Abstract
Sonography is an ideal, effective, noninvasive tool for evaluation of the spinal cord in neonatal and early infantile age groups owing to lack of ossification of the posterior elements of spine. Understanding normal anatomical appearances is a prerequisite for the interpretation of various pathologies of the spinal canal and its contents. This review elucidates normal appearances of the spinal cord in this age group, in both axial and sagittal planes. Usefulness of Doppler sonography is briefly discussed, and special emphasis is placed on normal anatomical variants that may mimic spinal abnormalities. Sonographic appearances of common intraspinal pathologies, both congenital and acquired, are exhaustively described. Key points regarding sonographic diagnosis of important spinal anomalies are emphasized and explained in detail. To conclude, spinal ultrasound is a reliable and widely available screening tool, albeit the usefulness of which is often underestimated.
Keywords: Myelomeningocele, spinal dysraphism, spinal sonography, split cord malformation
Introduction
In neonates and infants with suspected spinal and paraspinal anomalies, magnetic resonance imaging (MRI) was and remains the imaging gold standard. However, ultrasonography has recently witnessed tremendous improvement in image quality with the advent of new generation high frequency ultrasound scanners that have brought its diagnostic value on par with that of MRI[1] in certain conditions. Relative advantages of sonography over MRI include wide and cheap availability, no need for sedation or general anesthesia, and lack of vulnerability to artefacts due to patient movement, cerebrospinal fluid (CSF) pulsation, and vascular flow which can adversely affect MR image quality.[2]
In newborns and infants, the spinal arches are predominantly cartilaginous which provide an acoustic window allowing passage of the ultrasound beam. However, in older children, ultrasound suffers from diminished utility due to progressive ossification of the spinal arches.
Sonography is a well-established method for investigating the spinal canal, cord, and meningeal coverings and for characterizing nearly all spinal anomalies with high geometric resolution in the neonatal and infantile age groups.[3,4] The objective of this pictorial review is to present an educational exhibit of spinal sonography encompassing normal appearances, normal anatomical variants, and some common congenital and acquired spinal pathologies.
Indications
The American Institute of Ultrasound in Medicine (AIUM) guideline[5] lists the following indications for the ultrasound examination of the neonatal spine:
Lumbosacral stigmata known to be associated with spinal dysraphism
Evaluation of suspected defects such as cord tethering, diastematomyelia, hydromyelia, and syringomyelia
Spectrum of caudal regression syndrome (e.g., anal atresia or stenosis; sacral agenesis)
Detection of sequelae of injury (e.g., hematoma after spinal tap or birth injury; posttraumatic leakage of CSF; and sequelae of prior instrumentation, infection, or hemorrhage)
Visualization of hemorrhagic fluid within the spinal canal in neonates and infants with intracranial hemorrhage
Guidance for lumbar puncture
Postoperative assessment for cord re-tethering.
Amongst the spinal dysraphism, ultrasound is usually not preferred in the open spinal dysraphism because of risk of infection.
Technique
Sonography of the spine should be performed with a high frequency (7–12 MHz), high resolution linear transducer. Both axial and sagittal plane scanning is mandatory. The axial scanning can either be performed in a cranial to caudal direction or caudocranial direction. Localization of the conus medullaris is crucial for the detection of low-lying cord or high termination of cord. Location of conus should be interpreted in relation to the lumbar vertebral bodies. Sagittal scanning should be performed both in the median and paramedian planes.
Normal Appearances
Axial scan
The spinal cord is seen axially as a round or oval hypoechoic structure with central echogenicity within the anechoic subarachnoid space [Figure 1]. Paired dorsal and ventral nerve roots are seen to arise from the cord. The vertebral bodies and arches are seen ventral to the spinal cord as echogenic structures with distal acoustic shadowing [Figure 2]. The paravertebral muscles are seen below the level of L2 vertebra.[1,6]
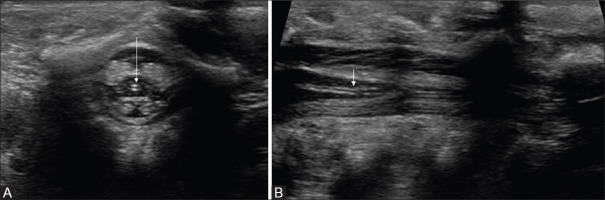
Figure 1 (A-C).
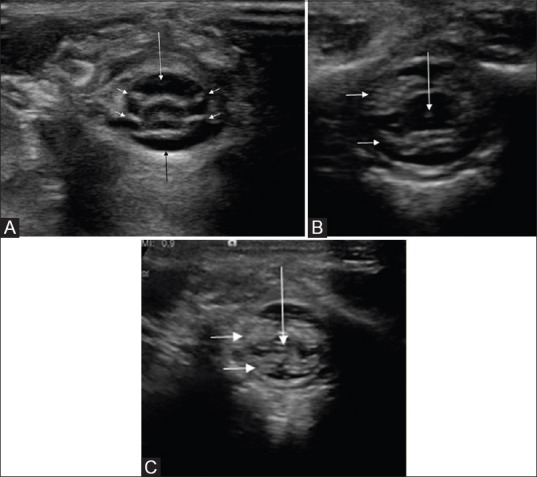
(A-C) Normal sequential axial sonogram of thoracolumbar spine. The spinal cord appears hypoechoic, covered by an echogenic pial lining, and surrounded by anechoic CSF spaces. Note that the ventral (black arrow) and dorsal (white arrow) CSF spaces are nearly equal in dimension. The nerve roots appear echogenic (small arrows). On a more caudal section (B), there is normal enlargement of cord at conus medullaris, which tapers distally. Hence, a more caudal section would reveal only a bunch of nerve roots as echogenic structure (block arrows), with a central hypoechoic filum terminale (arrow in C)
Figure 2.
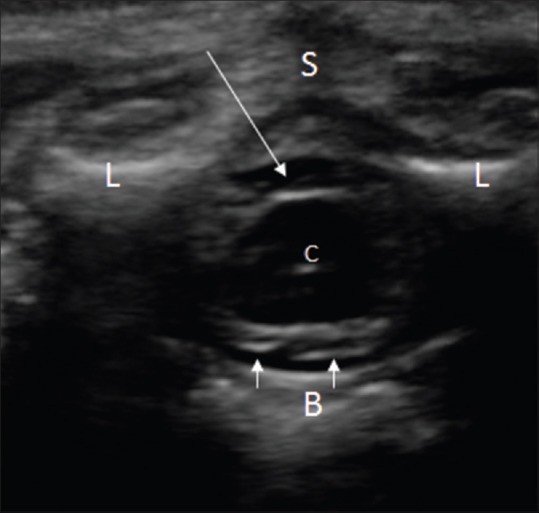
Normal conus medullaris on axial image. The spinal cord is seen as hypoechoic rounded structure, surrounded by anechoic CSF (arrow). The pial margin on the cord surface appears echogenic; the central canal (C) also appears echogenic. The ventral roots of spinal nerves appear as echogenic strands (small arrows). Note the echogenic laminae (L), unossified spinous process (S). and vertebral body (B) of a lumbar vertebra
The cord diameter is variable and is the largest at the cervical and lumbar levels, which are known as cervical and lumbar “enlargements” (which give rise to nerve roots of respective nerve plexuses). In terms of dorsal/ventral orientation, the cord normally lies a third-to-half way between the anterior and posterior walls of the spinal canal.[1]
Sagittal scan
The cervical cord and craniocervical junction can be difficult to evaluate on ultrasonography. On sequential scanning from cervical to lumbar level, the cervical and lumbar enlargements are well visualized. A systematic scanning from both the parasagittal to mid-sagittal planes is required [Figure 3]. While the parasagittal image is ideal for the evaluation of the paraspinal structures, the mid-sagittal image is ideal for the evaluation of the cord.[6,7]
Figure 3 (A-C).
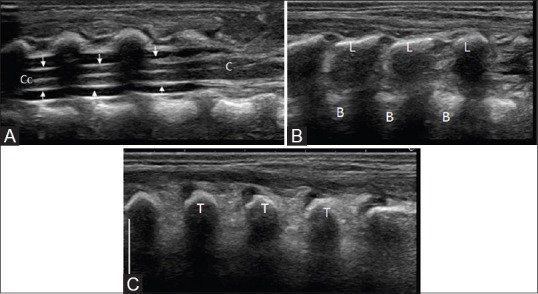
Mid-sagittal (A) to parasagittal (B) and further lateral (C) sagittal image of the thoracolumbar spine. The spinal cord is visualized as a hypoechoic tubular structure with a central echogenicity (central echo complex) representing the central canal (C, c). The pial lining is shown with small arrows. Note the enlargement of cord at the conus medulllaris (C). The arachnoid-dura mater complex of the thecal sac is represented by the echogenic borders of the spinal canal seen anterior and posterior to the CSF filled anechoic subarachnoid space. The spinous process of the vertebral bodies are visible in a mid-sagittal section (S), the laminae (L) and transverse processes (T) seen more laterally
The caudal end of the spinal cord is represented by the conus medullaris and filum terminale [Figure 4]. The conus is identified as the apex of the taper of the distal spinal cord, and its level is designated according to the adjacent intervertebral disk space or mid-vertebral body. For identifying the vertebral level, palpable landmarks such as the tip of the lowest rib and the iliac crest which correspond to the levels of the L2 and L5 vertebrae, respectively, may be used. Alternately, the lumbosacral junction may be identified by looking for the first clear angulation in the caudal spine with the L5 vertebral body lying immediately cranial to this level. In a healthy newborn, the tip of the conus medullaris is located between L1 and L2 vertebral levels. This method can be fallacious in cases of transitional vertebra. Another method of detection of vertebral level is to identify the coccyx and counting cranial to it.[6,7]
Figure 4 (A and B).
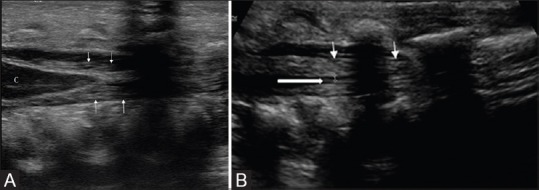
(A and B) Normal conus medullaris and filum terminale in sagittal section. The conus medullaris (c) tapers into filum terminale (block arrow, within the cursors in B); which appears as a cordlike structure surrounded by the echogenic cauda equina nerve roots (arrows)
The normal cord and nerve roots show pulsatile movements which must be specifically looked for to rule out cord tethering.
Normal sacrum in mid-sagittal scanning
Sacrum should be evaluated in the sagittal plane [Figure 5] for the presence of the spinal dysraphism. Because the posterior elements of the sacrum are unossified at this age, they appear hypoechoic.
Figure 5 (A and B).
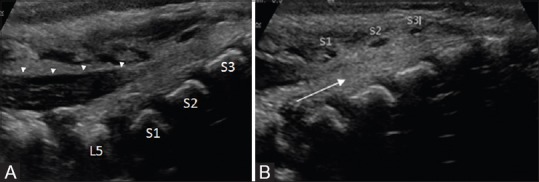
(A and B). Normal sacrum mid-sagittal view. The spinous processes are marked in B. The dura (arrowheads) ends at S1 level. Echogenic nerve roots are seen within the spinal canal. Beyond the dural attachment, the space within the sacral spinal canal is occupied by echogenic fatty tissue (arrow)
Doppler ultrasound imaging of the spinal cord
Although not routinely used for the evaluation of vascular anatomy and abnormality of spinal canal, Doppler ultrasound with the new generation scanners on a high frequency transducer can provide an insight of the vascular anatomy in considerable detail [Figure 6].
Figure 6 (A-C).
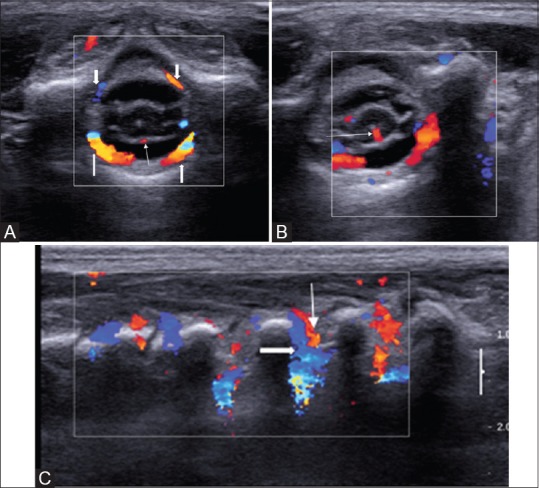
(A-C) Doppler USG of normal thoracic spine. Axial images with Doppler interrogation (A, B) show the anterior spinal artery (arrow in A); the sulco-commisural artery (arrow in B) and the ventral and dorsal dural arcades (block arrows). The parasagittal image (C) reveals the dorsal division of the dorsal spinal artery (arrow) with accompanying vein (block arrow) arising from the segmental arteries at multiple levels
Normal Variants
Many normal anatomical variants such as ventriculus terminalis and transient dilatation of the central canal are often incidentally detected on spinal ultrasonography (USG).
Ventriculus terminalis
Canalization and retrogressive differentiation of the caudal end of the developing spinal cord gives rise to a small, ependymal lined, oval, cystic structure (ventriculus terminalis) [Figure 7] located at the transition from the tip of the conus medullaris to the origin of the filum terminale.[8] This structure usually measures 8–10 mm in longitudinal diameter and 2–4 mm in transverse diameter.[8] This condition is asymptomatic and regresses in the first few weeks after birth.
Figure 7.
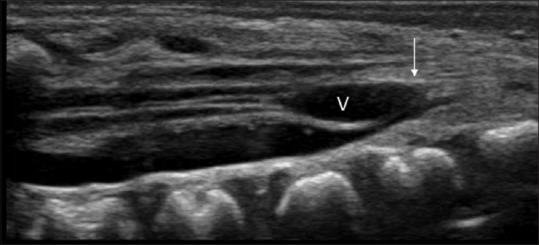
Ventriculus terminalis associated with low lying tethered cord in a neonate. Note the oval cystic structure (V) at the distal conus and filum; and nerve roots adherent to the posterior dura (arrow)
Transient dilatation of the central canal
Mild central canal dilatation [Figure 8] is an often detected incidental finding in many healthy newborns in the first few weeks of life which disappears later on. It should be differentiated from syringomyelia, which persists on follow-up imaging.
Figure 8 (A and B).
Axial (A) sagittal (B) sonogram of the distal conus medullaris and filum terminale revealing mild dilation of the central canal (arrow) in a healthy newborn
Filum terminale cysts
These refer to anechoic, centrally located, thin-walled unilocular cysts within the filum terminale, which appear spindle-shaped in the sagittal plane and round in the axial plane. When found isolated, they do not carry any clinical significance.
Pseudomass of the cauda equina
This entity may be seen in some cases due to nerve root clumping during sonographic examination in the decubitus position. On repeat sonography in prone position, it is no longer seen.
Dysmorphic coccyx
The coccyx, usually presenting with a dysmorphic dorsal curve at its tip, is a common sonographic finding in neonates and infants in whom a palpable lump is detected on clinical examination.
Pseudosinus tract
This refers to a residual cord such as echogenic fibrous structure extending from the coccyx to an overlying sacral dimple. These tracts, unlike true dermal sinus tracts, do not contain any fluid or reveal any associated mass.
Spinal Pathologies
Spinal pathologies can be broadly categorized as congenital malformations and acquired diseases. Congenital anomalies are attributed to missteps in embryological development of the spinal cord, which result in a diverse range of pathologies that present as myriad sonographic appearances. On the other hand, acquired intraspinal diseases following birth trauma or intraspinal extension of neurogenic tumors can also be detected with ultrasound.
Congenital anomalies
Open spinal dysraphism
Meningocele, myelomeningocele, myelocele, hemi-myelocele, hemi-myelomeningocele, etc, are included in the open spinal dysraphism group. These lesions are not skin covered. Myelomeningoceles constitute >98% of open spinal dysraphism.[9] In myelomeningocele [Figure 9], an expansion of the ventral subarachnoid space displaces the neural placode dorsally resulting in portions of the spinal cord, nerve roots, and leptomeninges lying within the sac; whereas in myelocele, the neural placode remains flush with the skin surface and there is no expansion of ventral CSF space.[9]
Figure 9 (A-C).
Lumbar myelomeningocele. Axial (A), sagittal (B, C) US images reveal herniation of the conus (arrow) with an expansion of ventral CSF space (asterisk). Sagittal T2-weighted MR image reveals the defect in posterior elements of upper lumbar vertebrae with herniation of neural tissue and CSF spaces (arrow)
Some authors caution against preoperative imaging for these anomalies owing to risk of infection or injury.[5,6] However, by observing strict aseptic precautions, including covering the probe with a sterile cover and using sterile gel, proper sonographic examination can be performed using the intact normal skin surrounding the parchment membrane of the lesion as the acoustic window. In addition to local examination, sonography is also useful in recognition of associated malformations such as Chiari II syndrome, tethered cord, hydromyelia/syringomyelia, and arachnoid cyst.[6] Ultrasound also finds an important role in post-repair cases because cord tethering is a common postoperative complication on account of postoperative scarring.
When myelomeningocele or myelocele is associated with split cord malformation (discussed later), they are termed as hemi-myelomeningocele/hemi-myelocele.
Closed spinal dysraphism
The major use of neonatal spinal sonography lies in the subgroup of closed spinal dysraphism. These entities can present with or without a back mass. Those with a back mass include meningocele, lipomyelocele, and lipomyelomeningocele [Figure 10]. Closed spinal dysraphism without a back mass includes dorsal dermal sinus, low lying tethered spinal cord, diastematomyelia, filar lipoma, fatty filum terminale. The cutaneous stigma includes sacral dimple, hyperpigmentation, sinus, or tuft of hair.
Figure 10 (A-C).
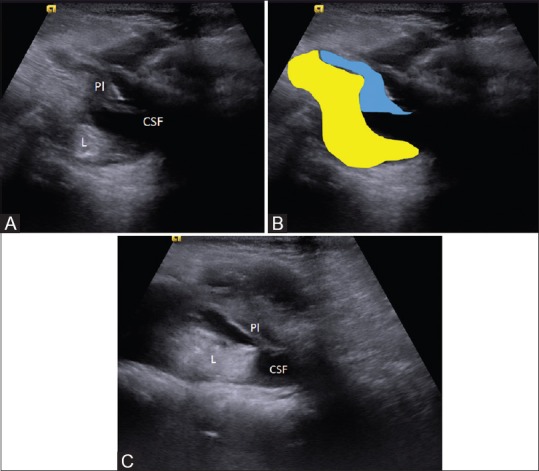
(A-C) Lipomyelomeningocele. Axial sonogram (A) and diagrammatic representation show a defect in the posterior element, intraspinal lipoma (colored in yellow). The placode (Pl, colored in blue)–lipoma interface lies outside the spinal canal; and the ventral CSF space (csf) is enlarged. Sagittal sonogram (C) shows the relationship of the lipomatous component, the placode, and the CSF space
-
Meningocele
Meningocele implies the herniation of a CSF filled meninges through a vertebral defect and usually does not contain any part of the spinal cord. On sonography, they appear as anechoic cystic mass, containing no neural tissue. Posterior meningoceles are more common and are mostly found in the lumbar location. Anterior meningoceles are more common in sacral region and can present as a presacral mass.[9] Lateral thoracic meningoceles may have syndromic association with neurofibromatosis-1.
-
Lipomyelomeningocele/lipomyelocele
These are skin covered closed spinal dysraphisms with a back mass. In both these entities, there is presence of a fatty mass in the subcutaneous tissue. The distinction between the two is established by the location of the lipoma–placode interface. In lipomyelocele, the lipoma–placode interface lies within the spinal canal, whereas in lipomyelomeningocele, there is expansion of CSF space and the interface lies outside the spinal canal.[9] The lipoma can be echogenic or isoechoic to subcutaneous fat on sonography.
-
Myelocystocele
Myelocystoceles are considered to be subtype of myelomeningoceles characterized by herniation of a dilated central canal through posterior midline spina bifida defect. They can be subclassified into terminal and nonterminal types. In terminal myelocystocele, there is a skin covered back mass with herniation of meninges through posterior spinal defect. In addition, there is a terminal syrinx communicating with the spinal central canal. In nonterminal myelocystocele, only the dilated central canal herniates through a posterior defect.
Closed spinal dysraphism without a back mass
In this subcategory of closed spinal dysraphism, the neural tissue is covered by the skin without any associated subcutaneous mass.[10]
Spina bifida occulta
This entity is characterized by the variable absence of several neural arches and various cutaneous abnormalities such as lipoma, hemangioma, cutis aplasia, dermal sinus, and hairy patch; it is often associated with low-lying conus and other spinal cord anomalies.
-
Fatty filum and filar fibrolipoma [Figure 11]
Fatty filum and filar lipomas occur due to persistent or de-differentiated fatty tissue secondary to spinal cord canalization anomalies.[1] Minimal fat in filum (fatty filum) is of no clinical significance as long as it is an isolated finding in a normal-size filum (<2 mm).[2,7]
When sonography depicts an echogenic fatty mass causing filum terminal thickening of >2 mm, it is referred to as filum terminale lipoma. This is a clinically significant disorder as it may be associated with myelomeningocele, tethered cord, and syringohydromelia, all of which can be easily diagnosed on sonography.
-
Tight filum terminale syndrome
Incomplete involution of the distal spinal cord during embryogenesis leads to abnormal thickening of filum terminale. Tight filum terminale syndrome is always associated with tethering of the spinal cord and abnormally positioned conus medullaris below L2-3.[11] Spinal ultrasound shows an abnormally thickened filum terminale (>2 mm at L5–S1 level) sometimes in combination with a centrally located small cyst or lipoma.[6]
-
Dorsal dermal sinus
A dorsal dermal sinus refers to an epithelium-lined tract that extends from the spinal cord, cauda equina, or arachnoid to the skin.[6] It usually manifests with cutaneous stigmata and is mostly found in the lumbosacral region in midline.[12,13] The entire tract can be visualized on spinal ultrasound. In the subcutaneous tissues, it is seen as a mildly hypoechoic track within the subcutaneous fat; on the other hand, in the CSF-filled subarachnoid space, it is easily visualized as a linear echogenic structure.[6]
-
Tethered cord
Incomplete regressive differentiation and failed involution of the terminal cord results in abnormal dorsal fixation of the spinal cord adjacent to the vertebral arches. Tethering of cord can be associated with other forms of spinal dysraphism or can be postoperative.[1,14]
In neonates, tethered cord is diagnosed on ultrasonography by abnormally dorsi-fixed spinal cord [Figure 12] (in prone position), presence of a low-lying conus medullaris (below the L2–L3 disk space), absence of normal pulsatile motion of the cord and nerve roots, and associated filum terminale thickening.[2,6,15] Assessment of normal nerve root motion is even more important in older children as the conus may be normally positioned but still be tethered (tight filum syndrome).[16]
Figure 11.
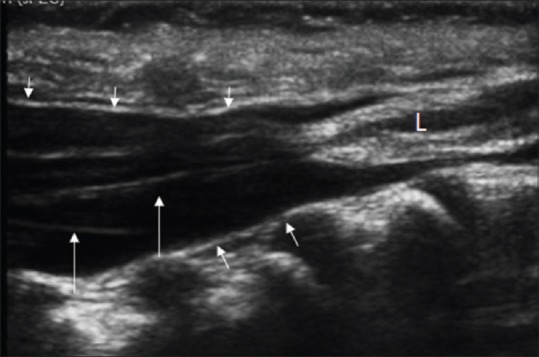
Filar lipoma. Sagittal spine sonogram shows a low lying conus with the pial covering, a thick filum with an echogenic mass at the filum (L). The dura (small arrows) extends to a level lower than normal when evaluated in respect to the sacral vertebrae. The nerve roots are shown with arrows
Figure 12.
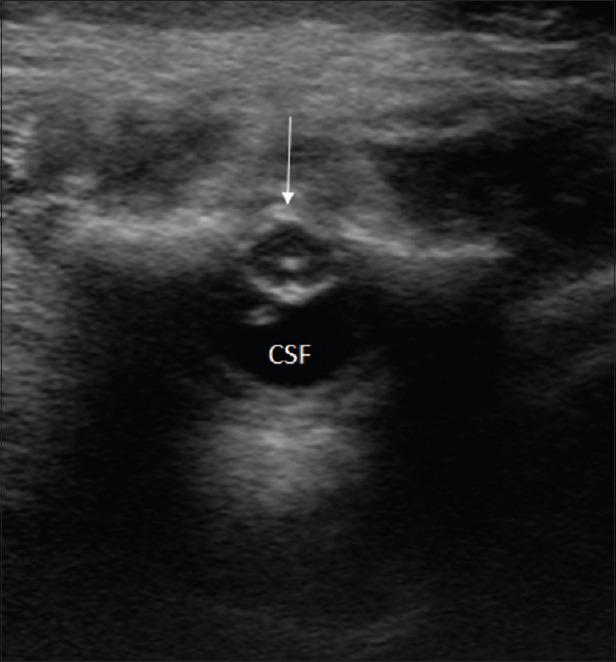
Tethered cord. Axial US image shows dorsal position of the conus (arrow) and an enlarged ventral CSF space (CSF)
Complex dysraphism
The disorders can result from abnormal midline notochordal integration resulting in split cord malformation or neuroenteric cyst. Abnormalities in notochord formation, as seen in sacral agenesis/dysgenesis or segmental spinal dysgenesis, represent another manifestation of this disorder.[9,10]
-
Split cord malformation [Figure 13]
Split cord malformation/diastematomyelia usually presents as a sagittal cleft in the thoracolumbar spinal cord, resulting in two hemicords (usually asymmetric), which generally reunite caudal to the cleft. Each hemicord has its own central canal and separate dorsal and ventral nerve roots. They can be divided into two types; either having a separate dural sheath for each cord or having a single sheath. The “stem” may be a bony, cartilaginous, or fibrous band.
Axial plane USG demonstrates both hemicords and echogenic spur in cross-section, each with a central canal and ipsilateral nerve roots. In addition, commonly associated malformations such as tethered cord and syringohydromelia (which must be specifically looked for) are also easily demonstrated on spinal ultrasonography.
-
Neuroenteric cyst
This is a complex dysraphism where there is a mucin secreting epithelium-lined cyst located in the posterior mediastinum and vertebral segmentation anomaly. Often they have a communicating tract and an intraspinal component of the cyst. The cyst shows a thick wall with alternate echogenic and hypoechoic layer (gut signature). The intraspinal component may be small and difficult to visualize on ultrasound alone [Figure 14].
-
Caudal regression syndrome
Caudal regression syndrome is attributed to abnormal mesodermal formation of the caudal cell mass.[6] The grade of deformity can vary from minimal to severe regression of the coccyx, sacrum, and lumbar spine, which consequently alters clinical presentation and sonographic appearances. On spinal USG, imaging appearance may comprise either a blunt, deformed conus medullaris that terminates above the normal level or an elongated conus which is tethered by a thickened filum terminale or intraspinal lipoma and ends below L1.
Figure 13.
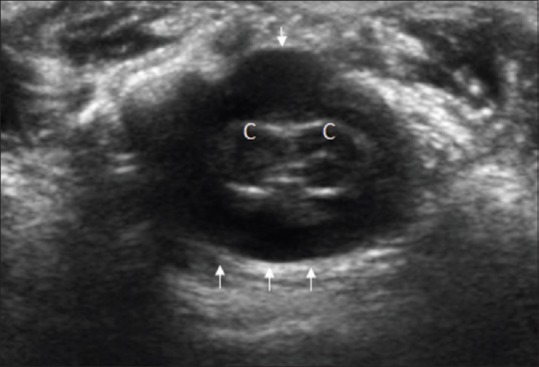
Split cord malformation. Axial sonographic image reveals splitting of spinal cord into two hemicords (c) with separate pial coverings. Single dural sheath is shown with small arrows
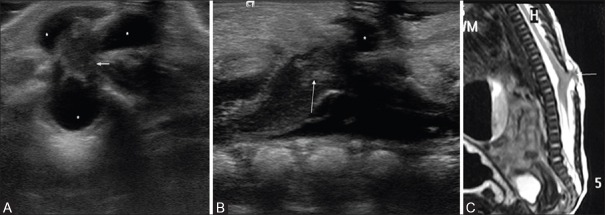
Figure 14 (A-E).
(A-E). Neuroenteric cyst. Axial sonographic image (A) reveals a cystic lesion (arrow) with echogenic walls in the posterior mediastinum and a defect in the vertebral body (also evident on CT volume rendered images (B). A more cranial sagittal (C) scanning in the cervical region revealed an intraspinal lesion (arrow), cranial to the level of vertebral cleft. MR images confirmed the findings. Axial T2-weighted TSE image (D) shows the intraspinal component (arrow). Three-dimensional volume rendered heavily T2-weighted MR image (E) shows the cyst (cy) with a beak-like extension to the upper thoracic spinal canal (arrow)
Acquired conditions
-
Spinal cord injury/Intraspinal hematoma
During delivery, excessive traction on the spinal cord can result in spinal cord transection or intraspinal hematoma formation. In such cases, sonography done shortly after birth reveals increased cord echogenicity due to edema, hemorrhage, or venous congestion with cord displacement by subdural/epidural hematoma. Follow-up scans are useful in tracking evolution/regression of hematoma as well as in evaluation of cord myelomalacia, which presents as persistently increased cord echogenicity.[17]
-
Neuroblastoma or other neurogenic tumors with intraspinal extension
Neurogenic tumors such as neuroblastoma or ganglioneuroblastoma may show intraspinal extension which can be visualized on sonography Figure 15.
-
Meningitis
Meningitis is an important cause of infantile morbidity and mortality, and its timely diagnosis and treatment is imperative to prevent neurological sequelae. Meningitis is essentially an inflammatory response of the pia-arachnoid mater and subarachnoid space CSF to the offending pathogen.[18] Thus, in the absence of any flow obstruction, infections in subarachnoid space can freely spread from the brain to spinal cord and vice versa. Spinal sonography performed in infants with suspected meningitis often reveals increased echogenicity and septations in the subarachnoid space CSF.[19] Real time sonography allows detection of decreased or absent pulsations of the spinal cord and nerve roots, which is adjunctive finding favoring meningitis.
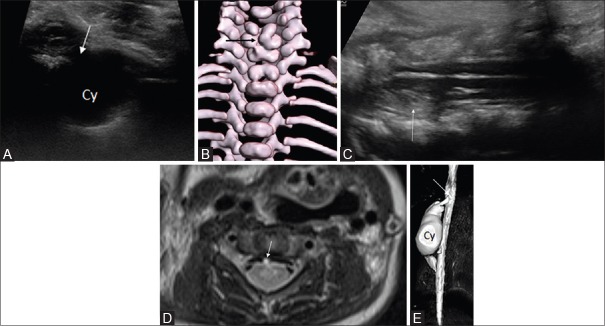
Figure 15 (A-D).
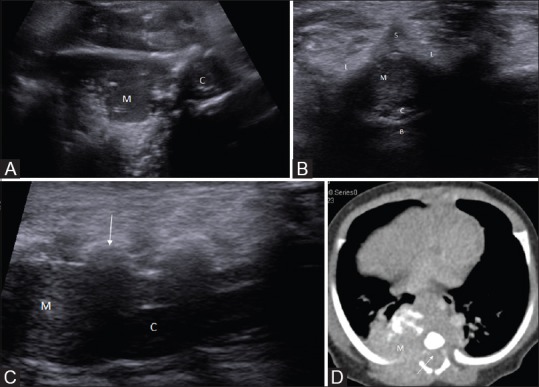
(A-D). Posterior mediastinal neuroblastoma with intraspinal extension. Axial sonogram reveals a large right paraspinal solid mass lesion (M) with intraspinal extension (in B-D). Axial and sagittal sonogram (B, C) show the compression of spinal cord (C) by the intraspinal tumor extension (M). The lamina and spinous processes are marked L, and S, respectively. CECT of thorax showed the enhancing mass having foci of calcification and intraspinal extension (arrows)
Therapeutic use
-
Caudal anesthesia
Caudal epidural block is a popular analgesic technique in pediatric patients for blocking lumbar and sacral nerve roots. The procedure involves injection of medication through the sacral hiatus, which is an inverted U-shaped opening in the dorsal sacral surface lying at the apex of an equilateral triangle formed with the two posterior superior iliac spines.[20] The sacral cornu, which flanks the rostral margin of the sacral hiatus on either side, acts as the surface landmark for identifying the sacral hiatus. Despite the high success rate of this landmark-based approach, difficulties are encountered in neonates owing to incompletely ossified posterior vertebral elements, which render palpation of the sacral cornu difficult.[21] It is here that ultrasound proves its utility by allowing easy identification of the sacral cornu and real time visualization of the injection procedure. The unreliability of the current surface landmark for caudal anesthesia, i.e., equilateral triangle renders the use of ultrasound essential in these procedures, particularly in cases where the sacral cornu are not palpable.[21] In neonates, ultrasound has been used to evaluate the cause of failed lumbar puncture and to estimate the likelihood of success of further lumbar puncture attempts.
Miscellaneous use
-
Lumbar puncture
Lumbar punctures are used for the diagnostic sampling of the CSF to evaluate for suspected infections, hemorrhage, neoplasm, or inflammatory disorders of the central nervous system. In addition, it also serves as a means of instilling therapeutic agents into the intrathecal space. Lumbar puncture in neonates and infants younger than 6 months may be technically difficult owing to incomplete ossification of posterior vertebral elements, thus making palpation of spinal landmarks a challenging affair. Ultrasound is exceptionally useful in such cases because it allows easy identification of relevant spinal landmarks and allows real time visualization of the entire procedure. This helps in drastically reducing instances of incorrect needle placement and decreasing incidence of traumatic lumbar punctures. In addition, sonographic spinal examination performed after an unsuccessful “blind” lumbar puncture helps in diagnosing the cause of failure and any resultant complications such as epidural or subarachnoid space hematoma, which can help in deciding whether or not to intervene further.[22]
Conclusion
Ultrasound is an inexpensive, easily performed, widely available, radiation free investigative technique, which is now considered to be the initial imaging modality of choice for investigating the spinal cord in neonates and infants up to 6 months of age. Its wide ranging diagnostic utility coupled with its high accuracy, especially in expert hands, plays a pivotal role in choosing the type and timing of therapeutic intervention. In addition, spinal sonography also carries therapeutic applications and is useful as an image guidance modality for certain procedures. However, despite all these advantages, spinal ultrasound remains an underutilized and often underestimated modality largely due to lack of awareness. Thus, popularization of spinal sonography for diagnostic and therapeutic uses in neonatal and early infantile population and spreading awareness regarding its merits is an urgent need of the hour.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol. 2002;75:384–92. doi: 10.1259/bjr.75.892.750384. [DOI] [PubMed] [Google Scholar]
- 2.Byrd SE, Darling CF, McLone DG. Developmental disorders of the pediatric spine. Radiol Clin North Am. 1991;29:711–52. [PubMed] [Google Scholar]
- 3.Cramer BC, Jequier SO, Gorman AM. Ultrasound of the neonatal craniocervical junction. Am J Neuroradiol. 1986;7:449–55. [Google Scholar]
- 4.Zieger M, Dorr U, Schulz RD. Pediatric spinal sonography. II. Malformations and mass lesions. Pediatr Radiol. 1988;18:105–11. doi: 10.1007/BF02387552. [DOI] [PubMed] [Google Scholar]
- 5.American Institute of Ultrasound in Medicine (AIUM). Practice Guideline for the Performance of an Ultrasound Examination of the Neonatal Spine. 2007 Oct; doi: 10.7863/jum.2012.31.1.155. Updated 2012. [DOI] [PubMed] [Google Scholar]
- 6.Unsinn KM, Geley T, Freund MC, Gassner I. US of the Spinal Cord in Newborns: Spectrum of Normal Findings, Variants, Congenital Anomalies and Acquired Diseases. Radiographics. 2000;20:923–38. doi: 10.1148/radiographics.20.4.g00jl06923. [DOI] [PubMed] [Google Scholar]
- 7.Barkovich AJ, Naidich TP. In: Congenital anomalies of the spine. Contemporary neuroimaging. Pediatric neuroimaging. 2nd ed. Norman D, editor. Vol. 1. New York, NY: Raven; 1995. pp. 477–540. [Google Scholar]
- 8.Sigal R, Denys A, Halimi P, Shapiro L, Doyon D, Boudghene F. Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatation. Am J Neuroradiol. 1991;12:733–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Rufener SL, Ibrahim M, Raybaud CA, Parmar HA. Congenital Spine and Spinal Cord Malformations—Pictorial Review. AJR Am J Roentgenol. 2010;194:S26–37. doi: 10.2214/AJR.07.7141. [DOI] [PubMed] [Google Scholar]
- 10.Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: A review of neuroradio-logical features with embryological correlations and proposal for a new classification. Neuroradiology. 2000;42:471–91. doi: 10.1007/s002340000325. [DOI] [PubMed] [Google Scholar]
- 11.Fitz CR, Harwood-Nash DC. The tethered conus. Am J Roentgenol. 1975;125:515–23. doi: 10.2214/ajr.125.3.515. [DOI] [PubMed] [Google Scholar]
- 12.Herman TE, Oser RF, Shackelford GD. Inter-gluteal dorsal dermal sinuses: The role of neonatal spinal sonography. Clin Pediatr. 1975;32:627–8. doi: 10.1177/000992289303201012. [DOI] [PubMed] [Google Scholar]
- 13.Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr. 1995;34:650–4. doi: 10.1177/000992289503401205. [DOI] [PubMed] [Google Scholar]
- 14.Lowe LH, Johanek AJ, Moore CW. Sonography of the Neonatal Spine: Part 2, Spinal Disorders. Am J Roentgenol. 2007;188:739–44. doi: 10.2214/AJR.05.2160. [DOI] [PubMed] [Google Scholar]
- 15.Hill CA, Gibson PJ. Ultrasound determination of the normal location of the conus medullaris in neonates. Am J Neuroradiol. 1995;16:469–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Selcuki M, Vatansever S, Inan S, Erdemli E, Bagdatoglu C, Polat A. Is a filum terminale with a normal appearance really normal? Childs Nerv Syst. 2003;19:3–10. doi: 10.1007/s00381-002-0665-1. [DOI] [PubMed] [Google Scholar]
- 17.Leadman M, Seigel S, Hollenberg R, Caco C. Ultrasound diagnosis of neonatal spinal epidural hemorrhage. J Clin Ultrasound. 1988;16:440–2. doi: 10.1002/jcu.1870160614. [DOI] [PubMed] [Google Scholar]
- 18.Baruah D, Gogoi N, Gogoi RK. Ultrasound evaluation of acute bacterial meningitis and its sequale in infants. Indian J Radiol Imaging. 2006;16:553–8. [Google Scholar]
- 19.Nepal P, Sodhi KS, Saxena AK, Bhatia A, Singhi S, Khandelwal N. Role of spinal ultrasound in diagnosis of meningitis in infants younger than 6 months. Eur J Radiol. 2015;84:469–73. doi: 10.1016/j.ejrad.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Senoglu N, Senoglu M, Oksuz H, Gumusalan Y, Yuksel KZ, Zencirci B, et al. Landmarks of the sacral hiatus for caudal epidural block: An anatomical study. Br J Anaesth. 2005;95:692–5. doi: 10.1093/bja/aei236. [DOI] [PubMed] [Google Scholar]
- 21.Mirjalili SA, Taghavi K, Frawley G, Craw S. Should we abandon landmark-based technique for caudal anesthesia in neonates and infants? Paediatr Anaesth. 2015;25:511–6. doi: 10.1111/pan.12576. [DOI] [PubMed] [Google Scholar]
- 22.Coley BD, Shiels WE, 2nd, Hogan MJ. Diagnostic and interventional ultrasonography in neonatal and infant lumbar puncture. Pediatr Radiol. 2001;31:399–402. doi: 10.1007/s002470100453. [DOI] [PubMed] [Google Scholar]