Abstract
Two obligate intracellular parasites, Trypanosoma cruzi, agent of Chagas disease, and Toxoplasma gondii, agent of toxoplasmosis, upregulate the mevalonate pathway of their host cells upon infection, suggesting that this host pathway could be a potential drug target. In this work we designed, synthesized and evaluated the effect of a number of compounds structurally related to WC-9 (4-phenoxyphenoxyethyl thiocyanate), a known squalene synthase inhibitor, on T. cruzi and T. gondii growth in tissue culture cells. The fluorine-containing derivatives 3-(3-fluorophenoxy) and 3-(4-fluorophenoxy)phenoxyethyl thiocyanates exhibited EC50 values of 1.6 µM and 4.9 µM, respectively, against tachyzoites of T. gondii, while they showed similar potency as WC-9 against intracellular T. cruzi (EC50 values 5.4 µM and 5.7 µM, respectively). In addition, 2-[3-(phenoxy)phenoxyethylthio]ethyl-1,1-bisphosphonate, which is a hybrid inhibitor containing 3-phenoxyphenoxy and bisphosphonate groups, has activity on T. gondii proliferation at sub micromolar levels (EC50 = 0.7 µM), suggesting combined inhibitory effect of the two functional groups.
Graphical Abstract
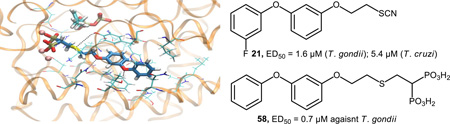
Compound 58 interacting at the active site of TgFPPS (IC50 = 0.076 µM) built by homology modeling.
1. Introduction
Obligate intracellular parasites depend on their host cell integrity to survive. They have evolved sophisticated strategies to manipulate their host and establish with them a close metabolic link in order to complete their development. A case in point is that of parasites like Trypanosoma cruzi, a trypanosomatid, and Toxoplasma gondii, an Apicomplexan parasite, which upon infection, upregulate host genes for the mevalonate pathway.[1–3] This up-regulation is probably in order to acquire cholesterol and other isoprenoids needed to accommodate an increasing intracellular parasite load and/or provide these lipids to be scavenged by the intracellular parasites.[3] T. cruzi is the agent of Chagas disease (American trypanosomiasis), the largest parasitic disease burden of the Americas.[4] Treatment of T. cruzi infection is with nifurtimox or benznidazole. Both these compounds are not FDA-approved drugs, and in the United States they are available only from CDC under investigational protocols. On the other hand, T. gondii is the agent of toxoplasmosis,[5] which affects a wide range of hosts, particularly humans and other warm-blooded animals.[6] Toxoplasmosis can be considered as one of the most prevalent parasitic diseases affecting almost one billion people worldwide.[7] This parasite can cause mortality among immune-compromised individuals such as AIDS patients and organ transplant recipients, as well as in congenitally infected children.[8] Toxoplasmosis may also lead to severe ocular disease in immune-competent patients.[9] The current chemotherapy for toxoplasmosis is also deficient as the available drugs may cause toxic side effects and they are not able to properly access the central nervous system. Another drawback of the present chemotherapy is its high cost.[10]
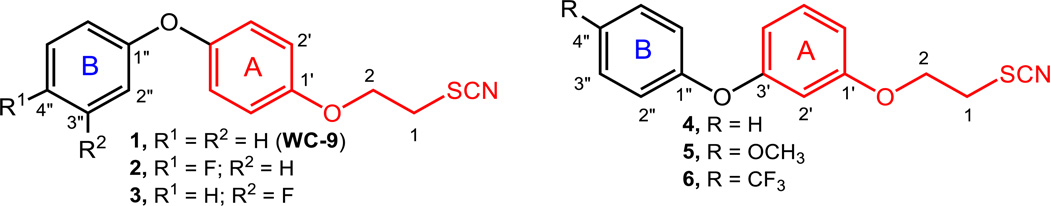
The up-regulation of the mevalonate pathway of the host by these intracellular parasites provides an additional potential drug target since its inhibition could affect the parasite and the host cell in which the parasite resides. T. gondii does not synthesize cholesterol and imports it from the host,[11] while it is also able to take up isoprenoids like farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP) synthesized by the host. As other trypanosomatids, T. cruzi has a strict requirement for specific endogenous sterols for survival although it can take up cholesterol from its mammalian host.[12,13] Appropriate ergosterol biosynthesis inhibitors can induce parasitological cure in both acute and chronic experimental models of Chagas disease.[14] 4-Phenoxyphenoxyethyl thiocyanate (compound 1; WC-9) is a potent inhibitor of the intracellular amastigote forms of T. cruzi.[15] WC-9 is a non-competitive inhibitor of T. cruzi squalene synthase (TcSQS) acting at low nanomolar concentration.[16] This enzyme catalyzes the first step in the sterol biosynthesis, which consists in the reductive dimerization of two molecules of farnesyl diphosphate to yield squalene. Additional synthetic derivatives of WC-9 (2–6) are shown in Figure 1.[15,17–21]
Figure 1.
Chemical structure of WC-9 and other closely related inhibitors of T. cruzi proliferation
It is interesting to note that T. gondii lacks the mevalonate pathway and uses the essential 1-deoxy-d-xylulose-5-phosphate (DOXP) pathway to make isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP).[22] As T. gondii does not synthesize cholesterol and imports it from the host,[11] it is reasonable to consider that inhibitors of the host SQS could eventually control T. gondii growth. Certainly, mevalonate pathway inhibitors modulate growth of different intracellular Apicomplexan parasites that are devoid of this pathway such as Babesia divergens,[23] Plasmodium falciparum,[23,24] Cryptosporidium parvum,[25] and T. gondii,[26] indicating that parasites lacking the mevalonate pathway are reliant on host precursors of isoprenoid biosynthesis. Interestingly, there is a synergistic effect when the mevalonate pathway of the host and the isoprenoid pathway of the parasite are targeted independently.[27] For example, zoledronic acid, a bisphosphonate that inhibits the T. gondii farnesyl diphosphate synthase (TgFPPS), and atorvastatin, an statin that inhibits the host 3-hydroxymethyl-glutaryl CoA-reductase, exhibit a marked synergism in the inhibition of T. gondii growth. [27]
2. Rationale
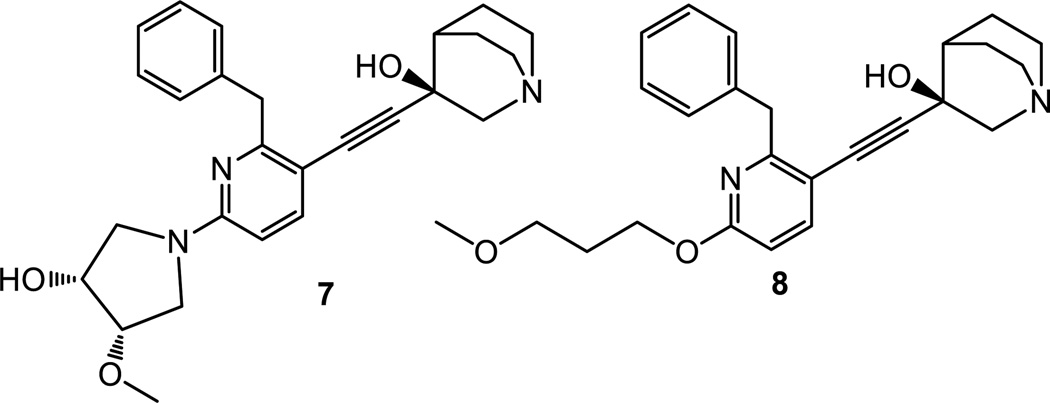
WC-9 is one of the few examples of a pharmacologically important lead compound possessing a thiocyanate group covalently bound to its main skeleton.[28] At the present time, there is no crystal structure available for the complex WC-9–TcSQS. However, an X-ray crystal structure of WC-9 with human SQS has been recently reported (pdb code 3WCD).[29] On the other hand, the X-ray crystal structure of the complexes E5700–TcSQS and ER-119884–TcSQS are available.[29] The quinuclidine derivatives E5700 (7) and ER-119884 (8) are potent inhibitors of T. cruzi growth acting as TcSQS inhibitors (Figure 2).[30,31] Both of these compounds are extremely potent inhibitors of the enzymatic activity of TcSQS exhibiting IC50 values of 0.84 nM and 3.5 nM, respectively.[31]
Figure 2.
Chemical structures of quinuclidine derivatives E5700 (7) and ER-119884 (8).
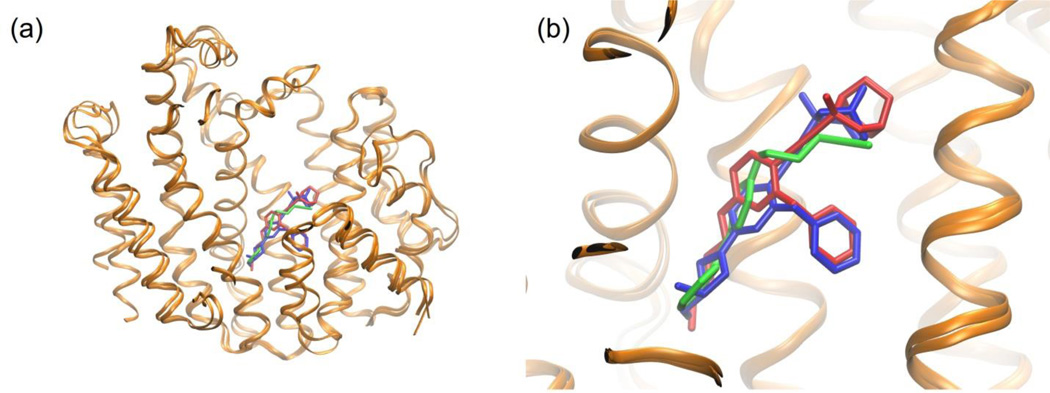
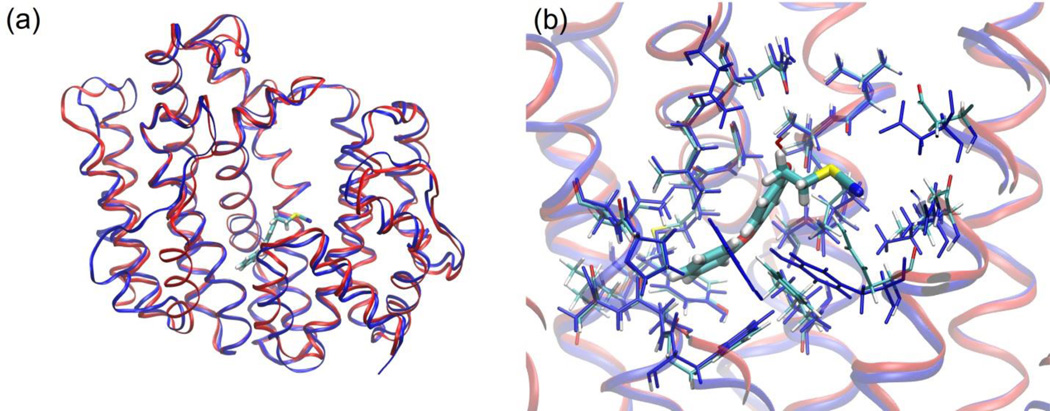
Figure 3 shows a superimposition of the E5700–TcSQS and ER-119884–TcSQS complexes with the crystal structure of the complex WC-9–human SQS. A high degree of similarity is observed between the T. cruzi and human protein structures. Furthermore, the quinoclidine inhibitors occupy the same binding site as WC-9. Given that these inhibitors were found to be mixed-type (7) and non-competitive (8),[32] they provide further evidence that WC-9 may in fact occupy the same binding site in T. cruzi SQS.
Figure 3.
(a) Superposition of the crystal structures of human SQS with WC-9 and T. cruzi SQS with 7 and 8. A high degree of similarity is observed between the protein chains. (b) Expansion of the structures showing that the quinuclidine derivatives 7 (red) and 8 (blue) occupy the same site S2 (homoallylic site) as WC-9 (green). The mechanisms of action of these compounds (7 mixed-type and 8 non-competitive) provide further evidence that WC-9 may indeed bind to the S2 site in T. cruzi SQS.
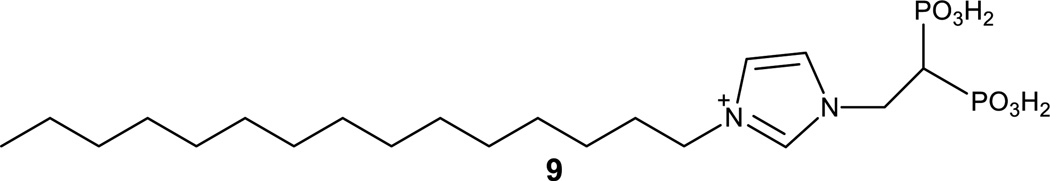
Interestingly, TcSQS activity is also inhibited by bisphosphonates.[33] Bisphosphonates are the main modulators of farnesyl diphosphate synthase (FPPS), a key enzyme of isoprenoid biosynthesis.[34] Moreover, several X-ray crystal structures of TcSQS are available with a number of bisphosphonates, such as BPH1344 (9) (pdb code 3WCG) (Figure 4).[29]
Figure 4.
Chemical structure of bisphosphonate known as BPH1344 (9).
Figure 5 shows a superposition of the WC-9–hsSQS complex (blue, NewRibbons representation) with the BPH1344-TcSQS complex (red), with the bisphosphonate deleted, showing the putative binding site that WC-9 could occupy in T. cruzi SQS.[29] As can be observed, the two structures show a high degree of similarity, except for the alpha helix 284 – 294 in T. cruzi which acquires a loop organization in the corresponding human SQS structure.[29] The X-ray crystal structure of WC-9 with dehydrosqualene synthase from Staphylococcus aureus, an enzyme very similar to SQS that catalyzes dehydrosqualene formation, is also available.[35]
Figure 5.
This Figure shows the binding site (amino acids within 4 Angstroms of the ligand) of WC-9 in the human SQS structure (amino acids shown in Licorice Representation, blue) (a), with the putative site it would occupy in T. cruzi SQS (amino acids in Licorice Representation with the Name color scheme) (b). A high degree of similarity is observed between the two binding sites. In terms of sequence, all amino acids in the binding sites are the same, except for Ser 256 in the T. cruzi vs. Cys 254 in the human enzyme.
At the present time, there is no a computer-assisted protocol to predict binding of WC-9 analogues to TcSQS. However, there are abundant structure activity relation (SAR) data available on T. cruzi and T. gondii cells that can be used to facilitate drug design.[17–21,36] In addition, there is strong evidence to state that the phenoxyethyl thiocyanate moiety of WC-9 (Figure 1) is the pharmacophore of this family of molecules. A question that emerges, whose answer is still pending, is whether the optimum substitution pattern will be at the C-4′ or the C-3′ position. The availability of the Buchwald coupling reaction allowing us to access a variety of WC-9 analogues encouraged us to go further in searching for better inhibitors against either T. cruzi or T. gondii cells.[37–40]
3. Results and Discussion
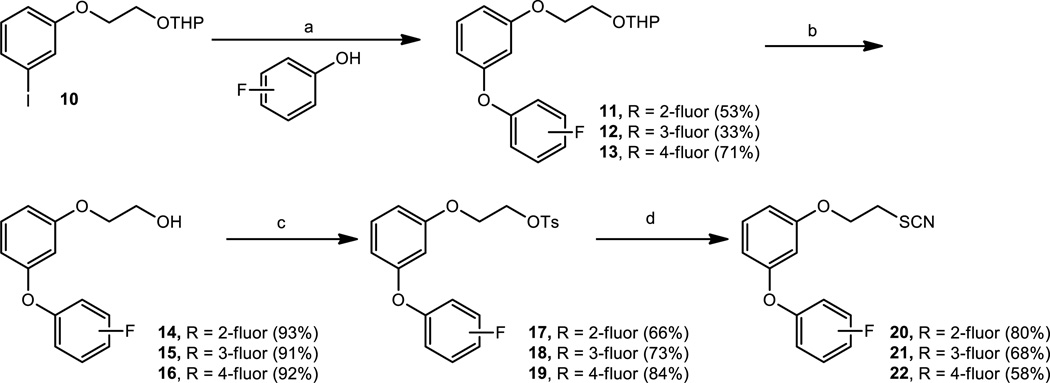
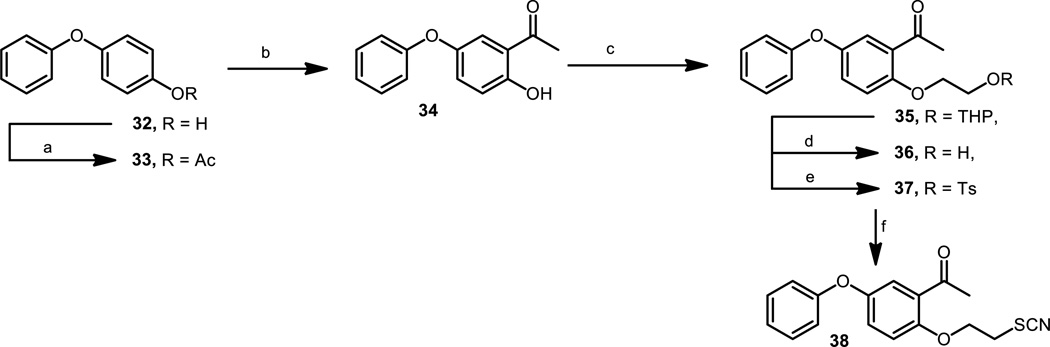
The introduction of a fluorine atom into the WC-9 structure to give rise to compounds 2 and 3 was a significant structural change, which was quite beneficial for the inhibitory action.[19] Therefore, it seemed of interest to study the biological activity of the fluorine–containing analogues of the regioisomer of WC-9, compound 4, envisioning compounds 11–13 as target molecules. Compound 10[20] was treated with 2-fluoro, 3-fluoro, and 4-fluorophenol under typical Buchwald coupling reaction conditions to generate tetrahydropyranyl derivatives 11, 12, and 13 in 53%, 33 and 71% yields, respectively. Each tetrahydropyranyl protecting group present in these compounds was cleaved by treatment with pyridinium p-toluenesulfonate producing the corresponding free alcohols 14, 15 and 16 in very good yields, which were tosylated to give 17, 18 and 19 in 66%, 73% and 84% yields, respectively. On treatment with potassium thiocyanate, in separate experiments, these compounds were converted into the target molecules 20, 21 and 22, respectively, as illustrated in Scheme 1.
Scheme 1.
Reagents and conditions: a) 5% CuI, 10% picolinic acid, (2-fluoro-, 3-fluoro-, 4-fluorophenol), K3PO4, DMSO, 80 ºC, 24 h; b) PPTs, CH3OH, rt, 16 h; c) ClTs, py, 0 ºC, 6 h; d) KSCN, DMF, 100 ºC, 6h.
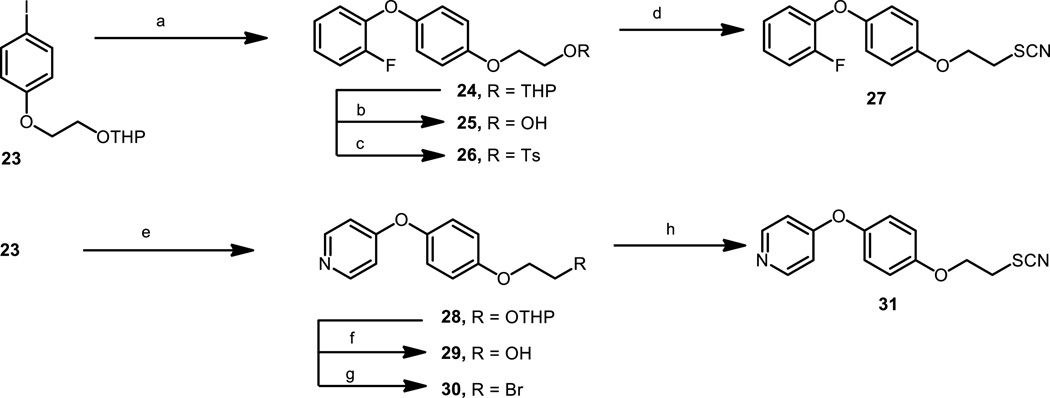
The fluoro derivatives of WC-9 at C-4″ and C-3″, namely 2 and 3, were more potent than our lead drug WC-9.[19] It seemed reasonable to also produce the fluorinated analogue at C-2″ (compound 27), which had not been prepared before. Therefore, Buchwald coupling reaction of 23[20] with 2-fluorophenol generated the tetrahydropyranyl derivative 24 in a low but reproducible yield, which on treatment with pyridinium 4-toluenesulfonate produced the free alcohol 25 in 64% yield. Tosylation of this compound to produce 26 followed by treatment with potassium thiocyanate resulted in the title compound 27 in 36% yield (Scheme 2).
Scheme 2.
Reagents and conditions: a) 5% CuI, 10% picolinic acid, 2-fluorophenol, K3PO4, DMSO, 80 ºC, 24 h, 37%; b) PPTs, CH3OH, rt, 24h, 62%; c) ClTs, py, rt, 24h, 92%; d) KSCN, DMF, 80 ºC, 6h, 36%; e) CuI, 10% picolinic acid, 4-hydroxypyridine, K3PO4, DMSO, 80 ºC, 24 h, 29%; f) PPTs, CH3OH, rt, 24h, 59%; g) NBS, Ph3P, 0ºC, 6h, 50%; h) KSCN, DMF, 80 ºC, 6h, 34%
An interesting structural variation was the replacement of the terminal phenyl group by a pyridyl group where the nitrogen atom occupied the 4″ position (compound 31). We have previously synthesized and evaluated the corresponding 2-pyridyl[21] and 3-pyridyl[20] derivatives; then, the availability of 31 would complete the corresponding SAR analysis. A straightforward synthesis of 31 was accomplished starting form 23,[20] which treated with 4-hydroxypyridine under typical coupling reaction generated 28. On treatment with pyridinium 4-toluenesulfonate, 28 was converted into alcohol 29, which was further transformed into 30 by reaction with N-bromosuccinimide and triphenylphosphine[41] in 52% yield. On reaction with potassium thiocyanate, 30 was transformed into the title compound 31 in 36% yield (Scheme 2).
Although a significant number of structural variations have been made on the structure of WC-9, only few of them correspond to substitutions at the A ring.[17] An interesting method to incorporate an acetyl group at the C-2′ is the photo-Fries rearrangement reaction.[42] Then, 4-phenoxyphenol (32) was acetylated to give 33 in excellent yield. 33 was irradiated at 254 nm to produce the corresponding photo-Fries rearranged product 34 in 39% yield. 34 was reacted with 2-bromoethyl tetrahydro-2H–pyran-2-yl ether via a Williamson etherification reaction to give 35 in 62% yield. This compound was deprotected by treatment with pyridinium 4-toluenesulfonate in methanol to generate free alcohol 36 (92% yield), which was treated with tosyl chloride in pyridine to give the expected tosylate 37 in 95% yield. This compound was further transformed into the thiocyanate derivative 38 by treatment with potassium thiocyanate in N,N-dimethylformamide at 80 ºC in 71% yield (Scheme 3).
Scheme 3.
Reagents and conditions: a) Ac2O, py, rt 16 h, 97%; b) hν, C6H12, rt, 12h, 39%; c) KOH, DMSO, BrCH2CH2OTHP, rt, 12h, 62%; d) PPTs, CH3OH, rt, 16h, 92%; e) ClTs, py, rt, 6h, 95%; f) KSCN, DMF, 80 ºC, 6h, 71%.
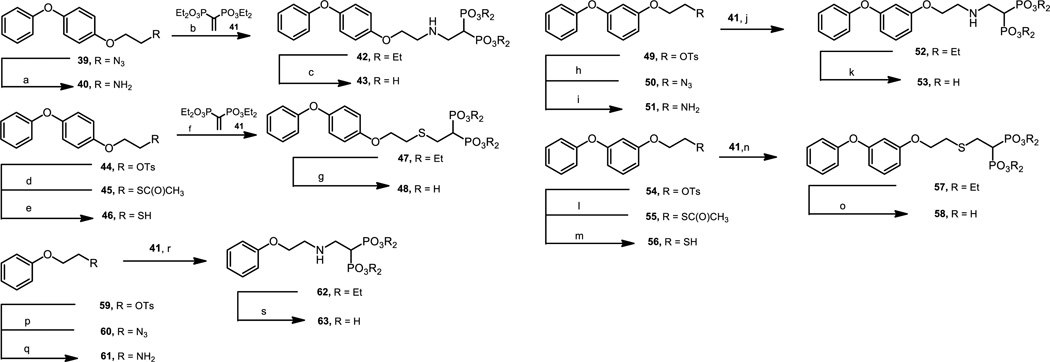
The concept of a hybrid drug where one compound possesses a dual mode of action is fascinating.[43–45] For this reason, hybrid molecules such as 43 and 48 were envisioned with the phenoxy unit targeting SQS and the bisphosphonate moiety acting against FPPS.[34] The production of 42 was straightforward employing the already described azide 39.[46] This compound was reduced to the corresponding amine 40 through a Staudinger reaction by treatment with triphenylphosphine in methylene chloride in 77% yield.[47] Michael-type addition of the resulting amine 40 with the Michael acceptor 41[48] yielded 42 in 82% yield, which after hydrolysis by treatment with refluxing hydrochloric acid produced the title compound 43. The hybrid compound 48 was straightforwardly prepared starting from the described tosylate 44.[15] Upon treatment of 48 with potassium thioacetate in N,N-dimethylformamide at 90 ºC 45 was produced, which when treated with lithium aluminum hydride in anhydrous tetrahydrofuran yielded the corresponding mercaptan 46 in good yields. Michael addition reaction of this resulting compound with 41 followed by hydrolysis of the phosphonate esters by treatment with bromotrimethylsilane and digestion with methanol produced the title compound 48. Following a quite similar approach, 31 was prepared from the already described tosylate 49,[20] which was transformed into azide 50 by treatment with sodium azide in N,N.dimethylformamide. Under a Staudinger-type conditions 50 was converted into amine 51 that was reacted with 41 to produce the Michael adduct 52. Hydrolysis of this compound by treatment with refluxing concentrated hydrochloric acid produced the title compound 53. The synthesis of 58 was also straightforward. The known tosylate 54[20] was reacted with potassium thioacetate via a SN2 reaction in N,N-dimethylformamide at 80 ºC to give rise to 55 in 83% yield. Hydrolysis of the acetyl group by treatment with potassium carbonate followed by reduction with zinc in glacial acetic acid generated the corresponding mercaptan 56 in 75% yield. Michael addition of this mercaptan followed by hydrolysis by treatment with bromotrimethylsilane and methanol digestion produced the title compound 58 in 65% yield. Similarly, 63 was obtained from the already described tosylate 59,[18] which was transformed into 60 by treatment with sodium azide. This compound was transformed into 61 that was reacted with 41 to yield 62. Treatment with refluxing concentrated hydrochloric acid resulted in the title compound 63 (Scheme 4).
Scheme 4.
Reagents and conditions: a) PPh3, CH2Cl2, rt, 4h, 77%; b) 41, CH2Cl2, NEt3, rt, 4h, 82%; c) HCl (c), reflux, 7h, 81%; d) CH3C(O)SK, DMF, 2h, 90 ºC, 83%; e) LiAlH4, THF, rt, 3h, 92%; f) 41, CH2Cl2, NEt3, rt, 4h, 82%; g) i. BrSi(CH3)3, CH2Cl2, rt, 24h, ii. CH3OH, rt, 12h, 69%; h) NaN3, DMF, 80 ºC, 2h, 90%; i) PPh3, THF, rt, 2h, 90%; j) 41, CH2Cl2, NEt3, rt, 24h, 97%; k) HCl (c), reflux, 7h, 35%; l) CH3C(O)SK, DMF, 2h, 80 ºC, 83%; m) i. K2CO3, CH3OH/H2O, 1h, rt, ii. Zn/AcOH, 3h, 110 ºC, 75%; n) 41, CH2Cl2, NEt3, rt, 16h, 75%; o) i. BrSi(CH3)3, CH2Cl2, rt, 24h, ii. CH3OH, rt, 12h, 65%; p) NaN3, DMF, 80 ºC, 2h, 81%; q) PPh3, THF, rt, 2h, 67%; r) 41, CH2Cl2, NEt3, rt, 24h, 90%; s) ) HCl (c), reflux, 7h, 81%.
As another structural variation, hydrophobic analogues of WC-9 such as 68 and 73, were also produced. Both of these compounds were easily prepared starting from β-naphthol (64) and α-naphthol (69), respectively. Therefore, each compound, in separate experiments, was treated with 2-bromoethyl tetrahydro-2H–pyran-2-yl ether under Williamson-type conditions to produce tetrahydropyranyl derivatives 65 and 70, respectively, which were easily deprotected by treatment with pyridinium 4-toluenesulfonate to yield the corresponding free alcohols 66–71, respectively. On treatment with excess of tosyl chloride, 66 and 71 were converted into tosylates 67 and 72, respectively. On reaction with potassium thiocyanate in N,N-dimethylformamide, 67 and 72 were converted into the title molecules 68 and 73, respectively as shown in Scheme 5.
Scheme 5.
Reagents and conditions: a) KOH, DMSO, BrCH2CH2OTHP, rt, 5 days, 48%; b) PPTs, CH3OH, rt, 24h, 85%; c) ClTs, py, rt, 0 ºC, 83%; d) KSCN, DMF, 80 ºC, 6h, 55%; f) KOH, DMSO, BrCH2CH2OTHP, rt, 5 days, 65%; g) PPTs, CH3OH, rt, 24h, 81%; e) ClTs, py, rt, 0 ºC, 90%; f) KSCN, DMF, 80 ºC, 6h, 58%.
Biological evaluation of these new compounds structurally related to WC-9 was promising. All fluorine-containing derivatives of compound 4, that is compounds 20–22, were effective inhibitors of tachyzoites of T. gondii exhibiting EC50 values of 3.1 µM, 1.6 µM, and 4.9 µM, respectively, with 20 and 21 being slightly more potent than 4 (EC50 = 4.0 µM).[20] The fluorinated compounds were also effective growth inhibitors of the intracellular form of T. cruzi (amastigotes), which is the clinically more relevant replicative form of the parasite. In fact, all of these compounds bearing a fluorine atom bound to the C-2″, C-3″, and C-4″ positions exhibited relatively similar EC50 values (7.0 µM, 5.4 µM, and 5.7 µM, respectively) compared to WC-9, used as a positive control, under the same assay conditions.[20] The fluorine analogue of WC-9 at C-2″, compound 27, which is the regioisomer of 2 and 3, was also a potent inhibitor of T. gondii tachyzoites growth having an EC50 value of 4.5 µM. Compound 27 was more potent than 2 (EC50 = 15.8 µM)[19] but exhibited quite similar efficacy as 3 (EC50 = 3.9 µM).[19] Quite unexpectedly, in spite of its regioisomers 2 and 3 being potent growth inhibitors of amastigotes of T. cruzi,[19] 27 was devoid of antiparasitic activity against these parasites. Pyridyl derivative 31 was free of antiparasitic activity against T. cruzi; however, 31 behaved as a growth inhibitor of T. gondii possessing an EC50 value of 7.9 µM. The introduction of an acetyl group at the C-2′ position giving rise to 38 resulted to be quite beneficial for the biological activity, as this compound showed a potent inhibitory action against T. gondii. In fact, its EC50 value was 2.4 µM. 38 was less effective against T. cruzi with an EC50 value of 11.3 µM. The hybrid molecules 53 and 58 were both devoid of activity against T. cruzi but they showed a strong inhibitory action against tachyzoites of T. gondii. The sulfur-containing analogue 58 showed a sub-micromolar activity against T. gondii having an EC50 value of 0.7 µM. In fact, the 3′-phenoxy substitution pattern found in 53 and 58 is more beneficial for biological activity against T. gondii than the 4′-phenoxy substitution present in 43 and 48. Compounds 53 and 58 were also recognized as potent inhibitors of the target enzyme TgFPPS exhibiting IC50 values of 0.040 µM and 0.076 µM, respectively. The simplified naphtyl derivatives 68 and 73 turned out to be potent inhibitors of T. gondii possessing EC50 values of 3.6 µM and 3.1 µM, respectively, but exhibited vanishing inhibitory action against T. cruzi. The results are presented in Table 1.
Table 1.
Biological activity of WC-9 analogues against T. gondii (tachyzoites), T. cruzi (amastigotes), and Vero cells.
| Compound |
T. gondii growth EC50 (µM) |
TgFPPS IC50 (µM) |
T. cruzi growth EC50 (µM) |
TcFPPS IC50 (µM) |
HhFPPS IC50 (µM) |
Cytotoxicity EC50 (µM) |
|---|---|---|---|---|---|---|
| 20 | 3.12 ± 0.37 | ND | 7.01 ± 0.51 | ND | ND | >60 |
| 21 | 1.63 ± 0.36 | ND | 5.38 ± 0.82 | ND | ND | >50 |
| 22 | 4.92 ± 1.94 | ND | 5.69 ± 0.47 | ND | ND | >55 |
| 27 | 4.54 ± 0.57 | ND | >10 | ND | ND | >55 |
| 31 | 7.86 ± 0.78 | ND | >10 | ND | ND | >200 |
| 38 | 2.42 ± 0.81 | ND | 11.3 ± 2.5 | ND | ND | 75.7 ± 7.3 |
| 43 | 5.90 ± 0.07 | 0.094 ± 0.020 | >10 | ND | >10 | >100 |
| 48 | > 10.0 | 0.362 ± 0.002 | >20 | 0.8 ± 0.12 | >15 | >200 |
| 53 | 2.40 ± 0.70 | 0.040 ± 0.002 | >10 | >10 | >10 | >50 |
| 58 | 0.67 ± 0.23 | 0.076 ± 0.019 | >10 | ND | >10 | >100 |
| 63 | > 10 | 0.180 ± 0.015 | >10 | 0.32 ± 0.04 | >10.0 | ND |
| 68 | 3.62 ± 0.38 | ND | >10 | ND | ND | ND |
| 73 | 3.13 ± 0.58 | ND | >10 | ND | ND | 7.5 |
| WC-9 | 4.8 ± 0.41[20] | ND | 5.0 ± 1.1[20] | ND | ND | 82.6 ± 7.3 |
| Benznidazole | ND | ND | 1.52 ± 0.10 | ND | ND | ND |
Values are means ± SD of results from at least three independent experiments, n = 3. ND, not determined
Given the high in vitro activity of hybrid derivatives of WC-9, we performed Molecular Dynamics-based computational studies to gain further insight on the interactions of this kind of compounds with the active site of FPPS. Taking 58 as a relevant example and as no crystal structure of TgFPPS is available, we constructed that structure using homology modeling based on the crystal structure of P. vivax FPPS (pdb id 3mav), the enzyme showing the highest degree of similarity with TgFPPS. We inserted 58, three Mg2+ ions and isopentenyl pyrophosphate as a co-ligand, by alignment with the TcFPPS alendronate complex structure (pdb id 1yhm). We carried out a 10 ns Molecular Dynamics simulation in explicit water, and performed a clustering analysis to obtain the most populated structure. Based on an implicit solvent energy decomposition calculation, we identified the key interactions present with the binding site. Tyr 303 forms a hydrogen bond with the sulfur at position 3 of 58, Gln 204 forms a hydrogen bond with the first bridging oxygen and Thr 200 forms an H-bond with the second bridging oxygen. π-π interactions are present between Tyr 303 and ring A and between Phe 131 and ring B. Hydrophobic interactions take place between ring B and Leu 307 and Asn 196. The high degree of specificity of these interactions is an indication of why the meta-substituted 58 shows a much higher potency than the para-substituted 48 (Figure 6). Further investigation of the ability of different isoprenoids to rescue the growth inhibition could help to pin down the actual target of the bisphosphonate.
Figure 6.
Interaction of 58 with TgFPPS. The structure was formed by homology modeling from P. vivax FPPS, and 58 (blue), three Mg ions (pink) and isopentenyl pyrophosphate (cyan) were inserted by alignment with the TcFPPS alendronate crystal structure (pdb id 1yhm). Residues at 4 Å from the ligand are shown in thick Line representation, with those contributing significantly to the binding energy in thin Licorice representation.
We cannot rule out that this inhibitory effect of WC-9 analogs is in part due to inhibition of the host squalene synthase and of other host enzymes of the mevalonate pathway. In this regard, microarray experiments have shown that 3-HMG-CoA reductase, diphosphomevalonate decarboxylase, and farnesyl diphosphate synthase are induced 24 h after T. gondii infection of human foreskin fibroblasts (HFF).[1] Squalene epoxidase, the second enzyme in the cholesterol biosynthesis pathway and one of the rate-limiting enzymes is up-regulated 5-fold following infection of either HFF[1] or porcine kidney epithelial cells line PK13,[2] suggesting that induction of mevalonate biosynthetic enzymes is necessary to increase cellular levels of squalene that could be scavenged by the parasite.[1] Similarly, 3-HMG-CoA reductase, diphosphomevalonate decarboxylase and farnesyl diphosphate synthase are induced several fold 24–72 hours postinfection of Macaca mulatta LLcMK2 kidney cells with T. cruzi.[3]
It can be concluded that most target compounds exhibited potent action against T. gondii proliferation and to a less extent some of them were also growth inhibitors of T. cruzi. The drug-like character of our lead compound (for instance it follows all Lipinki rules[49]: no hydrogen bond donors, three hydrogen bond acceptors, molecular weight is 271.3 and logP is 4.20) and other closely related analogues offer good chances for optimizing their chemical structure. The availability of the crystal structure of WC-9 with dehydrosqualene synthase from S. aureus together with crystallographic structure of this compound bound to human SQS and proper docking studies in homology models will allow the further production of more active WC-9 analogues. A more detailed computational study is under way to further understand the interactions of current inhibitors and guide the development of new compounds, which will be published elsewhere.
Experimental Section
General methods
The glassware used in air- and/or moisture-sensitive reactions was flame dried, and the reactions were performed under a dry argon atmosphere. Unless otherwise noted, chemicals were commercially available and were used without further purification. Anhydrous N,N-dimethylformamide and anhydrous dimethyl sulfoxide were used as supplied from Aldrich. Nuclear magnetic resonance spectra were performed by using a Bruker AM-500 MHz apparatus. Chemical shifts are reported in parts per million δ relative to tetramethylsilane. 13C NMR spectra were fully decoupled. High-resolution mass spectra were carried out by using a Bruker micrOTOF-Q II spectrometer, which is a hybrid quadrupole time of flight mass spectrometer with MS–MS capability. Melting points were determined by using a Fisher–Johns apparatus. Column chromatography was performed with E. Merck silica gel plates (Kieselgel 60, 230–400 mesh). Analytical thin-layer chromatography was performed by employing 0.2 mm coated commercial silica gel plates (E. Merck, DC-Aluminum sheets, Kieselgel 60 F254).
As judged from the homogeneity of the 1H, 13C, 19F and 31P NMR spectra and HPLC analyses of the title compounds employing a Beckmann Ultrasphere ODS-2 column 5 µM, 250 × 10 mm eluting with acetonitrile–water (9:1) at 3.00 mL/min with a refractive index detector indicated a purity >97%.
Syntheses
3-(2-Fluorophenoxy)phenoxyethyl Tetrahydro-2H-pyran-2-yl Ether (11)
A mixture of compound 10 (900 mg, 2.6 mmol), 2-fluorophenol (580 mg, 5.2 mmol), copper(I) iodide (49.2 mg, 0.26 mmol), 2-picolinic acid (63.6 mg, 0.52 mmol) and potassium phosphate tribasic (1.1 g, 5.2 mmol) under anhydrous conditions was evacuated and back-filled with argon. This sequence was repeated twice. Then, dimethyl sulfoxide was added (3.0 mL) and the reaction mixture was stirred at 90 ºC for 4 days. The mixture was cooled to room temperature and was partitioned between methylene chloride (20 mL) and water (20 mL). The aqueous layer was extracted with methane chloride (2 × 20 mL) and the combined organic phases were washed with brine (5 × 50 mL), dried (MgSO4) and the solvent was evaporated. The product was purified by column chromatography (silica gel) employing hexane–EtOAc (24:1) as eluent to produce 453 mg (53% yield) of pure compound 11 as a colorless oil.
3-(3-Fluorophenoxy)phenoxyethyl Tetrahydro-2H-pyran-2-yl Ether (12)
To a mixture of 10 (893 mg, 2.6 mmol), 3-fluorophenol (575 mg, 5.1 mmol), copper(I) iodide (48.9 mg, 0.26 mmol), 2-picolinic acid, (63.2 mg, 0.51 mmol), and potassium phosphate tribasic (1.092 g, 5.1 mmol) was added dimethyl sulfoxide was added (3.0 mL) as described for the preparation of 11. The reaction mixture was stirred at 90 ºC for 14 days. After the usual work-up the product was purified by column chromatography (silica gel) employing hexane–EtOAc (49:1) as eluent to produce 280 mg (33% yield) of 12 as a colorless oil.
3-(4-Fluorophenoxy)phenoxyethyl Tetrahydro-2H-pyran-2-yl Ether (13)
A mixture of 10 (877 mg, 2.5 mmol), 4-fluorphenol (565 mg, 5.0 mmol), copper(I) iodide (48.0 mg, 0.25 mmol), 2-picolinic acid, (62.0 mg, 0.50 mmol), and potassium phosphate tribasic (1.072 g, 5.0 mmol) was treated with dimethyl sulfoxide (3.0 mL) as described for the preparation of 11. The reaction mixture was stirred at 90 ºC for 4 days. The reaction was quenched as described for 11 and the product was purified by column chromatography (silica gel) employing hexane–EtOAc (24:1) as eluent to produce 594 mg (71% yield) of 13 as a colorless oil.
3-(2-Fluorophenoxy)phenoxyethanol (14)
A solution of 11 (426 mg, 1.3 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg). The reaction mixture was stirred at room temperature overnight. Then, water (50 mL) was added and the mixture was extracted with methylene chloride (3 × 50 mL). The combined organic layers were washed with brine (3 × 50 mL), dried (MgSO4), and the solvent was evaporated. The residue was purified by column chromatography eluting with hexane-EtOAc (85:15) to produce 297 mg (93% yield) of pure alcohol 14 as a colorless oil.
3-(3-Fluorophenoxy)phenoxyethanol (15)
A solution of 12 (269 mg, 0.81 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg) as depicted for the preparation of 14. The residue was purified by column chromatography eluting with hexane–EtOAc (83:17) to give 182 mg (91% yield) of alcohol 15 as a colorless oil.
3-(4-Fluorophenoxy)phenoxyethanol (16)
A solution of 13 (581 mg, 1.7 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg) as described for the preparation of 14. The crude product was purified by column chromatography (silica gel) eluting with hexane–EtOAc (41:9) to produce 399 mg (92% yield) of alcohol 16 as a colorless oil.
3-(2-Fluorophenoxy)phenoxyethyl 4-Toluenesulfonate (17)
A solution of alcohol 14 (253 mg, 0.95 mmol) in pyridine (3 mL) was treated with p-toluenesulfonyl chloride (546 mg, 2.9 mmol) and the mixture was stirred at room temperature for 4 h. Then, 5% HCl (50 mL) was added and the reaction mixture was stirred for an additional hour. The mixture was partitioned between methylene chloride (50 mL) and water (50 mL). The organic layer was washed with 5% HCl (3 × 50 mL) and water (3 × 50 mL). The organic phase was dried (MgSO4) and the solvent was evaporated. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (9:1) as eluent to produce 226 mg of 17 (66% yield) as a colorless oil.
3-(3-Fluorophenoxy)phenoxyethyl 4-Toluenesulfonate (18)
A solution of alcohol 15 (175 mg, 0.70 mmol) in pyridine (3 mL) was treated with 4-toluenesulfonyl chloride (402 mg, 2.1 mmol) and the mixture was stirred at room temperature for 4 h. The reaction was quenched as depicted for the preparation of 17. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (91:9) as eluent to produce 207 mg of 18 (73% yield) as a colorless oil.
3-(4-Fluorophenoxy)phenoxyethyl 4-Toluenesulfonate (19)
A solution of alcohol 16 (388 mg, 1.6 mmol) in pyridine (3.0 mL) was treated with 4-toluenesulfonyl chloride (894 mg, 4.7 mmol) as depicted for the preparation of 17. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (91:9) as eluent to produce 226 mg of 19 (84% yield) as a colorless oil.
3-(2-Fluorophenoxy)phenoxyethyl Thiocyanate (20)
A solution of tosylate 17 (381 mg, 0.98 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (460 mg, 4.7 mmol). The reaction mixture was heated at 100 ºC for 3 h. The mixture was allowed to cool to room temperature and water (20 mL) was added. The aqueous phase was extracted with methylene chloride (2 × 30 mL), and the combined organic layers were washed with brine (5 × 30 mL), water (2 × 30 mL), dried (MgSO4), and the solvent was evaporated. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (23:2) to produce 226 mg (80% yield) of 20 as a colorless oil.
3-(3-Fluorophenoxy)phenoxyethyl Thiocyanate (21)
A solution of 18 (197 mg, 0.49 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (237 mg, 2.4 mmol). The mixture was treated as described for the preparation of 20. The residue was purified by HPLC eluting with methanol–H2O (9:1) and employing a Bechman Ultrasphere 5 µM column (250 mm × 10 mm) to give rise to 107 mg (68% yield) of 21 as a colorless oil.
3-(4-Fluorophenoxy)phenoxyethyl Thiocyanate (22)
A solution of 19 (514 mg, 1.3 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (647 mg, 6.7 mmol). The reaction mixture was treated as depicted for 20. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (47:3) to produce 221 mg (58% yield) of 22 as a colorless oil.
4-(2-Fluorophenoxy)phenoxyethyl Tetrahydro-2H-pyran-2-yl Ether (24)
To a mixture of 23 (858 mg, 2.5 mmol), 2-fluorophenol (549.5 mg, 4.9 mmol), copper(I) iodide (46.0 mg, 0.24 mmol), 2-picolinic acid, (59.4 mg, 0.51 mmol), and potassium phosphate tribasic (1.026 g, 4.9 mmol) was added dimethyl sulfoxide was added (3.0 mL) as described for the preparation of 11. The reaction mixture was stirred at 90 ºC for 12 days. After the usual work-up the product was purified by column chromatography (silica gel) employing hexane–EtOAc (19:1) as eluent to produce 295 mg (37% yield) of 24 as a colorless oil.
4-(2-Fluorophenoxy)phenoxyethanol (25)
A solution of 12 (280 mg, 0.84 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg) as depicted for the preparation of 14. The residue was purified by column chromatography eluting with hexane–EtOAc (9:1) to give 124 mg (62% yield) of alcohol 25 as a colorless oil.
4-(2-Fluorophenoxy)phenoxyethyl 4-Toluenesulfonate (26)
A solution of alcohol 25 (110 mg, 0.44 mmol) in pyridine (3.0 mL) was treated with 4-toluenesulfonyl chloride (250 mg, 1.3 mmol) and the mixture was stirred at room temperature for 24 h. The reaction was quenched as depicted for the preparation of 17. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (19:1) as eluent to produce 163 mg of 26 (92% yield) as a colorless oil.
4-(2-Fluorophenoxy)phenoxyethyl Thiocyanate (27)
A solution of 26 (151 mg, 0.38 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (215.7 mg, 2.2 mmol). The reaction mixture was heated at 80 ºC for 6 h and was quenched as depicted for 20. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to produce 39.6 mg (36% yield) of 27 as a colorless oil.
4-(4-pyridyl)oxyphenoxyethyl Tetrahydro-2H-pyran-2-yl Ether (28)
To a mixture of 23 (2.145 g, 6.25 mmol), 4-hydroxypyridine (1.165 g, 12.3 mmol), copper(I) iodide (115.0 mg, 0.6 mmol), 2-picolinic acid, (149.2 mg, 1.28 mmol), and potassium phosphate tribasic (2.565 g, 12.3 mmol) was added dimethyl sulfoxide was added (10.0 mL) as described for the preparation of 11. The reaction mixture was stirred at 90 ºC for 12 days. After the usual work-up the product was purified by column chromatography (silica gel) employing hexane–EtOAc (1:1) as eluent to produce 572 mg (29% yield) of 28 as a colorless oil.
4-(4-pyridyl)oxyphenoxyethanol (29)
A solution of 28 (556 mg, 1.76 mmol) in methanol (10 mL) was treated with 4-toluenesulfonic acid (90 mg) as depicted for the preparation of 14. The residue was purified by column chromatography eluting with EtOAc–MeOH (49:1) to give 240.1 mg (59% yield) of alcohol 29 as a colorless oil.
4-(4-pyridyl)oxyphenoxyethyl Bromide (30)
To a mixture of N-bromosuccinimide (187.0 mg, 1.05 mmol) and triphenylphosphine (275.4 mg, 1.05 mmol) in anhydrous methylene chloride (20 mL) cooled at 0 ºC was treated with alcohol 29 (220.3 mg, 0.95 mmol) and the reaction mixture was stirred at 0 ºC for 6 h. The solvent was evaporated and the residue was purified by column chromatography (silica gel) eluting with a mixture of CH2Cl2–MeOH (99:1) to give 154.2 mg (50% yield) of pure 30 as a colorless oil.
4-(4-pyridyl)oxyphenoxyethyl Thiocyanate (31)
A solution of 30 (130 mg, 0.44 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (172.6 mg, 1.8 mmol). The reaction mixture was heated at 80 ºC for 6 h and was quenched as depicted for 20. The residue was purified by column chromatography (silica gel) eluting with CH2Cl2–MeOH (19:1) to produce 40.8 mg (34% yield) of 31 as a colorless oil.
4-Phenoxyphenyl Acetate (33)
A solution of 4-phenoxyphenol (32; 2.08 g, 11.2 mmol) in pyridine (5 mL) was treated with acetic anhydride (3 mL) and the reaction mixture was stirred at room temperature for 16 h. Then, water (10 mL) was added and the mixture was stirred for 1 h. The mixture was extracted with methylene chloride (2 × 30 mL). The combined organic layers were washed with an aqueous 1 N solution of hydrochloric acid (2 × 30 mL), water (2 × 30 mL), dried (MgSO4), and the solvent was evaporated. The product was purified by column chromatography (silica gel) eluting with a mixture of hexane–EtOAc (19:1) to give 2.478 g (97% yield) of 33 as a colorless oil.
2-Acetoxy-4-phenoxyphenol (34)
A solution of acetate 33 (257.8 mg, 1.13 mmol) in cyclohexane (200 mL), previously degassed with dry nitrogen, in a septum stopped quartz tube was irradiated with germicide lamps (4 × 20 W) centered at 254 nm at room temperature for 12 h. The solvent was evaporated and the residue was purified by column chromatography (silica gel) eluting with a mixture of hexane–EtOAc to produce 100.5 mg (39% yield) of pure 34 as a white solid.
2-Acetoxy-4-phenoxyphenoxyethyl Tetrahydro-2H-pyran-2-yl Ether (35)
A solution of 34 (457.1 g, 2.0 mmol) in dimethyl sulfoxide (5.0 mL) was treated with potassium hydroxide (450 mg, 8.0 mmol). The mixture was stirred at room temperature for 5 min. Then, bromoethyl tetrahydropyranyl ether (418 mg, 2.0 mmol) was added, and the reaction mixture was stirred at room temperature overnight. The mixture was partitioned between water (35 mL) and methylene chloride (35 mL). The aqueous phase was extracted with methylene chloride (2 × 30 mL). The combined organic layers were washed with a saturated solution of sodium chloride (5 × 30 mL), dried (MgSO4), and the solvent was evaporated. The product was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to yield 442 mg (62% yield) of 35 as a colorless oil.
2-Acetoxy-4-phenoxyphenoxyethanol (36)
A solution of 35 (420 mg, 1.2 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg) as depicted for the preparation of 14. The residue was purified by column chromatography eluting with hexane–EtOAc (83:17) to give 301 mg (92% yield) of alcohol 36 as a colorless oil.
2-Acetoxy-4-phenoxyphenoxyethyl 4-Toluenesulfonate (37)
A solution of alcohol 36 (280.2 mg, 1.03 mmol) in pyridine (3 mL) was treated with 4-toluenesulfonyl chloride (383 mg, 2.0 mmol) and the mixture was stirred at room temperature for 6 h. The reaction was quenched as depicted for the preparation of 17. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (9:1) as eluent to produce 417 mg of 37 (95% yield) as a colorless oil.
2-Acetoxy-4-phenoxyphenoxyethyl Thiocyanate (38)
A solution of 37 (348 mg, 0.82 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (321.6 mg, 3.3 mmol). The reaction mixture was heated at 80 ºC for 6 h and was quenched as depicted for 20. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (9:1) to produce 186.9 mg (71% yield) of 27 as a colorless oil.
4-Phenoxyphenoxyethyl amine (40)
A solution of azide 39 (1.020 g, 4.0 mmol) in tetrahydrofuran (20 mL) was treated with triphenylphosphine (2.308 g. 8.8 mmol). The reaction mixture was stirred at room temperature for 4 h. Then, water (2.0 mL) was added and the mixture was stirred for an additional hour. The solvent was evaporated and the residue was purified by column chromatography (silica gel) eluting with CH2Cl2–MeOH (19:1) to give 706 mg (77% yield ) of 40 as a colorless oil.
Tetraethyl 1-[(4-phenoxyphenoxyethylamino)ethyl]-1,1-bisphosphonate (42)
To a solution of 41 (300 mg, 1.5 mmol) in anhydrous methylene chloride (10 mL) was added amine 40 (345.1mg, 1.5 mmol) under an argon atmosphere. The reaction mixture was stirred at room temperature overnight. The solvent was evaporated to produce 651 mg (82% yield) of 42, which was used in next step without further purification.
1-[(4-Phenoxyphenoxyethylamino)ethyl]-1,1-bisphosphonic Acid (43)
Compound 42 (430.1 mg, 0.81 mmol) was treated with a concentrated aqueous solution of hydrochloric acid (3.0 mL). The resulting mixture was refluxed for 24 h. The solvent was evaporated and the residue was crystalized from H2O–ethanol (1:1) to give 274.1 mg (81% yield) of 52 as a white solid.
S-(2-(4-phenoxyphenoxy)ethyl) ethanethioate (45)
A solution of tosylate 44 (1.90 g, 4.9 mmol) in anhydrous N,N-dimethylformamide (10 mL) under argon atmosphere was treated with potassium thioacetate (1.23 g, 10.8 mmol). The reaction mixture was stirred at 90 ºC for 3 h. The solvent was evaporated and the residue was partitioned between water (50 mL) and methylene chloride (50 mL). The aqueous phase was extracted with methylene chloride (2 × 50 mL). The combined organic layers were dried (MgSO4), and the solvent was evaporated. The product was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to produce 1.383 g (98% yield) of pure 45 as a colorless oil.
2-(4-phenoxyphenoxy)ethanethiol (46)
To a mixture of lithium aluminum hydride (238 mg, 63 mmol) in anhydrous tetrahydrofuran (20.0 mL) cooled at 0 ºC was added a solution of compound 45 (1.35 g, 4.7 mmol) in tetrahydrofuran (8.0 mL). The reaction mixture was allowed to reach room temperature and was stirred for 60 min. The reaction was quenched by addition of ethyl acetate (10 mL). The mixture was partitioned between an aqueous saturated solution of sodium potassium tartrate (50 mL) and methylene chloride (50 mL). The aqueous layer was extracted with methylene chloride (2 × 50 mL). The combined organic layers were dried (MgSO4) and the solvent was evaporated. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (99:1) to give 1.064 g (92% yield) of 46 as a colorless oil.
Tetraethyl 1-[4-phenoxyphenoxyeth-1-ylthio)ethyl] 1,1-bisphosphonate (47)
To a solution of tetraethyl ethenylidenbisphosphonate (41; 300 mg, 1 mmol) in anhydrous dichloromethane (10 mL) was added triethylamine (139 µL, 101 mg, 1.0 mmol) and the corresponding mercaptan 24 (246 mg, 1.0 mmol). The reaction mixture was stirred at room temperature for 5h. Water (20 mL) was added, and the mixture was extracted with dichloromethane (3 × 10 mL). The combined organic layers were washed with brine (20 mL), dried (MgSO4), and the solvent was evaporated to produce 336 mg (59% yield) of tetraethyl ester 47. The product was used in the next step without further purification: colorless oil.
1-[4-Phenoxyphenoxyeth-1-ylthio)ethyl] 1,1-bisphosphonic acid (48)
A solution of the tetraethyl ester 47 (290 mg, 0.53 mmol) in anhydrous methylene chloride (10 mL) was treated with bromotrimethylsilane (10 equiv.) under an argon atmosphere. The reaction mixture was stirred at room temperature for 48 h. Then, methanol (1.0 mL) was added and the solvent was evaporated. The residue was dissolved in methanol (10.0 mL) and the mixture was stirred at room temperature for 24 h. The solvent was evaporated and the residue redissolved and evaporated in methanol four times, to complete the hydrolysis of remaining trimethylsilyl bromide and to remove the recently formed hydrobromic acid. The residue was purified by column chromatography (Silica gel C18-reversed phase) eluting with a mixture of water–methanol (9:1) to produce 159 mg (69% yield) of 48 as an amorphous solid.
3-Phenoxyphenoxyethylazide (50)
A solution of tosylate 49 (999.6 mg, 2.60 mmol) in anhydrous N,N-dimethylformamide (5 mL) was treated with sodium azide (845.1 mg, 13.0 mmol). The reaction mixture was stirred at 80 ºC for 2 h. Then, the mixture was allowed to cool to room temperature and water (50 mL) was added. The mixture was extracted with methylene chloride (3 × 20 mL) and the combined organic layers were washed with an aqueous saturated solution of sodium chloride (5 × 20 mL), water (2 × 20 mL) and dried (MgSO4). The solvent was evaporated and the product was purified by column chromatography (silica gel) using a hexane–EtOAc (97:3) as eluent to produce 590.1 mg (90% yield) of 50 as a colorless oil.
3-Phenoxyphenoxyethylamine (51)
A solution of 3-phenoxyphenoxyethyl azide (50, 545.3 mg, 2.1 mmol) in tetrahydrofuran (10 mL) was treated with triphenylphosphine (616.3 mg, 2.4 mmol). The reaction mixture was stirred 2 hours at room temperature. Then, water (20 mL) was added and the mixture was extracted with methylene chloride (3 × 20 mL). The combined organic phases were washed with water (2 × 20 mL), dried (MgSO4), and the solvent was evaporated. The product was purified by column chromatography (silica gel) eluting with a mixture of CH2Cl2–methanol (49:1) as eluent to produce 431.1 mg (89.5 yield) of 51 as a colorless oil.
Tetraethyl 1-[(3-phenoxyphenoxyethylamino)ethyl]-1,1-bisphosphonate (52)
To a solution of 41 (451.0 mg, 1.5 mmol) in anhydrous methylene chloride (10 mL) was added amine 51 (350.8 mg, 1.5 mmol) under an argon atmosphere. The reaction mixture was stirred at room temperature overnight. The solvent was evaporated to give 770 mg (97% yield) of 52, which was used in next step without further purification.
1-[(3-Phenoxyphenoxyethylamino)ethyl]-1,1-bisphosphonic Acid (53)
Compound 52 (411.2 mg, 0.78 mmol) was treated with a concentrated aqueous solution of hydrochloric acid (2 mL). The resulting mixture was refluxed for 24 h. The solvent was evaporated and the residue was crystalized from H2O–ethanol (1:1) to give 114 mg (35% yield) of 53 as an amorphous solid.
S-[3-Phenoxy)phenoxyethyl] ethanethioate (55)
A solution of 54 (660 mg, 1.7 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thioacetate (392 mg, 3.4 mmol). The reaction mixture was stirred at 80 ºC for 3 h. The mixture was allowed to cool to room temperature and water (20 mL) was added. The aqueous phase was extracted with methylene chloride (2 × 30 mL) and the combined organic layers were washed with brine (5 × 30 mL), water (2 × 30 mL), dried (MgSO4), and the solvent evaporated. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (49:1) to give 411 mg (83% yield) of 55 as a colorless oil.
3-(Phenoxy)phenoxyethyl mercaptan (56)
To a solution of 55 (957 mg, 3.3 mmol) in methanol (10 mL) potassium carbonate (anhydrous powder) were added while stirring, followed by water (3.0 mL) to obtain complete solution. The reaction mixture was stirred at room temperature for 40 min and the solvent was evaporated. The residue was dissolved in glacial acetic acid, and zinc (1.8 g, 28 mmol) was added. The reaction mixture was refluxed for 1 h. The mixture was allowed to cool to room temperature and was partitioned between water (30 mL) and methylene chloride (30 mL). The organic phase was washed with water (2 × 30 mL), dried (MgSO4), and the solvent was evaporated to yield 616 mg (75% yield) of 56 as a colorless oil, which was used in the next step without further purification.
Tetraethyl 2-[3-(Phenoxy)phenoxyethylthio]ethyl-1,1-bisphosphonate (57)
To a solution of tetraethyl ethenylidenbisphosphonate (41; 751 mg, 2.5 mmol) in anhydrous dichloromethane (10 mL) was added thiol 56 (616 mg, 2.5 mmol). The reaction mixture was stirred at room temperature for 1 day. Water (20 mL) was added, and the mixture was extracted with dichloromethane (3 × 10 mL). The combined organic layers were dried (MgSO4), and the solvent was evaporated. The residue was purified by column chromatography (silica gel) eluting with CH2Cl2–methanol (49:1) to produce 1.1 g (81% yield) of 57 as a colorless oil.
2-[3-(Phenoxy)phenoxyethylthio]ethyl-1,1-bisphosphonic Acid (58)
A solution of 57 (1.1 g, 2.0 mmol) in anhydrous methylene chloride (10 mL) was treated with bromotrimethylsilane (3.1 g, 20.2 mmol) under an argon atmosphere. The reaction mixture was stirred at room temperature for 48 h. Then, methanol (1.0 mL) was added and the solvent was evaporated. The residue was dissolved in methanol (8 mL) and the mixture was stirred at room temperature for 24 h. The solvent was evaporated and the residue redissolved/evaporated in methanol four times. The residue was purified by column chromatography (reverse phase) eluting with water–methanol (1:1) to produce 568 mg (65%) of a 58 as a yellow pale solid after lyophilization.
Phenoxyethylazide (60)
A solution of 59 (1.1054 g, 3.78 mmol) in anhydrous N,N-dimethylformamide (5 mL) was added sodium azide (1.2290 g, 18.9 mmol). The reaction mixture was stirred at 80 ºC for 2 h. Then, the mixture was allowed to cool to room temperature and water (50 mL) was added. The aqueous phase was extracted with methylene chloride (2 × 20 mL) and the combined organic layers were washed with an aqueous saturated solution of sodium chloride (5 × 20 mL), water (2 × 20 mL) and dried (MgSO4). The solvent was evaporated and the residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (97:3) to give 498.4 mg (81% yield) of 60 as a colorless oil.
Phenoxyethylamine (61)
A solution of azide 60 (463.6 mg, 2.84 mmol) in tetrahydrofuran (10 mL) was treated with triphenylphosphine (819.7 mg, 3.12 mmol) as depicted for the preparation of 40. The residue was purified by column chromatography (silica gel) eluting with a mixture of hexane–EtOAc (9:1) to afford 260.8 mg (67% yield) of 61 as a yellow pale oil.
Tetraethyl 1-[(Phenoxyethylamino)ethyl]-1,1-bisphosphonate (62)
To a solution of 41 (452.9 mg, 1.5 mmol) in anhydrous methylene chloride (10 mL) was added amine 61 (194.6 mg, 1.5 mmol) under an argon atmosphere. The reaction mixture was stirred at room temperature overnight. The solvent was evaporated to give 574.6 mg (88% yield) of 62, which was used in next step without further purification.
1-[(Phenoxyethylamino)ethyl]-1,1-bisphosphonic Acid (63)
Compound 62 (547.6 mg, 1.25 mmol) was treated with a concentrated aqueous solution of hydrochloric acid (4.0 mL). The mixture was refluxed for 24 h. The solvent was evaporated and the residue was crystalized from H2O–ethanol (1:1) to give 44.5 mg (11% yield) of 63 as a white solid.
2-(Naphthalen-2-yloxy)ethyl Tetrahydro-2H-pyran-2-yl Ether (65)
A solution of 64 (1.0121 g, 7.02 mmol) in dimethyl sulfoxide (10.0 mL) was treated with potassium hydroxide (877 mg, 15.6 mmol) and bromoethyl tetrahydropyranyl ether (1.7957 g, 8.6 mmol) as described for the preparation of 35. The product was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to produce 917.6 mg (48% yield) as a colorless oil.
2-(Naphthalen-2-yloxy)ethanol (66)
A solution of 65 (789.0 mg, 2.9 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg) as depicted for the preparation of 14. The residue was purified by column chromatography eluting with hexane–EtOAc (9:1) to give 463.5 mg (85% yield) of alcohol 66 as a colorless oil.
2-(Naphthalen-2-yloxy)ethyl 4-Toluenesulfonate (67)
A solution of alcohol 66 (450.3 mg, 2.45 mmol) in pyridine (5 mL) was treated with 4-toluenesulfonyl chloride (1.422 g, 7.5 mmol) and the mixture was stirred at room temperature for 4 h. The reaction was worked up as depicted for the preparation of 17. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (91:9) as eluent to produce 696.3 mg of 67 (83% yield) as a colorless oil.
2-(Naphthalen-2-yloxy)ethyl Thiocyanate (68)
A solution of 67 (464.1 mg, 1.35 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (531 mg, 5.5 mmol). The reaction mixture was treated as described for 20. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to produce 170.3 mg (55% yield) of 68 as a colorless oil.
2-(Naphthalen-1-yloxy)ethyl Tetrahydro-2H-pyran-2-yl Ether (70)
A solution of 69 (537.1 g, 3.82 mmol) in dimethyl sulfoxide (5.0 mL) was treated with potassium hydroxide (503 mg, 8.9 mmol) and bromoethyl tetrahydropyranyl ether (1.1157 g, 5.3 mmol) as described for the preparation of 35. The product was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to produce 675.7 mg (65% yield) as a colorless oil.
2-(Naphthalen-1-yloxy)ethanol (71)
A solution of 70 (562.8 mg, 2.07 mmol) in methanol (10 mL) was treated with pyridinium 4-toluenesulfonate (30 mg) as depicted for the preparation of 14. The residue was purified by column chromatography eluting with hexane–EtOAc (9:1) to give 315.6 mg (81% yield) of alcohol 71 as a colorless oil.
2-(Naphthalen-1-yloxy)ethyl 4-Toluenesulfonate (72)
A solution of alcohol 71 (326.1 mg, 1.77 mmol) in pyridine (5 mL) was treated with 4-toluenesulfonyl chloride (1.027 g, 5.4 mmol) and the mixture was stirred at room temperature for 4 h. The reaction was worked up as depicted for the preparation of 17. The product was purified by column chromatography (silica gel) employing a mixture of hexane–EtOAc (9:1) as eluent to produce 545.5 mg of 72 (90% yield) as a colorless oil.
2-(Naphthalen-1-yloxy)ethyl Thiocyanate (73)
A solution of 67 (445.4 mg, 1.29 mmol) in anhydrous N,N-dimethylformamide (3.0 mL) was treated with potassium thiocyanate (550 mg, 5.7 mmol). The reaction mixture was treated as described for 20. The residue was purified by column chromatography (silica gel) eluting with hexane–EtOAc (19:1) to yield 171.6 mg (58% yield) of 73 as a colorless oil.
Drug Screening
T. cruzi amastigote assays
These experiments were done as reported using tdTomato labeled trypomastigotes[50] with the modifications described by Recher et al., 2013.[51] EC50 values were determined by non-linear regression analysis using SigmaPlot.
T. gondii tachyzoites assays
Experiments on T. gondii tachyzoites were carried out as described previously[52] using T. gondii tachyzoites expressing red fluorescent protein[53] with the modifications described by Recher et al., 2013.[51] Plates were read with covered lids, and both excitation (544 nm) and emission (590 nm) were read from the bottom.
Cytotoxicity for Vero cells
The cytotoxicity was tested using the Alamar Blue™ assay as described by Recher et al., 2013.[51]
Computational Methods
Model building
The structure of TgFPPS was built using homology modeling using SwissModel,[54] based on the structure of P. vivax FPPS (pdb id 3mav), a protein showing the highest degree of similarity with TgFPPS. The constructed TgFPPS structure was aligned with the TcFPPS alendronate complex (pdb id 1yhm),[55] the structure of 58 was built on that of alendronate, Mg2+ ions and the co-ligand isopentenyl pyrophosphate were added. Charges for 58 and isopentenyl pyrophosphate were obtained using the RESP method at the Hartree Fock 6–31G* level, and the compounds were parameterized using the Generalized Amber Force Field. The complex was built using the tleap module in AmberTools 15.[56] The Amber FF14SB Force Field was used to parameterize the protein structure, the charge was neutralized by the addition of 7 Na+ ions and the complex was solvated with a box of TIP3P waters extending 10 Å beyond the complex.
Molecular Dynamics (MD) Simulations
Simulations were run using the sander module in AmberTools 15. The Mg and phosphate units of 58 were restrained during all simulations to their original coordinates using a 100 Kcal/mol harmonic potential. The complex was minimized for 1000 steps of steepest descent followed by 1000 steps of conjugate gradient. It was then heated from 0 K to 300 K for 20 ps using Langevin dynamics followed by an 80 ps optimization using the NPT ensemble. The production run consisted of a 10 ns molecular dynamics simulation using the NVT ensemble.
Energy calculations
a calculation of binding energy was obtained using the MMPBSA.py module in AmberTools 15. The Generalized Born Surface Area model was used with igb = 5 and the corresponding mbondi2 radii, and a salt concentration o f 0.1 M. 100 frames at 100 ps intervals were taken from the MD simulation. Pairwise energy decomposition was carried out with the ligand decomposed fragment-wise using an in-house modified version of MMPBSA.py.
Clustering analysis
A clustering analysis was performed with the cpptraj module in AmberTools 15 on the MD simulation stripped of solvent using the dbscan algorithm, with an epsilon of 0.75 Å for the residues at 4 Å of the ligand. The MD snapshot corresponding to the most populated cluster was minimized for 1000 steps of steepest descent followed by 1000 steps of conjugate gradient keeping the explicit waters for the minimization to assemble Figure 6.
Supplementary Material
Acknowledgments
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0797), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012 #0457), and the Universidad de Buenos Aires (20020130100223BA) to J.B.R., and the U.S. National Institutes of Health to R.D. (AI-107663) and S.N.J.M. (AI-102254). Technical assistance of Carolina Exeni is greatly appreciated.
Footnotes
Supporting Information: Physical data and spectral information of the target molecules and the corresponding intermediates as well as copies of the 1H NMR, 13C NMR, and 19F NMR spectra are included as supporting information.
References
- 1.Blader IJ, Manger ID, Boothroyd JC. J. Biol. Chem. 2001;276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 2.Okomo-Adhiambo M, Beattie CRA. Infect. Immun. 2006;74:4254–4265. doi: 10.1128/IAI.00386-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, Padmanabhan P, Ndegwa DM, Temanni MR, Corrada Bravo H, El-Sayed NM, Burleigh BA. PLoS Pathog. 2016;12:e1005511. doi: 10.1371/journal.ppat.1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liñares GEG, Ravaschino EL, Rodriguez JB. Curr. Med. Chem. 2006;13:335–360. doi: 10.2174/092986706775476043. [DOI] [PubMed] [Google Scholar]
- 5.Lüder CGK, Bohne W, Soldati D. Trends Parasitol. 2001;17:460–463. doi: 10.1016/s1471-4922(01)02093-1. [DOI] [PubMed] [Google Scholar]
- 6.Innes EAA. Zoonoses Public Health. 2010;57:1–7. doi: 10.1111/j.1863-2378.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- 7.Hill DE, Chirukandoth S, Dubey JP. Anim. Heal. Res. Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 8.Wong S-Y, Remington JS. Clin. Infect. Dis. 1994;18:853–862. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 9.Holland GN. Am. J. Ophthalmol. 2004;137:1–17. [PubMed] [Google Scholar]
- 10.Rodriguez JB, Szajnman SH, H S. Expert Opinion on Therapeutic Patents. 2012;22:311–333. doi: 10.1517/13543776.2012.668886. [DOI] [PubMed] [Google Scholar]
- 11.Coppens I, Sinai IAP, Joiner KA. J. Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckner FS, Urbina JA. Int. J. Parasitol. Drugs Drug Resist. 2012;2:236–242. doi: 10.1016/j.ijpddr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbina JA. Drugs Future. 2010;35:409–420. [Google Scholar]
- 14.Urbina JA, Concepcion JL, Rangel S, Visbal G, Lira R. Mol. Biochem. Parasitol. 2002;125:35–45. doi: 10.1016/s0166-6851(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 15.Cinque GM, Szajnman SH, Zhong L, Docampo R, Schvartzapel AJ, Rodriguez JB, Gros EG. J. Med. Chem. 1998;41:1540–1554. doi: 10.1021/jm970860z. [DOI] [PubMed] [Google Scholar]
- 16.Urbina JA, Concepcion JL, Montalvetti A, Rodriguez JB, Docampo R. Antimicrob. Agents Chemother. 2003;47:2047–2050. doi: 10.1128/AAC.47.6.2047-2050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szajnman SH, Yan W, Bailey BN, Docampo R, Elhalem E, Rodriguez JB. J. Med. Chem. 2000;43:1826–1840. doi: 10.1021/jm9905007. [DOI] [PubMed] [Google Scholar]
- 18.Elhalem E, Bailey BN, Docampo R, Ujváry I, Szajnman SH, Rodriguez JB. J. Med. Chem. 2002;45:3984–3999. doi: 10.1021/jm0201518. [DOI] [PubMed] [Google Scholar]
- 19.Liñares GG, Gismondi S, Codesido NO, Moreno SNJ, Docampo R, Rodriguez JB. Bioorg. Med. Chem. Lett. 2007;17:5068–5071. doi: 10.1016/j.bmcl.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elicio PD, Chao MN, Galizzi M, Li C, Szajnman SH, Docampo R, Moreno SNJ, Rodriguez JB. Eur. J. Med. Chem. 2013;69:480–489. doi: 10.1016/j.ejmech.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao MN, Matiuzzi CE, Storey M, Li C, Szajnman SH, Docampo R, Moreno SNJ, Rodriguez JB. ChemMedChem. 2015;10:1094–1108. doi: 10.1002/cmdc.201500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair SC, Brooks CF, Goodman CD, Strurm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SNJ, Striepen B. J. Exp. Med. 2011;208:1547–1559. doi: 10.1084/jem.20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grellier P, Valentin A, Millerioux V, Schrevel J, Rigomier D. Antimicrob. Agents Chemother. 1994;38:1144–1148. doi: 10.1128/aac.38.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pradines B, Torrentino-Madamet M, Fontaine A, Henry M, Baret E, Mosnier J, Briolant S, Fusai T, Rogier C. Antimicrob. Agents Chemother. 2007;51:2654–2655. doi: 10.1128/AAC.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bessoff K, Sateriale A, Lee KK, Huston CD. Antimicrob. Agents Chemother. 2013;57:1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortez E, Stumbo AC, Olieveira M, Barbosa HS, Carvalho L. Int. J. Antimicrob. Agents. 2009;33:184–185. doi: 10.1016/j.ijantimicag.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Li ZH, Ramakrishnan S, Striepen B, Moreno SNJ. PLoS Pathog. 2013;9:e1003665. doi: 10.1371/journal.ppat.1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzini P, Wermuth CG. Substituent Groups. In: Wermuth CG, Aldous D, Raboisson P, Rognan D, editors. The Practice of Medicinal Chemistry. Academic Press; 2015. pp. 348–349. [Google Scholar]
- 29.Shang N, Li Q, Ko TP, Chan HC, Li J, Zheng Y, Huang CH, Ren F, Chen CC, Zhu Z, Galizzi M, Li Z, Rodrigues-Poveda CA, Gonzalez-Pacanowska D, Veiga-Santos P, de Carvalho TMU, de Souza W, Urbina JA, Wang AHJ, Docampo R, Li K, Liu YL, Oldfield E, Guo RT. PLoS Pathog. 2014;10:e1004114. doi: 10.1371/journal.ppat.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbina JA, Concepcion JL, Caldera A, Payares G, Sanoja C, Otomo T, Hiyoshi H. Antimicrob. Agents Chemother. 2004;48:2379–2387. doi: 10.1128/AAC.48.7.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sealey-Cardona M, Cammerer S, Jones S, Ruiz-Pérez LM, Brun R, Gilbert IH, Urbina JA, González-Pacanowska D. Antimicrob. Agents Chemother. 2007;51:2123–2129. doi: 10.1128/AAC.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbina JA, Concepcion JL, Caldera A, Payares G, Sanoja C, Otomo T, Hiyoshi H. Antimicrob. Agents Chemother. 2004;48:2379–2387. doi: 10.1128/AAC.48.7.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrígues-Poveda CA, González-Pacanowska D, Szajnman SH, Rodríguez JB. Antimicrob. Agents Chemother. 2012;56:4483–4486. doi: 10.1128/AAC.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez JB, Falcone BN, Szajnman SH. Expert Opin. Drug Discov. 2016;11:307–320. doi: 10.1517/17460441.2016.1143814. [DOI] [PubMed] [Google Scholar]
- 35.Lin FY, Liu YL, Li K, Cao R, Zhu W, Axelson J, Pang R, Oldfield E. J. Med. Chem. 2012;55:4367–4372. doi: 10.1021/jm300208p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schvartzapel AJ, Zhong L, Docampo R, Rodriguez JB, Gros EG. J. Med. Chem. 1997;40:2314–2322. doi: 10.1021/jm9607616. [DOI] [PubMed] [Google Scholar]
- 37.Maiti D, Buchwald SL. J. Am. Chem. Soc. 2009;131:17423–17429. doi: 10.1021/ja9081815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruno NC, Buchwald SL. Org. Lett. 2013;15:2876–2879. doi: 10.1021/ol401208t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhayana B, Fors BP, Buchwald SL. Org. Lett. 2009;11:3954–3957. doi: 10.1021/ol9015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fors BP, Watson DA, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2008;130:13552–13554. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier R, Strych S, Trauner D. Org. Lett. 2014;16:2624–2637. doi: 10.1021/ol500800z. [DOI] [PubMed] [Google Scholar]
- 42.Miranda MA. In: CRC Handbook of Organic Photochemistry and Photobiology. Horspool WM, Song P-S, editors. Boca Raton: CRC Press Inc; 2004. pp. 1–11. [Google Scholar]
- 43.Meunier B. Acc. Chem. Res. 2008;41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, Huang W, Broering JM, Barron AE, Seo J. Bioorg. Med. Chem. Lett. 2015;25:2849–2852. doi: 10.1016/j.bmcl.2015.04.092. [DOI] [PubMed] [Google Scholar]
- 45.Dehnhardt CM, Venkatesan AM, Chen Z, Delos-Santos E, Ayral-Kaloustian S, Brooijmans N, Yu K, Hollander I, Feldberg L, Lucas J, Mallon R. Bioorg. Med. Chem. Lett. 2011;21:4773–4778. doi: 10.1016/j.bmcl.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 46.Ferrer-Casal M, Barboza AP, Szajnman SH, Rodriguez JB. Synthesis. 2013;45:2397–2404. [Google Scholar]
- 47.Saneyoshi H, Ochikubo T, Mashimo T, Hatano K, Ito Y, Abe H. Org. Lett. 2014;16:30–33. doi: 10.1021/ol402832w. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez JB. Synthesis. 2014;46:1129–1142. [Google Scholar]
- 49.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 50.Canavaci AMC, Bustamante JM, Padilla AM, Brandan CMP, Simpson LJ, Xu D, Boehlke CL, Tarleton RL. PLoS Negl. Trop. Dis. 2010;4:e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recher M, Barboza AP, Li Z-H, Galizzi M, Ferrer-Casal M, Szajnman SH, Docampo R, Moreno SNJ, Rodriguez JB. Eur. J. Med. Chem. 2013;60:431–440. doi: 10.1016/j.ejmech.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gubbels M, Li C, Striepen B. Antimicrob. Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agrawal S, van Dooren GG, Beatty WL, Striepen B. J. Biol. Chem. 2009;284:33683–33691. doi: 10.1074/jbc.M109.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Nat. Protoc. 2009;4:1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- 55.Gabelli SB, McLellan JS, Montalvetti A, Oldfield E, Docampo R, Amzel LM. Proteins. 2006;62:80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- 56.Case DA, Berryman JT, Betz RM, Cerutti DS, Cheatham TE, III, Darden TA, Duke RE, Giese TJ, Gohlke H, Goetz AW, Homeyer N, Izadi S, Janowski P, Kaus J, Kovalenko A, Lee TS, LeGrand S, Li P, Luchko T, Luo R, Madej B, Merz KM, Monard G, Needham P, Nguyen H, Nguyen HT, Omelyan I, Onufriev A, Roe DR, Roitberg A, Salomon-Ferrer R, Simmerling CL, Smith W, Swails J, Walker RC, Wang J, Wolf RM, Wu X, York DM, Kollman PA. AMBER 2015. San Francisco: University of California; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.