Abstract
Purpose
Care of children with spina bifida has significantly advanced in the last half century, resulting in gains in longevity and quality of life for affected children and caregivers. Bladder dysfunction is the norm in patients with spina bifida and may result in infection, renal scarring and chronic kidney disease. However, the optimal urological management for spina bifida related bladder dysfunction is unknown.
Materials and Methods
In 2012 the Centers for Disease Control and Prevention convened a working group composed of pediatric urologists, nephrologists, epidemiologists, methodologists, community advocates and Centers for Disease Control and Prevention personnel to develop a protocol to optimize urological care of children with spina bifida from the newborn period through age 5 years.
Results
An iterative quality improvement protocol was selected. In this model participating institutions agree to prospectively treat all newborns with spina bifida using a single consensus based protocol. During the 5-year study period outcomes will be routinely assessed and the protocol adjusted as needed to optimize patient and process outcomes. Primary study outcomes include urinary tract infections, renal scarring, renal function and bladder characteristics. The protocol specifies the timing and use of testing (eg ultrasonography, urodynamics) and interventions (eg intermittent catheterization, prophylactic antibiotics, antimuscarinic medications). Starting in 2014 the Centers for Disease Control and Prevention began funding 9 study sites to implement and evaluate the protocol.
Conclusions
The Centers for Disease Control and Prevention Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida began accruing patients in 2015. Assessment in the first 5 years will focus on urinary tract infections, renal function, renal scarring and clinical process improvements.
Keywords: pediatrics, spinal dysraphism, urinary bladder, neurogenic, urology
Spina bifida is the most common permanently disabling birth defect in the United States, occurring in approximately 3 of 10,000 live births.1,2 Urological complications are a major source of morbidity and may include urinary incontinence, recurrent urinary tract infections, chronic renal insufficiency and end-stage renal disease.3
SB care has significantly advanced in the last half century, resulting in gains in longevity and quality of life for patients and caregivers.4,5 However, the optimal urological care of SB related bladder dysfunction is currently unknown.6 There has been a recent trend toward proactive rather than reactive management of children with SB, although this shift is not well supported by evidence.4,5 This situation raises the possibility that urological care of children with SB can be further optimized, potentially leading to improvements in continence, prevention of chronic renal insufficiency and decreased need for surgery.
To define optimal SB management strategies, the Centers for Disease Control and Prevention recently initiated a collaborative effort with 9 SB centers around the United States, all of which currently participate in the National Spina Bifida Patient Registry and treat a minimum of 5 to 10 newborns yearly with SB. We present the methodological issues and rationale for the CDC Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida.
MATERIALS AND METHODS
Protocol Development and Design
Following a 2005 survey of SB centers by the Spina Bifida Association and CDC, the NSBPR was established to improve the consistency and quality of care of patients with SB. A secondary goal of the NSBPR was to establish an infrastructure to support SB clinical research.7–11
In 2012 the CDC convened a working group of pediatric urologists, nephrologists, clinical epidemiologists, methodologists, community advocates and CDC personnel to develop a standardized protocol to optimize urological care of children with SB from the newborn period through the first 5 years of life. The organizing committee evaluated potential study design options and determined that RCT designs would be impractical and unlikely to be effective due to the budget necessary for an adequate sample size and duration, the likelihood that this proposal would meet with the same accrual challenges that other recent pediatric RCTs have encountered,12,13 and significant concerns over whether a protocol based RCT could be effective due to issues of compliance and contamination.
An iterative quality improvement protocol was instead selected, wherein participating institutions would agree to prospectively treat all eligible newborns with SB using a single consensus based protocol that specifies the timing of subject followup visits, type and frequency of diagnostic testing procedures, and type and nature of any treatment related interventions. Conceptually similar models have been successfully used in common and rare pediatric conditions.14,15
A critical underlying concept of this design is that although the protocol represents current standard of care management as judged by the participating centers, it may need to be modified or customized to meet the needs of some patients. Therefore, each treating physician is free to deviate from the protocol if s/he feels that a deviation is warranted. Any deviations will then be analyzed to determine whether they suggest that the protocol should be modified for a subset of patients or for the entire population. If a modification to the protocol is deemed necessary, then the proposed modification will be evaluated by a steering committee composed of site investigators, CDC personnel and protocol developers. In 2014 the CDC solicited applications from SB centers to implement and evaluate the protocol, and 9 NSBPR centers were chosen.
Study Objectives
The primary objective of this effort is to deploy a standardized management protocol for newborns with MMC across multiple SB centers. The secondary objectives are to determine adherence to the proposed management protocol among providers, subjects and families of newborns with SB; to determine if the proposed management protocol is optimal to maximize urological function while minimizing morbidity; to characterize protocol deviations in case management by different providers and study sites, and to better define the longitudinal impact of SB on bladder and renal function.
Inclusion Criteria
The inclusion criteria for the study are 1) patient age 3 months or less if delivered at a study center, or if the patient transfers care to a protocol institution and the care has followed the protocol with no more than minor deviations since birth, 2) MMC form of SB, and 3) written informed consent by the parent or guardian to participate in the protocol and for the patient to be followed longitudinally. Patients may have undergone either prenatal or postnatal MMC closure.
Study Setting and Interventions
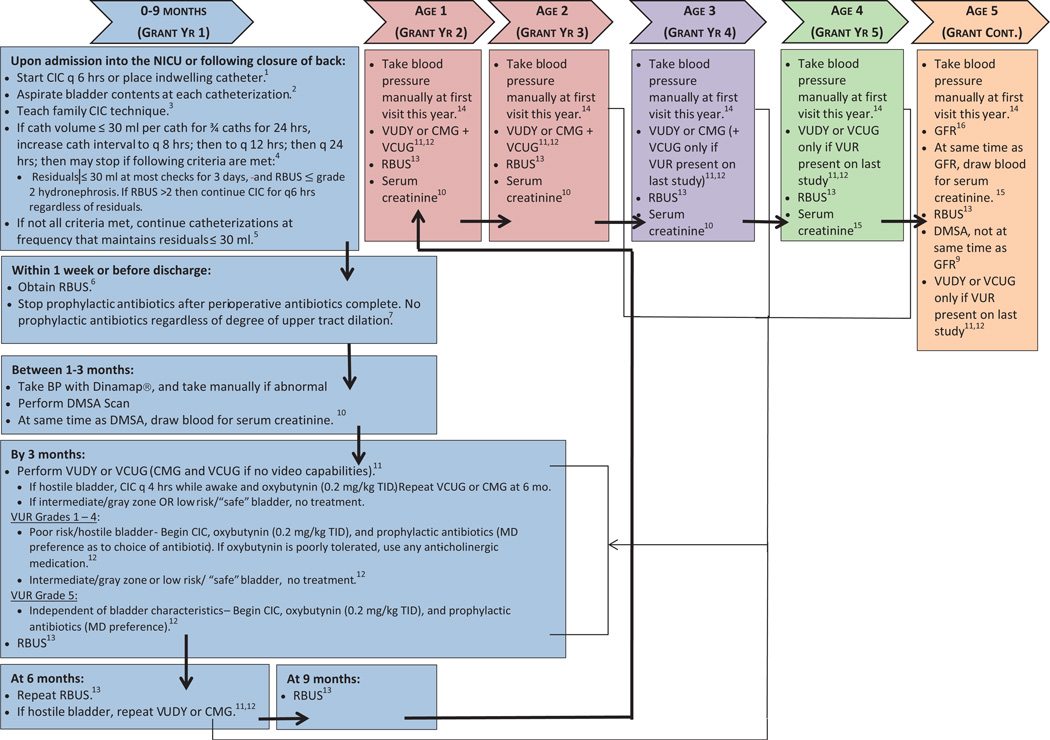
The recruitment and followup protocol is detailed in the figure. Patients may be enrolled during their initial hospital/neonatal intensive care unit stay or during a subsequent clinic visit. The protocol is approved by all study site institutional review boards.
figure.
Study schema. BP, blood pressure. cath, catheterization.CMG, cystometrogram. NICU, neonatal intensive care unit. q, every. TID, 3 times daily. VCUG, voiding cystourethrogram. VUDY, videourodynamics.
Urethral catheterization
Postnatally the bladder is initially drained via indwelling Foley catheter or intermittently catheterized. Once the infant can be moved from the prone position, CIC is initiated and performed every 6 hours to determine residual bladder volumes. Catheterization is continued until bladder volumes are less than 30 ml on the majority of catheterizations for 3 consecutive days with decreasing catheterization frequency. This age based approach was chosen over weight based formulas due to its simplicity (ie no calculations required) to increase the likelihood of successful implementation.16,17 If residual volumes are adequately low, CIC is stopped. If not, then catheterization is continued every 4 hours while the patient is awake. Previous NSBPR data demonstrate that 80% of all individuals with MMC undergo long-term CIC for bladder management.9 Therefore, parents/caregivers of all subjects will be taught intermittent catheterization techniques so that they are familiar and comfortable with the technique regardless of the initial bladder status. Standardized teaching materials were developed to assist with teaching. Continuation of CIC will be dictated by voiding efficiency through time.
Antimuscarinic medications
Oxybutynin is indicated for treatment of detrusor overactivity in patients with neurogenic bladder. A dose of 0.2 mg/kg oxybutynin orally given 3 times daily will be used for subjects noted to have a hostile bladder on urodynamic evaluation.18,19
Prophylactic antibiotics
Although commonly used in children with primary VUR,20 antibiotic use in VUR related to neurogenic bladder is controversial. Few published data exist to support the usefulness of prophylactic antibiotics among subgroups of patients with SB and VUR (eg those with dilating vs nondilating VUR).21,22 Thus, in this protocol antibiotics will be used only for subjects with grade V reflux or a hostile bladder. A dose of 15 mg/kg amoxicillin orally once daily will be administered through age 2 months. Thereafter, the treating physician may choose to use daily trimethoprim/sulfamethoxazole (2 ml/kg) or nitrofurantoin (1 to 2 mg/kg) suspensions.
RESULTS
Study Assessments
Planned interventions and assessments are outlined in the Appendix. A general physical examination will be performed at all visits, and yearly vital sign assessments will include height/length, weight and blood pressure. Recumbent length will be measured in infants and toddlers, and standing height and arm span for older subjects to determine their usefulness and interchangeability in the Schwartz formula for estimating GFR.23
Serum creatinine will be obtained yearly to assess renal function. If UTI is suspected, urinalysis and urine culture will be obtained. Defining criteria for UTI were reached by consensus (supplementary Appendix 1, http://jurology.com/). Standardized teaching materials on UTI diagnosis and treatment have been developed by the CDC in English and Spanish, and will be given to families and primary physicians.
Urodynamics
UDS determination of bladder function will be obtained at 3 months and yearly at ages 1 to 3 years (see figure). Videourodynamic testing is encouraged, although for sites without that capability a combination of voiding cystourethrogram and cystometrogram is an acceptable alternative. NSBPR and this protocol define 4 broad UDS classifications, including hostile bladder, intermediate risk, abnormal but safe and normal.
Hostile bladder is defined as end filling pressure or DLPP 40 cm H2O or greater, or NDO with detrusor sphincter dyssynergia.24 Patients with hostile bladder will be treated with CIC every 4 hours during waking hours and antimuscarinics, with repeat UDS 6 months later to assess treatment effectiveness. Treatment is not specifically recommended for patients with nonhostile bladder. Intermediate risk is defined as NDO, reduced compliance and end filling pressure or DLPP 25 to 39 cm H2O. Abnormal but safe is defined as end filling pressure or DLPP less than 25 cm H2O. Normal bladder is defined as normal capacity, compliance less than 15 cm H2O, no NDO, no detrusor sphincter dyssynergia and minimal post-void residual.
Renal and bladder ultrasound
RBUS will be obtained quarterly, then semiannually and then annually (see figure). Hydronephrosis will be graded according to the Society for Fetal Urology classification.25
Determination of GFR and renal scarring
Determination of renal function is planned at age 5 years using 99mTc-diethylenetriamine pentaacetic acid renal scan. To assess renal scarring, 2 DMSA scans will be performed at ages 3 months (baseline) and 5 years (to assess for acquired renal injury during the course of the study). Renal scarring will be graded via the RIVUR (Randomized Intervention for Children with Vesicoureteral Reflux) scale.26 More frequent testing (eg yearly) was not recommended due to concerns about cost and false-positive results.
As noted previously, creatinine will be used to estimate GFR via the Schwartz formula.23 Although several centers have reported using cystatin C,27,28 this test was not recommended due to the cost, the lack of availability at many study centers and the fact that it is not routinely used as a standard of care test. However, in the future cystatin C may become part of protocol assessments.
Statistical analysis
During the 5-year study period outcomes will be assessed quarterly and deviations from the protocol directly related to process measures, and patient outcomes will be documented (supplementary Appendix 2, http://jurology.com/). Since analyses will be dependent on deviations from the protocol, the power of the study to detect significant changes in the main outcomes will be monitored and associations will be tested only when adequate sample sizes have been reached. It is noteworthy that similar study designs have been successfully applied to monitoring health outcomes and minimizing resource utilization while maximizing guideline compliance.29
Planned Outcomes
Clinical outcomes of interest include UTI, degree of hydronephrosis and renal scarring compared to baseline, blood pressure, GFR and urodynamic findings (supplementary Appendix 2, http://jurology.com/). In addition, the protocol will examine process outcomes such as the proportion of patients who received study interventions as specified in the protocol, and the proportion of UTIs diagnosed and treated as specified in the protocol. We anticipate that longer term outcomes such as renal function and scarring or need for surgical intervention may require a lengthier period before a meaningful assessment can be performed. Continence measures will not yet be assessed since patients will not be older than 5 years. If funding remains available beyond the initial 5-year study period, these topics will be addressed once data have matured sufficiently.
Data Collection
The NSBPR uses a Web based EMR system to provide a reliable, standardized method for data collection (Ground Zero Software, Inc., Palm Springs, California).11 Because all newborn protocol sites participate in NSBPR, this system was adapted to include protocol variables. Ground Zero abstracts deidentified data elements from the EMR and securely transmits the data weekly to the CDC. To ensure data quality, systematic procedures have been implemented at each clinic site and at the CDC. The compiled data set goes through an automatic quality assurance/quality control system at the CDC, with guidance from CDC staff and the steering committee. As with NSBPR, each institution retains data ownership for its patients, while the CDC maintains data ownership of the overall data set.
DISCUSSION
Bladder dysfunction is the norm in children with MMC, and the impact of urological and renal issues on these children and their families is significant.4,5 To our knowledge, this is the first interventional protocol to prospectively define and evaluate the urological and renal management of children with MMC.
This study design is relatively uncommon in the pediatric urology literature. Unlike more typical RCTs or cohort studies, this protocol is flexible. As the study progresses, the protocol will change and (hopefully) improve. Furthermore, analytical strategies will vary depending on the outcome being studied. Clinical outcomes such as UTI development will need to be analyzed differently than protocol outcomes (eg whether the frequency of RBUS can be reduced in the first year of life). “Control” groups will vary as well but we anticipate that these will typically be defined using protocol deviations as a means to identify high (or low) risk groups.
Similar study designs have been used successfully in other fields in terms of improving outcomes and also reducing resource utilization.14,15 Developing this protocol involved significant negotiation and compromises among the development committee, CDC personnel and site investigators. For example several members believe strongly that antibiotic prophylaxis should be used in all patients with VUR, while others believe that there is insufficient evidence to provide for its use. Similarly several investigators believe that UDS and RBUS are used too frequently, while others hold the opposite view. We anticipate similar reactions from the broader pediatric urology community. Given the impossibility of unanimous agreement, we strove to achieve consensus to be able to obtain meaningful, well characterized data.
During the first 5 years of the study our goal is to address several aspects of urological care of children with MMC, including assessing the burden of UTIs, optimizing the impact of anthropometric measurements on renal estimates and improving the care delivery process (for example timing and number of urological studies and interventions). It is anticipated that the study will continue beyond 5 years, and as the protocol evolves through time we anticipate that the outcomes will shift to topics more relevant to older children, such as preservation of renal function, development of continence (assessed in NSBPR beginning at age 5 years) and need for surgical intervention. These goals will be accomplished in large part by studying the instructive outliers, for example subgroups of patients who respond poorly to protocol interventions or individuals who suffer potentially preventable complications of therapy.
This protocol should be considered in the context of its limitations. As a single arm trial, the lack of a defined control group may limit or even prevent some comparisons. However, this protocol design has previously been shown to be highly effective in the study of similar congenital conditions.15 It is thus useful to consider alternatives. A multiarm RCT would not be feasible due to budget limitations and a high likelihood of contamination between study arms. While an RCT can definitively address only its primary hypotheses, secondary analyses are often more useful for generating hypotheses than answering clinically relevant questions. By contrast, this protocol is specifically designed to be adaptable and includes the ability to address unforeseen issues that might arise during the course of the study.
CONCLUSIONS
The CDC Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida began accrual in 2015. This is the first prospective interventional protocol specifically designed to measure and optimize the urological management of newborns and young children with SB. Assessments in the first 5 years will focus on UTIs, renal function, renal scarring and clinical process improvements.
Supplementary Material
Acknowledgments
This project is supported by collaborative agreements with the Centers for Disease Control and Prevention (U01-DD001087). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. No other funding source supports this project.
Abbreviations and Acronyms
- CDC
Centers for Disease Control and Prevention
- CIC
clean intermittent catheterization
- DLPP
detrusor leak point pressure
- DMSA
dimercaptosuccinic acid
- EMR
electronic medical record
- GFR
glomerular filtration rate
- MMC
myelomeningocele
- NDO
neurogenic detrusor overactivity
- NSBPR
National Spina Bifida Patient Registry
- RBUS
renal and bladder ultrasound
- RCT
randomized controlled trial
- SB
spina bifida
- UDS
urodynamics
- UTI
urinary tract infection
- VUR
vesicoureteral reflux
APPENDIX
Planned Procedures and Interventions
| Age of child | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic procedure or intervention | Birth | 3 mos | 6 mos | 9 mos | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr |
| Foley catheter placement-intermittent catheterization* | X | ||||||||
| Teach family CIC technique† | X | ||||||||
| Medical history | X | X | X | X | X | X | X | X | X |
| Physical exam | X | X | X | X | X | X | X | X | X |
| Blood pressure | X | X | X | X | X | X | |||
| Serum creatinine | X | X | X | X | X | X | |||
| RBUS | X | X | X | X | X | X | X | X | X |
| Urodynamics‡ | X | X | X | X | |||||
| DMSA Nuclear Scan | X | X | |||||||
| GFR Nuclear Scan | X | ||||||||
| Antimuscarinic medication§ | |||||||||
| Prophylactic antibiotics‖ | |||||||||
Indwelling Foley urethral catheter is placed during initial neurosurgical closure of the spinal defect, typically in the first 24 hours of life. This is left in place until the infant can be moved from the prone position and clean intermittent catheterization initiated.
Families/caregivers will be taught CIC technique, and family/caregivers must demonstrate competency. CIC will be continued unless bladder is demonstrated to empty efficiently.
Urodynamics are to be performed routinely in all subjects in the first 3 months, at 12–15 months, 24–27 months, and 36–39 months. The study should be repeated at 6 months of age if initial study demonstrated that bladder is hostile by specific urodynamic parameters. The study should be repeated at 3, 4, and 5 years if bladder is hostile or if vesicoureteral reflux is present.
Antimuscarinic medication (oxybutynin 0.2 mg/kg/dose orally 3 times daily) to be prescribed if bladder hostility is diagnosed by urodynamics. Dosage may be increased as clinically indicated.
Prophylactic antibiotics to be prescribed if grade V VUR is present regardless of bladder hostility. For grades I–IV VUR, antibiotics are to be used only if bladder is hostile. Type and dosage of oral prophylactic antibiotic are not mandated, but typically consist of amoxicillin 15 mg/kg daily for first two months of life, then trimethoprim/sulfamethoxazole suspension (2 mg/kg) or nitrofurantoin (1–2 mg/kg) daily.
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
REFERENCES
- 1.Lloyd JC, Wiener JS, Gargollo PC, et al. Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol, suppl. 2013;190:1590. doi: 10.1016/j.juro.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang L, Bolen J, Valdez R, et al. Characteristics and survival of patients with end stage renal disease and spina bifida in the United States Renal Data System. J Urol. 2015;193:558. doi: 10.1016/j.juro.2014.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton DB, Brock JW, III, Joseph DB. Urologic management of spina bifida. Dev Disabil Res Rev. 2010;16:88. doi: 10.1002/ddrr.92. [DOI] [PubMed] [Google Scholar]
- 5.Snow-Lisy DC, Yerkes EB, Cheng EY. Update on urological management of spina bifida from prenatal diagnosis to adulthood. J Urol. 2015;194:288. doi: 10.1016/j.juro.2015.03.107. [DOI] [PubMed] [Google Scholar]
- 6.Wang HH, Lloyd JC, Wiener JS, et al. Nation-wide trends and variations in urological surgical interventions and renal outcome in patients with spina bifida. J Urol. 2016;195:1189. doi: 10.1016/j.juro.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicianno BE, Karmarkar A, Houtrow A, et al. Factors associated with mobility outcomes in a National Spina Bifida Patient Registry. Am J Phys Med Rehabil. 2015;94:1015. doi: 10.1097/PHM.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Ward E, Dicianno BE, et al. Factors associated with pressure ulcers in individuals with spina bifida. Arch Phys Med Rehabil. 2015;96:1435. doi: 10.1016/j.apmr.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawin KJ, Liu T, Ward E, et al. The National Spina Bifida Patient Registry: profile of a large cohort of participants from the first 10 clinics. J Pediatr. 2015;166:444. doi: 10.1016/j.jpeds.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schechter MS, Liu T, Soe M, et al. Sociodemographic attributes and spina bifida outcomes. Pediatrics. 2015;135:e957. doi: 10.1542/peds.2014-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thibadeau JK, Ward EA, Soe MM, et al. Testing the feasibility of a National Spina Bifida Patient Registry. Birth Defects Res A Clin Mol Teratol. 2013;97:36. doi: 10.1002/bdra.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoberman A, Greenfield SP, Mattoo TK, et al. RIVUR Trial Investigators: Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370:2367. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farias M, Jenkins K, Lock J, et al. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff (Millwood) 2013;32:911. doi: 10.1377/hlthaff.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathod RH, Farias M, Friedman KG, et al. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenit Heart Dis. 2010;5:343. doi: 10.1111/j.1747-0803.2010.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaefer M, Zurakowski D, Bauer SB, et al. Estimating normal bladder capacity in children. J Urol. 1997;158:2261. doi: 10.1016/s0022-5347(01)68230-2. [DOI] [PubMed] [Google Scholar]
- 17.Fairhurst JJ, Rubin CM, Hyde I, et al. Bladder capacity in infants. J Pediatr Surg. 1991;26:55. doi: 10.1016/0022-3468(91)90426-t. [DOI] [PubMed] [Google Scholar]
- 18.Kaefer M, Pabby A, Kelly M, et al. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomeningocele. J Urol. 1999;162:1068. doi: 10.1016/S0022-5347(01)68069-8. [DOI] [PubMed] [Google Scholar]
- 19.Kasabian NG, Bauer SB, Dyro FM, et al. The prophylactic value of clean intermittent catheterization and anticholinergic medication in newborns and infants with myelodysplasia at risk of developing urinary tract deterioration. Am J Dis Child. 1992;146:840. doi: 10.1001/archpedi.1992.02160190072024. [DOI] [PubMed] [Google Scholar]
- 20.Wang HH, Gbadegesin RA, Foreman JW, et al. Efficacy of antibiotic prophylaxis in children with vesicoureteral reflux: systematic review and meta-analysis. J Urol. 2015;193:963. doi: 10.1016/j.juro.2014.08.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen RA, Rushton HG, Belman AB, et al. Renal scarring and vesicoureteral reflux in children with myelodysplasia. J Urol. 1990;144:541. doi: 10.1016/s0022-5347(17)39517-4. [DOI] [PubMed] [Google Scholar]
- 22.Johnson HW, Anderson JD, Chambers GK, et al. A short-term study of nitrofurantoin prophylaxis in children managed with clean intermittent catheterization. Pediatrics. 1994;93:752. [PubMed] [Google Scholar]
- 23.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35:471. doi: 10.1002/nau.22751. [DOI] [PubMed] [Google Scholar]
- 25.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23:478. doi: 10.1007/BF02012459. [DOI] [PubMed] [Google Scholar]
- 26.Keren R, Carpenter MA, Hoberman A, et al. Rationale and design issues of the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) study. Pediatrics, suppl. 2008;122:S240. doi: 10.1542/peds.2008-1285d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox JA, Dudley AG, Bates C, et al. Cystatin C as a marker of early renal insufficiency in children with congenital neuropathic bladder. J Urol, suppl. 2014;191:1602. doi: 10.1016/j.juro.2013.09.093. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angoff GH, Kane DA, Giddins N, et al. Regional implementation of a pediatric cardiology chest pain guideline using SCAMPs methodology. Pediatrics. 2013;132:e1010. doi: 10.1542/peds.2013-0086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.