Abstract
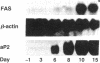
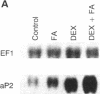
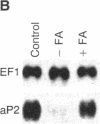
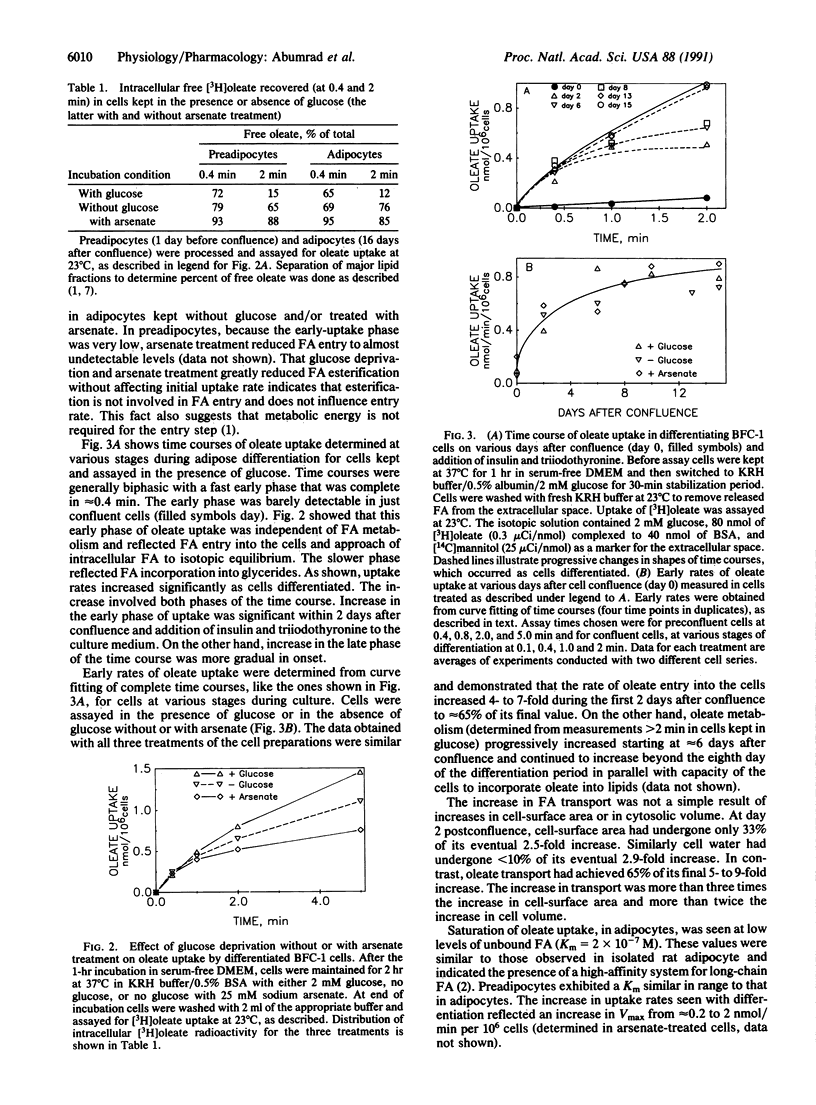
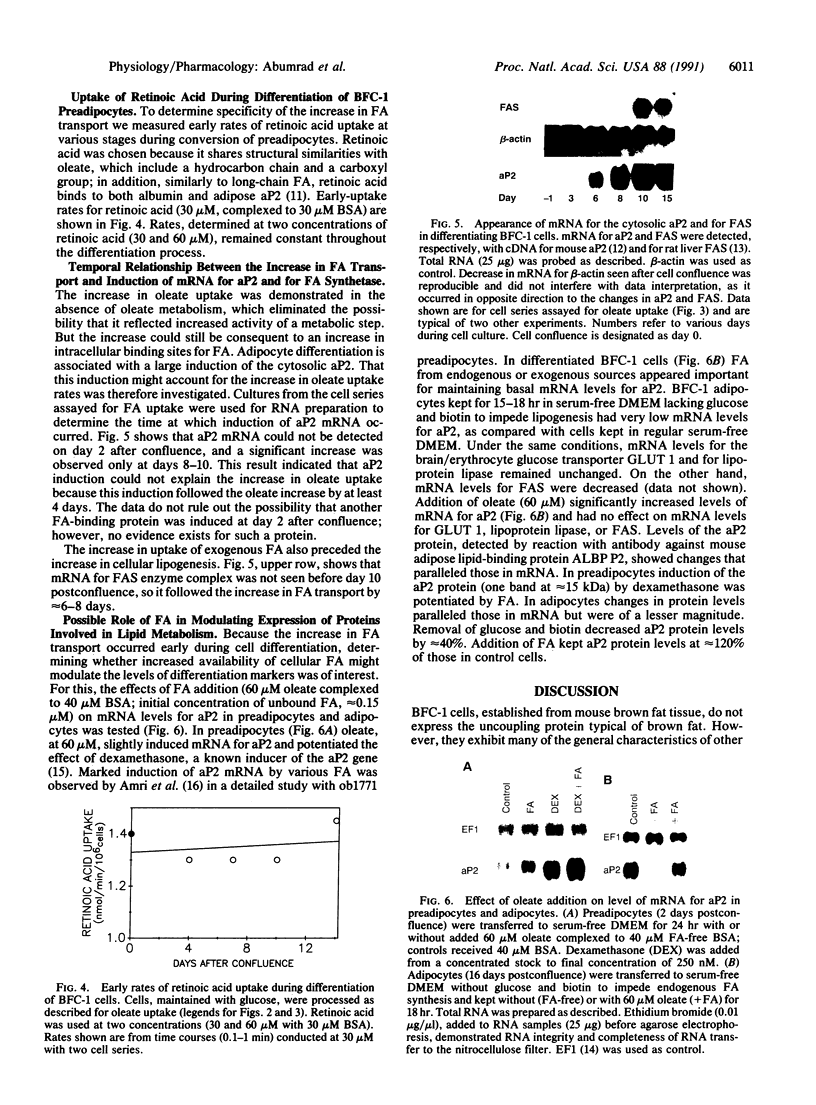
An increase in early rates of oleate uptake, which reflected fatty acid (FA) entry into the cells, was apparent 2-3 days after confluence of differentiating BFC-1 preadipocytes. The increase was measured in cells kept without glucose and with arsenate, where greater than 95% of intracellular radioactivity was recovered as free unesterified oleate. Uptake of retinoic acid, a molecule structurally similar to long-chain FA, remained unaltered during cell differentiation. Increase in oleate transport was related to increase in transport Vmax (determined under arsenate treatment) from 0.2 to 2 nmol/min per 10(6) cells, whereas Km remained unchanged (2 x 10(-7) M). Oleate transport was maximal at about day 6 after cell confluence (day 0), as FA metabolism (incorporation into lipids) began to gradually increase. The increase in transport preceded induction of mRNAs for both cytosolic FA-binding protein, which appeared at day 6, and for the FA synthase, which appeared at day 10. Data indicated that increases in activities of FA transport and of lipoprotein lipase, early during cell differentiation, favored increased availability of exogenous FA at a stage when endogenous FA synthesis is limited. This result would promote FA esterification and lipid deposition by supplying a rate-limiting substrate. Furthermore, oleate addition to BFC-1 preadipocytes at confluence potentiated the effect of dexamethasone in inducing mRNA for cytosolic FA-binding protein. In adipocytes, FA from exogenous or endogenous sources was necessary to maintain levels of cytosolic FA-binding protein mRNA. Thus, the increase in FA availability might contribute to, or modulate, induction of proteins necessary for preadipocyte differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Forest C., Regen D. M., Barnella U. S., Melki S. A. Metabolism of oleic acid in differentiating BFC-1 preadipose cells. Am J Physiol. 1991 Jul;261(1 Pt 1):E76–E86. doi: 10.1152/ajpendo.1991.261.1.E76. [DOI] [PubMed] [Google Scholar]
- Abumrad N. A., Harmon C. M., Barnela U. S., Whitesell R. R. Insulin antagonism of catecholamine stimulation of fatty acid transport in the adipocyte. Studies on its mechanism of action. J Biol Chem. 1988 Oct 15;263(29):14678–14683. [PubMed] [Google Scholar]
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Abumrad N. A., Perry P. R., Whitesell R. R. Insulin antagonizes epinephrine activation of the membrane transport of fatty acids. Potential site for hormonal suppression of lipid mobilization. J Biol Chem. 1986 Mar 5;261(7):2999–3001. [PubMed] [Google Scholar]
- Bernlohr D. A., Doering T. L., Kelly T. J., Jr, Lane M. D. Tissue specific expression of p422 protein, a putative lipid carrier, in mouse adipocytes. Biochem Biophys Res Commun. 1985 Oct 30;132(2):850–855. doi: 10.1016/0006-291x(85)91209-4. [DOI] [PubMed] [Google Scholar]
- Clarke S. D., Armstrong M. K., Jump D. B. Nutritional control of rat liver fatty acid synthase and S14 mRNA abundance. J Nutr. 1990 Feb;120(2):218–224. doi: 10.1093/jn/120.2.218. [DOI] [PubMed] [Google Scholar]
- Cook J. S., Lucas J. J., Sibley E., Bolanowski M. A., Christy R. J., Kelly T. J., Lane M. D. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988 May;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Hunt C. R., Spiegelman B. M. Developmentally regulated mRNAs in 3T3-adipocytes: analysis of transcriptional control. J Cell Biol. 1985 Feb;100(2):514–520. doi: 10.1083/jcb.100.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest C., Doglio A., Ricquier D., Ailhaud G. A preadipocyte clonal line from mouse brown adipose tissue. Short- and long-term responses to insulin and beta-adrenergics. Exp Cell Res. 1987 Jan;168(1):218–232. doi: 10.1016/0014-4827(87)90430-7. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Lauris V., Koch S., Crettaz M., Granner D. K. Acute and chronic regulation of phosphoenolpyruvate carboxykinase mRNA by insulin and glucose. Mol Endocrinol. 1989 May;3(5):840–845. doi: 10.1210/mend-3-5-840. [DOI] [PubMed] [Google Scholar]
- Klip A., Pâquet M. R. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990 Mar;13(3):228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- Matarese V., Bernlohr D. A. Purification of murine adipocyte lipid-binding protein. Characterization as a fatty acid- and retinoic acid-binding protein. J Biol Chem. 1988 Oct 5;263(28):14544–14551. [PubMed] [Google Scholar]
- Noguchi T., Inoue H., Tanaka T. Transcriptional and post-transcriptional regulation of L-type pyruvate kinase in diabetic rat liver by insulin and dietary fructose. J Biol Chem. 1985 Nov 15;260(26):14393–14397. [PubMed] [Google Scholar]
- Nunn W. D., Simons R. W. Transport of long-chain fatty acids by Escherichia coli: mapping and characterization of mutants in the fadL gene. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3377–3381. doi: 10.1073/pnas.75.7.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Goldstein J. L., Brown M. S. 5' end of HMG CoA reductase gene contains sequences responsible for cholesterol-mediated inhibition of transcription. Cell. 1985 Aug;42(1):203–212. doi: 10.1016/s0092-8674(85)80116-1. [DOI] [PubMed] [Google Scholar]
- Paulauskis J. D., Sul H. S. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem. 1989 Jan 5;264(1):574–577. [PubMed] [Google Scholar]
- Potter B. J., Sorrentino D., Berk P. D. Mechanisms of cellular uptake of free fatty acids. Annu Rev Nutr. 1989;9:253–270. doi: 10.1146/annurev.nu.09.070189.001345. [DOI] [PubMed] [Google Scholar]
- Rogers M. B., Watkins S. C., Gudas L. J. Gene expression in visceral endoderm: a comparison of mutant and wild-type F9 embryonal carcinoma cell differentiation. J Cell Biol. 1990 May;110(5):1767–1777. doi: 10.1083/jcb.110.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., John K., Fletcher J. E. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res. 1969 Jan;10(1):56–67. [PubMed] [Google Scholar]
- Suzuki H., Kawarabayasi Y., Kondo J., Abe T., Nishikawa K., Kimura S., Hashimoto T., Yamamoto T. Structure and regulation of rat long-chain acyl-CoA synthetase. J Biol Chem. 1990 May 25;265(15):8681–8685. [PubMed] [Google Scholar]
- Yan C., Wood E. A., Porter J. W. Characterization of fatty acid synthetase cDNA clone and its mRNA. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1235–1241. doi: 10.1016/0006-291x(85)90318-3. [DOI] [PubMed] [Google Scholar]