Abstract
Background:
Restricted diffusion within the splenium of the corpus callosum has been described by other authors in various conditions, however, restricted diffusion in the entire corpus callosum or isolated involvement of the splenium, genu, or body has been infrequently reported on magnetic resonance imaging (MRI) in neonatal hypoxic–ischemic encephalopathy. We report a series of cases showing different patterns of involvement.
Methods and Materials:
Perinatal imaging with MRI including diffusion-weighted imaging was performed in 40 neonates with hypoxic–ischemic encephalopathy, including 11 premature neonates. Sixteen out of 40 patients demonstrated restricted diffusion within the corpus callosum. Out of 16 patients, 9 showed restricted diffusion in the entire corpus callosum, 4 had isolated splenium involvement, 2 had body and splenium signal abnormality, and 1 showed diffusion restriction only in the genu.
Conclusions:
Changes in the corpus callosum were also associated with more severe clinical presentation of encephalopathy. Restricted diffusion within the corpus callosum in infants with hypoxic–ischemic encephalopathy is often associated with extensive brain injury and appears to be an early neuroradiologic marker of adverse neurologic outcome.
Keywords: Corpus callosum, HIE, MRI, restricted diffusion
Introduction
Hypoxic–ischemic encephalopathy (HIE) is a devastating event, which is a major cause of mortality and morbidity in newborns.[1] It is important to elicit the maturity of the neonate and the duration and severity of the insult because the pattern of injury varies.[2] The pattern of brain injury on magnetic resonance imaging (MRI) has been found to be a strong predictor of neurodevelopmental outcome in newborns with encephalopathy.[3] The pattern of injury is categorized into four types, i.e., involvement of the watershed areas, basal ganglia, total injury (maximal basal ganglia and watershed), and focal-multifocal injury (presence of strokes and/or white matter injury alone.[4] Restricted diffusion of the corpus callosum is infrequently reported in HIE. Transient reversible restricted diffusion within the splenium of the corpus callosum is a known radiological finding in various conditions that includes neonatal seizures, hemolytic-uremic syndrome with encephalopathy, neonatal hypoglycemia, antiepileptic drug therapy, epilepsy, viral encephalitis, demyelination disorders, and many other conditions.[5,6,7,8,9,10,11]
We studied the pattern of restricted diffusion within the corpus callosum on magnetic resonance imaging in neonates with HIE.
Materials and Methods
This study was conducted at the Department of Radio diagnosis and Imaging, Bharati Hospital and Research Center, Pune, Maharashtra between August 2013 and February 2016. We studied 40 neonates, 11 preterm, and term infants, with known or suspected hypoxic–ischemic injury. Patient's history, as well as the clinical staging by Sarnath, was recorded prior to MRI. This was a prospective, observational study conducted among patients of either sex. The study was carried out using Philips Achieva 1.5T MRI System with 8-channel dedicated pediatric head coil. Following sequences (time of relaxation/time of echo/flip angle/field of view) were acquired as a part of the study: Axial T1-weighted spin echo images (500/14/90/256/4 mm), axial spin echo T2-weighted images (4000/98/180/256), and isotropic diffusion-weighted images (b 0, 1000), with apparent diffusion coefficient (ADC) maps. Prior to the MRI study, the procedure was explained to the parents/guardians of the patient, and a written informed consent was obtained. Sedation was given as and when required, either orally (syrup Pedichloryl- Half hour before the study) or intravenously (ketamine/propofol), before the start of the study, as deemed appropriate by the attending anesthetist. A pediatrician/anesthetist experienced in resuscitation was always present during the imaging process.
Results
The study included different patterns of restricted diffusion in the corpus callosum according to the area involved. Restricted diffusion on DWI was noted in 16 out of 40 patients (40%). Signal abnormality in the rest of the brain was also documented.
Restricted diffusion in the entire corpus callosum was noted in 9 cases and was the most common finding in this category [Table 1]. Out of the 9 cases, 5 cases showed total (diffuse) pattern of involvement [Figure 1] of the rest of the brain, 3 cases showed watershed pattern [Figure 2], and 1 case showed basal ganglia thalamus pattern of involvement [Figure 3]. The splenium of the corpus callosum was the second most common area involved and was seen in four patients. Out of 4 patients, 2 showed total pattern of involvement and 1 patient each showed the focal-multifocal pattern and basal ganglia thalamus pattern [Figure 4]. Two patients showed combined involvement of the body and splenium, of these 1 patient showed total injury and the other showed focal-multifocal pattern [Figure 5]. Isolated involvement of the genu was noted in 1 case who had focal-multifocal pattern of involvement [Figure 6].
Table 1.
Clinical details and patterns of injury of infants with corpus callosum involvement
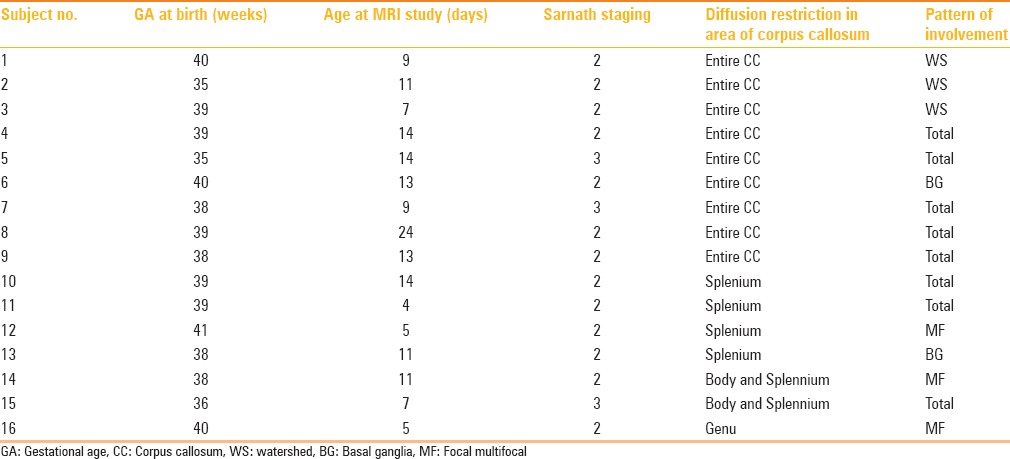
Figure 1 (A and B).
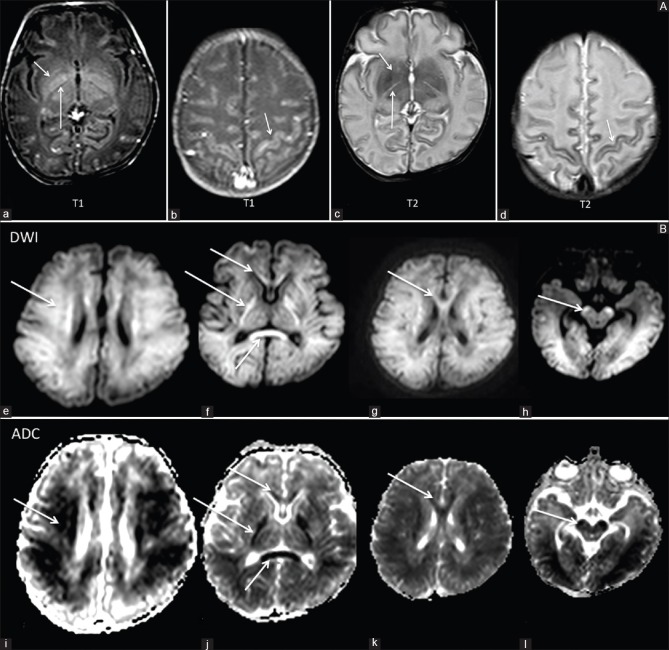
(A) A 7-day-old, full-term neonate with history of seizures. Area of signal abnormality noted in the lentiform nuclei, thalami (short arrow, a and c) and in the perirolandic gyri (arrow, b and d) bilaterally, which appeared hyperintense on T1WI and hypointense on T2WI, not showing restricted diffusion. Absent posterior limb sign was also seen (long arrow, a and c). (B) Areas of restriction diffusion were noted bilaterally in the fronto-parieto-temporo-occipital subcortical white matter and corona radiate (arrow e, i), posterior limbs of both the internal capsule (long arrow f, j), the entire corpus callosum (short arrow in f,g,j,k) and anteromedial midbrain (arrow h,I)
Figure 2 (A and B).
(A) A 20-day-old term neonate with history of asphyxia and two episodes of left-sided focal seizures. Multiple small areas of abnormal signal intensity appearing hyperintense on T2 and isointense on T1WI seen in the periventricular white matter bilaterally, predominantly in the frontal lobes (arrow, a-h). (B) Restricted diffusion was noted in the entire corpus callosum (arrow, I and j)
Figure 3.
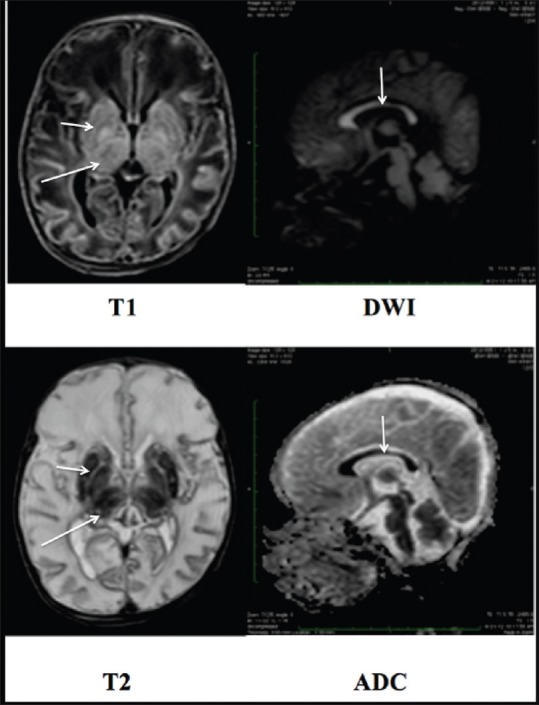
A 13-day-old, term neonate with respiratory distress, hypotonia, and seizures. MRI revealed abnormal signal intensity in the thalami and the basal ganglia, appearing hyperintense on T1WI and hypointense on T2WI images (long and short arrow). The entire corpus callosum showed restricted diffusion. (White arrow)
Figure 4 (A-G).
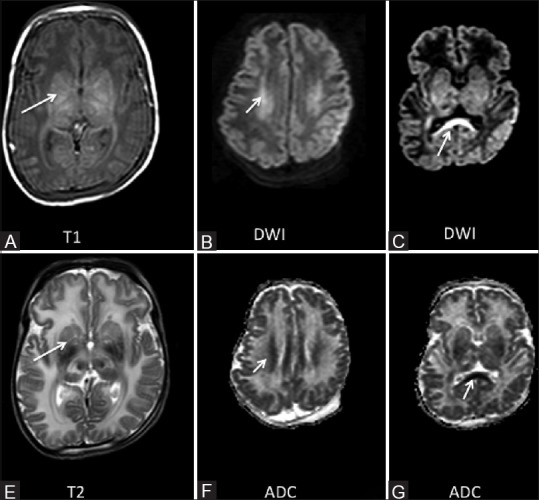
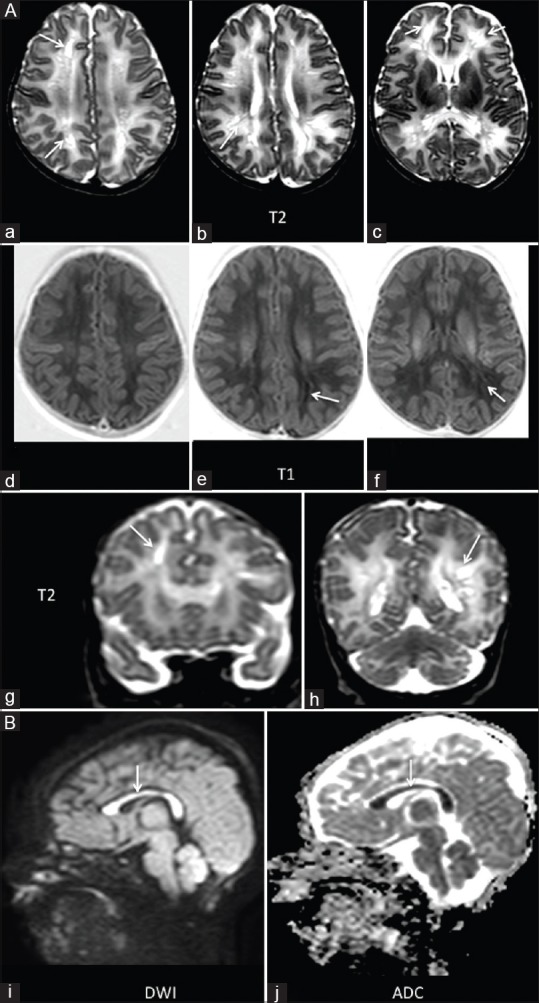
A 14-day-old term neonate born by LSCS for meconium aspiration came with tonic posturing. Abnormal signal intensity was noted in the lentiform nuclei and thalami (arrow A and E) appearing hyperintense on T1 and hypointense on T2 images. DWI images showed restriction of diffusion in the corona radiata (arrow, B and F) and the splenium of the corpus callosum (arrow C and G)
Figure 5 (A and B).
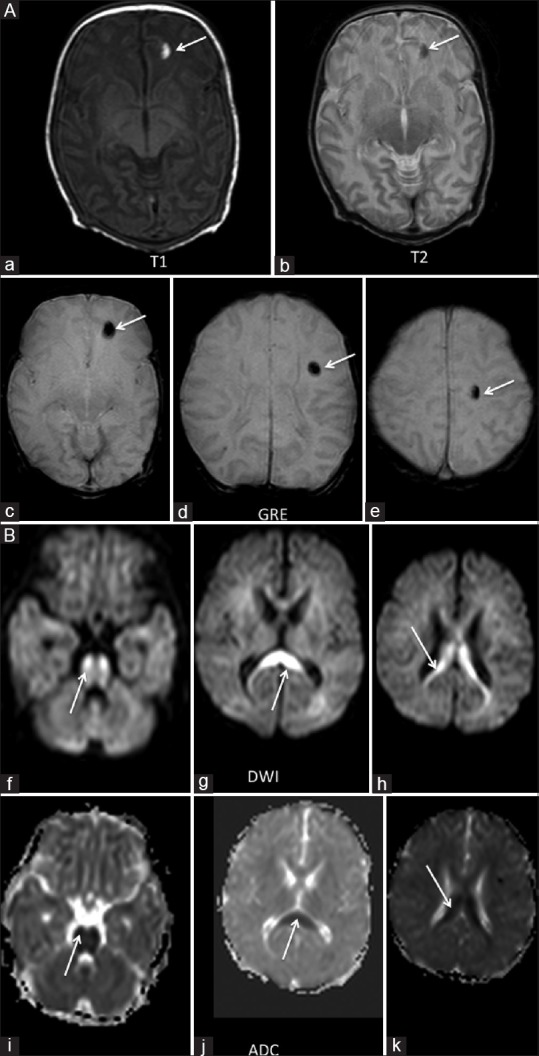
(A) An 11-day-old term neonate with history of seizures. Three focal areas of blooming were seen in the left frontal lobe on GRE images (arrow c-e). These areas appeared hypointense on T2WI and hyperintense on T1WI (arrow a and b), suggestive of a subacute hemorrhage. (B) The neonate also had areas of restricted diffusion in the corpus callosum involving the body (arrow h and k), splenium (arrow g and h) and the pons (arrow f and i)
Figure 6 (A-F).
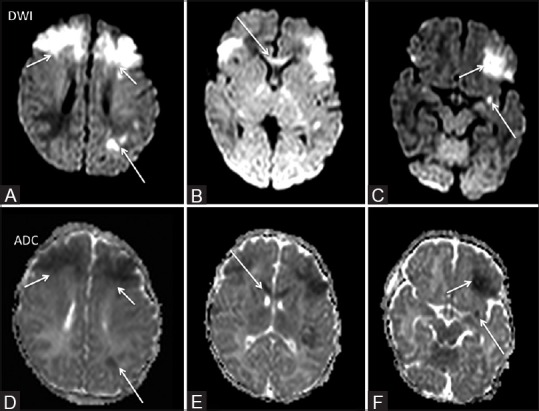
A 5-day-old term neonate a suspected case of HIE. Restricted diffusion was noted bilaterally in the high frontal region (short arrow A and D), left basifrontal region (short arrow F and C) and genu of corpus callosum (arrows B and E). Small focus of restricted diffusion was also noted in the left perisylvian region (long arrow F and C) and in the left parietal lobe (long arrow A and D)
Out of forty patients, 11 neonates were born premature. Three of these neonates showed corpus callosum injury. Out of these 3, 2 showed involvement of the entire corpus callosum and 1 had injury to the body and splenium. In our study, corpus callosum injury was found to be more common among term neonates (81%). According to the Sarnath staging for HIE, three patients who had stage three HIE had total brain injury on MRI. Two of them had total corpus callosum involvement and 1 had involvement of the body and splenium of corpus callosum.
In our study, we documented patients with restricted diffusion of the entire corpus callosum, as well as those with isolated involvement of the splenium and the genu. Corpus callosum injury was associated with more severe clinical presentations. Injuries were common among term neonates and most of the injuries were associated with total (maximal basal nuclei and watershed) pattern of brain injury (50%). Restricted diffusion of the entire corpus callosum, to our knowledge, has not been reported by any of the authors.
Discussion
The pathophysiologic effects of HIE are complex and evolve over time. The main underlying pathology in HIE is the Impaired cerebral blood flow[12] and oxygen delivery to the brain.[13] The pathological events of HIE occur in two phases, i.e., primary energy failure and secondary energy failure.
Initial reduction of cerebral blood flow ensues primary energy failure, which leads to decrease in oxygen and glucose. As a result, there is reduction in ATP and increase in lactate production.[14] Low ATP levels result in the depolarization of neurons due to the failure of Na/K pumps of the cell membranes, leading to excessive influx of the positively charged sodium ions.[15] This leads to the release of glutamate, which then binds to glutamate receptors allowing additional influx of intracellular calcium and sodium.[16] Effects of increased intracellular calcium leads to cerebral edema, ischemia, microvascular damage causing necrosis, and/or apoptosis.
Secondary energy failure is related to oxidative stress, excitotoxicity, and inflammation which occurs 6 to 48 hours after the initial injury.[13] The neuronal cell membranes are damaged due to overproduction of free radicals leading to necrosis or apoptosis. The neonatal brain lacks the ability to eliminate free radicals, and its increased susceptibility to free radicals lead to damage of the neuronal tissue.[17] Excessive levels of extracellular neurotransmitters, especially glutamate, causes excitotoxicity. Overstimulation of receptors by glutamate allows the additional influx of sodium and calcium into the neural cells.[16] Glutamate is used in hearing, vision, somatosensory functions, and in neuronal pathways of learning and memory.[18] Hence, glutamate excitotoxicity has a devastating effect in HIE and its sequelae. Inflammation also plays a significant role in the development of HIE-related brain injury, however, the exact mechanism still remains unknown.[19]
On MRI, restricted diffusion of the corpus callosum may represent acute cytotoxic edema within the affected area and could be a generalized response to the underlying hypoxic–ischemia. The corpus callosum affected by an acute process shows decrease in its ADC values, loses its anisotropic appearance, and shows restricted diffusion on diffusion-weighted images. Glutamate neurotoxicity is one of the important mechanisms of such an injury leading to cytotoxic edema. The corpus callosum as well as the basal ganglia contains an abundance of glutamate receptors, and are hence susceptible to glutamate neurotoxicity by hypoxic-ischemic injury.[20,21]
Diffuse brain injury in HIE is associated with severe seizures.[22] Seizures may also result in diffusion restriction within the corpus callosum.[11] Another factor associated with restricted diffusion in the corpus callosum includes anticonvulsant therapy.[23] In our study, some of these factors resulted in the involvement of the corpus callosum, whereas others did not.
A study by Takenouchi et al.[24] is the only published study to document diffusion restriction in the corpus callosum in HIE. Authors reviewed images of 34 infants in the study. Ten of the 34 (29%) infants demonstrated restricted diffusion within the splenium of the corpus callosum, with a significantly higher incidence of severe developmental delay or death, compared to infants without restricted diffusion in the splenium of the corpus callosum.
A study by Nagy et al.[25] has shown that corpus callosal injury in patients with a perinatal history of moderate hypoxic ischemia was associated with a worse neuropsychological performance. Thus, perinatal corpus callosal injury serves as an important marker of poor neurological outcome.
The limitations of our study include a relatively small sample size, as well as the fact that the patients were scanned only once. It remains unclear whether the changes represent a transient finding.
Conclusion
We present a series of neonates, both term and preterm, with HIE who underwent MRI. Restricted diffusion within the corpus callosum is a part of the spectrum of injury patterns in HIE, which is often associated with extensive brain injury. It serves as an early neuroradiologic marker of adverse neurologic prognosis that could help in appropriate clinical decision-making and patient management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wong DS, Poskitt KJ, Chau V, Miller SP, Roland E, Hill A, et al. Brain injury patterns in hypoglycemia in neonatal encephalopathy. AJNR Am J Neuroradiol. 2013;34:1456–61. doi: 10.3174/ajnr.A3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang BY, Castillo M. Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics. 2008;28:417–39. doi: 10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 3.Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, Gouyon JB, et al. Term neonate prognoses after perinatal asphyxia: Contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology. 2006;239:839–48. doi: 10.1148/radiol.2393050027. [DOI] [PubMed] [Google Scholar]
- 4.Chau V, Poskitt KJ, Sargent MA, Lupton BA, Hill A, Roland E, et al. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy. Pediatrics. 2009;123:319–26. doi: 10.1542/peds.2008-0283. [DOI] [PubMed] [Google Scholar]
- 5.Takanashi J, Barkovich AJ, Shiihara T, Tada H, Kawatani M, Tsukahara H, et al. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am J Neuroradiol. 2006;27:836–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T. Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. AJNR Am J Neuroradiol. 2006;27:1983–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P, Gogoi D, Vyas S, Khandelwal N. Transient splenial lesion: Further experience with two cases. Indian J Radiol Imaging. 2010;20:254–7. doi: 10.4103/0971-3026.73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra HS, Garg RK, Vidhate MR, Sharma PK. Boomerang sign: Clinical significance of transient lesion in splenium of corpus callosum. Ann Indian Acad Neurol. 2012;15:151–7. doi: 10.4103/0972-2327.95005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho JS, Ha SW, Han YS, Park SE, Hong KM, Han JH, et al. Mild encephalopathy with reversible lesion in the splenium of the corpus callosum and bilateral frontal white matter. J Clin Neurol. 2007;3:53–6. doi: 10.3988/jcn.2007.3.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Ali FA, Rowley HA, Bass N. Imaging patterns of neonatal hypoglycemia. AJNR Am J Neuroradiol. 1998;19:523–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota T, Kidokoro H, Ito M, Oe H, Hattori T, Kato Y, et al. Diffusion-weighted imaging abnormalities in the corpus callosum after neonatal seizure: A case report. Brain Dev. 2008;30:215–7. doi: 10.1016/j.braindev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Shalak L, Perlman JM. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev. 2004;80:125–41. doi: 10.1016/j.earlhumdev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Cotten CM, Shankaran S. Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev Obstet Gynecol. 2010;5:227–39. doi: 10.1586/eog.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanrahan JD, Sargentoni J, Azzopardi D, Manji K, Cowan FM, Rutherford MA, et al. Cerebral metabolism within 18 hours of birth asphyxia: A proton magnetic resonance spectroscopy study. Pediatr Res. 1996;39:584–90. doi: 10.1203/00006450-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Volpe J. Neurology of the Newborn. 5th ed. Philadelphia: Saunders; 2008. [Google Scholar]
- 16.Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Dev. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buonocore G, Groenendaal F. Anti-oxidant strategies. Semin Fetal Neonatal Med. 2007;12:287–95. doi: 10.1016/j.siny.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Fatemi A, Wilson MA, Johnston MV. Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol. 2009;36:835–58. doi: 10.1016/j.clp.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 20.Hassel B, Boldingh KA, Narvesen C, Iversen EG, Skrede KK. Glutamate transport, glutamine synthetase and phosphate-activated glutaminase in rat CNS white matter. A quantitative study. J Neurochem. 2003;87:230–7. doi: 10.1046/j.1471-4159.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- 21.Domercq M, Matute C. Expression of glutamate transporters in the adult bovine corpus callosum. Brain Res Mol Brain Res. 1999;67:296–302. doi: 10.1016/s0169-328x(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 22.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Prilipko O, Delavelle J, Lazeyras F, Seeck M. Reversible cytotoxic edema in the splenium of the corpus callosum related to antiepileptic treatment: Report of two cases and literature review. Epilepsia. 2005;46:1633–6. doi: 10.1111/j.1528-1167.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 24.Takenouchi T, Heier LA, Engel M, Perlman JM. Restricted diffusion in the corpus callosum in hypoxic-ischemic encephalopathy. Pediatr Neurol. 2010;43:190–6. doi: 10.1016/j.pediatrneurol.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Nagy Z, Lindstrom K, Westerberg H, Skare S, Andersson J, Hallberg B, et al. Diffusion tensor imaging on teenagers, born at term with moderate hypoxic-ischemic encephalopathy. Pediatr Res. 2005;58:936–40. doi: 10.1203/01.pdr.0000186516.85702.61. [DOI] [PubMed] [Google Scholar]