; Duan X. M.; Okada S.; Oikawa H.; Nakanishi H.; Vogel H.; Beljonne D.; Brédas J.-L. Fourier analysis of the femtosecond hyper-Rayleigh scattering signal from ionic fluorescent hemicyanine dyes. J. Opt. Soc. Am. B 2000, 17, 256–265. 10.1364/JOSAB.17.000256. [DOI] [Google Scholar]
- Ma H.; Liu S.; Luo J.; Suresh S.; Liu L.; Kang S. H.; Haller M.; Sassa T.; Dalton L. R.; Jen A. K.-Y. Highly efficient and thermally stable electro-optical dendrimers for photonics. Adv. Funct. Mater. 2002, 12, 565–574. . [DOI] [Google Scholar]
- Dalton L. Nonlinear optical polymeric materials: from chromophore design to commercial applications. Adv. Polym. Sci. 2002, 158, 1–86. 10.1007/3-540-44608-7_1. [DOI] [Google Scholar]
- Dalton L. R.; Harper A. W.; Robinson B. H. The role of London forces in defining noncentrosymmetric order of high dipole moment high hyperpolarizability chromophores in electrically poled polymeric thin films. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 4842–4847. 10.1073/pnas.94.10.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton L. R.; Steier W. H.; Robinson B. H.; Zhang C.; Ren A.; Garner S.; Chen A. T.; Londergan T.; Irwin L.; Carlson B.; Fifield L.; Phelan G.; Kincaid C.; Amend J.; Jen A. From molecules to opto-chips: organic electro-optic materials. J. Mater. Chem. 1999, 9, 1905–1920. 10.1039/a902659b. [DOI] [Google Scholar]
- Liakatas I.; Cai C.; Bösch M.; Jäger M.; Bosshard C.; Günter P.; Zhang C.; Dalton L. R. Importance of intermolecular interactions in the nonlinear optical properties of poled polymers. Appl. Phys. Lett. 2000, 76, 1368–1370. 10.1063/1.126034. [DOI] [Google Scholar]
- Luo J.; Haller M.; Ma H.; Liu S.; Kim T.-D.; Tian Y.; Chen B.; Jang S.-H.; Dalton L. R.; Jen A. K.-Y. Nanoscale architectural control and macromolecular engineering of nonlinear optical dendrimers and polymers for electro-optics. J. Phys. Chem. B 2004, 108, 8523–8530. 10.1021/jp036714o. [DOI] [Google Scholar]
- Robinson B. H.; Dalton L. R.; Harper A. W.; Ren A.; Wang F.; Zhang C.; Todorova G.; Lee M.; Aniszfeld R.; Garner S.; Chen A.; Steier W. H.; Houbrecht S.; Persoons A.; Ledoux I.; Zyss J.; Jen A. K.-Y. The molecular and supramolecular engineering of polymeric electro-optic materials. Chem. Phys. 1999, 245, 35–50. 10.1016/S0301-0104(99)00079-8. [DOI] [Google Scholar]
- Steier W. H.; Chen A.; Lee S.-S.; Garner S.; Zhang H.; Chuyanov V.; Dalton L. R.; Wang F.; Ren A. S.; Zhang C.; Todorova G.; Harper A.; Fetterman H. R.; Chen D.; Udupa A.; Bhattacharya D.; Tsap B. Polymer electro-optic devices for integrated optics. Chem. Phys. 1999, 245, 487–506. 10.1016/S0301-0104(99)00042-7. [DOI] [Google Scholar]
- Brédas J. L.; Meyers F.; Pierce B. M.; Zyss J. On the second-order polarizability of conjugated p-electron molecules with octupolar symmetry: the case of triaminotrinitrobenzene. J. Am. Chem. Soc. 1992, 114, 4928–4929. 10.1021/ja00038a082. [DOI] [Google Scholar]
- Cho B. R.; Park S. B.; Lee S. J.; Son K. H.; Lee S. H.; Lee M.-J.; Yoo J.; Lee Y. K.; Lee G. J.; Kang T. I.; Cho M.; Jeon S.-J. 1,3,5-tricyano-2,4,6-tris(vinyl)benzene derivatives with large second-order nonlinear optical properties. J. Am. Chem. Soc. 2001, 123, 6421–6422. 10.1021/ja0025595. [DOI] [PubMed] [Google Scholar]
- Hennrich G.; Asselberghs I.; Clays K.; Persoons A. Tuning octopolar nlo chromophores: synthesis and spectroscopic characterization of persubstituted 1,3,5-tris(ethynylphenyl)benzenes. J. Org. Chem. 2004, 69, 5077–5081. 10.1021/jo049279i. [DOI] [PubMed] [Google Scholar]
- Hennrich G.; Omenat A.; Asselberghs I.; Foerier S.; Clays K.; Verbiest T.; Serrano J. L. Liquid crystals from C3-symmetric mesogens for second-order nonlinear optics. Angew. Chem., Int. Ed. 2006, 45, 4203–4206. 10.1002/anie.200600019. [DOI] [PubMed] [Google Scholar]
- Kim H. M.; Cho B. R. Second-order nonlinear optical properties of octupolar molecules structure-property relationship. J. Mater. Chem. 2009, 19, 7402–7409. 10.1039/b906361g. [DOI] [Google Scholar]
- Lambert C.; Nöll G.; Schmälzlin E.; Meerholz K.; Bräuchle C. Synthesis, (non)linear optical and redox properties of a donor-substituted truxenone derivative. Chem. - Eur. J. 1998, 4, 2129–2135. . [DOI] [Google Scholar]
- Le Bozec H.; Le Bouder T.; Maury O.; Bondon A.; Ledoux I.; Deveau S.; Zyss J. Supramolecular octupolar self-ordering towards nonlinear optics. Adv. Mater. 2001, 13, 1677–1681. . [DOI] [Google Scholar]
- Le Floc’h V.; Brasselet S.; Zyss J.; Cho B. R.; Lee S. H.; Jeon S.-J.; Cho M.; Min K. S.; Suh M. P. High efficiency and quadratic nonlinear optical properties of a fully optimized 2D octupolar crystal characterized by nonlinear microscopy. Adv. Mater. 2005, 17, 196–200. 10.1002/adma.200400451. [DOI] [Google Scholar]
- Maury O.; Le Bozec H. Molecular engineering of octupolar NLO molecules and materials based on bipyridyl metal complexes. Acc. Chem. Res. 2005, 38, 691–704. 10.1021/ar020264l. [DOI] [PubMed] [Google Scholar]
- McDonagh A. M.; Humphrey M. G.; Samoc M.; Luther-Davies B.; Houbrechts S.; Wada T.; Sasabe H.; Persoons A. Organometallic complexes for nonlinear optics. 16. Second and third order optical nonlinearities of octopolar alkynylruthenium complexes. J. Am. Chem. Soc. 1999, 121, 1405–1406. 10.1021/ja982965c. [DOI] [Google Scholar]
- Verbiest T.; Clays K.; Samyn C.; Wolff J.; Reinhoudt D.; Persoons A. Investigations of the hyperpolarizability in organic molecules from dipolar to octopolar systems. J. Am. Chem. Soc. 1994, 116, 9320–9323. 10.1021/ja00099a058. [DOI] [Google Scholar]
- Wolff J. J.; Siegler F.; Matschiner R.; Wortmann R. Optimized two-dimensional NLO chromophores with a threefold symmetry axis. Angew. Chem., Int. Ed. 2000, 39, 1436–1439. . [DOI] [PubMed] [Google Scholar]
- Zyss J.; Dhenaut C.; Chauvan T.; Ledoux I. Quadratic nonlinear susceptibility of octupolar chiral ions. Chem. Phys. Lett. 1993, 206, 409–414. 10.1016/0009-2614(93)85574-8. [DOI] [Google Scholar]
- Ostroverkhov V.; Singer K. D.; Petschek R. G. Second-harmonic generation in nonpolar chiral materials: relationship between molecular and macroscopic properties. J. Opt. Soc. Am. B 2001, 18, 1858–1865. 10.1364/JOSAB.18.001858. [DOI] [Google Scholar]
- Bartholomew G. P.; Ledoux I.; Mukamel S.; Bazan G. C.; Zyss J. Three-dimensional nonlinear optical chromophores based on through-space delocalization. J. Am. Chem. Soc. 2002, 124, 13480–13485. 10.1021/ja0272179. [DOI] [PubMed] [Google Scholar]
- Tai O. Y.-H.; Wang C. H.; Ma H.; Jen A. K.-Y. Wavelength dependence of first molecular hyperpolarizability of a dendrimer in solution. J. Chem. Phys. 2004, 121, 6086–6092. 10.1063/1.1785777. [DOI] [PubMed] [Google Scholar]
- Stadler S.; Dietrich R.; Bourhill G.; Bräuchle C.; Pawlik A.; Grahn W. First hyperpolarizability measurements via hyper-Rayleigh scattering at 1500 nm. Chem. Phys. Lett. 1995, 247, 271–276. 10.1016/0009-2614(95)01234-6. [DOI] [Google Scholar]
- Stadler S.; Dietrich R.; Bourhill G.; Bräuchle C. Long-wavelength first hyperpolarizability measurements by hyper-Rayleigh scattering. Opt. Lett. 1996, 21, 251–253. 10.1364/OL.21.000251. [DOI] [PubMed] [Google Scholar]
- Schmälzlin E.; Meerholz K.; Stadler S.; Bräuchle C.; Patzelt H.; Oesterhelt D. Molecular first hyperpolarizabilities of retinal and its derivatives. Chem. Phys. Lett. 1997, 280, 551–555. 10.1016/S0009-2614(97)01168-8. [DOI] [Google Scholar]
- Büchert M.; Steenbock T.; Lukaschek C.; Wolff M. C.; Herrmann C.; Heck J. 2,2′-bipyridine-based dendritic structured compounds for second harmonic generation. Chem. - Eur. J. 2014, 20, 14351–14361. 10.1002/chem.201404001. [DOI] [PubMed] [Google Scholar]
- Silva T. J. L.; Mendes P. J.; Garcia M. H.; Robalo M. P.; Ramalho J. P. P.; Carvalho A. J. P.; Büchert M.; Wittenburg C.; Heck J. Benzo[c]thiophene chromophores linked to cationic fe and ru derivatives for NLO materials: synthesis characterization and quadratic hyperpolarizabilities. Eur. J. Inorg. Chem. 2013, 2013, 3506–3517. 10.1002/ejic.201300048. [DOI] [Google Scholar]
- Silva T. J. L.; Mendes P. J.; Santos A. M.; Garcia M. H.; Robalo M. P.; Ramalho J. P. P.; Carvalho A. J. P.; Büchert M.; Wittenburg C.; Heck J. Mono(η5-cyclopentadienyl)metal(II) complexes with thienyl acetylide chromophores: synthesis, electrochemical studies, and first hyperpolarizabilities. Organometallics 2014, 33, 4655–4671. 10.1021/om4001204. [DOI] [Google Scholar]
- DiMagno S. G.; Lin V. S.-Y.; Therien M. J. Facile elaboration of porphyrins via metal-mediated cross-coupling. J. Org. Chem. 1993, 58, 5983–5993. 10.1021/jo00074a027. [DOI] [Google Scholar]
- DiMagno S. G.; Lin V. S.-Y.; Therien M. J. Catalytic conversion of simple haloporphyrins into alkyl-, aryl-, pyridyl-, and vinyl-substituted porphyrins. J. Am. Chem. Soc. 1993, 115, 2513–2515. 10.1021/ja00059a060. [DOI] [Google Scholar]
- Heck R. F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. 10.1021/ar50136a006. [DOI] [Google Scholar]
- Kumada M. Nickel and palladium complex catalyzed cross-coupling reactions of organometallic reagents with organic halides. Pure Appl. Chem. 1980, 52, 669–679. 10.1351/pac198052030669. [DOI] [Google Scholar]
- Negishi E.; Luo F. T.; Frisbee R.; Matsushita H. Selective carbon-carbon bond formation via transition metal catalysis. 26. A regiospecific synthesis of carbo-substituted heteroaromatic derivatives via palladium-catalyzed cross coupling. Heterocycles 1982, 18, 117–122. 10.3987/S(B)-1982-01-0117. [DOI] [Google Scholar]
- Stille J. K. Palladium-catalyzed coupling reactions of organic electrophiles with organic tin compounds. Angew. Chem., Int. Ed. Engl. 1986, 25, 508–524. 10.1002/anie.198605081. [DOI] [Google Scholar]
- Takahashi S.; Kuroyama Y.; Sonogashira K.; Hagihara N. A convenient synthesis of ethynylarenes and diethynylarenes. Synthesis 1980, 1980, 627–630. 10.1055/s-1980-29145. [DOI] [Google Scholar]
- Ishizuka T.; Sinks L. E.; Song K.; Hung S.-T.; Nayak A.; Clays K.; Therien M. J. The roles of molecular structure and effective optical symmetry in evolving dipolar chromophoric building blocks to potent octopolar nonlinear optical chromophores. J. Am. Chem. Soc. 2011, 133, 2884–2896. 10.1021/ja105004k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno S. G.; Williams R. A.; Therien M. J. Facile synthesis of meso-tetrakis(perfluoroalkyl)porphyrins: spectroscopic properties and x-ray crystal structure of highly electron-deficient 5,10,15,20-tetrakis(heptafluoropropyl)porphyrin. J. Org. Chem. 1994, 59, 6943–6948. 10.1021/jo00102a017. [DOI] [Google Scholar]
- Goll J. G.; Moore K. T.; Ghosh A.; Therien M. J. Synthesis, structure, electronic spectroscopy, photophysics, electrochemistry, and x-ray photoelectron spectroscopy of highly-electron-deficient [5,10,15,20-tetrakis(perfluoroalkyl)porphinato]zinc(II) complexes and their free base derivatives. J. Am. Chem. Soc. 1996, 118, 8344–8354. 10.1021/ja9610904. [DOI] [Google Scholar]
- Moore K. T.; Fletcher J. T.; Therien M. J. Syntheses, NMR and EPR spectroscopy, electrochemical properties, and structural studies of [5,10,15,20-tetrakis(perfluoroalkyl)porphinato]iron(II) and -iron(III) complexes. J. Am. Chem. Soc. 1999, 121, 5196–5209. 10.1021/ja9816741. [DOI] [Google Scholar]
- Notaras E. G. A.; Fazekas M.; Doyle J. J.; Blau W. J.; Senge M. O. A2B2-type push–pull porphyrins as reverse saturable and saturable absorbers. Chem. Commun. 2007, 43, 2166–2168. 10.1039/B618996B. [DOI] [PubMed] [Google Scholar]
- LeCours S. M.; Philips C. M.; de Paula J. C.; Therien M. J. Synthesis, transient absorption, and transient resonance Raman spectroscopy of novel electron donor-acceptor complexes: [5,15-bis[(4 ′-nitrophenyl)ethynyl]-10,20-diphenylporphinato]copper(II) and [5-[[4 ′-(dimethylamino)phenyl]ethynyl]-15-[(4 ″-nitrophenyl)ethynyl]-10,20-diphenyl-porphinato]copper(II). J. Am. Chem. Soc. 1997, 119, 12578–12589. 10.1021/ja964436j. [DOI] [PubMed] [Google Scholar]
- Uyeda H. T. PhD Thesis; University of Pennsylvania: 2002. [Google Scholar]
- Damrauer N. H.; Boussie T. R.; Devenney M.; McCusker J. K. Effects of intraligand electron delocalization, steric tuning, and excited-state vibronic coupling on the photophysics of aryl-substituted bipyridyl complexes of Ru(II). J. Am. Chem. Soc. 1997, 119, 8253–8268. 10.1021/ja971321m. [DOI] [Google Scholar]
- Damrauer N. H.; Weldon B. T.; McCusker J. K. Theoretical studies of steric effects on intraligand electron delocalization: implications for the Franck-Condon state evolution of MLCT excited states. J. Phys. Chem. A 1998, 102, 3382–3397. 10.1021/jp9805095. [DOI] [Google Scholar]
- McCusker J. K. Femtosecond absorption spectroscopy of transition metal charge-transfer complexes. Acc. Chem. Res. 2003, 36, 876–887. 10.1021/ar030111d. [DOI] [PubMed] [Google Scholar]
- Winkler J. R.; Netzel T. L.; Creutz C.; Sutin N. Direct observation of metal-to-ligand charge-transfer (MLCT) excited states of pentaammineruthenium(II) complexes. J. Am. Chem. Soc. 1987, 109, 2381–2392. 10.1021/ja00242a023. [DOI] [Google Scholar]
- Kumble R.; Palese S.; Lin V. S.-Y.; Therien M. J.; Hochstrasser R. M. Ultrafast dynamics of highly conjugated porphyrin arrays. J. Am. Chem. Soc. 1998, 120, 11489–11498. 10.1021/ja981811u. [DOI] [Google Scholar]
- Oudar J. L. Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J. Chem. Phys. 1977, 67, 446–457. 10.1063/1.434888. [DOI] [Google Scholar]
- Oudar J. L.; Chemla D. S. Hyperpolarizabilities of nitroanilines and their relations to excited state dipole moment. J. Chem. Phys. 1977, 66, 2664–2668. 10.1063/1.434213. [DOI] [Google Scholar]
- Cyvin S. J.; Rauch J. E.; Decius J. C. Theory of hyper-raman effects (nonlinear inelastic light scattering) - selection rules and depolarization ratios for second-order polarizability. J. Chem. Phys. 1965, 43, 4083–4094. 10.1063/1.1696646. [DOI] [Google Scholar]
- Bersohn R.; Pao Y.-H.; Frisch H. L. Double-quantum light scattering by molecules. J. Chem. Phys. 1966, 45, 3184–3198. 10.1063/1.1728092. [DOI] [Google Scholar]
- Champagne B.; Pèrpete E. A.; van Gisbergen S. J. A.; Baerends E.-J.; Snijders J. G.; Soubra-Ghaoui C.; Robins K. A.; Kirtman B. Assessment of conventional density functional schemes for computing the polarizabilities and hyperpolarizabilities of conjugated oligomers: An ab initio investigation of polyacetylene chains. J. Chem. Phys. 1998, 109, 10489–10498. 10.1063/1.477731. [DOI] [Google Scholar]
- Jamorski C.; Casida M. E.; Salahub D. R. Dynamic polarizabilities and excitation spectra from a molecular implementation of time-dependent density-functional response theory: N2 as a case study. J. Chem. Phys. 1996, 104, 5134–5147. 10.1063/1.471140. [DOI] [Google Scholar]
- Karna S. P.; Prasad P. N.; Dupuis M. Nonlinear optical properties of p-nitroaniline: an ab initio time-dependent coupled perturbed hartree-fock study. J. Chem. Phys. 1991, 94, 1171–1181. 10.1063/1.460024. [DOI] [Google Scholar]
- Kirtman B.; Champagne B. Nonlinear optical properties of quasilinear conjugated oligomers, polymers and organic molecules. Int. Rev. Phys. Chem. 1997, 16, 389–420. 10.1080/014423597230181. [DOI] [Google Scholar]
- Tretiak S.; Chernyak V. Resonant nonlinear polarizabilities in the time-dependent density functional theory. J. Chem. Phys. 2003, 119, 8809–8823. 10.1063/1.1614240. [DOI] [Google Scholar]
- Tretiak S.; Mukamel S. Density matrix analysis and simulation of electronic excitations in conjugated and aggregated molecules. Chem. Rev. 2002, 102, 3171–3212. 10.1021/cr0101252. [DOI] [PubMed] [Google Scholar]
- van Gisbergen S. J. A.; Kootstra F.; Schipper P. R. T.; Gritsenko O. V.; Snijders J. G.; Baerends E. J. Density-functional-theory response-property calculations with accurate exchange-correlation potentials. Phys. Rev. A: At., Mol., Opt. Phys. 1998, 57, 2556–2571. 10.1103/PhysRevA.57.2556. [DOI] [Google Scholar]
- van Gisbergen S. J. A.; Snijders J. G.; Baerends E. J. Calculating frequency-dependent hyperpolarizabilities using time-dependent density functional theory. J. Chem. Phys. 1998, 109, 10644–10656. 10.1063/1.477762. [DOI] [Google Scholar]
- Champagne B.; Pèrpete E. A.; Jacquemin D.; van Gisbergen S. J. A.; Baerends E.-J.; Soubra-Ghaoui C.; Robins K. A.; Kirtman B. Assessment of conventional density functional schemes for computing the dipole moment and (hyper)polarizabilities of push-pull π-conjugated systems. J. Phys. Chem. A 2000, 104, 4755–4763. 10.1021/jp993839d. [DOI] [Google Scholar]
- Miura M.; Aoki Y.; Champagne B. Assessment of time-dependent density functional schemes for computing the oscillator strengths of benzene, phenol, aniline, and fluorobenzene. J. Chem. Phys. 2007, 127, 084103. 10.1063/1.2761886. [DOI] [PubMed] [Google Scholar]
- Kelley A. M. Frequency-dependent first hyperpolarizabilities from linear absorption spectra. J. Opt. Soc. Am. B 2002, 19, 1890–1900. 10.1364/JOSAB.19.001890. [DOI] [Google Scholar]
- Olivier J.-H.; Bai Y.; Uh H.; Yoo H.; Therien M. J.; Castellano F. N. Near infrared-to-visible photon upconversion enabled by conjugated porphyrinic sensitizers under low-power noncoherent illumination. J. Phys. Chem. A 2015, 119, 5642–5649. 10.1021/acs.jpca.5b03199. [DOI] [PubMed] [Google Scholar]
- Olbrechts G.; Strobbe R.; Clays K.; Persoons A. High-frequency demodulation of multi-photon fluorescence in hyper-Rayleigh scattering. Rev. Sci. Instrum. 1998, 69, 2233–2241. 10.1063/1.1148926. [DOI] [Google Scholar]
- Olbrechts G.; Wostyn K.; Clays K.; Persoons A. High-frequency demodulation of multiphoton fluorescence in long-wavelength hyper-Rayleigh scattering. Opt. Lett. 1999, 24, 403–405. 10.1364/OL.24.000403. [DOI] [PubMed] [Google Scholar]
- Campo J.; Painelli A.; Terenziani F.; Van Regemorter T.; Beljonne D.; Goovaerts E.; Wenseleers W. First hyperpolarizability dispersion of the octupolar molecule crystal violet: multiple resonances and vibrational and solvation effects. J. Am. Chem. Soc. 2010, 132, 16467–16478. 10.1021/ja105600t. [DOI] [PubMed] [Google Scholar]
- Woodford J. N.; Pauley M. A.; Wang C. H. Solvent dependence of the first molecular hyperpolarizability of p-nitroaniline revisited. J. Phys. Chem. A 1997, 101, 1989–1992. 10.1021/jp9639861. [DOI] [Google Scholar]
- Van Cleuvenbergen S.; Asselberghs I.; García-Frutos E. M.; Gómez-Lor B.; Clays K.; Pérez-Moreno J. Dispersion overwhelms charge transfer in determining the magnitude of the first hyperpolarizability in triindole octupoles. J. Phys. Chem. C 2012, 116, 12312–12321. 10.1021/jp3022997. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





