Abstract
Hydroxyphthioceranoic (HPA) and phthioceranoic (PA) acids are polymethylated long chain fatty acids with and without a hydroxyl group attached to the carbon next to the terminal methyl-branched carbon distal to the carboxylic end of the long-chain fatty acid, respectively. They are the major components of the sulfolipids found in the cell wall of Mycobacterium tuberculosis (M. tuberculosis) strain H37Rv. In this report, I describe CID linear ion-trap MSn mass spectrometric approaches combined with charge-reverse derivatization strategy toward characterization of these complex lipids which were released from sulfolipids by alkaline hydrolysis and sequentially derivatized to the N-(4-aminomethylphenyl) pyridinium (AMPP) derivatives. This method affords complete characterization of HPA and PA, including the location of the hydroxyl group and the multiple methyl side chains. The study also led to the notion that the hydroxyphthioceranoic acid in sulfolipid consists of 2 (for hC24) to 12 (for hC52) methyl branches, and among them 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid (hC40) is the most prominent, while phthioceranoic acids are the minor constituents. These results confirm our previous findings that sulfolipid II, a family of homologous 2-stearoyl(palmitoyl)-3,6,6′-tris(hydroxyphthioceranoy1)-trehalose 2′-sulfates is the predominant species, and sulfolipid I, a family of homologous 2-stearoyl(palmitoyl)-3-phthioceranoyl-6,6′-bis(hydroxyphthioceranoy1)-trehalose 2′-sulfates is the minor species in the cell wall of M. tuberculosis.
Keywords: HCD, charge-remote fragmentation, microbial lipids, lipidomics, Linear ion-trap, charge reversed derivatization, Mycobacterium tuberculosis.
Graphical Abstract
Introduction
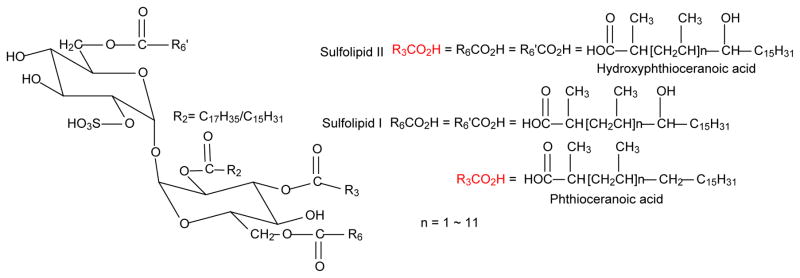
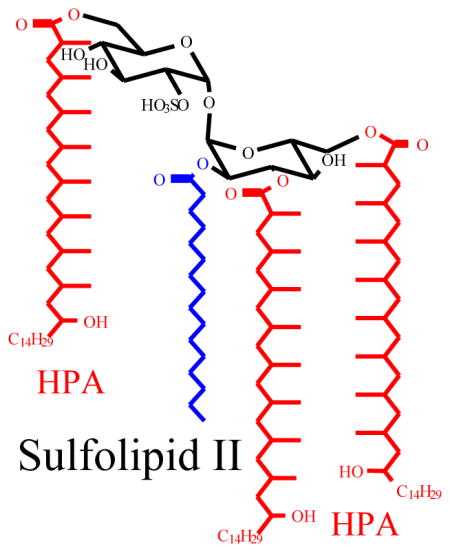
The family of sulfated acyl trehaloses defined as sulfolipids (SLs) were characterized by Goren and coworkers in their early studies on M. tuberculosis H37Rv [1–4]. The principal SLs were thought to be sulfolipid-I (SL-I), which is a homologous mixture of 2,3,6,6′-tetraacyl-α,α′-D-trehalose-2′-sulfate consisting of a pair of hydroxyphthioceranoic acid (HPA) located at 6, and 6′-position, and a nonhydroxylated phthioceranoic acids (PA) and a saturated fatty acid (16:0 or 18:0) located at the 3- and 2-position of the trehalose skeleton, respectively (Scheme 1). In addition to the major SL-I, minor species that were termed as SL-II (2-palmitoyl/stearoyl-3,6,6′-tris-hydroxyphthioceranyl-2′-sulfate), SL-I′ (2-palmitoyl/stearoyl-3,6-bis-phthioceranyl-6′-hydroxyphthioceranyl-2′-sulfate) and SL-II′ (2-palmitoyl/stearoyl-4,6,6′-tris-hydroxyphthioceranyl-2′-sulfate) were also reported [1–4]. However, recent studies with mass spectrometry including high resolution ESI linear ion-trap MSn and MALDI-TOF [5, 6] confirmed that the principal sulfolipid family is sulfolipid II, rather than sulfolipid I reported by Goren.
Scheme 1.
Structures of Sulfolipid I and II.
Both hydroxyphthioceranoic and phthioceranoic acids in sulfolipids are multiple methyl-branched long chain fatty acids. The traditional methods to define the structure require NMR, IR and GC/MS analysis, following alkaline solvolyses of the purified sulfolipid to the free acids, which were then derivatized to methyl esters [3]. Rhoades et al applied multiple stage mass spectrometric approach to locate the hydroxyl side chain of the hydroxyphthioceranoic acids, which were detected as [M − H]− ions formed by skimmer CAD on the intact sulfolipids. However, the location of the methyl side chains along the hydroxyphthioceranoic and phthioceranoic acids could not be assigned [5].
Towards sensitive quantitation and characterization of long chain fatty acid by ESI tandem quadrupole mass spectrometry, conversion of the free fatty acid to the N-(4-aminomethylphenyl) pyridinium (AMPP) derivative and detected as M+ ions was first described by Bollinger et al, followed by several groups [7–10]. This charge-reversed strategy also has been successfully applied to locate the methyl side chain of iso- and anteiso-long chain fatty acids in Listeria monocytogen cells [11]. In this report, similar charge-reversed strategy was used to convert HPA and PA to their AMPP derivatives. This is followed by ESI LIT MSn analysis of the derivatives to locate the hydroxyl and methyl side chains for unambiguous structural assignment of these complex long-chain fatty acids.
Materials and Methods
Materials
AMP+ Mass Spectrometry Kit (50 test) containing AMPP derivatizing reagent, n-butanol (HOBt), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), acetonitrile/DMF, solution, was purchased from Cayman Chemical Co. (Ann Harbor, MI). All other solvents (spectroscopic grade) and chemicals (ACS grade) were obtained from Sigma Chemical Co. (St. Louis, MO).
Sample preparation
M. tuberculosis strain H37Rv were grown and sulfolipids were extracted and isolated as previously described [5]. To the dry sulfolipid extract (200ug), 500 μL methanol and 500 μL tetrabutylammonium hydroxide (40 wt% solution in water) were added. The solution was heated at 75°C for 2 h, cooled to room temperature, and 2 mL water and 2 mL hexane were added, vortexed for 1 min, and centrifuged at 1200 x g for 2 min. The top layer containing hydroxyphthioceranoic and phthioceranoic acids was transferred to a centrifuge tube, dried under a stream of nitrogen, and AMPP derivative was made with the AMP+ Mass Spectrometry Kit, according to the manufacturer’s instruction. Briefly, the dried sample was resuspended in 20 μL ice-cold acetonitrile/DMF (4:1, v/v), and 20 μL of ice-cold 1 M EDCI (3-(dimethylamino)propyl)ethyl carbodiimide hydrochloride) in water was added. The vial was briefly mixed on a vortex mixer and placed on ice. To the vial, 10 μl of 5 mM N-hydroxybenzotriazole (HOAt) solution and 30 μl solution of 15 mM AMPP (in distilled acetonitrile) were added, mixed and heated at 65°C for 30 min. After cooling to room temperature, 1 ml water and 1 ml n-butanol were added. The final solution was vortexed for 1 min, centrifuged at 1200 x g for 3 min and the organic layer was transferred to another vial.
Mass spectrometry
Both high-resolution (R=100,000 at m/z 400) HCD and low-energy CID tandem mass spectrometric experiments were conducted on a Thermo Scientific (San Jose, CA) LTQ Orbitrap Velos mass spectrometer (MS) with Xcalibur operating system. Samples in methanol were infused (1.5 μL/min; ~ 1 pmol/μL) to the ESI source, where the skimmer was set at ground potential, the electrospray needle was set at 4.0 kV, and temperature of the heated capillary was 300°C. The automatic gain control of the ion trap was set to 5×104, with a maximum injection time of 100 ms. Helium was used as the buffer and collision gas at a pressure of 1×10−3 mbar (0.75 mTorr). The MSn experiments were carried out with an optimized relative collision energy ranging from 55–70% and with an activation q value at 0.25, and the activation time at 10 ms to leave a minimal residual abundance of precursor ion (around 20%). For HCD experiments, the collision energy was set at 60–70% and mass scanned from m/z 100 to the upper m/z value that covers the M+ ions. The mass selection window for the precursor ions was set at 1 Da wide to admit the monoisotopic ion to the ion-trap for collision-induced dissociation (CID) for unit resolution detection in the ion-trap or high resolution accurate mass detection in the Orbitrap mass analyzer. Mass spectra were accumulated in the profile mode, typically for 3–10 min for MSn spectra (n=2,3,4). MALDI-TOF spectrum of the same AMPP derivative of the hydroxyphthioceranoic and phthioceranoic acids was also obtained by an Applied Biosystem Voyager DE-STR instrument using α-cyano 4-hydroxycinnamic acid as matrix.
Nomenclature
To facilitate data interpretation, the following abbreviations as previously described were adopted [5, 12]. The abbreviation of the nonhydroxylated multiple methyl-branched phthioceranoic acids, for example, the 2,4,6,8,10,12,14,16-Octamethyl-dotriacontanoic acid is designated as C40-acid to reflect the fact that the structure represents a saturated C40 fatty acid with multiple methyl branches. For hydroxydotriacontanoic acids, e.g., 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid is designated as hC40-acid to reflect the fact that the compound is a saturated C40 fatty acid with multiple methyl side chains and one hydroxyl group attached at C-17. Therefore, the principal SL-II species (the position of the substituents on the trehalose backbone is adopted from the definition by Goren [13], which is a 2-stearoyl-3,6,6′-tris-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl-α,α′-D-trehalose-2′-sulfate) is designated as (18:0, hC40, hC40, hC40)-SL, signifying that the compound consists of one stearoyl and three 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl groups located at 2-, 3-, 6-and 6′-position of the trehalose backbone, respectively; while SL-I molecule such as 2-palmitoyl-3-2,4,6,8,10-Pentamethyl-pentaeicosanoyl-6.6′-bis-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl-α,α′-D-trehalose-2′-sulfate is designated as (16:0, C30, hC40, hC40)-SL.
Results and Discussion
Mass spectrometry of HPA and PA and their AMPP derivatives
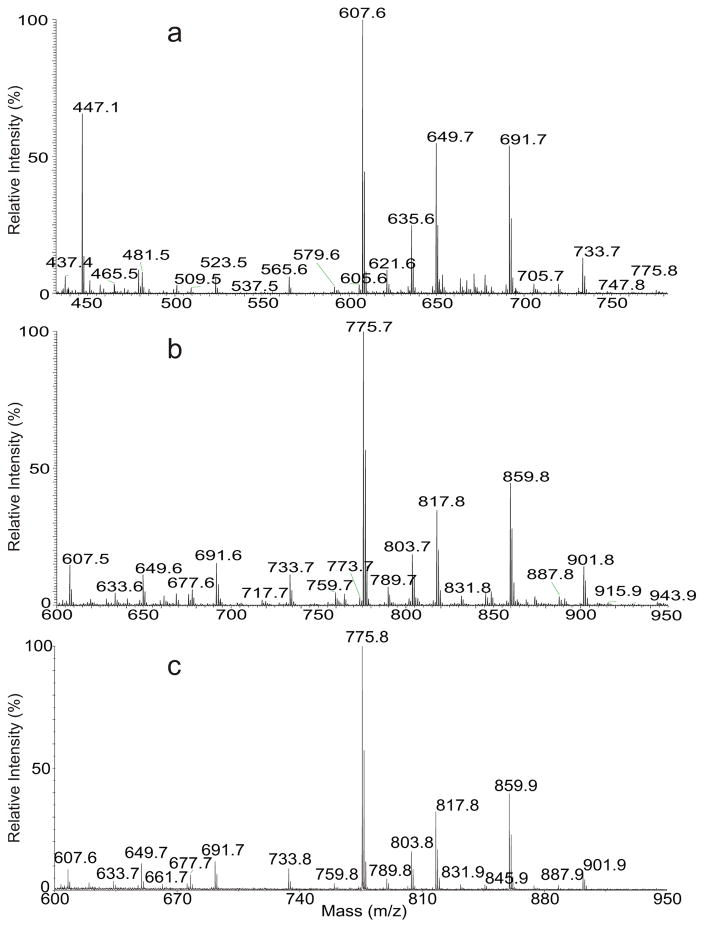
The full scan mass spectra of the released HPA and PA after hydrolysis are shown in Figure 1, in which Panel A represents the [M − H]− ions of the free acids and Panel b represent the [M]+ ions of corresponding AMPP derivative of the acids (Panel b) obtained by ESI. The profile of the MALDI-TOF spectrum of the acid-AMPP derivative (Panel c) is similar to that shown in Panel b, demonstrating the utility of fatty acid-AMPP derivative for sensitive and fast analysis by MALDI-TOF mass spectrometry. High resolution mass measurements on the [M − H]− ions (Table 1) indicate that two ion series were formed. The principal ion series belong to the hydroxyphthioceranoic acid family consisting of homologous ions from m/z 383 (hC24) to m/z 775 (hC52), with 2–12 methyl branches and a hydroxyl group attached to the carbon next to the C15, C16, or C17 alkyl chain terminal; while the minor ion series ranged from m/z 381 (C25) to m/z 675 (C46) belong to the phthioceranoic acid family with no hydroxyl group (Table 1). High resolution mass measurements on the M+ ions of the corresponding AMPP derivatives (with a terminal C5H5N+-C6H4-CH2NH- substituent) confirm the findings (Table 1). These results are consistent with the recent reports that sulfolipid II, which consists of three hydroxyphthioceranoyl substituents is the predominate sulfolipid family found in M. tuberculosis H37Rv, while sulfolipid I that possesses one phthioceranoyl and two hydroxyphthioceranoyl substituents is the minor species[5, 6], a reversal to the earlier findings by Goren [1–3]. The CID and HCD LIT MSn mass spectrometric approaches toward complete structural characterization of these hydrophthioceranoic and phthioceranoic acids as AMPP derivatives are described below.
Figure 1.
The full scan ESI mass spectrum of the hydroxyphthioceranoic and phthioceranoic acids released from alkaline hydrolysis of sulfolipids seen as the [M − H]− ions in the negative-ion mode (a), as the [M]+ ions of the AMPP derivative in positive-ion mode (b), and (c) the MALDI-TOF spectrum of the same AMPP derivative.
Table 1.
High resolution mass measurement of hydroxyphthioceranoic and phthioceranoic acids and their AMPP derivatives of sulfolipid hydrolysate
| Free acid | AMPP derivatives | terminal alkyl chain length |
# of methyl side chain |
structure type | Rel. Int. (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| measured m/z |
Theo. Mass Da | Deviat. (mDa) | Elemental Composition |
measured m/z |
Theo. Mass Da | Deviat. (mDa) | Elemental Composition | ||||
| 383.3527 | 383.3531 | −0.34 | C24 H47 O3 | 551.4563 | 551.4571 | −0.79 | C36 H59 O2 N2 | 17 | 2 | hC24 | 1.96 |
| 397.3684 | 397.3687 | −0.33 | C25 H49 O3 | 565.4722 | 565.4728 | −0.57 | C37 H61 O2 N2 | 15 | 3 | hC25 | 1.97 |
|
| |||||||||||
| 409.4048 | 409.4051 | −0.32 | C27 H53 O2 | 577.5082 | 577.5091 | −0.96 | C39 H65 O N2 | 15 | 4 | C27 | 2.64 |
|
| |||||||||||
| 411.3840 | 411.3844 | −0.33 | C26 H51 O3 | 579.4877 | 579.4884 | −0.73 | C38 H63 O2 N2 | 16 | 3 | hC26 | 0.91 |
|
| |||||||||||
| 423.4204 | 423.4208 | −0.35 | C28 H55 O2 | 591.5240 | 591.5248 | −0.83 | C40 H67 O N2 | 15 | 4 | C28 | 2.55 |
|
| |||||||||||
| 425.3997 | 425.4000 | −0.31 | C27 H53 O3 | 593.5033 | 593.5041 | −0.8 | C39 H65 O2 N2 | 17 | 3 | hC27 | 5.3 |
|
| |||||||||||
| 437.4361 | 437.4364 | −0.27 | C29 H57 O2 | 605.5397 | 605.5404 | −0.73 | C41 H69 O N2 | 16 | 4 | C29 | 7.08 |
|
| |||||||||||
| 439.4154 | 439.4157 | −0.29 | C28 H55 O3 | 607.5190 | 607.5197 | −0.72 | C40 H67 O2 N2 | 15 | 4 | hC28 | 1.31 |
|
| |||||||||||
| 451.4517 | 451.4521 | −0.37 | C30 H59 O2 | 619.5555 | 619.5561 | −0.6 | C42 H71 O N2 | 17 | 4 | C30 | 5.33 |
|
| |||||||||||
| 453.4310 | 453.4313 | −0.36 | C29 H57 O3 | 621.5348 | 621.5354 | −0.55 | C41 H69 O2 N2 | 16 | 4 | hC29 | 0.46 |
|
| |||||||||||
| 465.4674 | 465.4677 | −0.35 | C31 H61 O2 | 633.5713 | 633.5717 | −0.47 | C43 H73 O N2 | 15 | 5 | C31 | 2.4 |
|
| |||||||||||
| 467.4466 | 467.4470 | −0.36 | C30 H59 O3 | 635.5505 | 635.5510 | −0.53 | C42 H71 O2 N2 | 17 | 4 | hC30 | 0.88 |
|
| |||||||||||
| 479.4830 | 479.4834 | −0.32 | C32 H63 O2 | 647.5869 | 647.5874 | −0.48 | C44 H75 O N2 | 16 | 5 | C32 | 9.93 |
|
| |||||||||||
| 481.4623 | 481.4626 | −0.35 | C31 H61 O3 | 649.5662 | 649.5667 | −0.45 | C43 H73 O2 N2 | 15 | 5 | hC31 | 7.54 |
|
| |||||||||||
| 493.4986 | 493.4990 | −0.44 | C33 H65 O2 | 661.6026 | 661.6030 | −0.48 | C45 H77 O N2 | 17 | 5 | C33 | 1.01 |
|
| |||||||||||
| 495.4778 | 495.4783 | −0.46 | C32 H63 O3 | 663.5819 | 663.5823 | −0.41 | C44 H75 O2 N2 | 16 | 5 | hC32 | 0.67 |
|
| |||||||||||
| 507.5143 | 507.5147 | −0.4 | C34 H67 O2 | 675.6184 | 675.6187 | −0.29 | C46 H79 O N2 | 15 | 6 | C34 | 0.8 |
|
| |||||||||||
| 509.4935 | 509.4939 | −0.39 | C33 H65 O3 | 677.5977 | 677.5980 | −0.25 | C45 H77 O2 N2 | 17 | 5 | hC33 | 1.91 |
| 523.5092 | 523.5096 | −0.35 | C34 H67 O3 | 691.6134 | 691.6136 | −0.24 | C46 H79 O2 N2 | 15 | 6 | hC34 | 6.16 |
|
| |||||||||||
| 535.5458 | 535.5460 | −0.2 | C36 H71 O2 | 703.6497 | 703.6500 | −0.27 | C48 H83 O N2 | C36 | 0.8 | ||
|
| |||||||||||
| 537.5247 | 537.5252 | −0.47 | C35 H69 O3 | 705.6290 | 705.6293 | −0.3 | C47 H81 O2 N2 | 16 | 6 | hC35 | 0.35 |
|
| |||||||||||
| 549.5612 | 549.5616 | −0.43 | C37 H73 O2 | 717.6655 | 717.6656 | −0.15 | C49 H85 O N2 | 15 | 7 | C37 | 0.64 |
|
| |||||||||||
| 551.5404 | 551.5409 | −0.45 | C36 H71 O3 | 719.6447 | 719.6449 | −0.17 | C48 H83 O2 N2 | 17 | 6 | hC36 | 0.68 |
| 565.5561 | 565.5565 | −0.45 | C37 H73 O3 | 733.6604 | 733.6606 | −0.11 | C49 H85 O2 N2 | 15 | 7 | hC37 | 6.09 |
|
| |||||||||||
| 577.5924 | 577.5929 | −0.5 | C39 H77 O2 | 745.6966 | 745.6969 | −0.28 | C51 H89 O N2 | 17 | 7 | C39 | 0.35 |
|
| |||||||||||
| 579.5716 | 579.5722 | −0.53 | C38 H75 O3 | 747.6761 | 747.6762 | −0.15 | C50 H87 O2 N2 | 16 | 7 | hC38 | 0.53 |
|
| |||||||||||
| 591.6081 | 591.6086 | −0.45 | C40 H79 O2 | 759.7127 | 759.7126 | 0.11 | C52 H91 O N2 | 15 | 8 | C40 | 2.63 |
|
| |||||||||||
| 593.5873 | 593.5878 | −0.48 | C39 H77 O3 | 761.6920 | 761.6919 | 0.17 | C51 H89 O2 N2 | 17 | 7 | hC39 | 1.39 |
|
| |||||||||||
| 605.6237 | 605.6242 | −0.48 | C41 H81 O2 | 773.6920 | 773.7282 | 0.16 | C53 H93 O N2 | 16 | 8 | C41 | 0.45 |
|
| |||||||||||
| 607.6029 | 607.6035 | −0.62 | C40 H79 O3 | 775.7076 | 775.7075 | 0.06 | C52 H91 O2 N2 | 15 | 8 | hC40 | 100 |
|
| |||||||||||
| 619.6392 | 619.6399 | −0.67 | C42 H83 O2 | 787.7440 | 787.7439 | 0.14 | C54 H95 O N2 | 17 | 8 | C42 | 1.14 |
|
| |||||||||||
| 621.6185 | 621.6191 | −0.59 | C41 H81 O3 | 789.7232 | 789.7232 | 0.05 | C53 H93 O2 N2 | 16 | 8 | hC41 | 8.56 |
|
| |||||||||||
| 633.6549 | 633.6555 | −0.58 | C43 H85 O2 | 801.7597 | 801.7595 | 0.11 | C55 H97 O N2 | 15 | 9 | C43 | 2.82 |
|
| |||||||||||
| 635.6342 | 635.6348 | −0.57 | C42 H83 O3 | 803.7390 | 803.7388 | 0.16 | C54 H95 O2 N2 | 17 | 8 | hC42 | 25.17 |
|
| |||||||||||
| 647.6705 | 647.6712 | −0.64 | C44 H87 O2 | 815.7390 | 815.7752 | 0.15 | C56 H99 O N2 | 16 | 9 | C44 | 0.54 |
|
| |||||||||||
| 649.6497 | 649.6504 | −0.69 | C43 H85 O3 | 817.7546 | 817.7545 | 0.16 | C55 H97 O2 N2 | 15 | 9 | hC43 | 55.51 |
|
| |||||||||||
| 661.6861 | 661.6868 | −0.73 | C45 H89 O2 | 829.7910 | 829.7908 | 0.11 | C57 H101 O N2 | 17 | 9 | C45 | 1.07 |
|
| |||||||||||
| 663.6654 | 663.6661 | −0.7 | C44 H87 O3 | 831.7702 | 831.7701 | 0.1 | C56 H99 O2 N2 | 16 | 9 | hC44 | 5.52 |
|
| |||||||||||
| 675.7017 | 675.7025 | −0.8 | C46 H91 O2 | 843.8059 | 843.8065 | −0.6 | C58 H103 O N2 | 15 | 10 | C46 | 0.73 |
|
| |||||||||||
| 677.6810 | 677.6817 | −0.75 | C45 H89 O3 | 845.7852 | 845.7858 | −0.52 | C57 H101 O2 N2 | 17 | 9 | hC45 | 6.53 |
| 691.6965 | 691.6974 | −0.84 | C46 H91 O3 | 859.8008 | 859.8014 | −0.61 | C58 H103 O2 N2 | 15 | 10 | hC46 | 53.32 |
| 705.7122 | 705.7130 | −0.85 | C47 H93 O3 | 873.8165 | 873.7807 | −0.61 | C59 H105 O2 N2 | 16 | 10 | hC47 | 3.3 |
| 719.7278 | 719.7287 | −0.83 | C48 H95 O3 | 887.8320 | 887.8327 | −0.67 | C60 H109 O2 N2 | 17 | 10 | hC48 | 3.21 |
| 733.7435 | 733.7443 | −0.82 | C49 H97 O3 | 901.8478 | 901.8484 | −0.6 | C61 H111 O2 N2 | 15 | 11 | hC49 | 12.3 |
| 747.7591 | 747.7600 | −0.92 | C50 H99 O3 | 915.8634 | 915.8640 | −0.63 | C62 H111 O2 N2 | 16 | 11 | hC50 | 0.82 |
| 761.7747 | 761.7756 | −0.94 | C51 H101 O3 | 929.8791 | 929.8797 | −0.55 | C63 H113 O2 N2 | 17 | 11 | hC51 | 0.49 |
| 775.7903 | 775.7913 | −0.97 | C52 H103 O3 | 943.8938 | 943.8953 | −1.52 | C64 H115 O2 N2 | 15 | 12 | hC52 | 1.15 |
Characterization of hydroxyphthioceranoic acid-AMPP derivatives
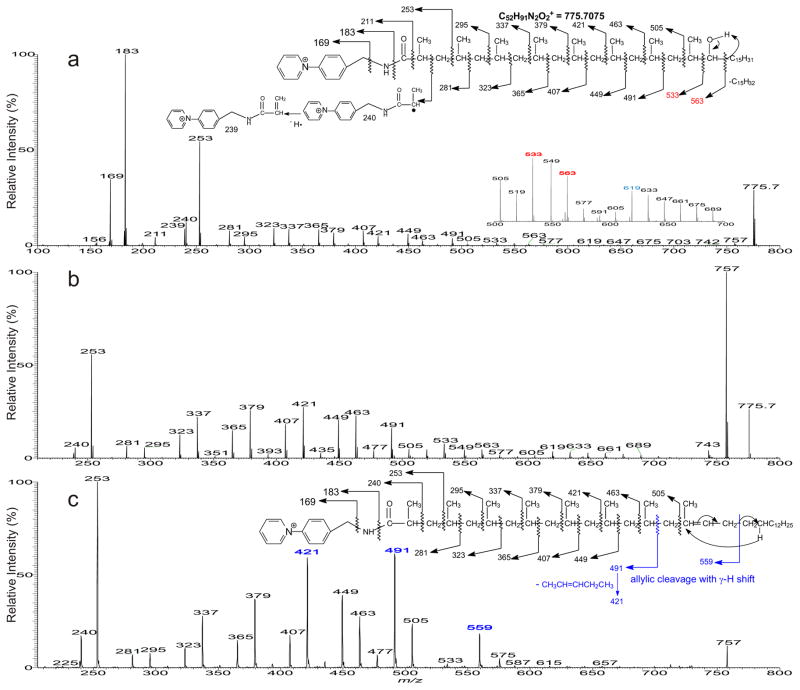
Both CID LIT MSn and the unique HCD MS2 feature of an Orbitrap were employed in these structural studies. As shown in Figure 2a, the HCD MS2 spectrum of the M+ ion of m/z 775 contained prominent ions at m/z 169 and 183, together with m/z 211 that are characteristic ions for the fatty acid-AMPP derivatives [7–9]. The spectrum also contained the ion series of m/z 239, 281, 323, 365, 407, 449, 491, and 533 arising from cleavages of the CH(CH3)-CH2 bonds, together with the ion series of 253, 295, 337, 379, 421, 463, and 505 arising from cleavage of CH2-CH(CH3) bonds along the acid-AMPP chain via charge-remote fragmentation processes, indicating the presence of the multiple methyl groups at 2, 4, 6, 8, 10, 12, 14, 16 of the fatty acid chain (Figure 2a, inset).
Figure 2.
The MS2 spectra of the [M]+ ion of the AMPP derivative of m/z 775 obtained with higher collision energy (HCD) (a), with low energy CID (b), and its MS3 spectrum of the ion of m/z 757 (775 → 757) (c).
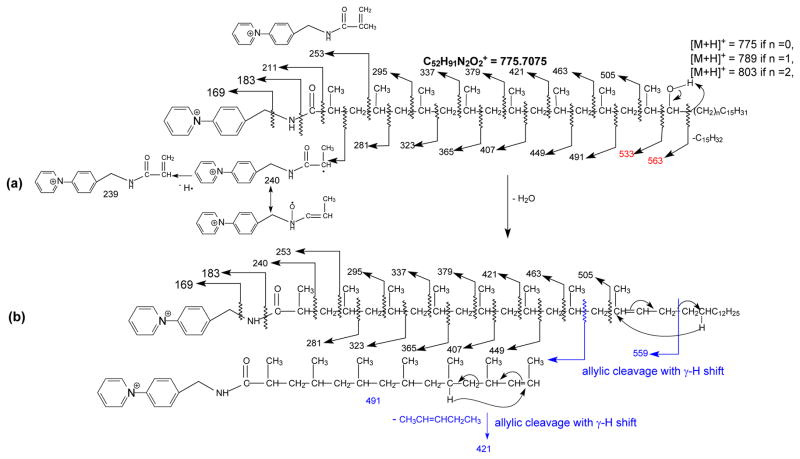
In addition to the above ions locating the methyl groups, ions at m/z 563 arising from cleavage of CH(OH)-C15H31 bond are also present. This ion is 30 Da (CH2O) heavier than the ion of m/z 533 that possesses the terminal methyl side chain, indicating that the hydroxyl side chain is attached to C-17 (Scheme 2a). These results point to the structure of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid (hC40), consistent with that reported by Goren [1–4]. In contrast, the CID MS2 spectrum of the ion of m/z 775 (Figure 2b) is dominated by the ion of m/z 757 arising from loss of H2O, together with the ion series that locate the methyl side chains at 2, 4, 6, 8, 10, 12, 14, 16, as well as the hydroxyl group at C-17 as seen in Figure 2a.
Scheme 2.
The fragmentation processes proposed for the C40-hydroxyphthioceranoic acid-AMPP derivative at m/z 775 under HCD (a), CID (a and b)
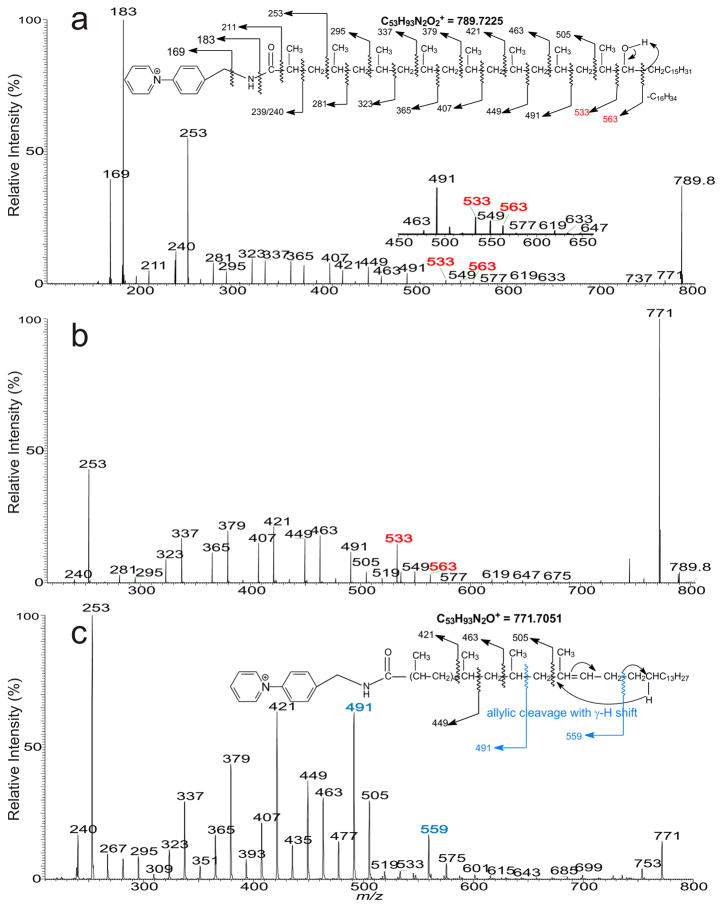
Further dissociation of the ion of m/z 757 (775 → 757; Figure 2c) gave rise to the ion series of m/z 281, 323, 365, 407, 449, and 491 indicating the presence of the methyl side chains at 2, 4, 6, 8, 10, 12, and 14; however, the ions at m/z 533 and 563, previously observed in Figure 2a and 2b are absent. The results indicate that the m/z 757 ion arising from a water loss, likely involves the participation of the hydrogen located at C-16 to form a 2, 4, 6, 8, 10, 12, 14-heptamethyl dotriacont-16-enoic acid (C40:1). The support of this proposed structure is recognized by the presence of the ion of m/z 559 (Figure 2c), arising from cleavage of the allylic bond distal from the cationic pyridinium charge site, via charge remote fragmentation with γ-H rearrangement as shown in Scheme 2b. Similar fragmentation process arising from cleavage of the allylic bond proximal to the charge site also results in the formation of the prominent 1-alkene ion at m/z 491, which undergoes further CRF with γ-H shift to yield the prominent ion of m/z 421 via loss of a CH3CH=CHCH2CH3 residue (Scheme 2b) [14, 15].
A distonic ion at m/z 240 and an abundant ion at m/z 253 were observed in the spectra shown in Figure 2a, 2b, and 2c. The former ion is most likely deriving from homolytic cleavage of the C2-C3 bond, while the latter ion may arise from cleavage of C3-C4 bond to form a stable 2-methyl prop-2-enamide cation (Scheme 2a). The assignments of these ions are consistent with the observation of the analogous distonic ion of m/z 226 and the prop-2-enamide cation at m/z 239 in the MS2 spectra of the palmitate-AMPP derivative released from the 2-palmitoyl substituent of HPA and PA (supplemental material, Figure s1), and of iso- and anteiso fatty acid-AMPP derivatives previously reported [11], and were confirmed by high resolution mass measurements (Table s1, supplemental material). Notably, this distonic ion of m/z 226 was not previously reported in the similar product-ion spectra obtained with a tandem quadrupole instrument [7]. The observation of m/z 240, analogous to m/z 226, also point to the notion that a methyl group is attached to C2, consistent with the assigned structure of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid.
Two pronounced ions at m/z 619 and 549 in the series (Panel 2a and 2b) are worth mentioning. Elemental compositions derived from high resolution mass measurement indicate that ions at m/z 619.5195 (calculated C41H67O2N2; 619.5197 Da) and 549.4409 (calculated C36H57O2N2:549.4415 Da) retain the two oxygen atoms (Table s1) of the precursor ion of m/z 775. MS3 on the ion of m/z 619 (775 → 619; supplemental material Figure s2) yielded the similar ion series of m/z 253, 295, 337, 379, 421, 463, and 505 that define the location of the multiple methyl chains, together with ions at m/z 561, 577 from losses of C3H6O and C3H6 residues (supported by HR mass measurement; data not shown) (Scheme s1), respectively. The results point to the notion that the ion may contain a terminal cyclic tetrahydropyran ring, which is likely formed by cyclization and cleavage of a C11H24 moiety (see the inset scheme in Figure s2 for fragmentation). Similar fragmentation process may also result in the formation of the ion of 549 by loss of a C16H34 residue. This structural information may be an indication of the location of the hydroxyl side chain; however, more studies are required to confirm this finding.
The HCD MS2 spectrum of the ion of m/z 789 (Figure 3a) and the CID MS2 spectrum of the ion of m/z 789 (Figure 3b) and its subsequent MS3 spectrum of m/z 771 (Figure 3c) all contained the identical ion series of m/z 239, 281, 323, 365, 407, 449, 491, and 533, as well as of m/z 253, 295, 337, 379, 421, 463, and 505 as seen earlier (Figure 2), defining the methyl side chains at 2, 4, 6, 8, 10, 12, 14, 16; while ions at m/z 563 indicate the presence of the hydroxyl group at C-17. These structural information led to the assignment of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxytritriacontanoic acid (hC41) in which a terminal C16-alkyl chain is attached to the distal (OH)CH-terminal. A series of the homologous ions consisting of a terminal C16-alkyl chain with various methyl side chains were observed at m/z 579, 621, 663, 705, 747, 789, 831, and 873 (Table 1). The structures of this minor ion series had not been previously reported by Goren [3].
Figure 3.
The MS2 spectra of the [M]+ ion of the AMPP derivative of m/z 789 obtained with HCD (a), with CID (b), and the sequential MS3 spectrum of m/z 771 (789 → 771) (c).
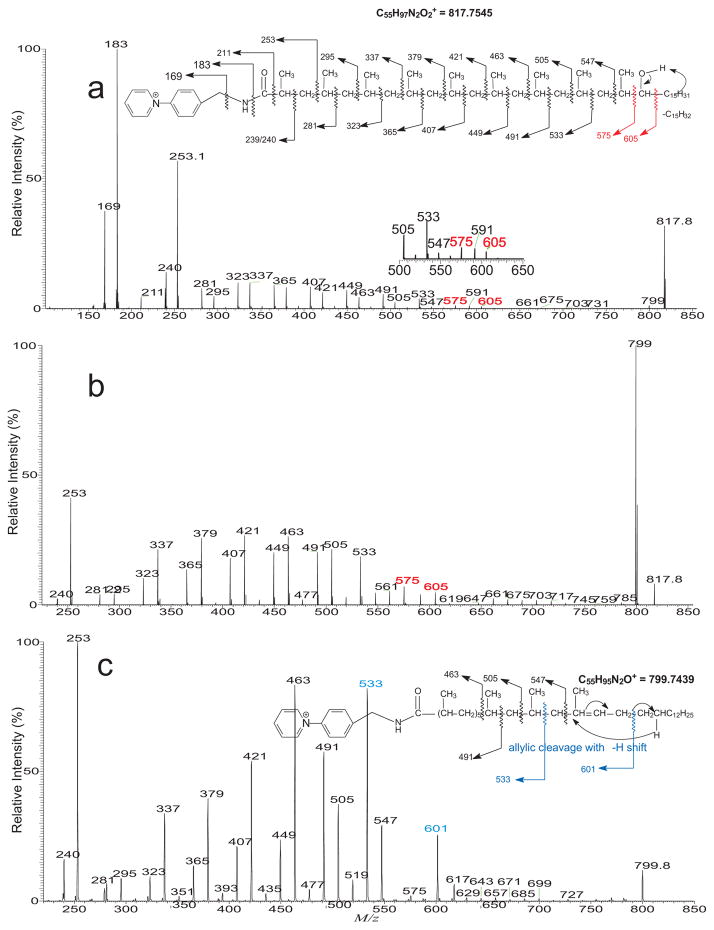
Assignments of the compounds possessing various methyl side chains are exemplified by the HCD MS2 spectrum of the ion of m/z 817 (Figure 4a), which comprises ions at m/z 239, 281, 323, 365, 407, 449, 491, 533 and 575, along with the ion at m/z 605. The results indicate the presence of the methyl side chains at 2, 4, 6, 8, 10, 12, 14, 16, 18 and the hydroxyl group at C-19, corresponding to 2,4,6,8,10,12,14,16,18-nonamethyl-19-hydroxytetratriacontanoic acid (inset). This structural assignment is further supported by the CID MS2 spectrum of m/z 817 (Figure 4b), and the MS3 spectrum of the ion of m/z 799 (817 → 799; Figure 4c), arising from loss of water. The spectrum contained the ion series of m/z 239, 281, 323, 365, 407, 449, 491, and 533, and of m/z 253, 295, 337, 379, 421, 463, 505, and 547 along with the ion of m/z 601 and 533 from allylic cleavages with γ-H shift, analogous to those seen in Figure 2c.
Figure 4.
The MS2 spectra of the ion of m/z 817 obtained with HCD (a), with low energy CAD (b), and its MS3 spectrum of the ion of m/z 799 (817 → 799) (c).
Characterization of phthioceranoic acid-AMPP derivatives
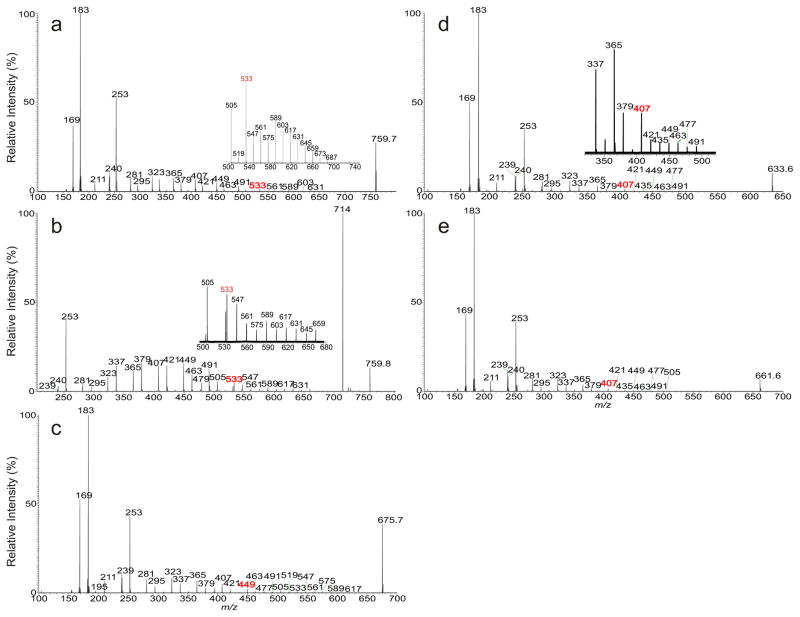
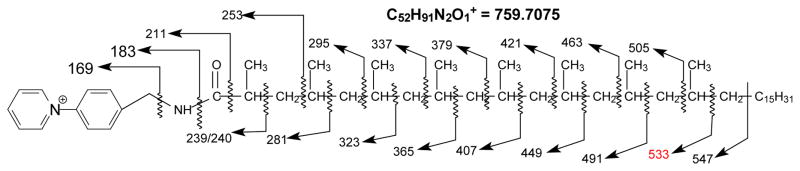
HCD and low energy CID tandem mass spectrometry toward characterization of AMPP derivative of phthioceranoic acid family was exemplified by the ion species at m/z 759, which gave rise to the HCD MS2 spectrum (Figure 5a) with feature ions of m/z 169, 183, and 211 along with the ion series of m/z 253, 295, 337, 379, 421, 463, and 505, and of m/z 239/240, 281, 323, 365, 407, 449, 491 and 533 that locate the multiple methyl side chains at 2, 4, 6, 8, 10, 12, 14, 16 of the fatty acid backbone (Scheme 3). The spectrum also contained ions at m/z 547, 561, 575, 589, 603, 617, 631, 645, 659, …, etc (Figure 5a, subset), arising from CRF cleavage of the C-C bonds of the n-alkyl terminal, indicating the attachment of a terminal n-hexaoctanyl (n-C16) residue. The above information gives assignment of 2,4,6,8,10,12,14,16-Octamethyl-dotriacontanoic acid structure (C40). Similar ions were also observed in the CID MS2 spectrum of the ion of m/z 759 (Figure 5b and inset), however, the spectrum is dominated by the ion of m/z 714, which is absent in Figure 5a. High resolution mass measurement of the ion (measured m/z: 714.7506 Da) failed to match an interpretable elemental composition, indicating that the ion may be artificial and the source of this artifact is unclear. Nevertheless, the results readily located the multiple methyl side chains and gave assignment of the C-40 phthioceranoic acid structure.
Figure 5.
The MS2 spectra of the ion of m/z 759 obtained with HCD (a), with low energy CAD (b), and the HCD MS2 spectra of the ions of m/z 675 (c), 633 (d) and of m/z 661 (e).
Scheme 3.
The fragmentation processes proposed for the C40-phthioceranoic acid-AMPP derivative at m/z 759
Similarly, the HCD mass spectrum of m/z 675 (Figure 5c) contained the ion series of m/z 239/240, 281, 323, 365, 407 and 449, and of m/z 253, 295, 337, 379, and 421 that locate the methyl groups at 2, 4, 6, 8, 10, and 12; together with ions at m/z 463, 477, 505, 519, 533, 547, … etc, that arise from cleavages of the terminal n-alkyl C-C bond. The results led to assignment of 2,4,6,8,10,12-hexamethyl-octaeicosanoic acid (C34), possessing a terminal n-C16 residue. The HCD mass spectra of m/z 633 (Figure 5d) and of 661 (Figure 5e) all contained the ion series of m/z 239/240, 281, 323, 365, and 407, along with the ions of 253, 295, 337, and 379 indicating the presence of the methyl side chains at 2, 4, 6, 8, and 10. These ions together with the ions of m/z 421, 435, 449, 463, 477, 491, 505, 519, 533, and etc, arising from CRF cleavages of the terminal n-alkyl chain, point to the notion that the former spectrum (Figure 5d) represents a 2,4,6,8,10-pentamethyl-hexaeicosanoic acid (C31); while the latter represents a 2,4,6,8,10-pentamethyl-octaeicosanoic acid structure (C33),in which the n-alkyl terminal is C2 longer (i.e., n-C18 chain).
Conclusions
Both the CID MSn and HCD tandem mass spectra of the AMPP derivatives of HPA and PA obtained with an Orbitrap provide structural information for complete characterization of their structures. Fragment ions arising from classical charge-remote fragmentations readily recognize the multiple methyl side chains and the hydroxyl groups. Although the sensitivity of the AMPP derivative of the hydroxyphthioceranoic and phthioceranoic acids was not evaluated in this study, a significant improvement in the detection by mass spectrometry was observed, as compared to that seen as the [M − H]− ions in the previous studies [5]. Thus, characterization of the minor species becomes feasible, and the structures including the minor phthioceranoic acid family and the low abundance ions such as 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxytritriacontanoic acid in the hydroxyphthioceranoic acid family can be determined. This latter species contains a terminal C16-alkyl chain and was not reported previously [1–3]. The observation of near equal abundances of palmitic and stearic acids in the hydrolysate (data not shown) is also consistent with the notion that sulfolipids consist of 2-palmitoyl/stearoyl substituent.
Supplementary Material
Acknowledgments
This research was supported by US Public Health Service Grants P41GM103422, P30DK020579, P30DK056341, and R21HL120760. The author thanks Dr. Elizabeth Rhoades of Cornell University for providing the sulfolipid samples.
Abbreviations
- ESI-MS
electrospray ionization-MS
- HRMS
high resolution mass spectrometry
- LIT
linear ion-trap
- HCD
higher energy collision induced dissociation
- HPA
hydroxyphthioceranoic acid
- PA
and phthioceranoic acid
- AMPP
N-(4-aminomethylphenyl) pyridinium
Footnotes
Supplementary materials are available.
References
- 1.Goren MB. Sulfolipid of Mycobacterium tuberculosis, strain H37Rv. I Purification and properties. Biochim Biophys Acta. 1970;210:116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- 2.Goren MB. Sulfolipid of Mycobacterium tuberculosis, strain H37Rv. II structure studies. Biochim Biophys Acta. 1970;210:127–138. doi: 10.1016/0005-2760(70)90068-8. [DOI] [PubMed] [Google Scholar]
- 3.Goren MB, Brokl O, Das BC, Lederer E. SulfolipidI of Mycobaterium tuberculosis, Strain H37Rv. Nature of the Acyl substituents. Biochemistry. 1971;10:72–81. doi: 10.1021/bi00777a012. [DOI] [PubMed] [Google Scholar]
- 4.Goren MB, Brokl O, Roller P, Fales HM, Das BC. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-I) Biochemistry. 1976;15:2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- 5.Rhoades ER, Streeter C, Turk J, Hsu FF. Characterization of Sulfolipids of Mycobacterium tuberculosis H37Rv by Multiple-Stage Linear Ion-Trap High-Resolution Mass Spectrometry with Electrospray Ionization Reveals That the Family of Sulfolipid II Predominates. Biochemistry. 2011;50:9135–9147. doi: 10.1021/bi2012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layre E, Cala-De Paepe D, Larrouy-Maumus G, Vaubourgeix J, Mundayoor S, Lindner B, et al. Deciphering sulfoglycolipids of Mycobacterium tuberculosis. J Lipid Res. 2011;52:1098–1110. doi: 10.1194/jlr.M013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K, Dilthey BG, Gross RW. Identification and Quantitation of Fatty Acid Double Bond Positional Isomers: A Shotgun Lipidomics Approach Using Charge-Switch Derivatization. Analytical Chemistry. 2013;85:9742–9750. doi: 10.1021/ac402104u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M, Turecek F, Gelb MH. Improved Sensitivity Mass Spectrometric Detection of Eicosanoids by Charge Reversal Derivatization. Anal Chem. 2010;82:6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Han RH, Han X. Fatty Acidomics: Global Analysis of Lipid Species Containing a Carboxyl Group with a Charge-Remote Fragmentation-Assisted Approach. Analytical Chemistry. 2013;85:9312–9320. doi: 10.1021/ac402078p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollinger JG, Rohan G, Sadilek M, Gelb MH. LC/ESI-MS/MS detection of FAs by charge reversal derivatization with more than four orders of magnitude improvement in sensitivity. J Lipid Res. 2013;54:3523–3530. doi: 10.1194/jlr.D040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatituri RV, Wolf B, Brenner M, Turk J, Hsu FF. Characterization of polar lipids of Listeria monocytogenes by HCD and low-energy CAD linear ion-trap mass spectrometry with electrospray ionization. Anal Bioanal Chem. 2015;407:2519–2528. doi: 10.1007/s00216-015-8480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill AH., Jr . In: Sphingolipids. Vance DE, Vance JE, editors. Elsevier; San Diego: 2008. [Google Scholar]
- 13.Goren MB, Brokl O, Das BC. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-I) Biochemistry. 1976;15:2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- 14.Hsu FF, Turk J. Elucidation of the double-bond position of long-chain unsaturated fatty acids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2008;19:1673–1680. doi: 10.1016/j.jasms.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu FF, Turk J. Structural characterization of unsaturated glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom. 2008;19:1681–1691. doi: 10.1016/j.jasms.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.