Abstract
Forkhead box (Fox) transcription factors are evolutionarily conserved in organisms ranging from yeast to humans. They regulate diverse biological processes both during development and throughout adult life. Mutations in many Fox genes are associated with human disease and, as such, various animal models have been generated to study the function of these transcription factors in mechanistic detail. In many cases, the absence of even a single Fox transcription factor is lethal. In this Primer, we provide an overview of the Fox family, highlighting several key Fox transcription factor families that are important for mammalian development.
KEY WORDS: Forkhead, Fox, Foregut development, Language acquisition, Pioneer factors, Transcription factors
Summary: This Primer provides an overview of the Fox family of transcription factors, highlighting the key family members that are important for mammalian development and discussing how they function.
Introduction
The forkhead (fkh) gene was originally identified in a random mutagenesis screen performed in Drosophila melanogaster (Weigel et al., 1989). This study showed that fkh is required for normal gut development, and that its absence results in a characteristic ʻforked head' appearance resulting from the homeotic transformation of the foregut into a head structure. Soon after this discovery, a number of related genes – termed Fox genes – were identified in multiple organisms, ranging from yeasts to humans.
The Drosophila Fkh protein is characterized by a winged-helix DNA-binding domain ∼100 residues long, termed the ‘forkhead box’. All Fox proteins share this distinctive DNA-binding domain but have divergent features and functions. Fox genes control a wide variety of biological functions and are broadly expressed both during development and in adult life. Their roles include, but are not limited to, the regulation of gastrulation (Ang and Rossant, 1994; Weinstein et al., 1994), stem cell and stem cell niche maintenance (Sackett et al., 2009; Aoki et al., 2016), the regulation of metabolism and cell cycle control (Hannenhalli and Kaestner, 2009). Indeed, Fox transcription factors are required for the normal specification, differentiation, maintenance and/or function of tissues such as the trophectoderm, liver, pancreas, ovaries, intestine, lung, kidney, prostate, brain, thyroid, skeletal and heart muscle, skeleton, vascular tissue and immune cells (Zhu, 2016).
Here, we first provide an overview of the Fox gene family and discuss how distinct Fox transcription factors regulate specific stages of development, tissue homeostasis and disease. Owing to their sheer number, we then concentrate on just four families: the FoxA factors and their role in the differentiation and maintenance of multiple cell types; FoxM1 and its control of the cell cycle; the FoxO group in regulating metabolism and longevity; and FoxP for its contribution to speech acquisition.
An overview of Fox transcription factors
The number of Fox genes currently cataloged varies widely among different organisms. Human and mouse both have 44, Drosophila 11, Caenorhabditis elegans 15, and Xenopus 45, the latter excluding alternate splice forms in all species and pseudogenes that were duplicated along with the rest of the Xenopus genome and expressed in exactly the same location as the original genes. Notably, Xenopus models contributed greatly to the initial description of Fox expression patterns in early embryogenesis (Pohl and Knöchel, 2005).
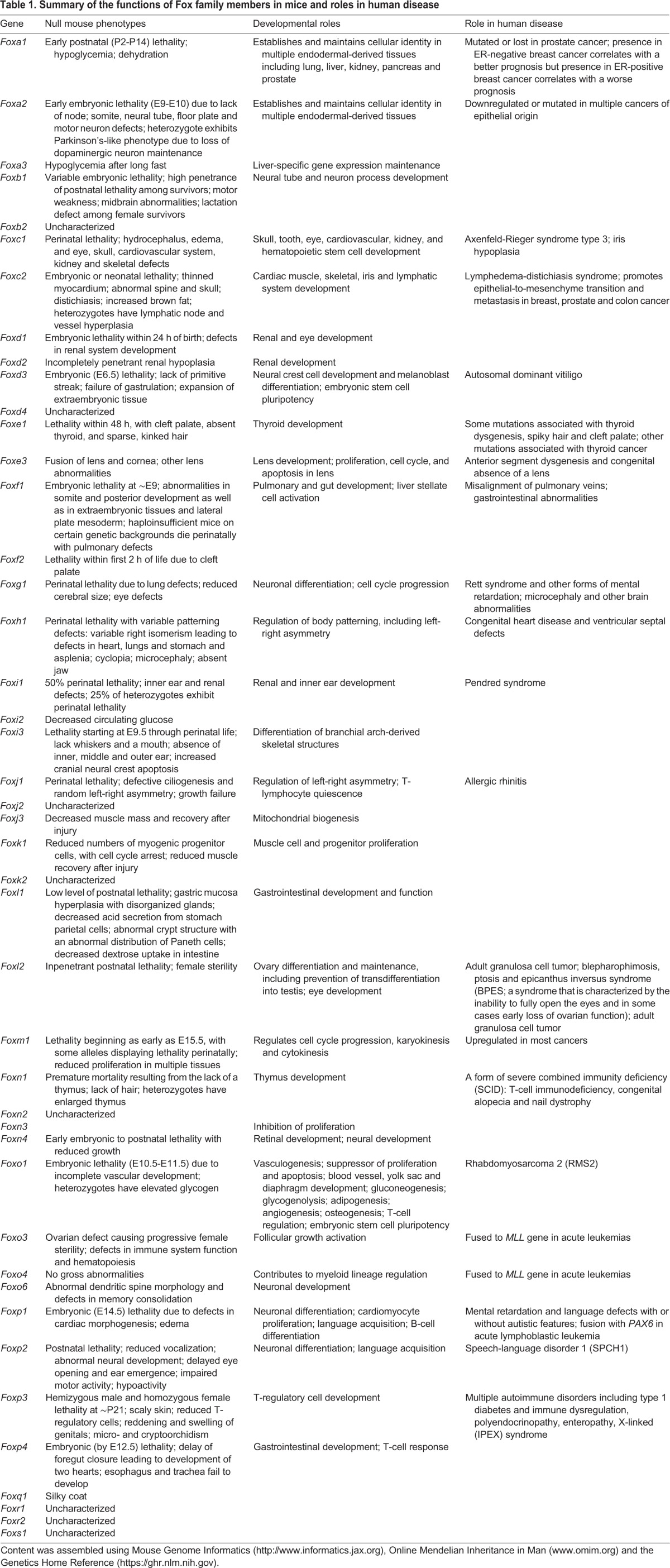
In mammals, Fox transcription factors are categorized into subclasses A to S (Fig. 1) based on sequence similarity within and outside of the forkhead box (Hannenhalli and Kaestner, 2009; Kaestner et al., 1999). In many cases, the homozygous deletion of just one Fox gene leads to embryonic or perinatal lethality and, in humans, mutations in or the abnormal regulation of Fox genes are associated with developmental disorders and diseases such as cancer (Halasi and Gartel, 2013; Li et al., 2015a; Wang et al., 2014b; Zhu et al., 2015; DeGraff et al., 2014; Halmos et al., 2004; Ren et al., 2015; Jones et al., 2015; Habashy et al., 2008), Parkinson's disease (Kittappa et al., 2007), autism spectrum disorder (Bowers and Konopka, 2012), ocular abnormalities (Acharya et al., 2011), defects in immune regulation and function (Mercer and Unutmaz, 2009) and deficiencies in language acquisition (Takahashi et al., 2009); see Table 1 for a comprehensive overview of Fox transcription factor expression patterns and their association with developmental disorders and disease.
Fig. 1.
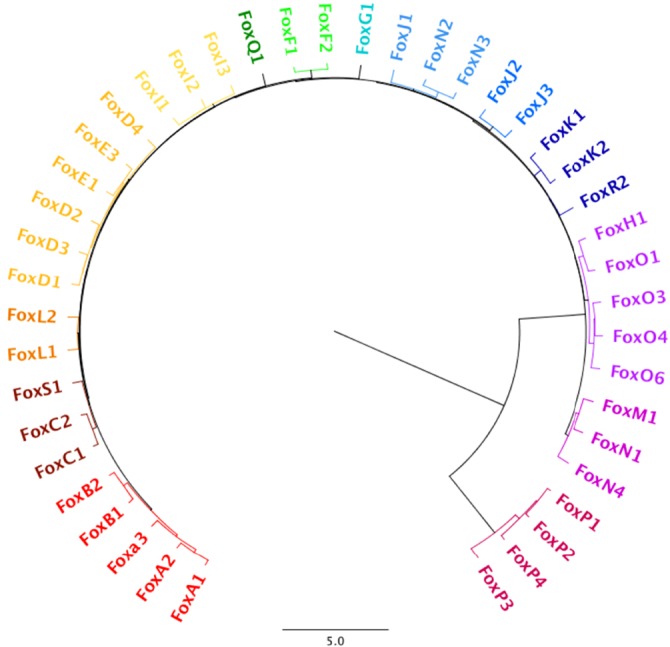
Phylogenetic tree of mouse Fox family members. The entire sequences of mouse Fox transcription factors were aligned pairwise using Geneious software. The following parameters were employed: global assignment with free end gaps, the Jukes-Cantor genetics distance model, and unweighted pair-group method with arithmetic mean. Differences with other phylogenetic trees of Fox transcription factors are likely the result of grouping by homology to the FKH DNA-binding domain only. Scale indicates the relative number of amino acid changes between proteins.
Table 1.
Summary of the functions of Fox family members in mice and roles in human disease
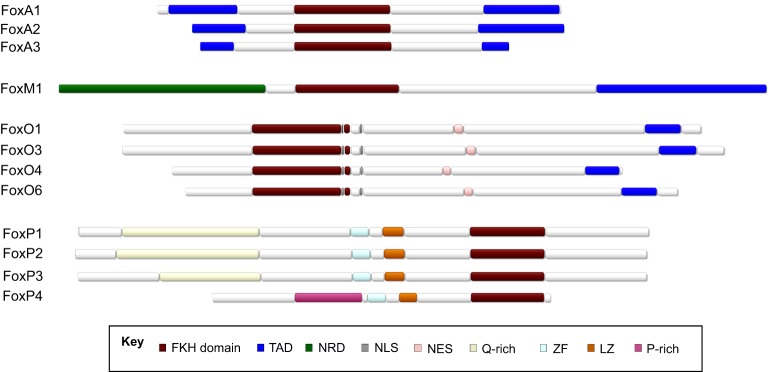
Distinct protein domains, expression patterns and post-translational modifications contribute to the divergent functions of Fox family members
Fox transcription factors bind a similar DNA sequence, albeit with different affinities, due to their highly conserved DNA-binding motif. How, then, do members of this large gene family have distinct roles? The divergent sequences outside of the conserved DNA-binding domain likely differentiate the function of these proteins, as do their distinct temporal and spatial gene activation patterns (Fig. 2).
Fig. 2.
The domain structure of selected Fox family members. Shown are the domain structures of mouse FoxA1-3, FoxM1, FoxO1, FoxO3, FoxO4, FoxO6 and FoxP1-4. TAD, transactivation domain; NRD, N-terminal repressor domain; NLS, nuclear localization signal; NES, nuclear export signal; ZF, zinc finger; LZ, leucine zipper.
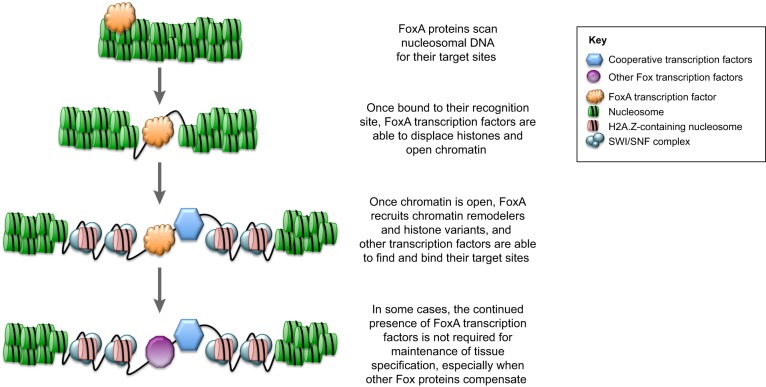
The binding domains of FoxA transcription factors, for example, have structural similarity to linker histones H1 and H5 (Clark et al., 1993; Zaret et al., 2010). This feature allows FoxA transcription factors to access closed chromatin (Fig. 3), thus allowing the recruitment of alternative histones and facilitating the subsequent binding of other transcription factors at nearby sites (Cirillo et al., 2002; Li et al., 2012b; Updike and Mango, 2006). Indeed, genome-wide mapping of FoxA binding sites and nucleosome architecture in the mouse liver has shown that FoxA binds both nucleosome-bound and nucleosome-free DNA targets with the same recognition site (Li et al., 2011). For this reason, FoxA family members have been termed ‘pioneer’ transcription factors (Cirillo et al., 2002). In this role, they help specify cell types by giving tissue-specific transcription factors access to their binding sites (Iwafuchi-Doi and Zaret, 2016). They also help maintain cell identity by acting as ‘place holders’ or ‘bookmarks’ at genes that are normally shut off during cell division; after cytokinesis is complete, cell type-specific genes previously marked by FoxA can be easily reactivated (Caravaca et al., 2013). Accordingly, a number of studies have shown that FoxA transcription factors are expressed in early development and play crucial roles in the development and homeostasis of various cells and tissues (see below).
Fig. 3.
FoxA proteins function as pioneer factors. The schematic depicts how FoxA transcription factors are able to function as pioneer factors that control gene expression via their interaction with chromatin.
Because of their actions as pioneer factors, FoxA proteins are required to facilitate the binding of many other transcription factors to their targets. This is especially evident for several nuclear receptors, such as androgen receptor (AR), glucocorticoid receptor (GR) and estrogen receptor (ER). This was first shown for the interaction between FoxA1 and AR in the prostate (Gao et al., 2003), then for ER and FoxA1 (Hurtado et al., 2011; Ross-Innes et al., 2012; Carroll et al., 2005; Lupien et al., 2008) and for FoxA2 and GR (Zhang et al., 2005). The consequences of this cooperative binding, particularly in the context of cancer, are discussed in detail below.
FoxM1 is best known for its role in regulating the cell cycle. Its forkhead DNA-binding domain (FHD) shares only 18% sequence identity with that of FoxA1, and FoxM1 has lower affinity for the forkhead consensus binding site than other Fox proteins (Littler et al., 2010). Some investigators have shown that, instead of directly interacting with the DNA of its proliferative targets, FoxM1 attaches to proteins in the DREAM complex – a larger conglomeration of proteins that prevents the transcription of proliferative targets in the quiescent state, but promotes expression of the same targets during the cell cycle (Chen et al., 2013). However, other reports have demonstrated direct binding of FoxM1 to its targets (Sanders et al., 2013).
Given its crucial function in cell cycle control, FoxM1 expression and activity must be tightly regulated. Indeed, Foxm1 mRNA and protein are only usually expressed during the cell cycle (Korver et al., 1997). The activity of FoxM1 is also tightly controlled by several post-transcriptional mechanisms (reviewed by Golson et al., 2010): (1) FoxM1 is generally excluded from the nucleus, but phosphorylation by MAPK induces its translocation; (2) an intramolecular repressive domain inhibits the binding of FoxM1 to DNA until phosphorylation by Chek2 (Chk2); (3) FoxM1 only recruits co-factors for gene transactivation when phosphorylated by Cdk1/2; and (4) FoxM1 is targeted to the proteasome when phosphorylated by a presently unknown kinase.
Like FoxM1, the activity of FoxO proteins – a group of proteins that inhibit the cell cycle and regulate lifespan and metabolism – is regulated post-translationally. Phosphorylation by Akt, for example, leads to the nuclear exclusion of FoxO1 and sequestration away from DNA by the 14-3-3 scaffolding protein (Tzivion et al., 2011). Unlike many other Fox transcription factors, FoxO factors contain both a nuclear export signal (NES) and a nuclear localization signal (NLS) (Tzivion et al., 2011). FoxO proteins help to activate transcription of their targets by recruiting the SWI/SNF chromatin remodeling complex (Riedel et al., 2013). These transcriptional targets include antioxidants, cell cycle inhibitors and metabolic genes; in many ways, FoxO proteins act counter to FoxM1.
Although most Fox transcription factors are strictly transcriptional activators, some, such as FoxP, have dual activator and repressor functions; indeed, members of the FoxP family are thought to act primarily as repressors (Zuo et al., 2007; Shu et al., 2001; Li et al., 2004a). FoxP family members can bind as homo- or heterodimers through their leucine zipper and zinc finger domains, and this interaction is required for FoxP family members to repress their targets, perhaps because a conformational change occurs upon binding that allows a portion of the N-terminal domain to recruit co-repressors such as histone deacetylases, the lysine acetyltransferase TIP60 (Kat5), and the SMRT complex (Li et al., 2007; Wang et al., 2003; Lal and Bromberg, 2009; Jepsen et al., 2008). The proline-rich track within the N-terminus of FoxP3 is essential for its function as a repressor (Xie et al., 2015), and this suggests that the glutamine-rich tracts in FoxP1, 2 and 4 are essential for their function. The structure of FoxP proteins when dimerized prevents them from binding the same strand of DNA on adjacent sites, suggesting that FoxP factors could also act to bring distal chromatin regions together (Stroud et al., 2006). Like FoxM1, recent reports have suggested that FoxP factors act in some cases by binding to other proteins rather than via direct interactions with DNA (Xie et al., 2015).
In addition to divergent protein domains, individual Fox factors have different binding partners and co-factors, which can influence both the specific DNA targets that are contacted and their downstream effects on transcriptional activity (Li et al., 2015b). Finally, many Fox transcription factors are expressed in distinct spatiotemporal patterns, allowing them to carry out distinct functions. However, it should be noted that, despite the well-known distinct roles of various families of Fox transcription factors, it is becoming clear that many of them regulate the same processes.
The FoxA family: regulators of development, differentiation and cell identity
Mammalian FoxA transcription factors were first identified for their DNA-binding properties in rat liver nuclear extracts and were thus originally named hepatocyte nuclear factor 3 (HNF3) α, β and γ (Costa et al., 1989). Of all mammalian Fox genes, Foxa2 shares the most homology with the original fkh gene discovered in Drosophila. Its homolog in the nematode C. elegans is pha-4 and, notably, studies of pha-4 mutants have helped further our understanding of FoxA function by identifying FoxA targets (Gaudet and Mango, 2002), establishing their interactions with nuclear hormone receptors (Ao et al., 2004), demonstrating the recruitment of RNA polymerase II (Hsu et al., 2015) and the histone variant H2A.Z (Updike and Mango, 2006), and characterizing the regulation of PHA-4 by the TOR pathway.
The expression of FoxA factors
In mammals, FoxA1, 2 and 3 exhibit overlapping but distinct expression patterns in a variety of developing and mature tissues (Friedman and Kaestner, 2006). In the mouse, Foxa2 is the first member of the FoxA family to be expressed. At embryonic day (E) 6.5, Foxa2 expression can be detected in the primitive streak and in the node, both of which are important for gastrulation. By E7.5, Foxa2 is expressed in the mesoderm and definitive endoderm (Monaghan et al., 1993; Ang et al., 1993). Its expression is then maintained in endoderm-derived tissues, such as the pancreas, liver, thyroid, prostate and lung, throughout development and in adulthood (Friedman and Kaestner, 2006), and is also observed in ectoderm-derived neural tissues, such as the ventral midbrain, including in dopaminergic neurons and the hypothalamus (Besnard et al., 2004).
During early embryogenesis, the expression of Foxa1 is similar to that of Foxa2, but following a short temporal delay. For example, Foxa1 can first be detected at E7.0 in the primitive streak, and then later in the notochord, neural plate, floor plate and neural tube (Monaghan et al., 1993). However, the expression of Foxa1 and of Foxa2 differ in the adult. Although Foxa1 is initially highly expressed in the developing pancreas, its expression falls to <10% of Foxa2 levels in alpha, beta and acinar cells in the adult pancreas (Bramswig et al., 2013). In addition, Foxa1 is more widely expressed than Foxa2 in several adult tissues, including the respiratory system, brain and gastrointestinal tract. Foxa1 is also expressed in some tissues that lack FoxA2 entirely, such as male reproductive organs, the ureter and bladder (Friedman and Kaestner, 2006).
The expression of Foxa3 is more restricted than that of Foxa1 or Foxa2, and is more specific to the foregut endoderm than other family members. Foxa3 expression can first be detected at E8.5 and is maintained strongly in the liver but also in the pancreas and intestine throughout adulthood (Monaghan et al., 1993). It is the mostly highly expressed FoxA transcription factor in the adult liver (Kaestner et al., 1994).
The role of FoxA transcription factors
The earlier expression of FoxA2 compared with FoxA1 and FoxA3 is reflected in the severity of phenotypes in mice lacking these factors. Mice null for Foxa2 die between E10 and E11, exhibiting defects in all three germ layers (Weinstein et al., 1994; Ang and Rossant, 1994). Heterozygotes for Foxa2 are viable but display a Parkinson's-like phenotype following aging (Kittappa et al., 2007). In contrast to Foxa2 null mice, those lacking Foxa1 survive until after birth, displaying lethality between postnatal day (P) 2 and 12 due to hypoglycemia and defects in kidney function (Behr et al., 2004; Kaestner et al., 1999); the hypoglycemia is likely to result from deficiencies in glucagon secretion, while the dehydration stems from abnormal kidney development (Behr et al., 2004; Kaestner et al., 1999). These mice also display defects in prostate morphogenesis (Gao et al., 2005). As might be expected from its limited expression pattern, among the FoxA family mice deficient for Foxa3 have the mildest phenotype. They are viable with a normal lifespan but do, however, display hypoglycemia after a prolonged fast because of defects in hepatic glucose production (Shen et al., 2001).
Since the embryonic lethality of Foxa2−/− mice precludes analysis of the role of FoxA2 in the organogenesis and function of many tissues, mice with conditional deletions of Foxa2 have been derived. Studies of these mutants reveal that FoxA2 on its own is not required for normal liver differentiation (Sund et al., 2000), although defects in lung morphogenesis are seen (Wan et al., 2004). In addition, severe defects in pancreatic islet formation and alpha and beta cell maturation that lead to alterations in glucose homeostasis are observed (Sund et al., 2000; Lantz et al., 2004). Interestingly, mice expressing an activated form of FoxA2 in neurons display an orexigenic (i.e. a stimulated appetite) phenotype (Silva et al., 2009). Although off-target effects of this mutated protein cannot be excluded, this study implicates FoxA2 in yet more aspects of metabolic regulation, namely food intake and energy output.
Cooperativity and compensation among FoxA transcription factors
The conditional deletion of genes encoding individual FoxA transcription factors revealed little requirement for any one FoxA family member in the liver (Lee et al., 2005b; Kaestner et al., 1999; Shen et al., 2001). Gross pancreatic morphology was also unaffected when only one FoxA transcription factor was missing (Lee et al., 2005b; Kaestner et al., 1999). These studies suggest either that FoxA family members are dispensable in these organs or, alternatively, that they can compensate for each other. Both in vivo and in vitro studies suggest that the latter possibility is likely: both FoxA1 and FoxA2 transcription factors must be suppressed to abolish expression of Muc2, which encodes a protein important for intestinal function (van der Sluis et al., 2008), and both Foxa1 and Foxa2 are upregulated in Foxa3 null livers (Shen et al., 2001). In addition, several different analyses using the conditional, simultaneous deletion of Foxa1 and Foxa2 demonstrated that these two transcription factors can compensate for each other in a number of tissues. For example, their combined absence results in complete liver agenesis (Lee et al., 2005a), near-complete pancreatic agenesis (Gao et al., 2008), and altered allocation of enteroendocrine cells within the intestine (Ye and Kaestner, 2009), but, as outlined above, deletion of only one FoxA transcription factor leads to a less severe phenotype in these tissues. Finally, mice with a late-gestation deletion of both Foxa1 and Foxa2 demonstrate upregulation of Foxa3, with FoxA3 also being observed to bind FoxA targets in the double-null animal that were bound only by FoxA1 and FoxA2 in wild-type mice (Iwafuchi-Doi et al., 2016).
FoxM1: a key cell cycle control factor
FoxM1 was identified nearly simultaneously by three groups and hence was originally given three names: Trident (Korver et al., 1997), hepatocyte nuclear factor 3/fork head homolog 11 (Ye et al., 1997) and WIN (winged helix protein in the INS1 cell line) (Yao et al., 1997). All three groups identified FoxM1 by homology to FoxA proteins, but whereas the Clevers group discovered it within the thymus, the Costa lab cloned it from the colon carcinoma cell line Caco-2, and the Wong group identified it in a cell line derived from an insulinoma.
FoxM1 is widely recognized for its role in driving the cell cycle (Fig. 4). Its direct transcriptional targets function at both the G1/S and G2/M transitions, as well as during karyokinesis and cytokinesis (reviewed by Wierstra, 2013; Wierstra and Alves, 2007). Accordingly, FoxM1 expression is mostly confined to actively replicating cells; its expression is thus broad in embryos and declines in differentiated tissue as well as with age (Ye et al., 1997).
Fig. 4.
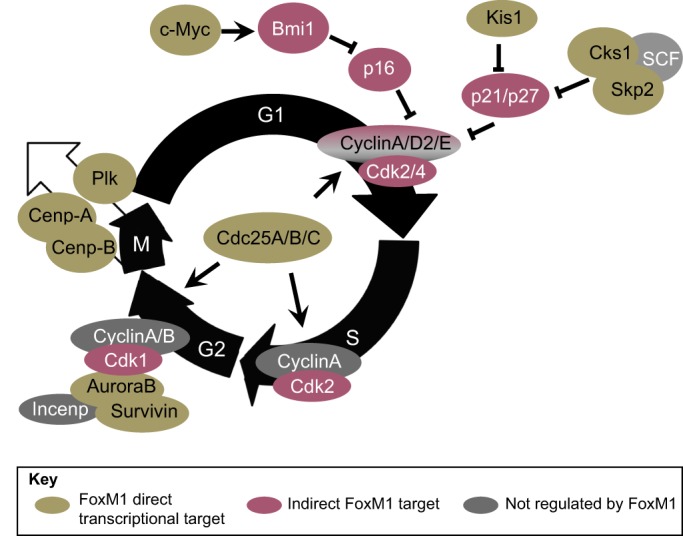
The role of FoxM1 in cell cycle regulation. FoxM1 regulates various factors involved in cell cycle regulation. No evidence for FoxM1 regulation exists for those cell cycle proteins shown in gray. Cyclin D2, but not A and E, is regulated by FoxM1. p16 is also known as Cdkn2a; SCF as Kitl; p21/p27 as Cdkn1a/Cdkn1b; and survivin as Birc5.
FoxM1 exists in various genera and species, including Xenopus, Danio, Mus, Rattus and H. sapiens, but not in C. elegans or Drosophila. The role of FoxM1 in Drosophila and C. elegans might be filled by other forkhead transcription factors, but at this time the only reports associating forkhead with proliferation in these two organisms is the increased replication in the absence of FoxO homologs. In humans, although one FOXM1 gene exists, splice variants produce three different FOXM1 proteins: FOXM1A, FOXM1B and FOXM1C. Both FOXM1B and FOXM1C are transcriptional activators, whereas FOXM1A is a transcriptional repressor. In mice, only one FoxM1 protein splice variant is produced; it is most similar to human FOXM1B and is likewise a transactivator.
The crucial role of FoxM1 in development is highlighted by studies perturbing its expression. For example, mice lacking FoxM1 display embryonic lethality between E14.5 and E16.5 due to failed liver and heart expansion (Krupczak-Hollis et al., 2004). Mice with conditional deletions of Foxm1 display profound proliferation defects in tissues such as the liver (Krupczak-Hollis et al., 2004), heart (Bolte et al., 2011), pancreas (Zhang et al., 2006), intestine (Yoshida et al., 2007), lungs (Kalin et al., 2008) and smooth muscle (Ustiyan et al., 2009). Furthermore, the early deletion of Foxm1 in either the heart (Bolte et al., 2011) or liver (Krupczak-Hollis et al., 2004) results in embryonic lethality, whereas mice lacking Foxm1 in smooth muscle cells (Ustiyan et al., 2009) or in the lung epithelium (Kalin et al., 2008) die perinatally.
Interest in FoxM1 was fueled by its prominent role in cancer. FoxM1 is highly expressed in a variety of tumor types and is associated with increased tumor aggressiveness (Laoukili et al., 2007). However, mouse models suggest that FoxM1 elevation by itself is not an initiating event in carcinogenesis. Mice constitutively expressing human FOXM1B in all tissues do not spontaneously develop tumors at least until 12 months of age (Kalinichenko et al., 2003; Wang et al., 2002; Yoshida et al., 2007). However, increased expression of FOXM1B, in addition to initiating mutations, leads to larger, more vascularized and more metastatic tumors (Yoshida et al., 2007; Kalinina et al., 2003). Conversely, mice lacking FoxM1 or with inhibited FoxM1 function in the tissue of interest show a reduced tumor load compared with controls when treated with carcinogens (Yoshida et al., 2007; Gusarova et al., 2007). Similar results have been observed in the lung, where increased FoxM1 activity hastens lung tumor growth in mice with induced Kras expression (Wang et al., 2010), while its absence inhibits lung tumorigenesis mediated by Kras (Wang et al., 2014a). Given this link between FoxM1 and tumor progression, anti-FoxM1 agents are being explored as potential cancer therapeutics (Box 1).
Box 1. Anti-FOXM1 agents as potential cancer therapies.
Owing to the association of FOXM1 with aggressive tumors, and because of direct evidence highlighting the importance of FoxM1 in tumor progression in mice, there is intense interest in developing anti-FOXM1 agents as therapies for a variety of cancers. The downregulation of FOXM1 by siRNA in vitro decreases DNA replication as well as angiogenic and metastatic potential (Wang and Gartel, 2011; Wu et al., 2010; Halasi and Gartel, 2012; Halasi et al., 2013). A number of proteasome inhibitors have been shown to decrease FOXM1 levels, probably by stabilizing an unidentified negative regulator of FOXM1 (Gartel, 2010), and many of these inhibitors are currently used in cancer treatments; most of their effects are likely to be due, at least in part, to FOXM1 downregulation. However, these drugs are also associated with serious side effects (Adams et al., 1999), so more specific inhibitors of FOXM1 have been sought. Recently, a high-throughput screen identified a small molecule, FDI-6, that makes direct contact with the DNA-binding domain of FOXM1, blocks the interactions of FOXM1 with its transcriptional targets, and suppresses the expression of those targets (Gormally et al., 2014). FDI-6 is specific enough that it does not affect the binding or function of other Fox transcription factors, and it also does not affect proteasomal activity. This novel molecule thus has exciting potential therapeutic applications for a variety of cancers.
FoxM1 also appears to be implicated in aging. FoxM1 levels decline with age in multiple tissues, and this decrease is accompanied by a decline in both baseline proliferation and replicative potential. The reactivation of FoxM1 activity in aged hepatocytes and pancreatic beta cells was able to rejuvenate replication in these tissues (Golson et al., 2015; Wang et al., 2002). In addition, transplanted aged FoxM1b-expressing hepatocytes were equally successful in repopulating recipient livers as hepatocytes from young mice (Brezillon et al., 2007). Currently, the transplantation of hepatocytes or beta cells can be used to treat liver diseases or type 1 diabetes, respectively; however, a shortage of both of these cell types for transplantation prevents their widespread application. Inducing FoxM1 expression transiently ex vivo could, therefore, expand the availability of transplantation-quality donor tissue by rejuvenating older donor tissue. However, as is the case for other proteins involved in the cell cycle, the benefits of activating or attenuating FoxM1 activity must be carefully considered. Drug treatments are currently not targeted to any one tissue, and activating FoxM1 endogenously to rejuvenate the replicative potential in a tissue of interest without targeting it to a specific cell type could potentially cause existing malignancies to become more prone to growth or metastasis. Conversely, inhibiting FoxM1 in cancer therapy could have negative impacts in tissues that are likely to require FoxM1 activity and exhibit high cellular turnover, such as the intestine.
FoxO factors: from cell cycle control to metabolism and longevity
FoxO proteins play multiple crucial roles: they regulate the cell cycle, apoptosis, metabolism and lifespan. Recently, interest in FoxO proteins has also been spurred on by their functions in mediating insulin and insulin-like signaling (Fig. 5) (reviewed by Golson et al., 2010). In short, insulin binding to the insulin receptor causes phosphorylation of insulin receptor substrate 1/2 (IRS1/2), resulting in a phosphorylation signaling cascade that involves phosphoinositide 3-kinase and Akt, and ultimately leads to the phosphorylation of FoxO. In the absence of insulin signaling, FoxO proteins reside in the nucleus; however, active insulin signaling causes them to translocate to the cytoplasm, where they are no longer able to bind or transactivate their targets. In addition, Akt-phosphorylated FoxO proteins are stabilized in the cytoplasm through interactions with 14-3-3 scaffolding proteins (reviewed by Tzivion et al., 2011). This stabilization can be disrupted by the phosphorylation of 14-3-3 by Jun kinase and the dephosphorylation of FoxO by protein phosphatase 2A. FoxO protein stability is also regulated by ERK1/2 (Mapk3/1), which phosphorylate FoxO proteins and target them for degradation (Yang et al., 2008).
Fig. 5.
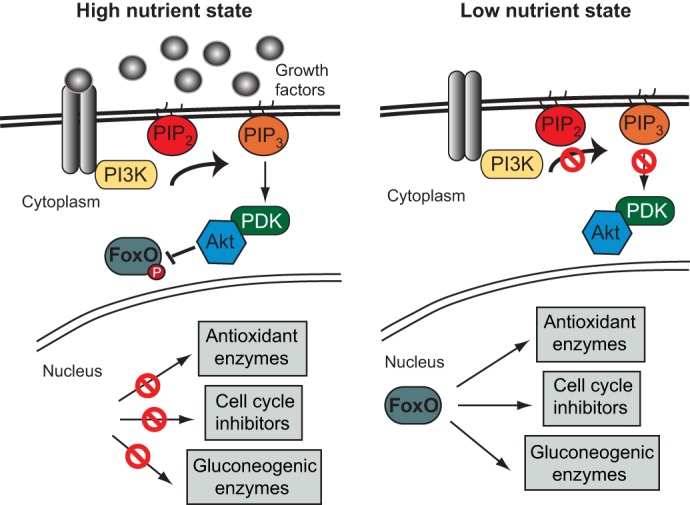
FoxO regulation in response to insulin signaling in mammals. The binding of growth factors to their receptors triggers a phosphorylation signaling cascade involving phosphoinositide 3-kinase (PI3K), phosphatidylinositol (4,5) bisphosphate (PIP2), phosphatidylinositol (3,4,5)-trisphosphate (PIP3), pyruvate dehydrogenase kinase (PDK) and Akt that ultimately results in the phosphorylation of FoxO. This phosphorylation confines FoxO to the cytoplasm. In the absence of growth factor signaling, including that of insulin, FoxO is thus present in the nucleus, where it upregulates anti-stress genes and cell cycle inhibitors. FoxO also regulates gluconeogenic genes in the mammalian liver to protect from hypoglycemia. Modified from Golson et al. (2010).
FoxO in C. elegans
The role of FoxO proteins in longevity was first revealed by studies in the nematode C. elegans. During periods of high stress, such as a low food supply, C. elegans enter a ‘dauer’ state that allows them to survive and also extend their lifespan (reviewed by Fielenbach and Antebi, 2008). In such times of nutrient shortage, expression of insulin receptor homologs such as DAF-2 is low. The sole C. elegans FoxO homolog, DAF-16, is therefore not phosphorylated and is thus able to enter the nucleus and transactivate its targets, a full list of which was recently published (Kaletsky et al., 2016). The effect of DAF-16 on longevity is primarily manifested in the intestine (Murphy et al., 2007; Libina et al., 2003), and its effects on lifespan are likely to be related to the regulation of genes involved in countering oxidative and thermal stress (Nemoto and Finkel, 2002).
DAF-16 also regulates multiple neuronal processes, including many different learning processes, sleep, morphology maintenance and axon regeneration (Kauffman et al., 2010; Tank et al., 2011; Toth et al., 2012; Murakami et al., 2005; Byrne et al., 2014; Nagy et al., 2014). A recent study delineated the neural targets of DAF-16 in C. elegans by comparing the transcriptomes of neurons that had been FACS sorted from wild type and insulin signaling-deficient (daf-16;daf-2) mutants (Kaletsky et al., 2016). Since many FoxO targets are conserved from C. elegans to humans (Webb et al., 2016), it has been proposed that understanding the mechanisms by which FoxO regulates aging in the nematode might provide a basis for extending human lifespan. However, given the fundamental differences in the aging process between these very distantly related species, caution is warranted.
FoxO in mammals
Mammals have four FoxO proteins: FoxO1, FoxO3, FoxO4 and FoxO6. FoxO6 shares the least sequence homology with other FoxO family members. FoxO1 is the most thoroughly studied of the family but, like FoxO1, FoxO3 and FoxO4 have roles in regulating lifespan and metabolism and in mediating cell cycle arrest.
Regulation of metabolism by FoxO1
FoxO1 is the most highly expressed FoxO family member in insulin-responsive tissues such as the liver, adipose tissue and skeletal muscle (Armoni et al., 2006). Consistent with this broad expression pattern, FoxO1 regulates multiple metabolic processes. In the liver, FoxO1 contributes to the activation of gluconeogenic targets (Nakae et al., 2002; Matsumoto et al., 2007; Titchenell et al., 2015; Haeusler et al., 2010), and deleting one copy of Foxo1 corrects the insulin resistance phenotype in insulin receptor-deficient mice (Nakae et al., 2002). In skeletal muscle, FoxO1 appears to control whether carbohydrates or lipids are used as fuel by regulating the expression of genes such as those encoding pyruvate dehydrogenase kinase and lipoprotein lipase (Bastie et al., 2005), while in adipose tissue it may protect white fat cells from the dysfunction associated with obesity (Subauste and Burant, 2007). FoxO1 is also a key player in regulating the differentiation of adipose tissue and skeletal muscle (Gross et al., 2008).
In addition to liver, muscle and adipose tissue, FoxO1 is highly expressed in insulin-secreting beta cells, where it may act in a protective capacity in times of stress. Several reports have indicated that FoxO proteins activate the expression of antioxidant enzymes, and FoxO1 protects beta cells against the effects of reactive oxygen species, since it and family members activate the expression of antioxidant enzymes (Kitamura et al., 2005; Kops et al., 2002; Nemoto and Finkel, 2002). FoxO1 has also been implicated in preventing beta cell de-differentiation during stress by suppressing neogenic genes, such as the early endocrine development factor neurogenin 3 (Neurog3), which is not normally expressed in the adult endocrine pancreas (Talchai et al., 2012). Protection by FoxO1 occurs in the presence of many metabolic stressors, such as multiple pregnancies, chemical ablation of beta cells, and when insulin resistance is conferred by leptin receptor (Lepr) deficiency. However, a contradictory study of mice deficient for Lepr in all tissues or for FoxO1 specifically in beta cells indicates that FoxO1 promotes the expression of the same genes (Kobayashi et al., 2012). The reason for this discrepancy is not apparent at this time, and both studies demonstrated more severe hyperglycemia in Lepr−/− mice lacking FoxO1 compared with those with FoxO1 expression. Previous work demonstrating the induction of the beta cell maturity marker MafA by FoxO1 also supports a role for FoxO1 in preventing de-differentiation (Kitamura et al., 2005). FoxO1 may thus protect beta cells from dysfunction during the period of insulin resistance that precedes type 2 diabetes.
A second role proposed for FoxO1 is in regulating beta cell mass. FoxO proteins have been shown to upregulate cell cycle inhibitors (Martins et al., 2015). In mice lacking one copy of the insulin receptor in all tissues, beta cell proliferation is reduced compared with that in wild-type mice, but deletion of one copy of Foxo1 partially rescues beta cell division (Nakae et al., 2002). Conversely, Foxo1 overexpression attenuates the increased beta cell mass normally observed in mice overexpressing the insulin receptor (Okamoto et al., 2006). However, contradictory reports have called the generality of these observations into question. Mice lacking Foxo1 either in beta cells or throughout the entire pancreas show no increase in beta cell proliferation either on a chow or high-fat diet (Kobayashi et al., 2012), and mice overexpressing FoxO1 fed a high-fat diet actually showed increased beta cell proliferation (Zhang et al., 2016). These results suggest that FoxO1 might inhibit beta cell replication only when the insulin receptor is completely absent, which is a unique and rare state.
FoxO in mammalian longevity
Like DAF-16 in C. elegans, FoxO transcription factors together with calorie restriction and insulin signaling have been reported to regulate lifespan in rodents (Mulvey et al., 2014; Shimokawa et al., 2015). For example, decreased insulin signaling correlates with human longevity (Kojima et al., 2004; Pawlikowska et al., 2009), as do several genetic variants in FOXO3 (Willcox et al., 2008; Pawlikowska et al., 2009; Flachsbart et al., 2009; Li et al., 2009). In mice, FoxO3, but not FoxO1, is required for an expanded lifespan due to calorie restriction (Shimokawa et al., 2015). However, a meta analysis calls this conclusion for calorie restriction into question (Swindell, 2012). First, in some mouse strains, life expectancy was actually shortened in cohorts with calorie restriction (Liao et al., 2010; Swindell, 2012). Second, ad libitum fed controls might not be an ideal comparison for rodents with calorie restriction; ad libitum access to normal chow leads to weight gain and associated detrimental health effects (Sohal and Forster, 2014). Mixed results have also been reported for non-human primates (Colman et al., 2009; Bodkin et al., 2003; Mattison et al., 2012), and studies correlating calorie restriction and longevity are hard to perform accurately in humans and thus far have been inconclusive (Heilbronn and Ravussin, 2003).
At present, the mechanisms by which FoxO3 or other FoxO transcription factors influence lifespan, if at all, in humans is still unknown. However, one could envision that the multiple processes in which FoxO plays a role, such as stress resistance, cell cycle regulation and control of apoptosis, could pleiotropically influence healthy aging.
The FoxP family: language acquisition and cognitive function
In mammals there are four FoxP genes (Foxp1-4), and the transcription factors that they encode have been shown to play a role in the development of multiple cell types, including cardiomyocytes (Wang et al., 2004), neurons (Shu et al., 2005), lung epithelial secretory cells (Li et al., 2012a) and regulatory T-cells (Fontenot et al., 2003). Accordingly, FoxP null mice display varying phenotypes. Foxp1−/− (Wang et al., 2004) and Foxp4−/− (Li et al., 2004b) mice display embryonic lethality by E14.5 due to a thinning of the cardiac ventricular myocardium. Foxp2−/− mice display severe cerebellar defects and motor impairment; they usually die by 3 weeks of age (Shu et al., 2005). Foxp3−/− mice have a defect in regulatory T-cells and escalated lymphocyte proliferation rates; these combined abnormalities lead to an immune attack on multiple cells and organs, including pancreatic beta cells, resulting in a disease resembling type 1 diabetes (Fontenot et al., 2003), and Foxp3−/− mice die by 4 weeks of age (Fontenot et al., 2003). Tissue-specific deletions of FoxP factors also result in developmental defects. For example, mice with a deletion of Foxp1 in neurons are viable but display a morphological change in the striatum and hippocampus (Bacon et al., 2015).
Members of the FoxP family, notably FoxP1 and FoxP2, have also generated interest because of their roles in regulating cognitive development processes such as speech acquisition. FoxP2 was the first of this transcription factor family to be associated with language deficits (Lai et al., 2001) but, more recently, FoxP1 has also been implicated in speech development (Horn et al., 2010; Hamdan et al., 2010). Mutations in both FOXP1 and FOXP2 are associated with other cognitive dysfunctions, such as autism spectrum disorder (ASD) and intellectual disability (Bacon and Rappold, 2012). However, disorders associated with the two genes do not have completely overlapping symptoms, indicating that the two transcription factors might regulate different but related brain functions. For example, language deficits caused by alterations in Foxp2 are generally accompanied by deficits in orofacial movements, whereas impairments in language acquisition associated with Foxp1 variants are always accompanied by another cognitive impairment (Bacon and Rappold, 2012). Currently, no known connections between cognitive function and Foxp3 or Foxp4 exist.
How FoxP1 and FoxP2 contribute to neural function has also been investigated in animal models. Normally, mouse pups subvocalize when separated from their mother; Foxp2−/− pups do not exhibit this behavior, and Foxp2+/− pups show a reduction (Shu et al., 2005). No alterations in vocalization were reported for mice with a brain-specific deletion of Foxp1; however, these mice exhibit abnormal cognitive and social behavior, echoing phenotypes in patients with FOXP1 mutations (Bacon et al., 2015).
In a striking example of evolutionary conservation, FoxP1 function has been studied in songbirds. Mouse subvocalization is innate, whereas human speech and birdsong share the necessity that they must be learned from older members of the species. After a FOXP2 mutation was identified in a family with inherited impaired speech acquisition, the expression patterns of Foxp1 and Foxp2 were investigated in zebra finch brains (Teramitsu et al., 2004). Similarities in the expression patterns of Foxp1 and Foxp2 were observed between songbird and human brains, and Foxp2 was upregulated during the period when adolescent finches actively learned song patterns (Haesler et al., 2007). When Foxp2 gene activity was suppressed during adolescence, the affected birds copied their tutors with less precision than controls. These studies highlight a remarkable conservation of function in Fox transcription factor function between disparate species.
Shared and cooperative roles for Fox transcription factors
Although Fox transcription factors have distinct expression patterns and are known for fulfilling different roles in regulating biological processes, many of these transcription factors often have similar roles. As mentioned above, FoxA and FoxO transcription factors both regulate glucose homeostasis and metabolism. However, FoxM1 (Golson et al., 2015) as well as FoxP1, FoxP2 and FoxP4 (Spaeth et al., 2015) were recently reported to contribute to these processes as well. Another overlap is the action of these proteins as pioneer factors; both FoxO (Hatta and Cirillo, 2007) and FoxE (Cuesta et al., 2007), in addition to members of the FoxA family, can act in this capacity.
Notably, a number of Fox transcription factors have been implicated in regulating cell proliferation. FoxM1 and FoxO, for example, have long been known to have opposing roles in the cell cycle and tumorigenesis. Many other Fox transcription factors, such as FoxC2, FoxE3, FoxF1, FoxF2, FoxN1 and FoxN3, also regulate proliferation (Tuteja and Kaestner, 2007b; Tuteja and Kaestner, 2007a). FoxA2 acts as a tumor suppressor by maintaining a differentiated state and decreasing metastasis in liver, lung and breast tissue (Tang et al., 2011; Wang et al., 2014b; Zhang et al., 2015), while FoxA1 acts as an oncogene in breast and prostate cancer (Yamaguchi et al., 2008; Imamura et al., 2012). Remarkably, in the liver, the FoxA proteins act as both repressors and promoters of hepatic carcinogenesis, dependent on the sex of the animals (Li et al., 2012c). Thus, while male mice deficient for both FoxA1 and FoxA2 develop fewer tumors, the situation is opposite in female mice, demonstrating the key role of FoxA factors in the action of the sex steroid hormone receptors.
Diverse Fox transcription factors also interact with estrogen receptor α (ERα; also known as ESR1) to regulate multiple aspects of tumorigenesis. In the MCF7 human breast cancer cell line, FOXA1 acts as a pioneer transcription factor and opens up chromatin, allowing the nearby binding of ERα (Hurtado et al., 2011). At cell cycle loci, FOXM1 can then displace FOXA1 and transactivate cell cycle genes. FOXM1 binds 70% of the same genes as FOXA1 in this breast cancer line (Sanders et al., 2013). Interestingly, FoxO transcription factors act as ERα status-dependent tumor suppressors in breast cancer. In ERα-positive breast cancer, FOXO3 is associated with less invasiveness, whereas in ERα-negative breast cancer, FOXO3 is associated with more invasive tumors (Sisci et al., 2013). Finally, the FoxA homolog PHA-4 contributes to increased longevity in C. elegans (Panowski et al., 2007). As with FoxO proteins, higher nutrient levels lead to the repression of PHA-4 activity and a decreased lifespan, and in this function FoxA is negatively regulated by the TOR pathway (Sheaffer et al., 2008).
Because many Fox transcription factors are required for life, they clearly are unable to compensate completely for each other. However, their highly related consensus binding sites suggest that they can substitute for each other to some degree. It is likely that some of the overlapping roles for Fox proteins reflect differences in expression patterns, such that one Fox protein might perform the same role in the gut that another performs in neural tissues. In other cases, it is likely that distinct Fox proteins act in somewhat divergent ways at the same loci, for example by recruiting different co-factors.
Conclusions
Fox transcription factors are remarkably well conserved DNA-binding proteins and regulators of gene expression. In mammals, diverse Fox factors share multiple roles. This ancient class of transcription factors contributes to the control of all aspects of development, from before gastrulation to the prevention of adult disease. Indeed, Fox transcription factors constitute a promising drug target considering their involvement in so many diseases. However, because they are transcription factors and hence are not accessible at the cell surface, or even generally in the cytoplasm, they have thus far largely remained unalterable, whether at the expression or activity level. Future studies to target Fox transcription factors should be aimed in the following directions: (1) the cell type-specific delivery of a drug payload that would be internalized; (2) gaining an understanding and then targeting of the regulatory mechanisms of Fox expression; (3) the identification and targeting of Fox target genes. Such approaches will, no doubt, further our understanding of Fox transcription factors in human biology and disease.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health (DK-P01-049120 to K.H.K.). Deposited in PMC for release after 12 months.
References
- Acharya M., Huang L., Fleisch V. C., Allison W. T. and Walter M. A. (2011). A complex regulatory network of transcription factors critical for ocular development and disease. Hum. Mol. Genet. 20, 1610-1624. 10.1093/hmg/ddr038 [DOI] [PubMed] [Google Scholar]
- Adams J., Palombella V. J., Sausville E. A., Johnson J., Destree A., Lazarus D. D., Maas J., Pien C. S., Prakash S. and Elliott P. J. (1999). Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 59, 2615-2622. [PubMed] [Google Scholar]
- Ang S.-L. and Rossant J. (1994). HNF-3 beta is essential for node and notochord formation in mouse development. Cell 78, 561-574. 10.1016/0092-8674(94)90522-3 [DOI] [PubMed] [Google Scholar]
- Ang S. L., Wierda A., Wong D., Stevens K. A., Cascio S., Rossant J. and Zaret K. S. (1993). The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development 119, 1301-1315. [DOI] [PubMed] [Google Scholar]
- Ao W., Gaudet J., Kent W. J., Muttumu S. and Mango S. E. (2004). Environmentally induced foregut remodeling by PHA-4/FoxA and DAF-12/NHR. Science 305, 1743-1746. 10.1126/science.1102216 [DOI] [PubMed] [Google Scholar]
- Aoki R., Shoshkes-Carmel M., Gao N., Shin S., May C. L., Golson M. L., Zahm A. M., Ray M., Wiser C. L., Wright C. V. E. et al. (2016). Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2, 175-188. 10.1016/j.jcmgh.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armoni M., Harel C., Karni S., Chen H., Bar-Yoseph F., Ver M. R., Quon M. J. and Karnieli E. (2006). FOXO1 represses peroxisome proliferator-activated receptor-Gamma1 and -Gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J. Biol. Chem. 281, 19881-19891. 10.1074/jbc.M600320200 [DOI] [PubMed] [Google Scholar]
- Bacon C. and Rappold G. A. (2012). The distinct and overlapping phenotypic spectra of FOXP1 and FOXP2 in cognitive disorders. Hum. Genet. 131, 1687-1698. 10.1007/s00439-012-1193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C., Schneider M., Le Magueresse C., Froehlich H., Sticht C., Gluch C., Monyer H. and Rappold G. A. (2015). Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol. Psychiatry 20, 632-639. 10.1038/mp.2014.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastie C. C., Nahle Z., McLoughlin T., Esser K., Zhang W., Unterman T. and Abumrad N. A. (2005). FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J. Biol. Chem. 280, 14222-14229. 10.1074/jbc.M413625200 [DOI] [PubMed] [Google Scholar]
- Behr R., Brestelli J., Fulmer J. T., Miyawaki N., Kleyman T. R. and Kaestner K. H. (2004). Mild nephrogenic diabetes insipidus caused by Foxa1 deficiency. J. Biol. Chem. 279, 41936-41941. 10.1074/jbc.M403354200 [DOI] [PubMed] [Google Scholar]
- Besnard V., Wert S. E., Hull W. M. and Whitsett J. A. (2004). Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr. Patterns 5, 193-208. 10.1016/j.modgep.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Bodkin N. L., Alexander T. M., Ortmeyer H. K., Johnson E. and Hansen B. C. (2003). Mortality and morbidity in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J. Gerontol. A Biol. Sci. Med. Sci. 58, B212-B219. 10.1093/gerona/58.3.B212 [DOI] [PubMed] [Google Scholar]
- Bolte C., Zhang Y., Wang I.-C., Kalin T. V., Molkentin J. D. and Kalinichenko V. V. (2011). Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PLoS ONE 6, e22217 10.1371/journal.pone.0022217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers J. M. and Konopka G. (2012). The role of the FOXP family of transcription factors in ASD. Dis. Markers 33, 251-260. 10.1155/2012/456787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig N. C., Everett L. J., Schug J., Dorrell C., Liu C., Luo Y., Streeter P. R., Naji A., Grompe M. and Kaestner K. H. (2013). Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J. Clin. Invest. 123, 1275-1284. 10.1172/JCI66514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezillon N., Lambert-Blot M., Morosan S., Couton D., Mitchell C., Kremsdorf D., Costa R. H., Gilgenkrantz H. and Guidotti J.-E. (2007). Transplanted hepatocytes over-expressing FoxM1B efficiently repopulate chronically injured mouse liver independent of donor age. Mol. Ther. 15, 1710-1715. 10.1038/sj.mt.6300232 [DOI] [PubMed] [Google Scholar]
- Byrne A. B., Walradt T., Gardner K. E., Hubbert A., Reinke V. and Hammarlund M. (2014). Insulin/IGF1 signaling inhibits age-dependent axon regeneration. Neuron 81, 561-573. 10.1016/j.neuron.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaca J. M., Donahue G., Becker J. S., He X., Vinson C. and Zaret K. S. (2013). Bookmarking by specific and nonspecific binding of Foxa1 pioneer factor to mitotic chromosomes. Genes Dev. 27, 251-260. 10.1101/gad.206458.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. S., Liu X. S., Brodsky A. S., Li W., Meyer C. A., Szary A. J., Eeckhoute J., Shao W., Hestermann E. V., Geistlinger T. R. et al. (2005). Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33-43. 10.1016/j.cell.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Chen X., Muller G. A., Quaas M., Fischer M., Han N., Stutchbury B., Sharrocks A. D. and Engeland K. (2013). The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Mol. Cell. Biol. 33, 227-236. 10.1128/MCB.00881-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo L. A., Lin F. R., Cuesta I., Friedman D., Jarnik M. and Zaret K. S. (2002). Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and Gata-4. Mol. Cell 9, 279-289. 10.1016/S1097-2765(02)00459-8 [DOI] [PubMed] [Google Scholar]
- Clark K. L., Halay E. D., Lai E. and Burley S. K. (1993). Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 364, 412-420. 10.1038/364412a0 [DOI] [PubMed] [Google Scholar]
- Colman R. J., Anderson R. M., Johnson S. C., Kastman E. K., Kosmatka K. J., Beasley T. M., Allison D. B., Cruzen C., Simmons H. A., Kemnitz J. W. et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201-204. 10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R. and Darnell J. E. Jr (1989). Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol. Cell. Biol. 9, 1415-1425. 10.1128/MCB.9.4.1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta I., Zaret K. S. and Santisteban P. (2007). The forkhead factor FoxE1 binds to the thyroperoxidase promoter during thyroid cell differentiation and modifies compacted chromatin structure. Mol. Cell. Biol. 27, 7302-7314. 10.1128/MCB.00758-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraff D. J., Grabowska M. M., Case T. C., Yu X., Herrick M. K., Hayward W. J., Strand D. W., Cates J. M., Hayward S. W., Gao N. et al. (2014). FOXA1 deletion in luminal epithelium causes prostatic hyperplasia and alteration of differentiated phenotype. Lab. Invest. 94, 726-739. 10.1038/labinvest.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N. and Antebi A. (2008). C. elegans Dauer formation and the molecular basis of plasticity. Genes Dev. 22, 2149-2165. 10.1101/gad.1701508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F., Caliebe A., Kleindorp R., Blanche H., von Eller-Eberstein H., Nikolaus S., Schreiber S. and Nebel A. (2009). Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. USA 106, 2700-2705. 10.1073/pnas.0809594106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J. D., Gavin M. A. and Rudensky A. Y. (2003). Foxp3 programs the development and function of Cd4+Cd25+ regulatory T cells. Nat. Immunol. 4, 330-336. 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Friedman J. R. and Kaestner K. H. (2006). The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. 63, 2317-2328. 10.1007/s00018-006-6095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., Zhang J., Rao M. A., Case T. C., Mirosevich J., Wang Y., Jin R., Gupta A., Rennie P. S. and Matusik R. J. (2003). The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol. Endocrinol. 17, 1484-1507. 10.1210/me.2003-0020 [DOI] [PubMed] [Google Scholar]
- Gao N., Ishii K., Mirosevich J., Kuwajima S., Oppenheimer S. R., Roberts R. L., Jiang M., Yu X., Shappell S. B., Caprioli R. M. et al. (2005). Forkhead Box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development 132, 3431-3443. 10.1242/dev.01917 [DOI] [PubMed] [Google Scholar]
- Gao N., LeLay J., Vatamaniuk M. Z., Rieck S., Friedman J. R. and Kaestner K. H. (2008). Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 22, 3435-3448. 10.1101/gad.1752608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel A. L. (2010). A new target for proteasome inhibitors: Foxm1. Expert Opin. Investig. Drugs 19, 235-242. 10.1517/13543780903563364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet J. and Mango S. E. (2002). Regulation of organogenesis by the Caenorhabditis elegans Foxa protein PHA-4. Science 295, 821-825. 10.1126/science.1065175 [DOI] [PubMed] [Google Scholar]
- Golson M. L., Misfeldt A. A., Kopsombut U. G., Petersen C. P. and Gannon M. (2010). High fat diet regulation of beta-cell proliferation and beta-cell mass. Open Endocrinol. J. 4, 66-77. 10.2174/1874216501004010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golson M. L., Dunn J. C., Maulis M. F., Dadi P. K., Osipovich A. B., Magnuson M. A., Jacobson D. A. and Gannon M. (2015). Activation of FoxM1 revitalizes the replicative potential of aged beta-cells in male mice and enhances insulin secretion. Diabetes 64, 3829-3838. 10.2337/db15-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormally M. V., Dexheimer T. S., Marsico G., Sanders D. A., Lowe C., Matak-Vinković D., Michael S., Jadhav A., Rai G., Maloney D. J. et al. (2014). Suppression of the FOXM1 transcriptional programme via novel small molecule inhibition. Nat. Commun. 5, 5165 10.1038/ncomms6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. N., van den Heuvel A. P. J. and Birnbaum M. J. (2008). The role of FoxO in the regulation of metabolism. Oncogene 27, 2320-2336. 10.1038/onc.2008.25 [DOI] [PubMed] [Google Scholar]
- Gusarova G. A., Wang I.-C., Major M. L., Kalinichenko V. V., Ackerson T., Petrovic V. and Costa R. H. (2007). A cell-penetrating ARF peptide inhibitor of FoxM1 in mouse hepatocellular carcinoma treatment. J. Clin. Invest. 117, 99-111. 10.1172/JCI27527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashy H. O., Powe D. G., Rakha E. A., Ball G., Paish C., Gee J., Nicholson R. I. and Ellis I. O. (2008). Forkhead-box A1 (Foxa1) expression in breast cancer and its prognostic significance. Eur. J. Cancer 44, 1541-1551. 10.1016/j.ejca.2008.04.020 [DOI] [PubMed] [Google Scholar]
- Haesler S., Rochefort C., Georgi B., Licznerski P., Osten P. and Scharff C. (2007). Incomplete and inaccurate vocal imitation after knockdown of Foxp2 in songbird basal ganglia nucleus area X. PLoS Biol. 5, e321 10.1371/journal.pbio.0050321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler R. A., Kaestner K. H. and Accili D. (2010). FoxOs function synergistically to promote glucose production. J. Biol. Chem. 285, 35245-35248. 10.1074/jbc.C110.175851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasi M. and Gartel A. L. (2012). Suppression of FOXM1 sensitizes human cancer cells to cell death induced by DNA-damage. PLoS ONE 7, e31761 10.1371/journal.pone.0031761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasi M. and Gartel A. L. (2013). FOX(M1) news--it is cancer. Mol. Cancer Ther. 12, 245-254. 10.1158/1535-7163.MCT-12-0712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasi M., Pandit B., Wang M., Nogueira V., Hay N. and Gartel A. L. (2013). Combination of oxidative stress and FOXM1 inhibitors induces apoptosis in cancer cells and inhibits Xenograft tumor growth. Am. J. Pathol. 183, 257-265. 10.1016/j.ajpath.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmos B., Basseres D. S., Monti S., D'alo F., Dayaram T., Ferenczi K., Wouters B. J., Huettner C. S., Golub T. R. and Tenen D. G. (2004). A transcriptional profiling study of CCAAT/enhancer binding protein targets identifies hepatocyte nuclear factor 3 beta as a novel tumor suppressor in lung cancer. Cancer Res. 64, 4137-4147. 10.1158/0008-5472.CAN-03-4052 [DOI] [PubMed] [Google Scholar]
- Hamdan F. F., Daoud H., Rochefort D., Piton A., Gauthier J., Langlois M., Foomani G., Dobrzeniecka S., Krebs M.-O., Joober R. et al. (2010). De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am. J. Hum. Genet. 87, 671-678. 10.1016/j.ajhg.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannenhalli S. and Kaestner K. H. (2009). The evolution of Fox genes and their role in development and disease. Nat. Rev. Genet. 10, 233-240. 10.1038/nrg2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta M. and Cirillo L. A. (2007). Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J. Biol. Chem. 282, 35583-35593. 10.1074/jbc.M704735200 [DOI] [PubMed] [Google Scholar]
- Heilbronn L. K. and Ravussin E. (2003). Calorie restriction and aging: review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 78, 361-369. [DOI] [PubMed] [Google Scholar]
- Horn D., Kapeller J., Rivera-Brugués N., Moog U., Lorenz-Depiereux B., Eck S., Hempel M., Wagenstaller J., Gawthrope A., Monaco A. P. et al. (2010). Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum. Mutat. 31, E1851-E1860. 10.1002/humu.21362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-T., Chen H.-M., Yang Z., Wang J., Lee N. K., Burger A., Zaret K., Liu T., Levine E. and Mango S. E. (2015). Recruitment of RNA polymerase II by the pioneer transcription factor PHA-4. Science 348, 1372-1376. 10.1126/science.aab1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado A., Holmes K. A., Ross-Innes C. S., Schmidt D. and Carroll J. S. (2011). FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat. Genet. 43, 27-33. 10.1038/ng.730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y., Sakamoto S., Endo T., Utsumi T., Fuse M., Suyama T., Kawamura K., Imamoto T., Yano K., Uzawa K. et al. (2012). FOXA1 promotes tumor progression in prostate cancer via the insulin-like growth factor binding protein 3 pathway. PLoS ONE 7, e42456 10.1371/journal.pone.0042456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M. and Zaret K. S. (2016). Cell fate control by pioneer transcription factors. Development 143, 1833-1837. 10.1242/dev.133900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M., Donahue G., Kakumanu A., Watts J. A., Mahony S., Pugh B. F., Lee D., Kaestner K. H. and Zaret K. S. (2016). The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol. Cell 62, 79-91. 10.1016/j.molcel.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K., Gleiberman A. S., Shi C., Simon D. I. and Rosenfeld M. G. (2008). Cooperative regulation in development by SMRT and FOXP1. Genes Dev. 22, 740-745. 10.1101/gad.1637108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Wade M., Nakjang S., Chaytor L., Grey J., Robson C. N. and Gaughan L. (2015). FOXA1 regulates androgen receptor variant activity in models of castrate-resistant prostate cancer. Oncotarget 6, 29782-29794. 10.18632/oncotarget.4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Hiemisch H., Luckow B. and Schütz G. (1994). The HNF-3 gene family of transcription factors in mice: gene structure, cDNA sequence, and mRNA distribution. Genomics 20, 377-385. 10.1006/geno.1994.1191 [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Katz J., Liu Y., Drucker D. J. and Schutz G. (1999). Inactivation of the winged helix transcription factor HNF3ALPHA affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 13, 495-504. 10.1101/gad.13.4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R., Lakhina V., Arey R., Williams A., Landis J., Ashraf J. and Murphy C. T. (2016). The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature 529, 92-96. 10.1038/nature16483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin T. V., Wang I.-C., Meliton L., Zhang Y., Wert S. E., Ren X., Snyder J., Bell S. M., Graf L. Jr, Whitsett J. A. et al. (2008). Forkhead Box M1 transcription factor is required for perinatal lung function. Proc. Natl. Acad. Sci. USA 105, 19330-19335. 10.1073/pnas.0806748105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichenko V. V., Gusarova G. A., Tan Y., Wang I.-C., Major M. L., Wang X., Yoder H. M. and Costal R. H. (2003). Ubiquitous expression of the Forkhead Box M1b transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J. Biol. Chem. 278, 37888-37894. 10.1074/jbc.M305555200 [DOI] [PubMed] [Google Scholar]
- Kalinina O. A., Kalinin S. A., Polack E. W., Mikaelian I., Panda S., Costa R. H. and Adami G. R. (2003). Sustained hepatic expression of FoxM1B in transgenic mice has minimal effects on hepatocellular carcinoma development but increases cell proliferation rates in preneoplastic and early neoplastic lesions. Oncogene 22, 6266-6276. 10.1038/sj.onc.1206640 [DOI] [PubMed] [Google Scholar]
- Kauffman A. L., Ashraf J. M., Corces-Zimmerman M. R., Landis J. N. and Murphy C. T. (2010). Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol. 8, e1000372 10.1371/journal.pbio.1000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y. I., Kitamura T., Kruse J.-P., Raum J. C., Stein R., Gu W. and Accili D. (2005). Foxo1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2, 153-163. 10.1016/j.cmet.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Kittappa R., Chang W. W., Awatramani R. B. and McKay R. D. G. (2007). The Foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 5, e325 10.1371/journal.pbio.0050325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Kikuchi O., Sasaki T., Kim H.-J., Yokota-Hashimoto H., Lee Y.-S., Amano K., Kitazumi T., Susanti V. Y., Kitamura Y. I. et al. (2012). FoxO1 as a double-edged sword in the pancreas: analysis of pancreas- and beta-cell-specific Foxo1 knockout mice. Am. J. Physiol. Endocrinol. Metab. 302, E603-E613. 10.1152/ajpendo.00469.2011 [DOI] [PubMed] [Google Scholar]
- Kojima T., Kamei H., Aizu T., Arai Y., Takayama M., Nakazawa S., Ebihara Y., Inagaki H., Masui Y., Gondo Y. et al. (2004). Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp. Gerontol. 39, 1595-1598. 10.1016/j.exger.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Kops G. J. P. L., Dansen T. B., Polderman P. E., Saarloos I., Wirtz K. W. A., Coffer P. J., Huang T.-T., Bos J. L., Medema R. H. and Burgering B. M. T. (2002). Forkhead transcription factor Foxo3a protects quiescent cells from oxidative stress. Nature 419, 316-321. 10.1038/nature01036 [DOI] [PubMed] [Google Scholar]
- Korver W., Roose J. and Clevers H. (1997). The winged-helix transcription factor trident is expressed in cycling cells. Nucleic Acids Res. 25, 1715-1719. 10.1093/nar/25.9.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupczak-Hollis K., Wang X., Kalinichenko V. V., Gusarova G. A., Wang I.-C., Dennewitz M. B., Yoder H. M., Kiyokawa H., Kaestner K. H. and Costa R. H. (2004). The mouse Forkhead Box M1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev. Biol. 276, 74-88. 10.1016/j.ydbio.2004.08.022 [DOI] [PubMed] [Google Scholar]
- Lai C. S. L., Fisher S. E., Hurst J. A., Vargha-Khadem F. and Monaco A. P. (2001). A Forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519-523. 10.1038/35097076 [DOI] [PubMed] [Google Scholar]
- Lal G. and Bromberg J. S. (2009). Epigenetic mechanisms of regulation of Foxp3 expression. Blood 114, 3727-3735. 10.1182/blood-2009-05-219584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz K. A., Vatamaniuk M. Z., Brestelli J. E., Friedman J. R., Matschinsky F. M. and Kaestner K. H. (2004). Foxa2 regulates multiple pathways of insulin secretion. J. Clin. Invest. 114, 512-520. 10.1172/JCI21149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoukili J., Stahl M. and Medema R. H. (2007). Foxm1: at the crossroads of ageing and cancer. Biochim. Biophys. Acta 1775, 92-102. 10.1016/j.bbcan.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Lee C. S., Friedman J. R., Fulmer J. T. and Kaestner K. H. (2005a). The initiation of liver development is dependent on Foxa transcription factors. Nature 435, 944-947. 10.1038/nature03649 [DOI] [PubMed] [Google Scholar]
- Lee C. S., Sund N. J., Behr R., Herrera P. L. and Kaestner K. H. (2005b). Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev. Biol. 278, 484-495. 10.1016/j.ydbio.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Li S., Weidenfeld J. and Morrisey E. E. (2004a). Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol. Cell. Biol. 24, 809-822. 10.1128/MCB.24.2.809-822.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhou D., Lu M. M. and Morrisey E. E. (2004b). Advanced cardiac morphogenesis does not require heart tube fusion. Science 305, 1619-1622. 10.1126/science.1098674 [DOI] [PubMed] [Google Scholar]
- Li B., Samanta A., Song X., Iacono K. T., Bembas K., Tao R., Basu S., Riley J. L., Hancock W. W., Shen Y. et al. (2007). Foxp3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. USA 104, 4571-4576. 10.1073/pnas.0700298104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang W.-J., Cao H., Lu J., Wu C., Hu F.-Y., Guo J., Zhao L., Yang F., Zhang Y.-X. et al. (2009). Genetic association of Foxo1a and Foxo3a with longevity trait in Han Chinese populations. Hum. Mol. Genet. 18, 4897-4904. 10.1093/hmg/ddp459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Schug J., Tuteja G., White P. and Kaestner K. H. (2011). The nucleosome map of the mammalian liver. Nat. Struct. Mol. Biol. 18, 742-746. 10.1038/nsmb.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang Y., Zhang Y., Lu M. M., DeMayo F. J., Dekker J. D., Tucker P. W. and Morrisey E. E. (2012a). Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development 139, 2500-2509. 10.1242/dev.079699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Gadue P., Chen K., Jiao Y., Tuteja G., Schug J., Li W. and Kaestner K. H. (2012b). Foxa2 and H2a.Z mediate nucleosome depletion during embryonic stem cell differentiation. Cell 151, 1608-1616. 10.1016/j.cell.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Tuteja G., Schug J. and Kaestner K. H. (2012c). Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 148, 72-83. 10.1016/j.cell.2011.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. M.-C., Gocheva V., Oudin M. J., Bhutkar A., Wang S. Y., Date S. R., Ng S. R., Whittaker C. A., Bronson R. T., Snyder E. L. et al. (2015a). Foxa2 and Cdx2 cooperate with Nkx2-1 to inhibit lung adenocarcinoma metastasis. Genes Dev. 29, 1850-1862. 10.1101/gad.267393.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang W., Wang J., Malovannaya A., Xi Y., Li W., Guerra R., Hawke D. H., Qin J. and Chen J. (2015b). Proteomic analyses reveal distinct chromatin-associated and soluble transcription factor complexes. Mol. Syst. Biol. 11, 775 10.15252/msb.20145504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.-Y., Rikke B. A., Johnson T. E., Diaz V. and Nelson J. F. (2010). Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92-95. 10.1111/j.1474-9726.2009.00533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N., Berman J. R. and Kenyon C. (2003). Tissue-specific activities of C. elegans Daf-16 in the regulation of lifespan. Cell 115, 489-502. 10.1016/S0092-8674(03)00889-4 [DOI] [PubMed] [Google Scholar]
- Littler D. R., Alvarez-Fernandez M., Stein A., Hibbert R. G., Heidebrecht T., Aloy P., Medema R. H. and Perrakis A. (2010). Structure of the FoxM1 DNA-recognition domain bound to a promoter sequence. Nucleic Acids Res. 38, 4527-4538. 10.1093/nar/gkq194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M., Eeckhoute J., Meyer C. A., Wang Q., Zhang Y., Li W., Carroll J. S., Liu X. S. and Brown M. (2008). FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132, 958-970. 10.1016/j.cell.2008.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R., Lithgow G. J. and Link W. (2015). Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell 15, 196-207. 10.1111/acel.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Pocai A., Rossetti L., DePinho R. A. and Accili D. (2007). Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 6, 208-216. 10.1016/j.cmet.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Mattison J. A., Roth G. S., Beasley T. M., Tilmont E. M., Handy A. M., Herbert R. L., Longo D. L., Allison D. B., Young J. E., Bryant M. et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318-321. 10.1038/nature11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer F. and Unutmaz D. (2009). The biology of FoxP3: a key player in immune suppression during infections, autoimmune diseases and cancer. Adv. Exp. Med. Biol. 665, 47-59. 10.1007/978-1-4419-1599-3_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan A. P., Kaestner K. H., Grau E. and Schutz G. (1993). Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development 119, 567-578. [DOI] [PubMed] [Google Scholar]
- Mulvey L., Sinclair A. and Selman C. (2014). Lifespan modulation in mice and the confounding effects of genetic background. J. Genet. Genomics 41, 497-503. 10.1016/j.jgg.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Bessinger K., Hellmann J. and Murakami S. (2005). Aging-dependent and -independent modulation of associative learning behavior by insulin/insulin-like growth factor-1 signal in Caenorhabditis elegans. J. Neurosci. 25, 10894-10904. 10.1523/JNEUROSCI.3600-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. T., Lee S.-J. and Kenyon C. (2007). Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104, 19046-19050. 10.1073/pnas.0709613104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy S., Tramm N., Sanders J., Iwanir S., Shirley I. A., Levine E. and Biron D. (2014). Homeostasis in C. elegans sleep is characterized by two behaviorally and genetically distinct mechanisms. Elife 3, e04380 10.7554/eLife.04380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J., Biggs W. H. III, Kitamura T., Cavenee W. K., Wright C. V. E., Arden K. C. and Accili D. (2002). Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 32, 245-253. 10.1038/ng890 [DOI] [PubMed] [Google Scholar]
- Nemoto S. and Finkel T. (2002). Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295, 2450-2452. 10.1126/science.1069004 [DOI] [PubMed] [Google Scholar]
- Okamoto H., Hribal M. L., Lin H. V., Bennett W. R., Ward A. and Accili D. (2006). Role of the forkhead protein Foxo1 in beta cell compensation to insulin resistance. J. Clin. Invest. 116, 775-782. 10.1172/JCI24967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski S. H., Wolff S., Aguilaniu H., Durieux J. and Dillin A. (2007). PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447, 550-555. 10.1038/nature05837 [DOI] [PubMed] [Google Scholar]
- Pawlikowska L., Hu D., Huntsman S., Sung A., Chu C., Chen J., Joyner A. H., Schork N. J., Hsueh W.-C., Reiner A. P. et al. (2009). Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8, 460-472. 10.1111/j.1474-9726.2009.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl B. S. and Knöchel W. (2005). Of Fox and frogs: Fox (fork head/winged helix) transcription factors in Xenopus development. Gene 344, 21-32. 10.1016/j.gene.2004.09.037 [DOI] [PubMed] [Google Scholar]
- Ren H., Zhang P., Tang Y., Wu M. and Zhang W. (2015). Forkhead box protein A1 is a prognostic predictor and promotes tumor growth of gastric cancer. Onco Targets Ther. 8, 3029-3039. 10.2147/OTT.S91035 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Riedel C. G., Dowen R. H., Lourenco G. F., Kirienko N. V., Heimbucher T., West J. A., Bowman S. K., Kingston R. E., Dillin A., Asara J. M. et al. (2013). Daf-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat. Cell Biol. 15, 491-501. 10.1038/ncb2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes C. S., Stark R., Teschendorff A. E., Holmes K. A., Ali H. R., Dunning M. J., Brown G. D., Gojis O., Ellis I. O., Green A. R. et al. (2012). Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389-393. 10.1038/nature10730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett S. D., Li Z., Hurtt R., Gao Y., Wells R. G., Brondell K., Kaestner K. H. and Greenbaum L. E. (2009). Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 49, 920-929. 10.1002/hep.22705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D. A., Ross-Innes C. S., Beraldi D., Carroll J. S. and Balasubramanian S. (2013). Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol. 14, R6 10.1186/gb-2013-14-1-r6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer K. L., Updike D. L. and Mango S. E. (2008). The target of rapamycin pathway antagonizes PHA-4/FoxA to control development and aging. Curr. Biol. 18, 1355-1364. 10.1016/j.cub.2008.07.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Scearce L. M., Brestelli J. E., Sund N. J. and Kaestner K. H. (2001). Foxa3 (hepatocyte nuclear factor 3gamma) is required for the regulation of hepatic GLUT2 expression and the maintenance of glucose homeostasis during a prolonged fast. J. Biol. Chem. 276, 42812-42817. 10.1074/jbc.M106344200 [DOI] [PubMed] [Google Scholar]
- Shimokawa I., Komatsu T., Hayashi N., Kim S.-E., Kawata T., Park S., Hayashi H., Yamaza H., Chiba T. and Mori R. (2015). The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell 14, 707-709. 10.1111/acel.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W., Yang H., Zhang L., Lu M. M. and Morrisey E. E. (2001). Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J. Biol. Chem. 276, 27488-27497. 10.1074/jbc.M100636200 [DOI] [PubMed] [Google Scholar]
- Shu W., Cho J. Y., Jiang Y., Zhang M., Weisz D., Elder G. A., Schmeidler J., De Gasperi R., Sosa M. A. G., Rabidou D. et al. (2005). Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc. Natl. Acad. Sci. USA 102, 9643-9648. 10.1073/pnas.0503739102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. P., von Meyenn F., Howell J., Thorens B., Wolfrum C. and Stoffel M. (2009). Regulation of adaptive behaviour during fasting by hypothalamic Foxa2. Nature 462, 646-650. 10.1038/nature08589 [DOI] [PubMed] [Google Scholar]
- Sisci D., Maris P., Cesario M. G., Anselmo W., Coroniti R., Trombino G. E., Romeo F., Ferraro A., Lanzino M., Aquila S. et al. (2013). The estrogen receptor alpha is the key regulator of the bifunctional role of FoxO3a transcription factor in breast cancer motility and invasiveness. Cell Cycle 12, 3405-3420. 10.4161/cc.26421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S. and Forster M. J. (2014). Caloric restriction and the aging process: a critique. Free Radic. Biol. Med. 73, 366-382. 10.1016/j.freeradbiomed.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth J. M., Hunter C. S., Bonatakis L., Guo M., French C. A., Slack I., Hara M., Fisher S. E., Ferrer J., Morrisey E. E. et al. (2015). The FOXP1, FOXP2 and FOXP4 transcription factors are required for islet alpha cell proliferation and function in mice. Diabetologia 58, 1836-1844. 10.1007/s00125-015-3635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud J. C., Wu Y., Bates D. L., Han A., Nowick K., Paabo S., Tong H. and Chen L. (2006). Structure of the forkhead domain of FOXP2 bound to DNA. Structure 14, 159-166. 10.1016/j.str.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Subauste A. R. and Burant C. F. (2007). Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am. J. Physiol. Endocrinol. Metab. 293, E159-E164. 10.1152/ajpendo.00629.2006 [DOI] [PubMed] [Google Scholar]
- Sund N. J., Ang S.-L., Sackett S. D., Shen W., Daigle N., Magnuson M. A. and Kaestner K. H. (2000). Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol. Cell. Biol. 20, 5175-5183. 10.1128/MCB.20.14.5175-5183.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell W. R. (2012). Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res. Rev. 11, 254-270. 10.1016/j.arr.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Takahashi K. and Liu F.-C. (2009). Foxp genes, neural development, speech and language disorders. Adv. Exp. Med. Biol. 665, 117-129. 10.1007/978-1-4419-1599-3_9 [DOI] [PubMed] [Google Scholar]
- Talchai C., Xuan S., Lin H. V., Sussel L. and Accili D. (2012). Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223-1234. 10.1016/j.cell.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Shu G., Yuan X., Jing N. and Song J. (2011). FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res. 21, 316-326. 10.1038/cr.2010.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank E. M. H., Rodgers K. E. and Kenyon C. (2011). Spontaneous age-related neurite branching in Caenorhabditis elegans. J. Neurosci. 31, 9279-9288. 10.1523/JNEUROSCI.6606-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I., Kudo L. C., London S. E., Geschwind D. H. and White S. A. (2004). Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J. Neurosci. 24, 3152-3163. 10.1523/JNEUROSCI.5589-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titchenell P. M., Chu Q., Monks B. R. and Birnbaum M. J. (2015). Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat. Commun. 6, 7078 10.1038/ncomms8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M. L., Melentijevic I., Shah L., Bhatia A., Lu K., Talwar A., Naji H., Ibanez-Ventoso C., Ghose P., Jevince A. et al. (2012). Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans nervous system. J. Neurosci. 32, 8778-8790. 10.1523/JNEUROSCI.1494-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja G. and Kaestner K. H. (2007a). SnapShot: forkhead transcription factors II. Cell 131, 192 10.1016/j.cell.2007.09.016 [DOI] [PubMed] [Google Scholar]
- Tuteja G. and Kaestner K. H. (2007b). Snapshot: forkhead transcription factors I. Cell 130, 1160 10.1016/j.cell.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Tzivion G., Dobson M. and Ramakrishnan G. (2011). FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta 1813, 1938-1945. 10.1016/j.bbamcr.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Updike D. L. and Mango S. E. (2006). Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA. PLoS Genet. 2, e161 10.1371/journal.pgen.0020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustiyan V., Wang I.-C., Ren X., Zhang Y., Snyder J., Xu Y., Wert S. E., Lessard J. L., Kalin T. V. and Kalinichenko V. V. (2009). Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus. Dev. Biol. 336, 266-279. 10.1016/j.ydbio.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis M., Vincent A., Bouma J., Korteland-Van Male A., Van Goudoever J. B., Renes I. B. and Van Seuningen I. (2008). Forkhead box transcription factors Foxa1 and Foxa2 are important regulators of Muc2 mucin expression in intestinal epithelial cells. Biochem. Biophys. Res. Commun. 369, 1108-1113. 10.1016/j.bbrc.2008.02.158 [DOI] [PubMed] [Google Scholar]
- Wan H., Kaestner K. H., Ang S.-L., Ikegami M., Finkelman F. D., Stahlman M. T., Fulkerson P. C., Rothenberg M. E. and Whitsett J. A. (2004). Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131, 953-964. 10.1242/dev.00966 [DOI] [PubMed] [Google Scholar]
- Wang M. and Gartel A. L. (2011). The suppression of FoxM1 and its targets in breast cancer xenograft tumors by siRNA. Oncotarget 2, 1218-1226. 10.18632/oncotarget.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Krupczak-Hollis K., Tan Y., Dennewitz M. B., Adami G. R. and Costa R. H. (2002). Increased hepatic forkhead box M1b (Foxm1b) levels in old-aged mice stimulated liver regeneration through diminished P27Kip1 protein levels and increased Cdc25B expression. J. Biol. Chem. 277, 44310-44316. 10.1074/jbc.M207510200 [DOI] [PubMed] [Google Scholar]
- Wang B., Lin D., Li C. and Tucker P. (2003). Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J. Biol. Chem. 278, 24259-24268. 10.1074/jbc.M207174200 [DOI] [PubMed] [Google Scholar]
- Wang B., Weidenfeld J., Lu M. M., Maika S., Kuziel W. A., Morrisey E. E. and Tucker P. W. (2004). Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development 131, 4477-4487. 10.1242/dev.01287 [DOI] [PubMed] [Google Scholar]
- Wang I.-C., Zhang Y., Snyder J., Sutherland M. J., Burhans M. S., Shannon J. M., Park H. J., Whitsett J. A. and Kalinichenko V. V. (2010). Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev. Biol. 347, 301-314. 10.1016/j.ydbio.2010.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I.-C., Ustiyan V., Zhang Y., Cai Y., Kalin T. V. and Kalinichenko V. V. (2014a). Foxm1 transcription factor is required for the initiation of lung tumorigenesis by oncogenic Kras(G12D.). Oncogene 33, 5391-5396. 10.1038/onc.2013.475 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhu C.-P., Hu P.-F., Qian H., Ning B.-F., Zhang Q., Chen F., Liu J., Shi B., Zhang X. et al. (2014b). Foxa2 suppresses the metastasis of hepatocellular carcinoma partially through matrix metalloproteinase-9 inhibition. Carcinogenesis 35, 2576-2583. 10.1093/carcin/bgu180 [DOI] [PubMed] [Google Scholar]
- Webb A. E., Kundaje A. and Brunet A. (2016). Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15, 673-685. 10.1111/acel.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Küttner F., Seifert E. and Jäckle H. (1989). The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 57, 645-658. 10.1016/0092-8674(89)90133-5 [DOI] [PubMed] [Google Scholar]
- Weinstein D. C., Ruiz I Altaba A., Chen W. S., Hoodless P., Prezioso V. R., Jessell T. M. and Darnell J. E. Jr. (1994). The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell 78, 575-588. 10.1016/0092-8674(94)90523-1 [DOI] [PubMed] [Google Scholar]
- Wierstra I. (2013). The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv. Cancer Res. 118, 97-398. 10.1016/B978-0-12-407173-5.00004-2 [DOI] [PubMed] [Google Scholar]
- Wierstra I. and Alves J. (2007). FOXM1, a typical proliferation-associated transcription factor. Biol. Chem. 388, 1257-1274. 10.1515/bc.2007.159 [DOI] [PubMed] [Google Scholar]
- Willcox B. J., Donlon T. A., He Q., Chen R., Grove J. S., Yano K., Masaki K. H., Willcox D. C., Rodriguez B. and Curb J. D. (2008). FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 105, 13987-13992. 10.1073/pnas.0801030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.-F., Liu C., Tai M.-H., Liu D., Lei L., Wang R.-T., Tian M. and Lü Y. (2010). Knockdown of Foxm1 by siRNA interference decreases cell proliferation, induces cell cycle arrest and inhibits cell invasion in MHCC-97H cells in vitro. Acta Pharmacol. Sin 31, 361-366. 10.1038/aps.2010.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Stubbington M. J. T., Nissen J. K., Andersen K. G., Hebenstreit D., Teichmann S. A. and Betz A. G. (2015). The regulatory T cell lineage factor Foxp3 regulates gene expression through several distinct mechanisms mostly independent of direct DNA binding. PLoS Genet. 11, e1005251 10.1371/journal.pgen.1005251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Ito E., Azuma S., Honma R., Yanagisawa Y., Nishikawa A., Kawamura M., Imai J.-I., Tatsuta K., Inoue J.-I. et al. (2008). FoxA1 as a lineage-specific oncogene in luminal type breast cancer. Biochem. Biophys. Res. Commun. 365, 711-717. 10.1016/j.bbrc.2007.11.064 [DOI] [PubMed] [Google Scholar]
- Yang J.-Y., Zong C. S., Xia W., Yamaguchi H., Ding Q., Xie X., Lang J.-Y., Lai C.-C., Chang C.-J., Huang W.-C. et al. (2008). Erk promotes tumorigenesis by inhibiting FOXO3A via MDM2-mediated degradation. Nat. Cell Biol. 10, 138-148. 10.1038/ncb1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K.-M., Sha M., Lu Z. and Wong G. G. (1997). Molecular analysis of a novel winged helix protein, WIN. Expression pattern, DNA binding property, and alternative splicing within the DNA binding domain. J. Biol. Chem. 272, 19827-19836. 10.1074/jbc.272.32.19827 [DOI] [PubMed] [Google Scholar]
- Ye D. Z. and Kaestner K. H. (2009). Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology 137, 2052-2062. 10.1053/j.gastro.2009.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H., Kelly T. F., Samadani U., Lim L., Rubio S., Overdier D. G., Roebuck K. A. and Costa R. H. (1997). Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol. Cell. Biol. 17, 1626-1641. 10.1128/MCB.17.3.1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Wang I. C., Yoder H. M., Davidson N. O. and Costa R. H. (2007). The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology 132, 1420-1431. 10.1053/j.gastro.2007.01.036 [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Caravaca J. M., Tulin A. and Sekiya T. (2010). Nuclear mobility and mitotic chromosome binding: similarities between pioneer transcription factor FoxA and linker histone H1. Cold Spring Harb. Symp. Quant. Biol. 75, 219-226. 10.1101/sqb.2010.75.061 [DOI] [PubMed] [Google Scholar]
- Zhang L., Rubins N. E., Ahima R. S., Greenbaum L. E. and Kaestner K. H. (2005). Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2, 141-148. 10.1016/j.cmet.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Zhang H., Ackermann A. M., Gusarova G. A., Lowe D., Feng X., Kopsombut U. G., Costa R. H. and Gannon M. (2006). The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol. Endocrinol. 20, 1853-1866. 10.1210/me.2006-0056 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yang C., Gao W., Chen T., Qian T., Hu J. and Tan Y. (2015). FOXA2 attenuates the epithelial to mesenchymal transition by regulating the transcription of E-cadherin and ZEB2 in human breast cancer. Cancer Lett. 361, 240-250. 10.1016/j.canlet.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Zhang T., Kim D. H., Xiao X., Lee S., Gong Z., Muzumdar R., Calabuig-Navarro V., Yamauchi J., Harashima H., Wang R. et al. (2016). FoxO1 plays an important role in regulating beta-cell compensation for insulin resistance in male mice. Endocrinology 157, 1055-1070. 10.1210/en.2015-1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. (2016). Forkhead box transcription factors in embryonic heart development and congenital heart disease. Life Sci. 144, 194-201. 10.1016/j.lfs.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Zhu C.-P., Wang J., Shi B., Hu P.-F., Ning B.-F., Zhang Q., Chen F., Chen W.-S., Zhang X. and Xie W.-F. (2015). The transcription factor FOXA2 suppresses gastric tumorigenesis in vitro and in vivo. Dig. Dis. Sci. 60, 109-117. 10.1007/s10620-014-3290-4 [DOI] [PubMed] [Google Scholar]
- Zuo T., Liu R., Zhang H., Chang X., Liu Y., Wang L., Zheng P. and Liu Y. (2007). FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J. Clin. Invest. 117, 3765-3773. 10.1172/jci32538 [DOI] [PMC free article] [PubMed] [Google Scholar]