ABSTRACT
Host location in bed bugs is poorly understood. Of the primary host-associated cues known to attract bed bugs – CO2, odors, heat – heat has received little attention as an independent stimulus. We evaluated the effects of target temperatures ranging from 23 to 48°C on bed bug activation, orientation and feeding. Activation and orientation responses were assessed using a heated target in a circular arena. All targets heated above ambient temperature activated bed bugs (initiated movement) and elicited oriented movement toward the target, with higher temperatures generally resulting in faster activation and orientation. The distance over which bed bugs could orient toward a heat source was measured using a 2-choice T-maze assay. Positive thermotaxis was limited to distances <3 cm. Bed bug feeding responses on an artificial feeding system increased with feeder temperature up to 38 and 43°C, and declined precipitously at 48°C. In addition, bed bugs responded to the relative difference between ambient and feeder temperatures. These results highlight the wide range of temperatures that elicit activation, orientation and feeding responses in bed bugs. In contrast, the ability of bed bugs to correctly orient towards a heated target, independently of other cues, is limited to very short distances (<3 cm). Finally, bed bug feeding is shown to be relative to ambient temperature, not an absolute response to feeder blood temperature.
KEY WORDS: Cimex lectularius, Cimicidae, Host attraction, Sensory cues, Thermal orientation, Thermotaxis
Summary: Bed bugs utilize heat to locate and feed on hosts, and show a characteristic increase in the number of responders and decrease in response time with increasing target temperature.
INTRODUCTION
Heat is a common sensory cue used across diverse taxa, from microscopic organisms such as bacteria (Paster and Ryu, 2008) and protozoans (Poff and Skokut, 1977) to a wide range of animals including insects (Dillon et al., 2009), fish (Reynolds, 1977), reptiles (De Cock Buning, 1983) and mammals (Leonard, 1974). Heat cues serve a multitude of functions, such as indicating the presence of appropriate habitats (Graham, 1958; Holsapple and Florentine, 1972; Leonard, 1974), signaling the need to initiate estivation (Finch and Collier, 1985) and mediating orientation to hosts (Bullock and Cowles, 1952; Lazzari and Núñez, 1989b; Lees, 1948; Peterson and Brown, 1951), prey (De Cock Buning, 1983) and thermogenic flowers (Ivancic et al., 2008; Seymour and Schultze-Motel, 1997; Wang and Zhang, 2015).
Many arthropods can detect heat to locate hosts and flowers, including hematophagous mosquitoes (Peterson and Brown, 1951), kissing bugs (Lazzari and Núñez, 1989b), ticks (Lees, 1948), fleas (Osbrink and Rust, 1985), bed bugs (Rivnay, 1932) and some pollinators (Seymour and Schultze-Motel, 1997). However, the ability to perceive heat and the manner in which heat is used vary widely among species. Most species orient to heat over short distances (Lazzari and Núñez, 1989b), with only the buprestid Melanophila acuminate known to be capable of positive thermotaxis over long distances of several kilometers, through the use of specialized infra-red receptors (Evans, 1964; Schmitz and Bleckmann, 1998). Mosquitoes typically use CO2 and visual cues at long distances, which subsequently guide them close enough to the host to utilize odors, humidity and heat to locate a landing site (van Breugel et al., 2015). Lazzari and Núñez (1989b) found the kissing bug Triatoma infestans to be capable of infrared heat detection, quite different from other arthropods, which primarily rely on conduction and convection (Khan et al., 1966, 1968). When orienting toward a heat source, kissing bugs were observed to use both telotaxis (orientation using a single sensory structure over longer distances) and tropotaxis (orientation using dual sensory structures over short distances) (Lazzari, 2009). Although thermotaxis for host location has received some attention in hematophagous insects, it has traditionally been evaluated in combination with other host cues (Grossman and Pappas, 1991; Khan et al., 1968; Takken and Verhulst, 2013). Only recently have researchers elucidated the integration of heat with CO2, odors and vision in host location by a mosquito (van Breugel et al., 2015).
Bed bugs (Cimex lectularius Linnaeus) are hematophagous ectoparasites that feed on endothermic hosts (Usinger, 1966). They require a blood meal at each of their five nymphal instars to molt and as adults to survive and reproduce (Usinger, 1966). Bed bugs are typically found living in close proximity to their host, but not directly on their host (i.e. unlike lice and some fleas). Host seeking appears to be highly mediated by a circadian rhythm, with unfed bed bugs showing increased activity levels at night (Reis and Miller, 2011; Romero et al., 2010). When searching for a host, bed bugs actively orient to three host-related stimuli: body odors (Harraca et al., 2012), CO2 (Anderson et al., 2009) and heat (Rivnay, 1932). Since its discovery as an attractant, heat has been evaluated through trap catch assays designed to understand bed bug orientation and improve detection and monitoring of infestations (Anderson et al., 2009; Singh et al., 2012; Wang et al., 2009). However, such endpoint assays that measure the accumulation of insects at a site provide little information about thermotactic strategies and the direct role of heat alone in bed bug host searching and feeding behavior.
The purpose of this study was to evaluate the effects of heat on bed bug activation, orientation and feeding. In addition, we aimed to empirically assess the distance over which bed bugs detect a heated target and orient toward it, and how changes in ambient temperature affect bed bug feeding behavior. We used behavioral assays and an artificial feeding system to evaluate the effects of different temperatures on bed bug behavior.
MATERIALS AND METHODS
Experimental animals
A laboratory colony of bed bugs was used for all experiments. This strain, Harold Harlan (HH), was collected in 1973 in Ft Dix, NJ, USA. Another strain, Winston Salem (WS), originally collected from Winston Salem, NC, USA, in 2008, was also used for some feeding experiments.
Bed bugs were reared on a 12 h:12 h light:dark cycle at 27°C and 50±5% relative humidity. All bed bugs were fed defibrinated rabbit blood in an artificial feeding system similar to that described by Montes et al. (2002), which used a circulating water bath (B. Braun Biotech Inc., Allentown, PA, USA) to warm the blood to 38°C. Bed bugs fed through an artificial membrane (Nescofilm, Karlan, Cottonwood, AZ, USA). All experiments used only adult males that were 7–10 days post-feeding and hence likely motivated to seek hosts. Additionally, all experiments were conducted during the scotophase (under red-light) and at 25°C and 50±5% relative humidity, unless otherwise stated. All temperatures (including experimental apparatuses) were checked using a BAT-12 microprobe thermometer (Physitemp Instruments, Inc., Clifton, NJ, USA).
Activation in response to heat
A single HH bed bug was allowed to acclimate for 3 days in a plastic Petri dish (90 mm diameter, Fisher Scientific, Waltham, MA, USA) lined with a filter paper floor and a paper tent (8 mm×30 mm) at the edge of the dish. This period was long enough for most bugs to shelter in the tent, although longer times were granted if after 3 days bugs were found moving during the scotophase. During the scotophase, a copper coil (i.d.=1.5 mm, coiled diameter=3 cm) heated or cooled via a circulating water bath (RM6 Thermostat, Brinkmann Instruments Inc., Delran, NJ, USA) to 23, 28, 33, 38, 43 or 48°C was carefully introduced into the arena, in line with the opening of the paper tent (where a single bed bug was quiescent) at a distance of 40 mm from the bug. The bed bug was observed and time to first movement was recorded. Each trial lasted for up to 5 min; after this time, bugs that did not move were scored as unresponsive. At least 12 replicates were performed for each coil temperature.
Orientation in response to heat
HH bed bugs were placed into individual glass vials (7.5 ml) 24 h prior to the start of the experiment. Next, each bed bug was introduced into a large plastic Petri dish arena (141 mm diameter) by inverting the glass vial along the edge of the arena wall. The arena was lined with a filter paper floor and contained the same copper coil as before in the center of the arena (45 mm from the glass vial). The coil was set to one of six temperatures: 23, 28, 33, 38, 43 or 48°C. Each bed bug was allowed 5 min to acclimate to the arena within the glass vial. The glass vial was then carefully removed and bed bug behavior was recorded using a Sony Handycam video recorder (HDR-XR260, Sony Corporation, Minato, Tokyo, Japan) until the bed bug contacted the copper coil (5 min maximum time limit). The time taken to reach the coil was recorded. Digital videos were analyzed for a suite of behaviors using the video analysis software Ctrax (Branson et al., 2009), with corrections to videos made with Matlab (The Mathworks, Natick, MA, USA). The following parameters were calculated for each video: average velocity, angular speed, average distance to the wall, total distance traveled and angle to arena center. At least 14 replicates were performed at each coil temperature.
Effect of distance on orientation to heat
The distance over which bed bugs were capable of detecting heat was evaluated with two-choice assays. Each HH bed bug was placed in a glass vial (7.5 ml) 24 h prior to the start of the experiment. A bed bug was introduced at the base of a vertically oriented T-shaped two-choice arena constructed by adhering a paper substrate to a Plexiglas backing. The dimensions of the T-maze were as follows: 20 mm wide base which narrowed to a width of 3 mm over 50 mm. The two side arms of the T-maze were each 75 mm long and 20 mm wide. The copper coil was heated to 38°C and placed at one side of the ‘T’ 10, 30 or 50 mm from the choice point. The heated side was alternated left and right to account for any position biases and a control assay was run with the coil set to room temperature (25°C) to account for vibrational effects. A choice was recorded when the bed bug moved 10 mm in one direction at the top of the ‘T’. All assays were conducted under red light and at least 30 replicates were performed for each distance.
Feeding in response to heat
The effects of both feeder temperature and ambient temperature on bed bug feeding were evaluated. Each HH or WS bed bug was placed individually into a glass vial (7.5 ml) ∼24 h prior to the start of the experiment. The vial contained a paper ramp leading up to a nylon mesh lid (0.3 mm mesh size; BioQuip Products, Rancho Dominguez, CA, USA), which permitted feeding but retained the bug in the vial. The vial was placed under the artificial feeding system for 30 min at one of six feeder temperatures (measured by a thermocouple inserted directly into the blood): 23, 28, 33, 38, 43 or 48°C. The number of fully engorged bed bugs was recorded at each temperature. A sample size of 36 bugs was used for each temperature, with bugs fed individually.
Bed bugs from both populations fed at feeder temperatures below the ambient temperature. To confirm this observation and control for any olfactory or gustatory cues the blood may have provided, we repeated the above feeding experiment at 23 and 38°C using only a solution of 1 mmol l−1 ATP in PBS, previously confirmed by Romero and Schal (2014) to be sufficient to elicit feeding in bed bugs. In addition, all surfaces were sterilized using ethanol prior to feeding.
To evaluate the interaction between ambient temperature and feeder temperature, the same feeding methods previously described were used, but the room was maintained at 25°C, heated to 30°C or cooled to 20°C. In addition, only feeder temperatures that produced intermediate levels of feeding at an ambient temperature of 25°C were used, i.e. 23, 28 and 33°C. The number of fully engorged bugs was recorded for each ambient temperature–feeder temperature combination. At least 36 bugs were used for each ambient temperature–feeder temperature combination, with bugs fed individually.
Data analysis
The proportion of bugs that activated and oriented were analyzed using logistic regression. Only those bugs that responded were analyzed further. Regression analysis was used to understand the effects of target temperature on activation, time to reach the coil and all additional orientation parameters (velocity, angular speed, distance to the wall, total distance traveled). Angular data were evaluated using circular statistics. At each temperature, the length and angle of the mean vector of orientation were calculated for the bed bugs that reached the coil. Significance of mean vectors was determined at each temperature using the Rayleigh test (Batschelet, 1981). Chi-square tests were used to evaluate the effects of distance on bed bug orientation. Logistic regression was used to evaluate all feeding data. In addition, a Chi-square test of independence was used to determine the effects of ambient temperature and feeder temperature on bed bug feeding. All statistics were implemented in SAS 9.4 (SAS Institute, Cary, NC, USA) and PAST (Hammer et al., 2001).
RESULTS
Activation in response to heat
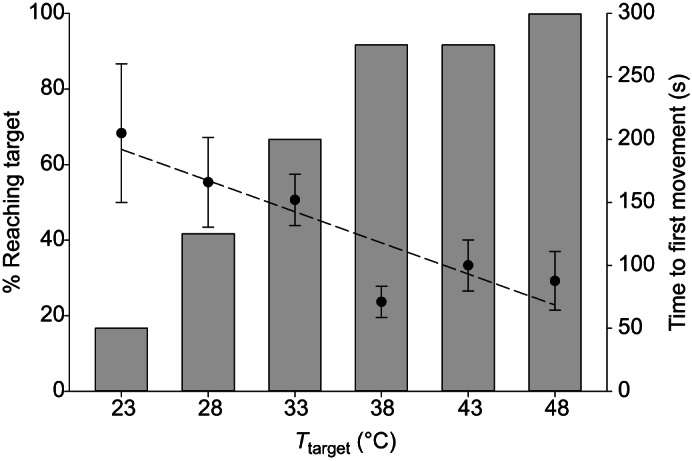
The responses of HH bed bugs at a distance of 40 mm from a cooled or heated target were significantly affected by the temperature of the target, with two distinct treatment effects. First, the percentage of bugs responding increased with target temperature (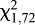 =33.09, P<0.0001; Fig. 1), and second, activation latency (time to first movement) was negatively related with target temperature (F1,4=14.91, P=0.0181, r2=0.7884; Fig. 1, dashed line).
=33.09, P<0.0001; Fig. 1), and second, activation latency (time to first movement) was negatively related with target temperature (F1,4=14.91, P=0.0181, r2=0.7884; Fig. 1, dashed line).
Fig. 1.
 |
Orientation in response to heat
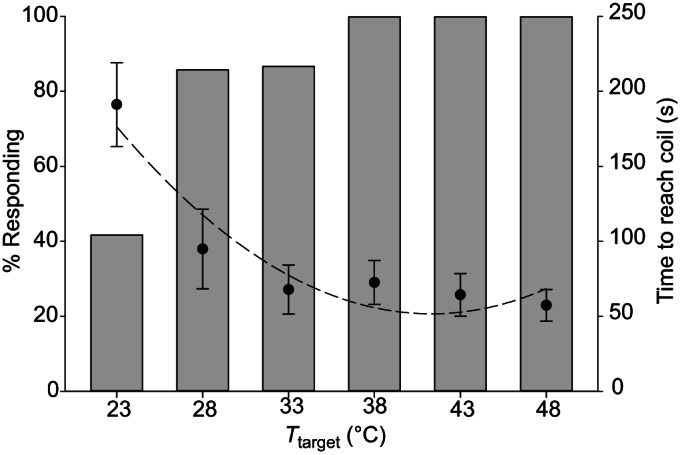
Orientation to the target over a distance of 40 mm was significantly affected by target temperature. The number of HH bugs that reached the target coil increased with temperature, with all bugs reaching the coil at target temperatures ≥38°C (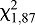 =24.19, P<0.0001; Fig. 2). Higher temperatures resulted in significantly shorter response times for those bugs that reached the coil, although the relationship was non-linear (F2,3=12.56, P=0.0348, r2=0.8933; Fig. 2, dashed line).
=24.19, P<0.0001; Fig. 2). Higher temperatures resulted in significantly shorter response times for those bugs that reached the coil, although the relationship was non-linear (F2,3=12.56, P=0.0348, r2=0.8933; Fig. 2, dashed line).
Fig. 2.
 |
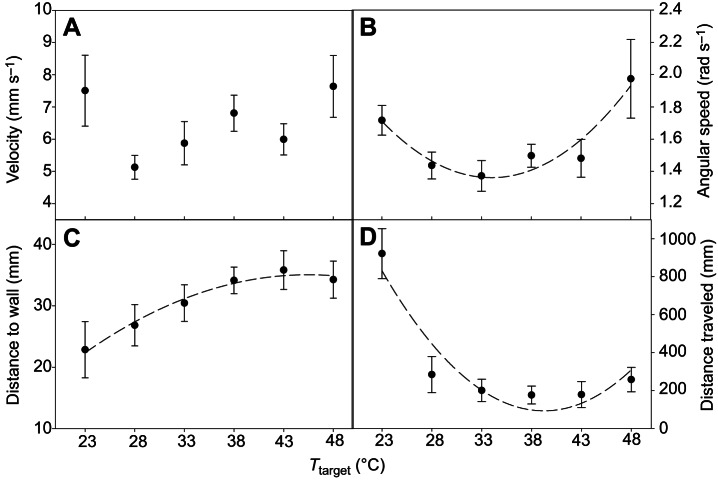
Walking velocity did not show a significant relationship with target temperature (Fig. 3A; F2,3=1.59, P=0.3374, r2=0.5153). Average angular speed increased with temperature in a curvilinear fashion (Fig. 3B; F2,3=14.32, P=0.0292, r2=0.9052). The average distance a bug was located relative to the wall throughout the experiment showed a curvilinear relationship with temperature (Fig. 3C; F2,3=74.79, P=0.0028, r2=0.9803). The total distance traveled before reaching the coil was related to temperature in a curvilinear fashion (Fig. 3D; F2,3=12.38, P=0.0355, r2=0.8920).
Fig. 3.
 |
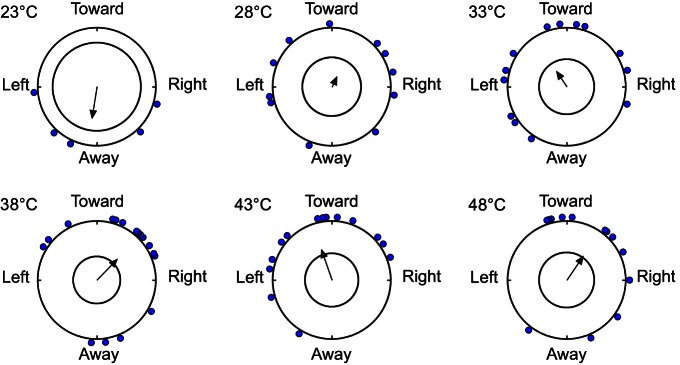
In addition, coil temperature significantly affected the orientation angle of each HH bed bug relative to the center of the arena (where the coil was located). At lower temperatures [23°C (r=0.5513, N=5, P=0.2282), 28°C (r=0.1896, N=12, P=0.6590) and 33°C (r=0.3155, N=13, P=0.2792)], bed bugs did not exhibit a significant directional orientation (Fig. 4). At higher temperatures, however [38°C (r=0.5016, N=18, P=0.0090), 43°C (r=0.5418, N=14, P=0.0137) and 48°C (r=0.4937, N=13, P=0.0389)], bed bugs oriented significantly toward the respective targets (Fig. 4).
Fig. 4.
Bed bug orientation angles to heated or cooled targets. Mean orientation angle for each HH strain bed bug that responded within 5 min is displayed on the circumference of the circle representing the arena, with the grand mean of all bed bugs that responded indicated by the arrow. The interior circle at each temperature represents the respective α<0.05 (Rayleigh test), and significant deviation from random orientation is indicated by arrows that cross the interior circle (P<0.05).
Orientation distance in response to a heated target
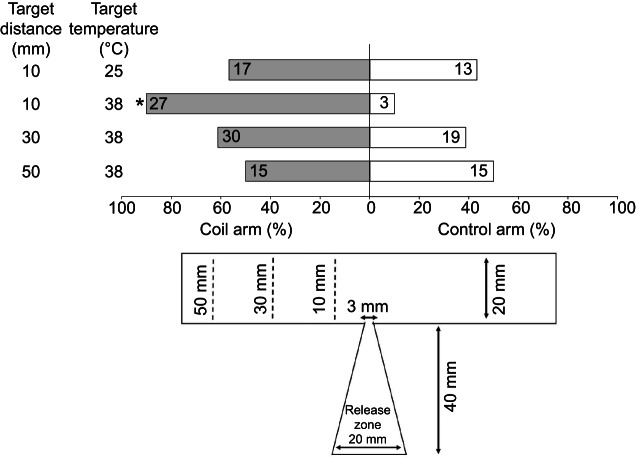
HH bed bugs had no significant side bias, orienting equally to the two arms of the T-maze when the coil was 10 mm away from the T-junction, with water at ambient temperature (25°C) circulating through the coil (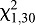 =0.5333, P=0.4652; Fig. 5). Bed bugs showed a significant preference for the side with the heated coil (38°C) when it was located 10 mm away from the T-junction (
=0.5333, P=0.4652; Fig. 5). Bed bugs showed a significant preference for the side with the heated coil (38°C) when it was located 10 mm away from the T-junction (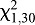 =19.20, P<0.0001), but no significant preference at 30 mm (
=19.20, P<0.0001), but no significant preference at 30 mm (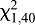 =2.47, P=0.1161) or 50 mm (
=2.47, P=0.1161) or 50 mm (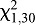 =0.0000, P=1.0000) (Fig. 5).
=0.0000, P=1.0000) (Fig. 5).
Fig. 5.
Bed bug orientation over distance in a T-arena. Effects of distance on the ability of HH strain bed bugs to correctly orient toward a heated coil (38°C) based on a 2-choice T-maze assay, as depicted at the bottom of the figure. An asterisk indicates significant choice of one arm of the T-arena (coil or control) based on the Chi-square test (P<0.05). Sample sizes are indicated in the bars.
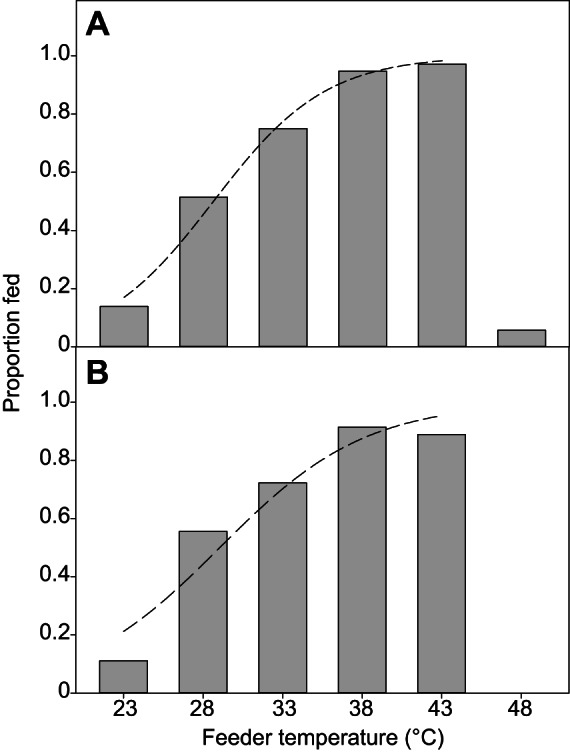
Feeding in response to feeder blood temperature
Blood (feeder) temperature significantly and similarly affected feeding in two bed bug strains (Fig. 6). As the feeder temperature increased, the proportion of HH bed bugs that fed increased, with the greatest percentage feeding at 38–43°C; bed bugs did not feed when the blood was at 48°C (Fig. 6). Excluding 48°C from the analysis, the proportion of HH bed bugs that fed was significantly related to temperature (logistic regression, 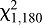 =85.19, P<0.0001; Fig. 6A). The proportion of WS bed bugs that fed similarly increased with feeder temperature (logistic regression,
=85.19, P<0.0001; Fig. 6A). The proportion of WS bed bugs that fed similarly increased with feeder temperature (logistic regression, 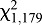 =64.35, P<0.0001; Fig. 6B).
=64.35, P<0.0001; Fig. 6B).
Fig. 6.
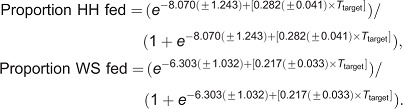 |
Because a small proportion of both HH and WS bed bugs fed at a feeder temperature of 23°C, we performed an additional set of assays being particularly careful to avoid contamination by human odors. At 23°C, 3 out of 28 (11%) individually housed bed bugs fed on 1 mmol l−1 ATP in PBS from a surface-sterilized feeder, while at 38°C, 26 out of 29 (90%) bugs fed. These results confirm that some, albeit few, bed bugs engorge on blood cooled below ambient temperature.
Ambient temperature also significantly affected bed bug feeding (Fig. 7). At ambient temperatures of 20 and 25°C, the proportion of HH bugs that fed increased monotonically as blood feeder temperature increased from 23 to 33°C, with the interactive term (ambient temperature×feeder temperature) not significant (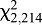 =0.82, P=0.6625). At any given feeder temperature, more bugs fed at an ambient temperature of 20°C than at 25°C (
=0.82, P=0.6625). At any given feeder temperature, more bugs fed at an ambient temperature of 20°C than at 25°C (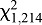 =10.83, P=0.0010). Feeding responses at a high ambient temperature of 30°C were substantially different, with no clear relationship with feeder temperature, and a high percentage of bugs (>62%) feeding at all feeder temperatures. Feeding increased as feeder temperature increased from 28 to 33°C. However, at a feeder temperature of 23°C, more than twice as many bugs fed at an ambient temperature of 30°C than at either 20 or 25°C (
=10.83, P=0.0010). Feeding responses at a high ambient temperature of 30°C were substantially different, with no clear relationship with feeder temperature, and a high percentage of bugs (>62%) feeding at all feeder temperatures. Feeding increased as feeder temperature increased from 28 to 33°C. However, at a feeder temperature of 23°C, more than twice as many bugs fed at an ambient temperature of 30°C than at either 20 or 25°C (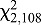 =37.59, P<0.0001).
=37.59, P<0.0001).
Fig. 7.
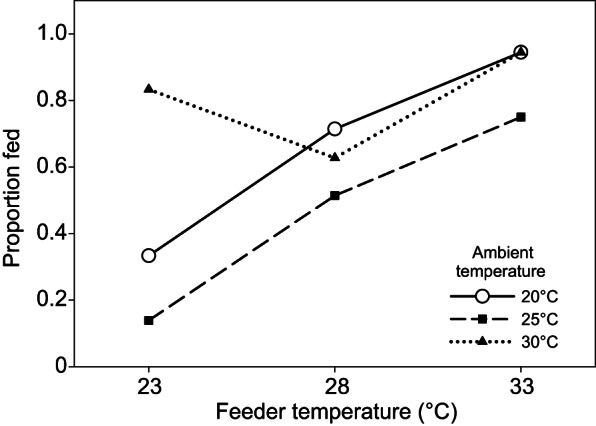
Bed bug feeding in relation to feeder and ambient temperature. Effects of feeder (blood) temperature (23, 28, 33°C) on the proportion of HH strain bed bugs feeding at three different ambient temperatures: 20, 25, 30°C. A minimum sample size of N=36 bugs was used for each ambient temperature–feeder temperature tested.
DISCUSSION
Many hematophagous insects respond to body heat as part of their orientation response to warm-blooded hosts (Lazzari and Núñez, 1989b; Lees, 1948; Peterson and Brown, 1951). However, few studies have investigated the activation of quiescent insects in response to heated targets alone. This is understandable, as most hematophagous arthropods detect their hosts at some distance and initiate their orientation toward the host using both chemosensory and visual cues. Some arthropods, however, including bed bugs and other cimicids, shelter in close proximity to their host, often within bat roosts or bird nests (Usinger, 1966), and it is plausible that under these ecological conditions, heat emanating from the host may play an important function in early steps of the orientation process. Indeed, heated targets, representing a host, significantly activated quiescent bed bugs at an ambient temperature of 25°C. When the target was cooled to 23°C, only one out of 12 bed bugs moved. These results show that targets above ambient temperature, including temperatures that cause thermal stress and death upon prolonged exposure (Benoit et al., 2009; Kells and Goblirsch, 2011; Pereira et al., 2009), can quickly activate bed bugs from an arrested state.
Orientation toward a target was significantly and characteristically affected by the temperature of the target. At target temperatures ≥28°C, bed bugs oriented toward the heated coil, with >85% of the bugs contacting the coil within 5 min. The time taken to reach the coil decreased as temperature increased. Even with the exclusion of bugs that did not reach the coil, there was still a precipitous decline in response time between 23°C (2°C below ambient) and 28°C (3°C above ambient). These results show that bed bugs are well adapted to detect even small changes in host temperature relative to ambient temperature. Our results also confirm Rivnay's (1932) findings, which were based on very few replicates, showing that bed bugs can detect temperature differences of 2°C. When the cat flea (Ctenocephalides felis) was tested over a similar range of temperatures (27–50°C), response rates and preference for a heated target also increased (Osbrink and Rust, 1985).
Several orientation parameters varied with the temperature of a target positioned in the center of the arena. Average distance to the wall of the circular arena was positively related to target temperature, increasing with temperature up to 38°C and then leveling off thereafter. The angular speed and total distance traveled by bed bugs that reached the target was positively related to target temperatures ≥38°C, with the highest values observed at the extremes of the measured temperatures (23 and 48°C). As expected, at low target temperatures, bed bugs remained closer to the arena wall and traveled farther before reaching the target than at higher target temperatures. As the coil temperature approached 48°C, angular speed increased, despite bugs reaching the coil at a similar time to that for all intermediate temperatures (28–43°C). Angular speed quantifies the observed ‘circling’ behavior that is typical of bed bugs in response to this unusually high temperature. Bed bugs approached the coil heated to 48°C, circled it and were slow to make contact with it. Rivnay (1932) reported that target temperatures ≥45°C repelled bed bugs, and it is possible that he considered this circling behavior a manifestation of repellency, although the small size of our heat coil probably resulted in less heat emission and ultimately in a more approachable stimulus. Despite this circling behavior, response time to a 48°C target remained low. In addition, orientation angle relative to the center of the arena was only significantly affected by temperatures ≥38°C, showing that even though bed bugs can locate a target heated above ambient temperature, higher target temperatures result in a more direct orientation toward the target.
There are three important caveats for interpreting these results: the size of the heat source, the size of the arena and their spatial alignment. Although similar assays with larger arenas and larger heated targets might produce quantitatively different results, we expect the overall patterns to reflect our results. The spatial alignment of the arena and heated target might be more important. All our experiments were conducted on a horizontal plane with minimal air movement, so it is unlikely that convection was a factor in bed bug orientation behavior. Yet, convection currents play a major role in heat detection in mosquitoes (Peterson and Brown, 1951), and their preference for vertically oriented flight has inspired the design of vertical olfactometers (Feinsod and Spielman, 1979). Moreover, in all our experiments, the heat coil remained in contact with the substrate, so it is unclear whether bed bugs responded to conductive or radiant heat, with radiant heat inducing responses in the closely related hematophagous hemipteran Triatoma infestans (Lazzari, 2009; Lazzari and Núñez, 1989b).
Although bed bugs detected heat over a distance of at least 4 cm, as indicated by faster activation at higher target temperatures, directed movement towards a heated target was limited to shorter distances (<3 cm). In the T-maze assay, bed bugs walked up to the junction of the ‘T’, and then chose a side with the 38°C heated target positioned at various distances from this junction. Bed bugs showed a clear preference for the target when the heated coil was 10 mm from the T-junction, a marginal (although not significant) preference at 30 mm (61%, P=0.1161), but no preference when the coil was 50 mm away. These results are not surprising in the context of bed bug ecology. Because C. lectularius (along with many other cimicids) shelter in relatively close proximity to their host, typically in locations where the host rests at night (Usinger, 1966), long-range host orientation may not be as important as it is for other hematophagous insects. Therefore, host temperature may serve as an important short-range cue under these conditions, although long-range attraction to larger bodies of heat should be evaluated. Other host-associated cues known to attract bed bugs, such as CO2 (Wang et al., 2009) and body odors (Aak et al., 2014; Liu and Liu, 2015; Rivnay, 1932), are more likely to serve as long-range attractants.
Most bed bugs fully engorged at a broad range of feeder blood temperatures from 28 to 43°C, with >88% feeding at 38 and 43°C, and few bugs feeding at the extreme temperatures (23 and 48°C). In all feeding assays, we used rabbit blood, which is highly phagostimulatory to bed bugs (Romero and Schal, 2014). Therefore, it is reasonable to conclude that the propensity to feed in our assays was related to feeder temperature and not to the quality of the blood. Most interesting was the observation that some bed bugs fed on blood set to 23°C, 2°C below ambient temperature. Although we were careful to eliminate human and blood odorants and tastants from the membrane surface, it was possible that blood constituents permeated the feeder membrane. To control for this, we repeated the assays at 23 and 38°C with a sterilized membrane and replaced blood with PBS fortified with ATP. Romero and Schal (2014) reported low levels of engorgement on PBS solutions equilibrated at 37°C, but the addition of ATP, a constituent of human blood, induced nearly 100% of bugs to feed. As with blood, some bed bugs (11%) fed on the PBS-ATP solution set to 23°C in our assays. Based on these results and behavioral observations, we suggest that bed bugs naturally probe all surfaces and if conditions are permissive (e.g. membranous surface), some bugs will penetrate the surface and begin feeding if they taste phagostimulants such as ATP. While these observations also predict that heat alone might increase the rate of probing, it is also possible that the cooler temperature of the glass feeder resulted in a localized increase in relative humidity, a cue that guides other hematophagous insects to hosts (van Breugel et al., 2015). In triatomine bugs, heat is a primary stimulus that triggers biting (Lazzari and Núñez, 1989a), but triatomines are not known to bite targets set below ambient temperature, and neither do tsetse flies, Glossina pallidipes (Chappuis et al., 2013). Specifically, in triatomines, feeding initiation (biting) appears to be a function of only surface temperature, while the number of bugs that fully engorge and the intake rate are controlled by blood temperature (Lazzari and Núñez, 1989a). This does not appear to be the case in bed bugs, with heat not required to induce feeding, although feeding initiation does increase with increasing surface/blood temperature. In both triatomines and tsetse flies, other sensory cues modulate the probing response. The triatomine Rhodnius prolixus bites more when a heated metal surface is covered with latex, suggesting that the decision to probe/bite integrates thermal and mechanical cues (Ferreira et al., 2011). In G. pallidipes, skin temperature and humidity synergistically increase the biting response (Chappuis et al., 2013). Mosquito feeding is also affected by temperature, although not shown independently of host odors (Grossman and Pappas, 1991; Willis, 1958).
The feeding response of bed bugs is complicated by the interaction of feeder (host) temperature with ambient temperature. Significantly more bed bugs fed at an ambient temperature of 20°C than at 25°C at each feeder temperature (23, 28, 33°C). These results suggest that at ambient temperatures <30°C, feeding responses in bed bugs depend on the relative difference between ambient and feeder temperatures. However, at an ambient temperature of 30°C, 83% of bugs fed at a feeder temperature of 23°C (7°C below ambient). At the same feeder temperature, only 33% and 14% of bugs fed at ambient temperatures of 20 and 25°C, respectively. This differential feeding response is likely the result of increased movement and thus increased probing of the artificial membrane at high temperatures, although this idea has not been tested. We hypothesize that feeding at low temperatures may relate to the evolutionary history of feeding on bats and even reptiles, whose body temperatures are often close to or only slightly above the ambient temperature (Hock, 1951).
Understanding the activational and appetitive responses of bed bugs to heat should facilitate the development and deployment of traps, other monitoring devices, and direct control approaches. Our results suggest that traps and baits that only use heat to attract bed bugs will need to be deployed at very high density to overcome their limited active space of <3 cm. Nevertheless, thermally attractive devices may be highly effective if placed near bed bug sheltering sites or along their foraging paths. While some traps may or may not integrate heat with chemoattractants to attract bed bugs, whereas other traps do not (Anderson et al., 2009; Singh et al., 2012; Wang et al., 2009), heat appears to be important in stimulating probing and feeding in bed bugs, an obligatory step in the development of an artificial bait.
To our knowledge, this is the first comprehensive report showing heat to be responsible for activating bed bugs from an arrested state, orienting them toward a heat source, and modulating feeding responses based on both feeder and ambient temperatures. These results should facilitate the design of monitoring devices and ultimately the development of an artificial liquid bait. Future studies should investigate the sensory structures that bed bugs use to perceive heat (and cold), their distribution on the body of the bed bug, and the genes that encode the thermal receptors. The recently sequenced C. lectularius genome (Benoit et al., 2016; Rosenfeld et al., 2016) should expedite these investigations. In addition, future work should elucidate the interactions of host-produced heat with other host cues, including CO2 and body odors, and the orientation patterns of bed bugs at different spatial alignments with the host.
Acknowledgements
We thank Richard G. Santangelo for assistance with experimental animals during this study and Ayako Wada-Katsumata for assistance in preparing figures for the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Z.C.D. and C.S. designed the study and wrote the manuscript. Z.C.D. and R.M. performed the experiments. Z.C.D. analyzed the data.
Funding
This research was supported in part by the Blanton J. Whitmire endowment, the Pest Management Foundation of the National Pest Management Association, the US Department of Housing and Urban Development Healthy Homes program (NCHHU0017-13), the Alfred P. Sloan Foundation (2013-5-35 MBE), the National Science Foundation (IOS-1456973), and an award from the National Institute of Environmental Health Sciences (P30ES025128) to the Center for Human Health and the Environment. Z.C.D. was supported by the David R. Nimocks, Jr Fellowship and the North Carolina Pest Management Association Fellowship, and scholarships from the Entomological Society of America. Deposited in PMC for release after 12 months.
References
- Aak A., Rukke B. A., Soleng A. and Rosnes M. K. (2014). Questing activity in bed bug populations: male and female responses to host signals. Physiol. Entomol. 39, 199-207. 10.1111/phen.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. F., Ferrandino F. J., McKnight S., Nolen J. and Miller J. (2009). A carbon dioxide, heat and chemical lure trap for the bedbug, Cimex lectularius. Med. Vet. Entomol. 23, 99-105. 10.1111/j.1365-2915.2008.00790.x [DOI] [PubMed] [Google Scholar]
- Batschelet E. (1981). Circular Statistics in Biology. London: Academic press. [Google Scholar]
- Benoit J. B., Lopez-Martinez G., Teets N. M., Phillips S. A. and Denlinger D. L. (2009). Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med. Vet. Entomol. 23, 418-425. 10.1111/j.1365-2915.2009.00832.x [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Adelman Z. N., Reinhardt K., Dolan A., Poelchau M., Jennings E. C., Szuter E. M., Hagan R. W., Gujar H., Shukla J. N. et al. (2016). Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nat. Commun. 7, 10165 10.1038/ncomms10165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson K., Robie A. A., Bender J., Perona P. and Dickinson M. H. (2009). High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451-457. 10.1038/nmeth.1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock T. H. and Cowles R. B. (1952). Physiology of an infrared receptor: the facial pit of pit vipers. Science 115, 541-543. 10.1126/science.115.2994.541-a [DOI] [PubMed] [Google Scholar]
- Chappuis C. J. F., Béguin S., Vlimant M. and Guerin P. M. (2013). Water vapour and heat combine to elicit biting and biting persistence in tsetse. Parasites Vectors 6, 240 10.1186/1756-3305-6-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cock Buning T. (1983). Thermal sensitivity as a specialization for prey capture and feeding in snakes. Am. Zool. 23, 363-375. 10.1093/icb/23.2.363 [DOI] [Google Scholar]
- Dillon M. E., Wang G., Garrity P. A. and Huey R. B. (2009). Thermal preference in Drosophila. J. Therm. Biol. 34, 109-119. 10.1016/j.jtherbio.2008.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. G. (1964). Infra-red receptors in Melanophila acuminata DeGeer. Nature 202, 211 10.1038/202211a0 [DOI] [PubMed] [Google Scholar]
- Feinsod F. M. and Spielman A. (1979). An olfactometer for measuring host-seeking behavior of female Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 15, 282-285. 10.1093/jmedent/15.3.282 [DOI] [Google Scholar]
- Ferreira R. A., Pereira M. H. and Lorenzo M. G. (2011). Substrate texture properties induce triatomine probing on bitten warm surfaces. Parasites Vectors 4, 111 10.1186/1756-3305-4-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch S. and Collier R. H. (1985). Laboratory studies on aestivation in the cabbage root fly (Delia radicum). Entomol. Exp. Appl. 38, 137-143. 10.1111/j.1570-7458.1985.tb03510.x [DOI] [Google Scholar]
- Graham W. M. (1958). Temperature preference determinations using Tribolium. Anim. Behav. 6, 231-237. 10.1016/0003-3472(58)90056-3 [DOI] [Google Scholar]
- Grossman G. L. and Pappas L. G. (1991). Human skin temperature and mosquito (Diptera: Culicidae) blood feeding rate. J. Med. Entomol. 28, 456-460. 10.1093/jmedent/28.3.456 [DOI] [PubMed] [Google Scholar]
- Hammer Ø., Harper D. and Ryan P. (2001). PAST-PAlaeontological STatistics, ver. 1.89. Palaeontol. Electron. 4, 1-9. [Google Scholar]
- Harraca V., Ryne C., Birgersson G. and Ignell R. (2012). Smelling your way to food: can bed bugs use our odour? J. Exp. Biol. 215, 623-629. 10.1242/jeb.065748 [DOI] [PubMed] [Google Scholar]
- Hock R. J. (1951). The metabolic rates and body temperatures of bats. Biol. Bull. 101, 289-299. 10.2307/1538547 [DOI] [Google Scholar]
- Holsapple J. G. and Florentine G. J. (1972). Thermal perception by the red flour beetle, Tribolium castaneum. Ann. Entomol. Soc. Am. 65, 1388-1390. 10.1093/aesa/65.6.1388 [DOI] [Google Scholar]
- Ivancic A., Roupsard O., Garcia J. Q., Melteras M., Molisale T., Tara S. and Lebot V. (2008). Thermogenesis and flowering biology of Colocasia gigantea, Araceae. J. Plant Res. 121, 73-82. 10.1007/s10265-007-0129-5 [DOI] [PubMed] [Google Scholar]
- Kells S. A. and Goblirsch M. J. (2011). Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2, 412-422. 10.3390/insects2030412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A., Maibach H., Strauss W. G. and Fenley W. R. (1966). Quantitation of effect of several stimuli on the approach of Aedes aegypti. J. Econ. Entomol. 59, 690-694. 10.1093/jee/59.3.690 [DOI] [PubMed] [Google Scholar]
- Khan A., Maibach H. I. and Strauss W. G. (1968). The role of convection currents in mosquito attraction to human skin. Mosq. News 28, 462-464. [Google Scholar]
- Lazzari C. R. (2009). Orientation towards hosts in haematophagous nsects: an integrative perspective. In Advances in Insect Physiology, Vol. 37 (ed. Stephen J. S. and Jeacuterocircme C.), pp. 1-58. New York: Academic Press. [Google Scholar]
- Lazzari C. R. and Núñez J. A. (1989a). Blood temperature and feeding behavior in Triatoma infestans (Heteroptera: Reduviidae). Entomol. Gen. 14, 183-188. 10.1127/entom.gen/14/1989/183 [DOI] [Google Scholar]
- Lazzari C. R. and Núñez J. A. (1989b). The response to radiant heat and the estimation of the temperature of distant sources in Triatoma infestans. J. Insect Physiol. 35, 525-529. 10.1016/0022-1910(89)90060-7 [DOI] [Google Scholar]
- Lees A. (1948). The sensory physiology of the sheep tick, Ixodes ricinus L. J. Exp. Biol. 25, 145-207. [Google Scholar]
- Leonard C. M. (1974). Thermotaxis in golden hamster pups. J. Comp. Physiol. Psychol. 86, 458 10.1037/h0036135 [DOI] [PubMed] [Google Scholar]
- Liu F. and Liu N. (2015). Human odorant reception in the common bed bug, Cimex lectularius. Sci. Rep. 5, 15558 10.1038/srep15558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes C., Cuadrillero C. and Vilella D. (2002). Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J. Med. Entomol. 39, 675-679. 10.1603/0022-2585-39.4.675 [DOI] [PubMed] [Google Scholar]
- Osbrink W. L. A. and Rust M. K. (1985). Cat flea (Siphonaptera: Pulicidae): factors influencing host-finding behavior in the laboratory. Ann. Entomol. Soc. Am. 78, 29-34. 10.1093/aesa/78.1.29 [DOI] [Google Scholar]
- Paster E. and Ryu W. S. (2008). The thermal impulse response of Escherichia coli. Proc. Natl. Acad. Sci. USA 105, 5373-5377. 10.1073/pnas.0709903105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R. M., Koehler P. G., Pfiester M. and Walker W. (2009). Lethal effects of heat and use of localized heat treatment for control of bed bug infestations. J. Econ. Entomol. 102, 1182-1188. 10.1603/029.102.0342 [DOI] [PubMed] [Google Scholar]
- Peterson D. G. and Brown A. W. A. (1951). Studies of the responses of the female Aedes mosquito. Part III. The response of Aedes aegypti (L.) to a warm body and its radiation. Bull. Entomol. Res. 42, 535-541. 10.1017/S0007485300028935 [DOI] [Google Scholar]
- Poff K. L. and Skokut M. (1977). Thermotaxis by pseudoplasmodia of Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 74, 2007-2010. 10.1073/pnas.74.5.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M. D. and Miller D. M. (2011). Host searching and aggregation activity of recently fed and unfed bed bugs (Cimex lectularius L.). Insects 2, 186-194. 10.3390/insects2020186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W. W. (1977). Temperature as a proximate factor in orientation behavior. J. Fish Res. Board Can. 34, 734-739. 10.1139/f77-114 [DOI] [Google Scholar]
- Rivnay E. (1932). Studies in tropisms of the bed bug Cimex lectularius L. Parasitology 24, 121-136. 10.1017/S0031182000020461 [DOI] [Google Scholar]
- Romero A. and Schal C. (2014). Blood constituents as phagostimulants for the bed bug, Cimex lectularius L. J. Exp. Biol. 217, 552-557. 10.1242/jeb.096727 [DOI] [PubMed] [Google Scholar]
- Romero A., Potter M. F. and Haynes K. F. (2010). Circadian rhythm of spontaneous locomotor activity in the bed bug, Cimex lectularius L. J. Insect Physiol. 56, 1516-1522. 10.1016/j.jinsphys.2010.04.025 [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. A., Reeves D., Brugler M. R., Narechania A., Simon S., Durrett R., Foox J., Shianna K., Schatz M. C., Gandara J. et al. (2016). Genome assembly and geospatial phylogenomics of the bed bug Cimex lectularius. Nat. Commun. 7, 10164 10.1038/ncomms10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H. and Bleckmann H. (1998). The photomechanic infrared receptor for the detection of forest fires in the beetle Melanophila acuminata (Coleoptera: Buprestidae). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 182, 647-657. 10.1007/s003590050210 [DOI] [Google Scholar]
- Seymour R. S. and Schultze-Motel P. (1997). Heat-producing flowers. Endeavour 21, 125-129. 10.1016/S0160-9327(97)80222-0 [DOI] [Google Scholar]
- Singh N., Wang C., Cooper R. and Liu C. (2012). Interactions among carbon dioxide, heat, and chemical lures in attracting the bed bug, Cimex lectularius L. (Hemiptera: Cimicidae). Psyche 102, 1580-1585. [Google Scholar]
- Takken W. and Verhulst N. O. (2013). Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433-453. 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- Usinger R. L. (1966). Monograph of Cimicidae (Hemiptera, Heteroptera). College Park, MD: Entomological Society of America. [Google Scholar]
- van Breugel F., Riffell J., Fairhall A. and Dickinson M. H. (2015). Mosquitoes use vision to associate odor plumes with thermal targets. Curr. Biol. 25, 2123-2129. 10.1016/j.cub.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. and Zhang Z. (2015). Floral thermogenesis: an adaptive strategy of pollination biology in Magnoliaceae. Commun. Integr. Biol. 8, e992746 10.4161/19420889.2014.992746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Gibb T., Bennett G. W. and McKnight S. (2009). Bed Bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical Lure. J. Econ. Entomol. 102, 1580-1585. 10.1603/029.102.0423 [DOI] [PubMed] [Google Scholar]
- Willis E. R. (1958). The use of artificial heat to induce mosquitoes to feed on cold-blooded animals. Ann. Entomol. Soc. Am. 51, 257-261. 10.1093/aesa/51.3.257 [DOI] [Google Scholar]