Abstract
Defensins are a class of immune peptides with a broad range of activities against bacterial, fungal and viral pathogens. Besides exerting direct anti-microbial activity via dis-organization of bacterial membranes, defensins are also able to neutralize various unrelated bacterial toxins. Recently, we have demonstrated that in the case of human α- and β-defensins, this later ability is achieved through exploiting toxins’ marginal thermodynamic stability, i.e. defensins act as molecular anti-chaperones unfolding toxin molecules and exposing their hydrophobic regions and thus promoting toxin precipitation and inactivation [Kudryashova et al. (2014) Immunity 41, 709–721]. Retrocyclins (RCs) are humanized synthetic θ-defensin peptides that possess unique cyclic structure, differentiating them from α- and β-defensins. Importantly, RCs are more potent against some bacterial and viral pathogens and more stable than their linear counterparts. However, the mechanism of bacterial toxin inactivation by RCs is not known. In the present study, we demonstrate that RCs facilitate unfolding of bacterial toxins. Using differential scanning fluorimetry (DSF), limited proteolysis and collisional quenching of internal tryptophan fluorescence, we show that hydrophobic regions of toxins normally buried in the molecule interior become more exposed to solvents and accessible to proteolytic cleavage in the presence of RCs. The RC-induced unfolding of toxins led to their precipitation and abrogated activity. Toxin inactivation by RCs was strongly diminished under reducing conditions, but preserved at physiological salt and serum concentrations. Therefore, despite significant structural diversity, α-, β- and θ-defensins employ similar mechanisms of toxin inactivation, which may be shared by anti-microbial peptides from other families.
Keywords: bacterial toxins, defensins, retrocyclins, structural plasticity, thermodynamic instability, unfolding
INTRODUCTION
Defensins are small cysteine-rich peptides used throughout animal and plant kingdoms as a frontline innate immune defence against broad-spectrum bacterial, fungal and viral pathogens [1]. In primates, β- and θ-defensins are produced by various leucocytes and epithelial cells, whereas the production of α-defensins is limited to neutrophils and Paneth cells of the small intestine. In humans, production of α- and β-defensins can be either constitutive or inducible [2]; however, synthesis of human θ-defensin is blocked at the protein level due to the presence of a premature stop codon in the mRNA transcript of the θ-defensin pseudogene [3]. Given the anti-HIV-1 activity of θ-defensins [4], their evolutionary loss was suggested to be a contributing factor to HIV-1 susceptibility in humans [5].
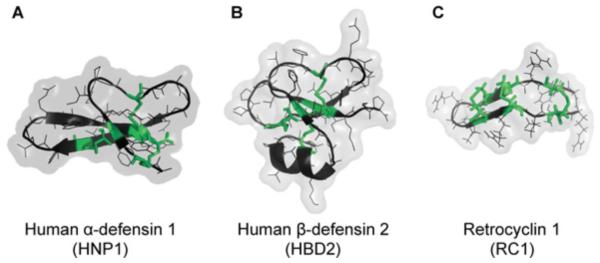
All defensins share three characteristic intramolecular disulfide bonds and are divided into α-, β- and θ-subfamilies based on the pattern of these bonds, size of the molecule and the nature of the peptide (linear in α- and β-defensins compared with cyclic in θ-defensins; Figure 1). The secondary and tertiary structures of α- and β-defensins are conserved despite their variable primary sequence. Three disulfide bonds stabilize the three antiparallel β-sheets of α- and β-defensins (Figures 1A and 1B). The later primarily differ from each other by the length of the N-terminal α-helix segment (Figure 1B). Initially discovered in old-world primates [6], θ-defensins are the most recently evolved and the only cyclic peptides known to be produced by animals [7]. They are formed by head-to-tail ligation of the translation products from either one gene or two separate genes resulting in an 18-resudue cyclic θ-defensin peptide [6]. Intramolecular disulfide bonds of θ-defensins cross-linking the cyclic peptide backbone are arranged in a unique cyclic cystine ladder motif (Figure 1C) [8]. Products from several θ-defensin genes can be paired together in different combinations diversifying the subfamily [9].
Figure 1. Structure of α-, β- and θ-defensins.
(A) Human α-defensin 1. (B) Human β-defensin 2. (C) RC-1. Images were generated by PyMOL (http://www.pymol.org/) using PDB structures: 3GNY (A), 1FD3 (B), and 1HVZ (C). Characteristic cysteines connected via disulfide bonds are coloured in green. Note that even though structures of monomeric defensins are shown, in solution defensins have a tendency to form dimers or higher-order oligomers.
With an anticipation of high therapeutic potential, human θ-defensin was synthesized based on the sequence encoded by the human θ-defensin pseudogenes and named retrocyclin-1 (RC-1) [10]. It was found that synthetic RCs share anti-bacterial activities with other natural defence peptides. Similar to α-defensins, they effectively kill bacteria by permeabilizing bacterial cell membranes [11]. Remarkable anti-viral properties of RCs were predicted based on their similarities to primate θ-defensins and attributed to their ability to bind both viral and host membrane glycoproteins involved in viral entry [4,12,13]. Finally, similar to α- and β-defensins, RCs were shown to inhibit several unrelated bacterial toxins, {e.g., Bacillus anthracis toxin [14], Gardnerella vaginalis toxin [15] and Listeria monocytogenes listeriolysin O (LLO) [16]}, but the mechanisms of selective inhibition of toxins (without affecting host proteins) remained enigmatic.
Recently, we showed that human defensins from α- and β-subfamilies act as molecular anti-chaperones, i.e. take advantage of low thermodynamic stability of bacterial toxins to promote their unfolding [17]. Indispensable for pore forming or passing through the host membrane, the thermodynamic instability of toxins is efficiently exploited by defensins; destabilization by co-folding with defence peptides leads to toxin partial unfolding and precipitation via exposed hydrophobic regions. The defensin-promoted exposure of hydrophobic regions of toxins may increase their immunogenicity [18] and susceptibility to proteolytic cleavage and degradation [17].
Because the mechanisms of toxin inactivation by RCs remained unaddressed in previous studies, the present work is aimed to bridge this gap in our understanding of innate immune mechanisms and to investigate whether RCs neutralize bacterial effector proteins by mechanisms similar to those employed by other families of human defensins.
EXPERIMENTAL
Retrocyclins and proteins
Synthesis of RC-1 (a gift of Dr Robert Lehrer, UCLA) [10] and RC-101 [19] was described previously. Actin cross-linking domains (ACD) of MARTX (multi-functional auto-processing repeats-in-toxin) toxins from Vibrio cholerae and Aeromonas hydrophila (ACDVc and ACDAh) and a construct that spans all four effector domains of MARTXVc in their natural orientation (4dMARTXVc), were expressed in Escherichia coli and purified as described previously [20]. Clostridium difficile toxin A and B glucosyltransferase domains (TcdA– and TcdB–GTD) were expressed in Bacillus megaterium cells (provided by Dr Lacy, Vanderbilt University) and purified as described [21]. Purification procedures for Bacillus anthracis protective antigen (PA) [22], LLO and Streptococcus pneumoniae pneumolysin (PLY) [23,24] were published previously. LFnACD, a fusion construct of the N-terminus of anthrax lethal factor (LFn) and ACD, was purified as described [25]. Skeletal muscle actin preparation from rabbit skeletal muscle acetone powder [26] (Pel-Freez Biologicals) and human plastin isoform 3 (PLS3) [27] were described previously. Human IgG and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were obtained from Sigma–Aldrich.
Limited proteolysis
Limited proteolysis procedure was conducted as described previously [17]. Briefly, 5 μM ACDVc protein was mixed with 25 μM RC-1 or RC-101 in 20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2 buffer and cleaved by chymotrypsin (1:100 w/w ratio to protein) or thermolysin (1:200 w/w ratio) at 30 °C. Protein samples were resolved on SDS/PAGE. Total protein content (sum of all bands in a lane) was quantified using ImageJ software (http://rsb.info.nih.gov/ij/) [28].
Acrylamide collisional quenching of tryptophan fluorescence
Tryptophan fluorescence quenching experiments were performed using a multifunctional plate reader (Tecan) with excitation and emission wavelengths 295 and 328 nm respectively. ACDVc was diluted in PBS (pH 7.4) to 2 μM with or without addition of5 fold mole excess of RC-101 and titrated with increasing amounts of freshly prepared acrylamide solution in PBS. Data were presented as Stern–Volmer plots, where the ratios of fluorescence intensities (F0 /F) in the absence (F0) and presence (F) of a given quencher (acrylamide) concentration were plotted against quencher concentration [29].
Differential scanning fluorimetry
Temperature melting profiles of 10 μM proteins in PBS (pH 7.4) in the presence of SYPRO Orange (SO) dye (Invitrogen) were obtained using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) as described previously [20].
Precipitation assay
Precipitation of ACDVc and ACDAh was assessed by differential ultra-centrifugation. Prior to centrifugation, both ACDVc and ACDAh (5 μM final concentrations) were incubated in the absence or presence of RC-101 (at 1:1, 1:2, 1:3 or 1:5 molar ratios to proteins) for 30 min at 30 °C in a buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl. For reducing condition experiments, all the components (buffer solution, both proteins and RC-101) were supplemented with 10 mM tris(2-carboxyethyl)phosphine (TCEP) before mixing. Aliquots of the samples with and without 25 μM RC-101, in the presence or absence of TCEP, were withdrawn immediately prior to centrifugation for the ACD cross-linking activity assay (see below). All samples were centrifuged using a TLA-100 rotor in an Optima TL-100 ultracentrifuge (Beckman Coulter) at 280 000 g for 30 min at 4 °C. The supernatant and pellet fractions were resolved on SDS/PAGE. Gels were analysed using ImageJ software (http://rsb.info.nih.gov/ij/).
ACD cross-linking activity assay
ACD cross-linking activity assay was described in detail previously [30]. Briefly, cross-linking of 10 μM actin by ACD in a buffer containing 50 mM HEPES, pH 7.5, 0.2 mM CaCl2, 0.5 mM ATP, 2 mM MgCl2 was monitored by SDS/PAGE. To initiate the cross-linking, aliquots withdrawn from the pelleting assays were added to actin at a final ACD concentration of 25 nM. In a separate set of experiments, 4dMARTXVc (25 nM) was used to cross-link actin (5 μM) in the absence or presence of RC-101 (300 nM).
Cell culture
Cell culture experiments were conducted in triplicates essentially as described [17]. Briefly, LFN ACD was mixed with PA (final concentrations 5 and 12.5 nM respectively) and added to complete Dulbecco’s Modified Eagle’s medium (DMEM) containing 10 % FBS. Then RC-101 (5 and 10 μM final concentrations) was added. The mixture was incubated for 20 min at 37 °C and used to replace the medium on the monolayers of rat small intestinal epithelial cell (IEC-18) line (A.T.C.C.). Phase contrast microphotographs were taken using a Nikon inverted microscope Eclipse Ti-E (Nikon).
Statistical analysis
Data were analysed using Microsoft Excel and KaleidaGraph software. Error bars represent S.E.M. Statistical significance was determined by two-tailed Student’s t test: P-values less than 0.05 were considered statistically significant.
RESULTS
Retrocyclins facilitate unfolding of ACDVc
RC-1 is a synthetic peptide corresponding to a putative ancestral human peptide encoded by θ-defensin pseudogene [10], whereas RC-101 is an RC-1 analogue that differs by a single amino acid substitution of arginine to lysine [19]. We assessed the effects of both RCs on unfolding of ACD, an actin cross-linking effector toxin, which is expressed as a part of larger toxins MARTX (Vibrio and Aeromonas spp.) [31] and valine-glycine repeat protein G1 (VgrG1) (V. cholerae) [32]. Previously we have demonstrated that similar to many other bacterial toxins, ACD from MARTX toxins of both V. cholerae and A. hydrophila (ACDVc and ACDAh respectively) have low thermodynamic stability and, at the physiological temperature of the human body, exist in equilibrium between fully folded and partially unfolded (molten globule) states [20]. Therefore, ACD can serve as a good model protein to study protein unfolding mediated by defensins [17].
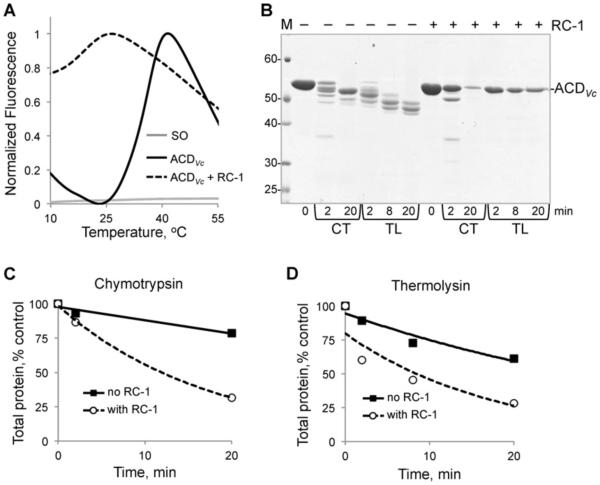
Effects of RC-1 on ACDVc unfolding were assessed by differential scanning fluorimetry (DSF) [33] and limited proteolysis (Figure 2). In the presence of RC-1, hydrophobic regions of ACDVc got exposed to solution at lower temperatures, compared with ACDVc alone, as detected by an enhanced fluorescence of SO dye sensitive to a hydrophobic environment (Figure 2A). Next, the addition of RC-1 brought notable changes to the ACDVc cleavage pattern upon limited proteolysis of this toxin by chymotrypsin and thermolysin (Figure 2B). In the absence of RC-1, cleavage by both proteases resulted in formation of stable proteolytic products of ACDVc and the total protein content was not substantially reduced, suggesting that the cleavage occurred at a few places in the flexible loops of the toxin. In contrast, whereas moderately inhibiting the disappearance of the full size ACDVc toxin band, RC-1 caused dramatic reduction in the amount of total protein due to the almost complete lack of stable proteolytic fragments, which were highly pronounced in the absence of RC-1 (Figures 2B–2D). This suggests that similarly to α-defensin HNP1 (human neutrophil peptide 1), RC-1 promoted toxin unfolding exposing additional sites, proteolytic cleavage of which generates randomly fragmented polypeptides spread throughout the gel lane [17].
Figure 2. Effects of RC-1 on ACDVc toxin.
(A) DSF of ACDVc (final concentration 10 μM) was carried out in the presence (dashed line) or absence (solid black line) of 5 fold mole excess of RC-1 (50 μM). SO dye alone (solid grey line). (B) Limited proteolysis of 8.7 μM ACDVc in the absence and presence of 5 fold mole excess RC-1 (44 μM). Samples were incubated at 30 °C in the presence of chymotrypsin (CT) or thermolysin (TL) for the indicated periods of time and subjected to SDS/PAGE. Position of the full-length ACDVc is indicated on the gel. M, molecular mass ladder (values in kDa). (C and D) Quantification of total protein content (sum of all bands in a lane) for CT (C) and TL (D) proteolysis.
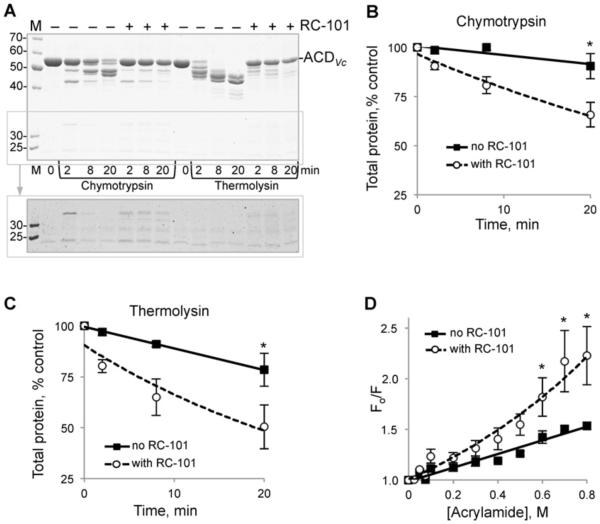
The effects of RC-101 on limited proteolysis of ACDVc were similar to those imposed by RC-1 (Figures 3A–3C). Namely, (i) high molecular mass ACDVc cleavage products were not accumulated (Figure 3A); (ii) the detectable amount of total protein in a lane was decreased, compared with ACDVc in the absence of RC-101 (Figures 3B and 3C); (iii) accumulation of low molecular mass products due to fragmentation of ACDVc at the additional sites was more prominent in the presence of RC-101, especially after increasing contrast of gel images (Figure 3A, boxed area).
Figure 3. Effects of RC-101 on ACDVc toxin.
(A) Limited proteolysis of 5 μM ACDVc in the absence and presence of 5 fold mole excess RC-101 (25 μM). Samples were incubated at 30 °C in the presence of chymotrypsin or thermolysin for the indicated periods of time and subjected to SDS/PAGE. Position of the full-length ACDVc is indicated on the gel. M, molecular mass ladder (values in kDa). Contrast was adjusted for the lower part of the gel (boxed) to reveal additional fragmentation of ACDVc in the presence of RC-101. (B and C) Amount of total protein (sum of all bands) ina lane was quantified and plotted against time for chymotrypsin (B) or thermolysin (C) cleavage. Error bars represent S.E.M., *P < 0.05. (D) Stern–Volmer plots obtained for collisional acrylamide quenching of tryptophan fluorescence of 2 μM ACDVc in the absence (solid line) and presence (dotted line) of 5 fold mole excess of RC-101 (10 μM). Error bars represent S.E.M., *P < 0.05.
Collisional quenching of intrinsic tryptophan fluorescence by acrylamide showed higher accessibility of at least some of the eight tryptophan residues of ACDVc to quencher (acrylamide) in the presence of RC-101, which does not have any tryptophan residues and therefore does not contribute to intrinsic fluorescence of the analysed samples (Figure 3D). We have demonstrated previously that tryptophan quenching of the mammalian proteins and unaffected toxins is not increased in the presence of defensins [17]. Therefore, our data strongly suggest that both RC-1 and RC-101 promote unfolding of ACDVc.
RC-101 induces precipitation and abrogates activity of ACDVc and ACDAh
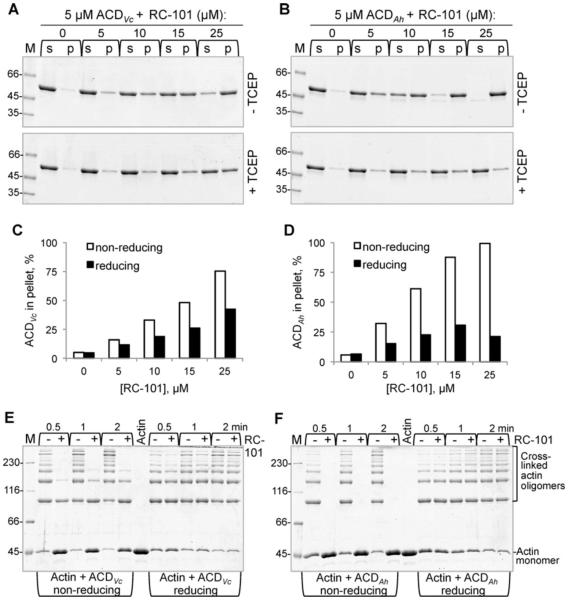
Precipitation of bacterial toxins in the presence of defensins is well recognized as one of the key mechanisms of toxin inactivation by these peptides [34]. Therefore, we tested whether the observed unfolding of ACD by RC-101 is accompanied by toxin precipitation by analysing toxin band redistribution between supernatant and pellet fractions upon ultra-centrifugation. We have demonstrated previously that both ACD orthologues from V. cholerae and A. hydrophila have low thermodynamic stability, with ACDAh being notably less stable than ACDVc [17,20]. Accordingly, under non-reducing conditions, addition of RC-101 induced precipitation of both toxins, although precipitation of ACDAh was more pronounced (Figures 4A and 4B, upper panels; Figures 4C and 4D). In the presence of reducing agent (TCEP), precipitation of both toxins was strongly reduced (Figures 4A and 4B, lower panels, Figures 4C and 4D), confirming that native conformation of the peptide, stabilized by three disulfide bonds, is essential for its specific activity.
Figure 4. Effects of RC-101 on precipitation and activity of ACD toxins.
(A and B) Precipitation of 5 μM ACDVc (A) and ACDAh (B) in the presence of increasing concentrations of RC-101 (0–25 μM) in the absence (upper panels) and presence (lower panels) of 10 mM TCEP. Following ultra-centrifugation, supernatant (s) and pellet (p) fractions were resolved on SDS/PAGE. M, molecular mass ladder (values in kDa). (C and D) Amount of protein in the supernatant and pellet fractions for ACDVc (C) and ACDAh (D) in the absence (non-reducing) or presence of TCEP (reducing) was quantified. (E and F) Actin cross-linking activity of ACD in the samples from (A) and (B) taken before ultra-centrifugation (as described in the ‘Experimental’ section) was analysed by SDS/PAGE. ACDVc (E) and ACDAh (F) incubated in the absence or presence of 25 μM RC-101 with or without of TCEP were added at a final concentration of ACD 25 nM to 10 μM actin and incubated for the indicated periods of time. Actin, actin alone; M, molecular mass ladder (values in kDa).
Catalytic activity of ACD can be monitored in vitro by detecting the accumulation of covalently cross-linked actin species using SDS/PAGE [30]. We found that activity of both enzymes is severely abrogated (or even completely blocked in the case of ACDAh) following their incubation with RC-101 under non-reducing conditions, but nearly unaffected when ACD and RC-101 were incubated in the presence of TCEP (Figures 4E and 4F).
Bacterial toxins from several major families are destabilized by RC-101
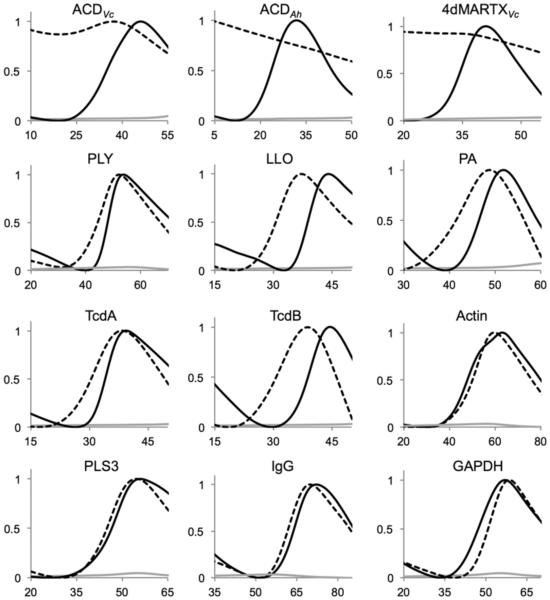
Next, we utilized DSF to test whether the ability of RC-101 to promote toxin unfolding extends to other toxins from different, unrelated families: pore forming toxin (anthrax toxin PA), cholesterol-dependent cytolysins (CDCs) from L. monocytogenesis and Streptococcus pneumoniae (LLO and PLY), enzymatic C. difficile toxins (GTDs of TcdA and TcdB) and effector domains of the MARTXVc and MARTXAh toxins (ACDVc, ACDAh and 4dMARTXVc construct with all effector domains fused together). The DSF analysis demonstrated that similarly to other defensins, RC-101 is able to further destabilize and promote unfolding of thermodynamically marginally stable bacterial toxins (Figure 5). Toxins, which have been previously demonstrated to be less susceptible to inactivation by defensins (TcdA [35] and PLY [34]), were less affected by RC-101 (Figure 5) and HNP1 [17] alike. Importantly, none of the tested mammalian proteins was affected by RC-101 in a comparable manner (Figure 5).
Figure 5. RC-101 potentiates thermal unfolding of toxins from several major families.
DSF melting profiles of proteins (final concentration 10 μM) in 20 mM HEPES, pH 7.5, 150 mM NaCl in the presence (dashed line) or absence (solid black line) of 5 fold mole excess of RC-101. SO dye alone (solid grey line). Axes for all plots: x, temperature (°C); y, normalized fluorescence.
RC-101 inhibits ACD activity in vitro and in cell culture under physiologically relevant conditions
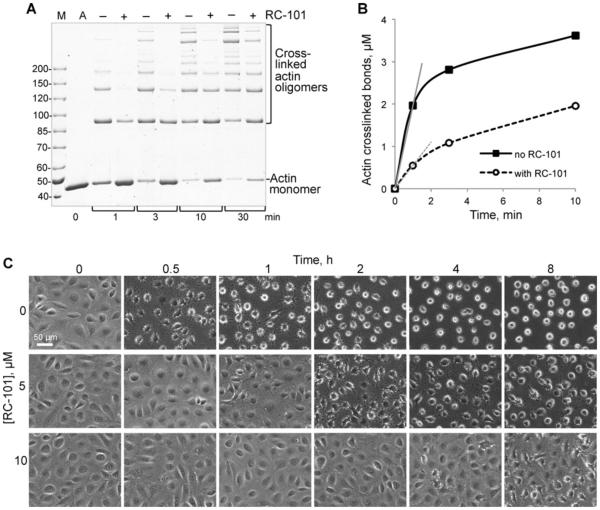
Next, we addressed the question whether the RC-101 effect on toxins would lead to toxins’ inactivation under physiologically relevant conditions. To this end, the ability of ACD to cross-link actin in vitro and to cause cytotoxic cell rounding effects in cell culture were examined in the presence and absence of defensin [17]. For the cell culture experiments, the anthrax toxin delivery machinery (PA and LFN) was employed to deliver the LFN-fusion construct of ACDVc [25] into the cytoplasm of IEC-18 rat intestinal epithelial cells. We found that RC-101 reduced the rate of actin cross-linking imposed by the ACD domain of the 4dMARTXVc construct by 3.6-fold (Figures 6A and 6B). Moreover, addition of micromolar (5 and 10 μM) concentrations of RC-101 to nanomolar amounts of the LFN ACD–PA complex in a serum-containing medium notably protected the cells from the cytotoxicity inflicted by LFN ACD (Figure 6C). This confirms the potency of the RC under physiological salt and serum concentrations.
Figure 6. RC-101 inhibits ACD activity in vitro and protects cells from ACD toxicity.
(A) Cross-linking of 5 μM actin by 25 nM 4dMARTXVc in the absence or presence of 300 nM RC-101 was monitored by SDS/PAGE. A, actin alone; M, molecular mass ladder (values in kDa). (B) ACD cross-linking activity of 4dMARTXVc was expressed as the concentration of formed isopeptide bonds and plotted against time. Initial reaction rates were calculated using linear slopes of the curves (grey lines) for the initial 1-min period. (C) IEC-18 intestinal epithelial cells were treated with a complex of LFN ACD and PA (5 and 12.5 nM final concentrations respectively). RC-101 was added at 5 or 10 μM to the mixture of LFN ACD and PA diluted in serum-containing medium and applied to the cells. Images were taken at the indicated time points. Scale bar is 50 μm.
DISCUSSION
Defensins are a class of cysteine-rich anti-microbial peptides produced not only by fungi, plants and animals, but also by prokaryotes (e.g., laterosporulin class IId bacteriocin [36]) and, therefore represent some of the most ancient and most universal immune molecules on the planet. In higher eukaryotes, defensins are potent immuno-modulators [37]. A major anti-microbial mechanism of defensins is manifested via their direct interaction with the bacterial membrane, leading to its disorganization and compromised integrity [38]. Another major mechanism of the anti-microbial activity of defensins involves interaction with bacterial proteins, either cell-associated (e.g., secretion portals [39,40], fimbrial adhesins [41]) or secreted (e.g., toxins [34,35,42,43]). It is easy to recognize that both anti-microbial mechanisms are important and complementary. Many bacterial toxins are extremely potent and can be lethal to the host in small doses if not promptly neutralized. Therefore, inactivation of toxins in solution, before they have a chance to reach the cells, is essential, but not sufficient if bacteria remain active and produce more toxins. On the other hand, killing and eradicating bacteria from the body might not be sufficient if even trace amounts of toxins are released. Furthermore, toxins can remain active in the body for days hiding in intraluminal vesicles (ILV) and then can be released from initially affected cells in exosomes, even when the causative pathogen has been eradicated [44]. Therefore, both defensin mechanisms are essential.
The list of bacterial proteins neutralized by defensins is being updated every year since the first report on inactivation of a bacterial toxin by defensin in 2005 [45]. Therefore, it is increasingly clear that defensins can recognize and neutralize many unrelated proteins of bacterial nature, proteins with different properties, specific activities and structure. Recently we proposed that the key feature recognized by human α- and β-defensins is thermodynamic instability of the bacterial toxins essential for their ability to undergo dramatic conformational perturbations upon formation of membrane pores or upon unfolding for transition through such pores [17]. Now we demonstrate that retrocyclins, which belong to the family of θ-defensins, also exploit thermodynamic instability as a primary target to promote unfolding, susceptibility to proteolysis and precipitation of bacterial toxins. Future studies can help to uncover the detailed mechanisms of unfolding/co-folding of each individual toxin with the defensins. Given that toxin unfolding by defensins results in precipitation, which precludes the use of solution methods for structural studies, we anticipate that the magic angle spinning (MAS) solid-state NMR (MAS–NMR) technique [46] can be particularly helpful in future studies.
θ-defensins are likely to be more potent under in vivo conditions due to the overall higher stability and longer lifetime of cyclic peptides in serum and under condition of protease attack [47]. Therefore, initially introduced as promising anti-viral humanized synthetic peptides, analogous to those that are still active in old-world primates [6] and widely proposed as a topical microbicide [48–50], RCs should be seriously considered as first-line antidotes against a broad range of bacterial toxins. We predict that the newly discovered mechanism of RC anti-toxin activity will further promote their development as therapeutic agents.
The evolutionary loss of θ-defensins was suggested to be a contributing factor to HIV-1 susceptibility in humans [5]. This surprising evolutionary loss occurred after the divergence from orangutans, which have six intact and one defective θ-defensin genes [3]. Interestingly, all multiple human θ-defensin pseudogenes, as well as all the pseudogenes of chimpanzees, gorillas and bonobos, contain the identical non-sense mutation (the same as is in the defective θ-defensin gene from orangutans), which therefore is probably acquired from their common ancestor [3]. Since, the θ-defensin pseudogenes are genetically clustered with α-defensins, the apparently enigmatic duplication of these pseudogenes in humans is likely to be linked to expansion of the fully functional α-defensin genes [51]. In fact, one of the most credible explanations for the enigmatic evolutionary loss of functional and highly potent θ-defensins is that they have been replaced by somewhat less potent, but less energetically costly and more robustly produced α-defensins [7]. It is also plausible that human θ-defensins were traded for a yet unknown evolutionarily advantageous acquisition, with which they were in conflict, similarly to the pseudogeneization of uricase linked to improvements in fructose metabolism [52]. Finally, as a rather bold speculation one might suggest that a moderately increased vulnerability and exposure to viruses may benefit species via mechanisms of accelerated evolution. Even though viral infections are often devastating at the organismal level, by promoting horizontal gene transfer and provoking major genomic rearrangements viruses also play a critical and occasionally highly beneficial role in evolution of species. For example, the acquisition of genes encoding syncytins, proteins derived from the envelope protein of endogenous retroviral elements, enabled mammals to develop placenta and fetal-maternal tolerance and thereby fundamentally influenced the emergence of the entire Mammalia class [53,54]. Furthermore, loss of defensins could force our ancestors to develop alternative anti-viral strategies, some of which (such as inactivation of a gene responsible for synthesis of N-glycolylneuraminic acid, a common receptor for many pathogens) could beneficially affect brain development [55,56]. If the later scenarios are correct, than re-introduction of θ-defensins to the human population (either at the genetic or at the pharmaceutical levels) might bring all the benefits of increased resistance to viral and bacterial pathogens without negative side effects.
Given that structurally diverse α-, β- and θ-defensins employ similar mechanisms of toxin inactivation, our findings suggest that defensins from other organisms may share the ability of human defensins to interfere with the folding-unfolding equilibrium of marginally stable bacterial toxins. In this regard, it will be very interesting and important to ascertain whether human and non-human anti-microbial peptides from other families can, at least partially, employ similar strategies in their anti-microbial defence mechanisms.
ACKNOWLEDGEMENTS
We thank Dr Robert I. Lehrer (UCLA) for a generous gift of RC-1 peptide, Dr D. Borden Lacy (Vanderbilt University) for donating B. megaterium cells expressing TcdA– and TcdB–GTD constructs and Dr Irina Artsimovitch (OSU) for providing access to a real-time PCR instrument.
FUNDING
This work was supported by The Ohio State University start-up fund (to D.S.K.); and the National Institutes of Health [grant numbers R01NIAID107250 (to S.S.) and NIH AI072732 (to W.L.)].
Abbreviations
- 4dMARTXVc
four effector domains of MARTXVc fused in their natural orientation
- ACD
actin cross-linking domain of MARTX and VgrG1 toxins
- CDC
cholesterol-dependent cytolysin
- DSF
differential scanning fluorimetry
- HNP1
human neutrophil peptide 1
- LFN
N-terminus of Bacillus anthracis lethal factor
- LLO
Listeria monocytogenes lysteriolysin O
- MARTX
multi-functional auto-processing repeats-in-toxin
- MAS
magic angle spinning
- PA
Bacillus anthracis protective antigen
- PLY
Streptococcus pneumoniae pneumolysin
- RC
retrocyclin
- SO
SYPRO Orange
- TcdA– and TcdB–GTD
Clostridium difficile toxin A and B glucosyltransferase domains
- TCEP
tris(2-carboxyethyl)phosphine
- VgrG1
valine-glycine repeat protein G1
Footnotes
The structure for human α-defensin 1, human β-defensin 2 and retrocyclin-1 will appear in the PDB under accession codes 3GNY, 1FD3 and 1HVZ respectively.
AUTHOR CONTRIBUTION
Elena Kudryashova designed the experiments, produced proteins, acquired and analysed data and wrote the manuscript. Stephanie Seveau produced CDCs and Wuyuan Lu synthesized RC-101. Dmitri Kudryashov co-ordinated the project, designed the experiments, analysed data and wrote the manuscript.
REFERENCES
- 1.Zhao L, Lu W. Defensins in innate immunity. Curr. Opin. Hematol. 2014;21:37–42. doi: 10.1097/MOH.0000000000000005. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser V, Diamond G. Expression of mammalian defensin genes. J. Leukoc. Biol. 2000;68:779–784. PubMed. [PubMed] [Google Scholar]
- 3.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–1654. doi: 10.1016/j.peptides.2003.07.023. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 4.Gallo SA, Wang W, Rawat SS, Jung G, Waring AJ, Cole AM, Lu H, Yan X, Daly NL, Craik DJ, et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 2006;281:18787–18792. doi: 10.1074/jbc.M602422200. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Munk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR, Cole AM. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retroviruses. 2003;19:875–881. doi: 10.1089/088922203322493049. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer RI, Cole AM, Selsted ME. θ-Defensins: cyclic peptides with endless potential. J. Biol. Chem. 2012;287:27014–27019. doi: 10.1074/jbc.R112.346098. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conibear AC, Rosengren KJ, Harvey PJ, Craik DJ. Structural characterization of the cyclic cystine ladder motif of θ-defensins. Biochemistry. 2012;51:9718–9726. doi: 10.1021/bi301363a. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Leonova L, Kokryakov VN, Aleshina G, Hong T, Nguyen T, Zhao C, Waring AJ, Lehrer RI. Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 2001;70:461–464. PubMed. [PubMed] [Google Scholar]
- 10.Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1813–1818. doi: 10.1073/pnas.052706399. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran D, Tran P, Roberts K, Osapay G, Schaal J, Ouellette A, Selsted ME. Microbicidal properties and cytocidal selectivity of rhesus macaque theta defensins. Antimicrob. Agents Chemother. 2008;52:944–953. doi: 10.1128/AAC.01090-07. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Leikina E, Delanoe-Ayari H, Melikov K, Cho MS, Chen A, Waring AJ, Wang W, Xie Y, Loo JA, Lehrer RI, Chernomordik LV. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 2005;6:995–1001. doi: 10.1038/ni1248. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Mulakala C, Ward SC, Jung G, Luong H, Pham D, Waring AJ, Kaznessis Y, Lu W, Bradley KA, Lehrer RI. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 2006;281:32755–32764. doi: 10.1074/jbc.M603614200. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooven TA, Randis TM, Hymes SR, Rampersaud R, Ratner AJ. Retrocyclin inhibits Gardnerella vaginalis biofilm formation and toxin activity. J. Antimicrob. Chemother. 2012;67:2870–2872. doi: 10.1093/jac/dks305. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett E, Lehrer RI, Pratikhya P, Lu W, Seveau S. Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. 2011;13:635–651. doi: 10.1111/j.1462-5822.2010.01563.x. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Kudryashova E, Quintyn R, Seveau S, Lu W, Wysocki VH, Kudryashov DS. Human defensins facilitate local unfolding of thermodynamically unstable regions of bacterial protein toxins. Immunity. 2014;41:709–721. doi: 10.1016/j.immuni.2014.10.018. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlgraf KG, Pingel LC, Dietrich DE, Brogden KA. Defensins as anti-inflammatory compounds and mucosal adjuvants. Future Microbiol. 2010;5:99–113. doi: 10.2217/fmb.09.104. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen SM, Rudolph DL, Wang W, Cole AM, Waring AJ, Lal RB, Lehrer RI. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses. 2004;20:1157–1165. doi: 10.1089/aid.2004.20.1157. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Kudryashova E, Heisler D, Zywiec A, Kudryashov DS. Thermodynamic properties of the effector domains of MARTX toxins suggest their unfolding for translocation across the host membrane. Mol. Microbiol. 2014;92:1056–1071. doi: 10.1111/mmi.12615. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Chumbler NM, Farrow MA, Lapierre LA, Franklin JL, Haslam DB, Goldenring JR, Lacy DB. Clostridium difficile toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathogens. 2012;8:e1003072. doi: 10.1371/journal.ppat.1003072. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wesche J, Elliott JL, Falnes PO, Olsnes S, Collier RJ. Characterization of membrane translocation by anthrax protective antigen. Biochemistry. 1998;37:15737–15746. doi: 10.1021/bi981436i. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Arnett E, Vadia S, Nackerman CC, Oghumu S, Satoskar AR, McLeish KR, Uriarte SM, Seveau S. The pore-forming toxin listeriolysin O is degraded by neutrophil metalloproteinase-8 and fails to mediate Listeria monocytogenes intracellular survival in neutrophils. J. Immunol. 2014;192:234–244. doi: 10.4049/jimmunol.1301302. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordero CL, Kudryashov DS, Reisler E, Satchell KJ. The actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J. Biol. Chem. 2006;281:32366–32374. doi: 10.1074/jbc.M605275200. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. PubMed. [PubMed] [Google Scholar]
- 27.Lyon AN, Pineda RH, Hao le T, Kudryashova E, Kudryashov DS, Beattie CE. Calcium binding is essential for plastin 3 function in Smn-deficient motoneurons. Hum. Mol. Genet. 2014;23:1990–2004. doi: 10.1093/hmg/ddt595. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eftink MR, Ghiron CA. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976;15:672–680. doi: 10.1021/bi00648a035. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 30.Kudryashova E, Kalda C, Kudryashov DS. Glutamyl phosphate is an activated intermediate in actin crosslinking by actin crosslinking domain (ACD) toxin. PLoS One. 2012;7:e45721. doi: 10.1371/journal.pone.0045721. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satchell KJ. Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu. Rev. Microbiol. 2011;65:71–90. doi: 10.1146/annurev-micro-090110-102943. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Durand E, Derrez E, Audoly G, Spinelli S, Ortiz-Lombardia M, Raoult D, Cascales E, Cambillau C. Crystal structure of the VgrG1 actin cross-linking domain of the Vibrio cholerae type VI secretion system. J. Biol. Chem. 2012;287:38190–38199. doi: 10.1074/jbc.M112.390153. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senisterra GA, Finerty PJ., Jr High throughput methods of assessing protein stability and aggregation. Mol. Biosyst. 2009;5:217–223. doi: 10.1039/b814377c. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Lehrer RI, Jung G, Ruchala P, Wang W, Micewicz ED, Waring AJ, Gillespie EJ, Bradley KA, Ratner AJ, Rest RF, Lu W. Human alpha-defensins inhibit hemolysis mediated by cholesterol-dependent cytolysins. Infect. Immun. 2009;77:4028–4040. doi: 10.1128/IAI.00232-09. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giesemann T, Guttenberg G, Aktories K. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology. 2008;134:2049–2058. doi: 10.1053/j.gastro.2008.03.008. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Singh PK, Chittpurna A, Sharma V, Patil PB, Korpole S. Identification, purification and characterization of laterosporulin, a novel bacteriocin produced by Brevibacillus sp. strain GI-9. PLoS One. 2012;7:e31498. doi: 10.1371/journal.pone.0031498. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansour SC, Pena OM, Hancock RE. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35:443–450. doi: 10.1016/j.it.2014.07.004. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 38.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Vega LA, Caparon MG. Cationic antimicrobial peptides disrupt the Streptococcus pyogenes ExPortal. Mol. Microbiol. 2012;85:1119–1132. doi: 10.1111/j.1365-2958.2012.08163.x. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kandaswamy K, Liew TH, Wang CY, Huston-Warren E, Meyer-Hoffert U, Hultenby K, Schroder JM, Caparon MG, Normark S, Henriques-Normark B, et al. Focal targeting by human beta-defensin 2 disrupts localized virulence factor assembly sites in Enterococcus faecalis. Proc. Natl. Acad. Sci. U.S.A. 2013;110:20230–20235. doi: 10.1073/pnas.1319066110. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietrich DE, Xiao X, Dawson DV, Belanger M, Xie H, Progulske-Fox A, Brogden KA. Human alpha- and beta-defensins bind to immobilized adhesins from Porphyromonas gingivalis. Infect. Immun. 2008;76:5714–5720. doi: 10.1128/IAI.00997-08. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim C, Gajendran N, Mittrucker HW, Weiwad M, Song YH, Hurwitz R, Wilmanns M, Fischer G, Kaufmann SH. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4830–4835. doi: 10.1073/pnas.0500508102. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim C, Slavinskaya Z, Merrill AR, Kaufmann SH. Human alpha-defensins neutralize toxins of the mono-ADP-ribosyltransferase family. Biochem. J. 2006;399:225–229. doi: 10.1042/BJ20060425. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5:986–996. doi: 10.1016/j.celrep.2013.10.019. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim C, Gajendran N, Mittrucker HW, Weiwad M, Song YH, Hurwitz R, Wilmanns M, Fischer G, Kaufmann SH. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4830–4835. doi: 10.1073/pnas.0500508102. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan S, Suiter CL, Hou G, Zhang H, Polenova T. Probing structure and dynamics of protein assemblies by magic angle spinning NMR spectroscopy. Acc. Chem. Res. 2013;46:2047–2058. doi: 10.1021/ar300309s. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howell SM, Fiacco SV, Takahashi TT, Jalali-Yazdi F, Millward SW, Hu B, Wang P, Roberts RW. Serum stable natural peptides designed by mRNA display. Sci. Rep. 2014;4:6008. doi: 10.1038/srep06008. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sassi AB, Cost MR, Cole AL, Cole AM, Patton DL, Gupta P, Rohan LC. Formulation development of retrocyclin 1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob. Agents Chemother. 2011;55:2282–2289. doi: 10.1128/AAC.01190-10. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkataraman N, Cole AL, Ruchala P, Waring AJ, Lehrer RI, Stuchlik O, Pohl J, Cole AM. Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 2009;7:e95. doi: 10.1371/journal.pbio.1000095. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sassi AB, Bunge KE, Hood BL, Conrads TP, Cole AM, Gupta P, Rohan LC. Preformulation and stability in biological fluids of the retrocyclin RC-101, a potential anti-HIV topical microbicide. AIDS Res. Therapy. 2011;8:27. doi: 10.1186/1742-6405-8-27. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng DQ, Li Y, Huang JF. Molecular evolution of the primate α-/θ-defensin multigene family. PLoS One. 2014;9:e97425. doi: 10.1371/journal.pone.0097425. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kratzer JT, Lanaspa MA, Murphy MN, Cicerchi C, Graves CL, Tipton PA, Ortlund EA, Johnson RJ, Gaucher EA. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. U.S.A. 2014;111:3763–3768. doi: 10.1073/pnas.1320393111. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mangeney M, Renard M, Schlecht-Louf G, Bouallaga I, Heidmann O, Letzelter C, Richaud A, Ducos B, Heidmann T. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20534–20539. doi: 10.1073/pnas.0707873105. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T. Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120507. doi: 10.1098/rstb.2012.0507. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am. J. Physical Anthropol. 2001;Suppl 33:54–69. doi: 10.1002/ajpa.10018. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]