Abstract
Hepatocyte apoptosis plays important roles in both the removal of external microorganisms and the occurrence and development of liver diseases. Different conditions, such as virus infection, fatty liver disease, hepatic ischemia reperfusion, and drug-induced liver injury, are accompanied by hepatocyte apoptosis. This review summarizes recent research on the mechanism of hepatocyte apoptosis involving the classical extrinsic and intrinsic apoptotic pathways, endoplasmic reticulum stress, and oxidative stress-induced apoptosis. We emphasized the major causes of apoptosis according to the characteristics of different liver diseases. Several concerns regarding future research and clinical application are also raised.
Keywords: hepatocyte apoptosis, viral hepatitis, fatty liver disease, hepatic ischemia reperfusion injury, drug-induced liver injury, signaling pathways
Introduction
As the basic unit of life, cells constitute the human body. Their proliferation and death must be tightly regulated to maintain normal organ function. Hepatocytes, the major liver cell type, have a strong ability to regenerate when they face damaging factors, such as ischemia, virus infection, and alcohol. On the other hand, several cell death modalities are noted in hepatocytes, including apoptosis, necroptosis, necrosis, and autophagic-dependent cell death. Cell apoptosis, sometimes called programmed cell death, is a common physiological process that is controlled by multiple genes and involves numerous biological events. Apoptosis is a cellular self-destruction method to remove old and damaged cells to protect cells from external disturbances and maintain homeostasis. Necroptosis, or programmed necrosis, is morphologically characterized by necrotic cell death and involves autophagy.1 Necrosis is generally uncontrolled and occurs as a passive tissue damage process characterized by cell membrane damage, cell swelling, and cellular disruption leading to inflammation.2 Autophagy is an intracellular catabolic process that delivers cytoplasmic components to lysosomes for degradation. Autophagic activity may regulate hepatocyte apoptosis via the mitochondrial pathway.3
Hepatocyte apoptosis plays an important protective role in the removal of external microorganisms by self-destruction. On the other hand, apoptosis also contributes to both chronic and acute liver diseases, such as viral hepatitis, alcoholic and nonalcoholic liver disease, cholestatic disorders, and ischemia reperfusion injury. Hepatocyte apoptosis plays such an important role in liver injury that understanding the molecular mechanism and regulating factors of how hepatocyte apoptosis proceeds is crucial for the treatment of liver diseases.4 Compared with other types of cell death, apoptosis is an energy-requiring process that can be altered. Therefore, apoptosis monitors the scope and extent of cell death, thus protecting the liver from both infection and hepatitis. In acute liver failure patients, caspase activation and apoptosis involve in spontaneous recovery.5 Hepatocyte apoptosis also correlates with liver disease severity and participates in the progress of hepatic fibrosis.6
This review aims to provide an up-to-date summary of the relationship between hepatocyte apoptosis and different liver diseases and their underling mechanisms. Readers should keep in mind that liver diseases often involve different modes of cell death and that a change of cell death pathways due to genetic or nongenetic alterations may influence disease progression. For example, hepatocyte necroptosis contributes to high-fat diet-induced liver injury together with the apoptosis pathway. Absence of a key necroptotic mediator, receptor-interacting protein 3 (RIP3), causes aggravated liver inflammation, hepatocyte apoptosis, and fibrosis.7
Hepatocyte Apoptotic Pathways
Hepatocyte apoptosis involves two fundamental pathways: the extrinsic pathway, which transmits death signals by the death receptor (DR), and the intrinsic pathway, which is initiated by intracellular stimuli.
The extrinsic apoptotic pathway is activated by the binding of the death ligand to DRs on the plasma membrane. Death ligands belong to tumor necrosis factor (TNF) superfamily, including FasL, TNF-α, and TNF-related apoptosis-inducing ligand (TRAIL). Accordingly, DR includes Fas, TNF-receptor 1, and TRAIL-R. The binding of the death ligand to DR induces trimerization and a conformational change of DR. This change recruits cytoplasmic adaptor proteins (such as Fas-associated protein with death domain [FADD]), and the latter recruits apoptosis signaling molecules (such as caspase-8). The DR, adaptor protein, and associated apoptosis signaling molecule form the death-inducing signaling complex (DISC), thus leading to the activation of the effector caspase cascade (which typically involves caspase-3, -6, and -7).8–10 Cellular FADD-like interleukin-1β (IL-1β) converting enzyme inhibitory protein (c-FLIP) can inhibit DISC and prevent apoptosis.11
The intrinsic apoptosis pathway is characterized by the release of cytochrome c or other caspase-activating factors from the mitochondria intermembrane space into the cytoplasm.12 This release is mediated by the mitochondrial permeability transition pore. Then, in the cytoplasm, a complex named the apoptosome is formed and activates caspase-9, which further activates the effector caspase cascade.13 The mitochondria-mediated intrinsic apoptosis pathway is regulated by Bcl-2 family proteins, including proapoptotic (Bid, Bax, Bak) and antiapoptotic proteins (Bcl-2, Bcl-xL).14 Bcl-2, Bcl-xL, and other antiapoptotic proteins bind to the outer membrane of the mitochondria, decreasing their permeability and inhibiting the release of cytochrome c into the cytoplasm and the gathering of Bax and Bak. TNF-α binding to its receptor results in the cleavage of Bid into a truncated form and causes Bax oligomerization and insertion into mitochondria, thus initiating apoptosis.15
In hepatocytes, the apoptotic signals from DR are typically not powerful enough to initiate the effector caspase cascade, so the mitochondria-mediated pathway is generally used to amplify it.8,10 This amplification is extremely important for TNF-α-mediated hepatocyte apoptosis.16 When intrinsic apoptosis pathways are blocked by a caspase-9 inhibitor, TNF-α-induced apoptosis is abolished.16 In fact, unlike the Fas ligand, TNF-α alone fails to induce liver injury in normal hepatocytes.17 The concurrent activation of the antiapoptotic factor nuclear factor (NF)-kappa B is probably the underlying mechanism.18 Rapid activation of NF-kappa B induces c-FLIP expression and weakens caspase-8 activation.19
Mitochondrial dysfunction can initiate hepatocyte apoptosis, and other intracellular organelles can also trigger apoptosis through the mitochondrial-dependent mechanism.14 Both TNF-α and TRAIL signaling involve lysosomal protease release into the cytosol and lead to mitochondrial dysfunction.20,21 Alterations to calcium homeostasis, protein overload, or misfolding will induce endoplasmic reticulum (ER) stress. Prolonged ER stress activates Bax and trigger apoptosis.22–24 ER stress is actually the third pathway of apoptosis in addition to the extrinsic and intrinsic pathways.24 Oxidative stress caused by overproduction of reactive oxygen species (ROS) is another apoptosis inducer. Mitochondrial dysfunction seems to precede ROS accumulation.25 Excessive ROS can further cause oxidative damage to mitochondrial DNA (mtDNA), proteins, and phospholipids and induce hepatocyte apoptosis.
Although the caspase cascade plays an essential role in hepatocyte apoptosis, the wide-ranging caspase inhibitor Z-VAD.fmk fails to prevent apoptosis in some experimental settings. Apoptosis induction without caspase cascade activation or when caspases are inhibited is called caspase-independent apoptosis. One example of caspase-independent apoptosis is ROS-induced mitochondrial damage, as mentioned above. A second example is apoptosis induced by the release of apoptosis-inducing factor (AIF) from mitochondria into the cytosol.26 The translocation of AIF from the cytosol to the nucleus directly triggers chromatin condensation and DNA cleavage.27 TNF-α may also cause caspase-independent apoptosis when caspase is inhibited.
In addition to apoptosis, other cell death modalities also participate in hepatocyte death and liver injury through crosstalk and overlap, yielding highly heterogeneous death processes.28 DR activation may also activate necroptosis under certain conditions, such as a specific virus infection, which leads to the interaction between RIP1 and RIP3.29
Activation of similar apoptotic pathways can be observed in different types and phases of liver diseases. On the other hand, each disease exhibits specific features of hepatocyte apoptosis due to the different initiating factors and disease states. In the following sections, the mechanism of hepatocyte apoptosis is discussed based on the types of liver diseases.
Hepatocyte Apoptosis and Viral Hepatitis
Viral hepatitis, a main cause of hepatic cirrhosis, is an infectious disease with high incidence. There are five types of viral hepatitis according to their pathogens: hepatitis A, B, C, D, and E. Virus-induced hepatocyte apoptosis can be caused by the immune response through the release of perforin/granzyme and the expression of specific viral protein products and cell membrane receptors related to apoptosis.30–34 Among all types of viral hepatitis, hepatitis B virus (HBV) and hepatitis C virus (HCV) are the most common and serious.
Perforin/granzyme-mediated apoptosis in viral hepatitis
Cytotoxic T lymphocytes (CTL) play crucial roles in the clearance of viral infection by perforin/granzyme B- and Fas/Fas ligand (FasL)-based mechanisms. Liver tissues with more severe inflammation typically exhibit higher Fas expression in the cytoplasm of hepatocytes. When virus-specific T-cells recognize the viral antigen and become activated, they express FasL to convey the apoptotic death signal to Fas-positive hepatocytes.34 Perforin expression by T-cells during the late acute phase is associated with virus clearance in chimpanzees.35 In the livers of chronic hepatitis B patients, the messenger RNA (mRNA) expression rates of FasL, perforin, and granzyme B were 79.2%, 62.5%, and 33.3%, respectively. The expression correlated with alanine aminotransferase levels, histologic activity index, and apoptosis.36
Inhibition of granzyme B expression might contribute to sustained virus infection. Stimulating hepatocytes with interferon (IFN)-α induces resistance to CTL killing in vitro by upregulating the expression of the granzyme B inhibitor, proteinase inhibitor 9 (PI-9).37 PI-9 expression has also been observed in liver tissue biopsies from patients with chronic HCV infection.37
HBV and apoptosis
HBV infection can result in various outcomes depending on replication of the virus and host immune response. Acute hepatitis B often causes massive hepatocyte damage followed by the clearance of the virus, regaining health in most cases. During acute hepatitis B, DR on the hepatocyte membrane is activated, and significant apoptosis is observed.38 On the contrary, chronic hepatitis B is more complicated. A variety of stages exist in these patients, including the inactive carrier state, chronic hepatitis, cirrhosis, and hepatic decompensation. The progress of chronic HBV infection typically links with different phases of the immune response: first, immune tolerance, followed by immune clearance and low/nonreplication, and finally, immune escape.39 Apoptotic cell death contributes to virus clearance, whereas dysregulated apoptosis facilitates immune escape.
HBV influences the host response by its unique protein products. The HBV genome contains four open-reading frames: preS/S, polymerase, preCore/core, and X. Among them, the HBV X (HBx) gene is the smallest but most important. The HBx protein is a double-edged sword in regulating apoptosis.40,41 The overall effect of HBx is to establish and maintain the chronic infection state. Its pro- or antiapoptotic action depends on several factors, such as the different phases of infection, amount of HBx expression, and cellular microenvironment. Activations of survival and proliferation signaling pathways account for its antiapoptotic property, while a change in the mitochondrial membrane potential is important for its apoptotic property. At the early stage of HBV infection, HBx activates AKT and inhibits apoptosis. The balance between virus replication and cell survival may cause the failure to remove infected cells and benefit persistent HBV infection.42,43 At the late stages of HBV infection, HBx becomes more proapoptotic and may accelerate HBV-induced liver damage. In addition, Rawat et al30 indicated that HBx works differently in diverse hepatic cells.
The proapoptotic effect of HBx involves the activation of both the extrinsic and the intrinsic pathways. The DR-mediated extrinsic apoptosis pathway is upregulated by HBx by facilitating the activation of caspase-8 by c-FLIP inhibition.11 HBx also dose-dependently increases TRAIL mRNA transcription and protein expression in hepatocytes.44 HBx causes a loss of the mitochondrial membrane potential and increases the release of cytochrome c to the cytoplasm from mitochondria.45 HBx sensitizes the apoptotic response to oxidative stress and activates caspase-3.46,47 Interaction with heat shock protein 60 may also contribute to its proapoptotic effect.48
Many studies have reported the antiapoptotic role of HBx. HBx inhibits apoptosis mediated by the Fas–FasL system.41 This effect may be related to the inhibition of caspase-8 and caspase-3 activation, as well as cytochrome c release.41 Upregulation of the SAPK/JNK pathway by HBx exerts a survival mechanism in hepatocytes undergoing Fas-mediated apoptosis.41 Pan et al49 suggested that the inhibition of HBx in Fas-mediated apoptosis is related to the activation and translocation of NF-kappa B into the nucleus. This translocation triggers target gene transcription and thus inhibits hepatocyte apoptosis. Serum deprivation, protein kinase C inhibition, or topoisomerase inhibition-induced apoptosis is mitigated by phosphatidylinositol 3-kinase pathway activation in HBx-transformed Chang liver cells.50 Increased telomerase activity also plays a role.51 HBx is antiapoptotic in normal hepatocytes by NF-kappa B activation52 and hepatic progenitor cells by Wnt/β-catenin pathway activation.53
As mentioned above, the seemingly contradictory effect of HBx on apoptosis is the comprehensive outcome of several factors, such as the different phases of HBV infection, NF-kappa B status,52 and/or the amount of HBx.54 When NF-kappa B is stimulated, HBx inhibits the apoptotic pathways. On the contrary, inhibition of NF-kappa B activation makes HBx proapoptotic.52 Zhai et al54 reported that moderate HBx overexpression inhibits apoptosis, whereas massive overexpression promotes apoptosis. Interestingly, the amount of HBx affects the subcellular distribution of NF-kappa B. Low HBx expression stimulates nuclear translocation of NF-kappa B; thus, apoptosis is inhibited. In contrast, higher HBx expression relocates a portion of NF-kappa B into the cytoplasm. This relocation antagonizes the antiapoptotic effect of NF-kappa B and leads to enhanced apoptosis.54 Knoll et al55 found that HBx is proapoptotic in human hepatocellular carcinoma (HCC) cell lines, but antiapoptotic in normal cells. This cell type specificity is due to different interactions between p53 and HBx. The association with Ras activity also plays a role in the functional shift of HBx.56
Throughout the HBV genome, there are naturally occurring mutations that may favor virus replication and alter apoptosis.57 An HBx variant, carboxyl-terminus-truncated HBx, is frequently found in HCC. This tumorigenic variant downregulates growth arrest-specific 2-induced p53-mediated apoptosis.58
Factors other than the products of the HBV genome may influence the outcome of HBV infection. Cellular inhibitor of apoptosis proteins are endogenous inhibitors of apoptosis. They favor viral persistence by weakening TNF signaling and restrict the clearance of infected cells.59
HCV and apoptosis
HCV is a leading cause of chronic viral hepatitis. HCV infection may result in progressive liver fibrosis, cirrhosis, and HCC. Hepatocyte apoptosis plays a major role in disease progression. In acute HCV infection, intensive hepatocyte apoptosis helps to eliminate the virus. In chronic HCV, hepatocyte apoptosis is accompanied by cell proliferation and ensures persistent infection, as observed in chronic HBV patients.60 An important difference is noted between HBV and HCV infection: HCV-induced apoptosis is not strongly correlated with the amount of virus but is rather immune-oriented.61 By studying liver biopsy specimens from chronic HCV patients, hepatocyte apoptosis correlates with histology grading and infiltration of CD8-positive immune cells.62 Hepatocyte apoptosis is elevated in HCV patients with fibrotic liver damage, as evidenced by the increased serum caspase activity.63
The HCV genome encodes a pre-polyprotein, which can be cleaved into three independent structural proteins (core protein, E1, and E2) as well as six nonstructural proteins.64 Among them, the core protein is the most important structural protein because it regulates hepatocyte apoptosis. The HCV core protein may be either pro- or antiapoptotic. This inconsistency derives from several influencing factors: genetic heterogeneity caused quasi-species HCV core proteins, activation of different signaling pathways, and diverse experimental settings.60 Similar as the dual regulatory role of HBx in hepatocyte apoptosis, activations of survival and proliferation signaling pathways are responsible for its antiapoptotic property, while a change in the mitochondrial membrane potential is usually seen when HCV core appears to be apoptotic.
The antiapoptotic effect of the HCV core protein correlates with its ability to enhance NF-kappa B expression.65 The core protein blocks TNF-α-mediated apoptosis signaling by sustaining the expression of c-FLIP, thus inhibiting caspase-8 activation.66 The core protein also inhibits Fas-mediated apoptosis by inhibiting the release of cytochrome c and activating caspase-9, -3, and -7.67 The direct interaction between the core protein and DNA-binding domain of Smad3 results in the inhibition of the transforming growth factor-β-induced apoptotic pathway.68 Moreover, the HCV core protein downregulates p21 and inhibits curcumin-induced apoptosis by microRNA-345 targeting human hepatoma cells.69 Several studies note that tumor suppressor p53 may be a target of the core. The proapoptotic effect of ROS is abolished when the p14–MDM2 (mouse double minute 2)-p53 pathway is repressed by the core through p14 promoter hypermethylation.70 Elevated sirt1 expression is another mechanism of p53 attenuation.71
The HCV core protein can also be proapoptotic. It augments TRAIL-mediated apoptosis by enhancing Bid cleavage and activating the mitochondria apoptosis signaling pathway.72 Core enhances the signal transduction of Fas and TNF-R1 by interacting with them.73 Researchers found that Core connects with the death domain of FADD73 and enhances FADD-mediated apoptosis.74 In terms of TNF-α signaling, Core does not directly interact with the death domain of TNF-R1-associated death domain protein (TRADD) but rather interferes with the binding of TRADD to TNF-R1 and inhibits signaling transduction.74 Furthermore, Core disrupts mitochondria function by direct physical interactions75 and indirect ROS production.76 Based on these findings, Core-induced apoptosis is mitochondria dependent.61
Another two structural proteins, E1 and E2, also influence hepatocyte apoptosis. E1 increases apoptosis mainly through its C-terminal transmembrane domain.77 This hydrophobic region alters membrane permeability and activates apoptosis.77 E2 inhibits DR-induced apoptosis in hepatoma cells by inhibiting the release of mitochondrial cytochrome c.78 However, another group observed its proapoptotic effect with the mechanism of inducing mitochondria-related and caspase-dependent apoptosis in the same cell line.79 A study also demonstrated that the proapoptotic effect of the nonstructural protein of HCV by causing mitochondrial damage.80
In addition to viral hepatitis, noninfectious liver diseases also threaten the health of human beings. Some of these diseases are quite common in civilized society. Next, we discuss the mechanism of hepatocyte apoptosis in three noninfectious liver diseases, including fatty liver disease due to either metabolic disorders or alcohol abuse, hepatic ischemia reperfusion injury (HIRI) that typically occurs during surgery, and drug-induced liver injury (DILI) after medication.
Hepatocyte Apoptosis and Fatty Liver Disease
Hepatocyte steatosis, the accumulation of fat inside the cell, is a typical pathological change observed in fatty liver diseases. The biochemical changes in the pathogenesis of fatty liver include reduced fatty acids oxidation, increased fatty acids transportation into the liver, and enhanced fatty acid synthesis.81 According to the etiology, fatty liver disease is typically divided into two categories: alcoholic liver diseases (ALDs) and nonalcoholic fatty liver diseases (NAFLDs). Both of these conditions exhibit remarkable hepatocyte apoptosis.82 On the other hand, there is a synergistic effect between nonalcoholic and alcoholic fatty liver injury.83 Although steatosis marks the onset of fatty liver disease, it may progress to fibrosis, cirrhosis, and HCC.84 Both intrinsic and extrinsic factors are involved in steatosis-induced apoptosis. Among all the apoptotic pathways, oxidative stress is of great importance in both ALDs and NAFLDs. Metabolic alterations, such as a high-fat state, inhibit the activity of electron transport chain complexes and elongate the life of electron transport intermediates, thus facilitating ROS generation.81
Bacterial endotoxins absorbed from the gastrointestinal tract into portal circulation are increased in ALD patients, suggesting the influence of the gut–liver axis in ALD. Both ethanol85,86 and a high-fat diet87 can disrupt the intestinal epithelial barrier and increase intestinal permeability. Lipo-polysaccharide (LPS) is a bacterial endotoxin that can be absorbed and enters the liver. LPS activates Toll-like receptor 4 and augments the secretion of TNF-α by the liver and thus promotes hepatocyte apoptosis.88 The administration of Lactobacillus johnsonii can lower intestinal permeability and thus reduces serum LPS levels in NAFLD mice.89
Alcoholic liver diseases
Excess alcohol intake increases the risk of ALD. Long-term drinking typically results in ALD with obvious damage to liver function and distinct increases in hepatocyte apoptosis.90 An in vivo rat model showed that hepatic apoptosis increased over the duration of alcohol intake.22 The number of apoptotic cells in liver specimens from ALD patients is highest in grade 4 steatohepatitis compared with lower grades.90 Although the detailed mechanism of hepatocyte apoptosis during ALD is diverse, it is widely accepted that overproduction of ROS is the major cause of alcoholic hepatocyte injury through the mechanism of mitochondrial damage and ER stress.91,92
Alcohol and its metabolites cause lipid peroxide to induce oxidative stress. The later not only exhausts mitochondrial antioxidant defender glutathione (GSH) but also activates Fas/FasL and the downstream apoptotic signaling pathway.93,94 Hepatocyte Fas expression was moderate to strong in ALD patients compared with only minimal Fas expression in control groups.90 GSH depletion sensitizes hepatocytes to TNF-α-induced cell death.95 Redundant ROS is harmful to the function of mitochondria by damaging the respiratory chain and releasing the pro-oxidant, proapoptotic protein cytochrome P450 2E1.96
Alcohol induces ER stress in hepatocytes.97,98 Alcohol feeding elevates mRNA levels of ER chaperones and caspase-12.99 Caspase-12 is a key element in ER stress-related apoptosis.98 Chronic alcohol feeding reduces the activity of cystathionine beta-synthase and results in hyperhomocysteinemia. The latter induces ER stress and activates caspase-12.22 ER stress is also involved in the development of ALD. IFN regulatory factor 3, a transcription factor that regulates the innate immune response, links ER stress with the proapoptotic protein Bax and contributes to hepatocyte apoptosis.23
In addition to oxidative stress and ER stress, inhibition of survival genes, calcium overload, and the immune response are involved in ALD-induced apoptosis. Alcohol enhances nuclear translocation and activation of transglutaminase 2 and inactivates the transcription factor Sp1, thus inhibiting the expression of the survival gene c-Met.100 Alcohol elevates store-operated Ca2+ entry and leads to intracellular Ca2+ overload and apoptosis.101 Alcohol also causes accumulation of secretory IgM in the liver, which activates complement, increases the expression of inflammatory cytokines, and leads to bid-dependent apoptosis.102
Based on the above information, appropriate treatment for ALD includes alimentary and drug therapy. Acetylcysteine indirectly increases the content of GSH in hepatocyte and is helpful to clear apoptosis-inducing radicals.103 The results from clinical trials support the use of TNF-α inhibitors, such as infliximab and pentoxifylline. These inhibitors significantly inhibit apoptosis caused by the TNF-α signal.104,105 The administration of S-Adenosylmethionine may be a potential therapy for ALD. The mechanism involves reduced expression of TNF-α and increased expression of the anti-inflammatory cytokine IL-10.106
Nonalcoholic fatty liver disease
High-fat food and metabolic disorders endangered people worldwide with NAFLD. This disease refers to pathological changes, including nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Active caspase-3 and -7 and strong Fas expression is present in specimens from NASH patients. In addition, the number of apoptotic hepatocytes is significantly increased and correlates well with disease severity.107 Furthermore, there is a positive correlation between hepatocyte apoptosis and hepatic fibrosis.107 Three risk factors inseparably correlate with hepatocyte apoptosis in NAFLD. The first risk factor is dysregulated hepatic lipid accumulation. The second risk factor is cellular stress derived from oxidative, metabolic, and cytokine alterations. The third risk factor is mitochondrial dysfunction.108 Increased plasma caspase-3 generates cytokeratin-18 fragments and soluble Fas. An elevated circulating level of these two biomarkers is observed in NASH patients and correlates well with liver histopathology.109
The progression of NAFLD is explained by the “two-hit theory”. The first strike is dysregulated hepatic lipid accumulation and steatosis caused by metabolic alterations; the second strike is mitochondrial dysfunction caused by metabolic, oxidative, and cytokine stress.110 Antioxidants, such as vitamin E and betaine, reduce the level of transaminase by mitigating cellular oxidative stress and inhibiting hepatocyte apoptosis.81 Cytokines, such as TNF-α, IFN-α, IL-4, and IL-13, activate their downstream pathways and lead to hepatocyte apoptosis.108,111
Mitochondrial dysfunction plays a key role in hepatocyte apoptosis of NAFLD.81 It disrupts the balance of fat metabolism in hepatocytes, induces oxidative stress, and increases ROS production. ROS overproduction may damage mitochondrial proteins, phospholipids, and mtDNA.112,113 Hepatocyte mtDNA depletion leads to the decreased expression of polypeptides encoded by mtDNA and increases mitochondrial dysfunction.81,114
ER stress is also involved in apoptosis in NAFLD.115 Unsaturated fatty acid treatment reverses ER stress and inhibits apoptosis.116
Hepatocyte Apoptosis and Hepatic Ischemia Reperfusion Injury (HIRI)
As a biochemical factory, normal liver function is highly dependent on the oxygen supply. Reduced oxygen availability often occurs among inpatients during liver surgery, such as liver transplantation or hepatic lobectomy. These situations may block blood flow to the hepatic pedicle and induce HIRI.
HIRI is a key factor of postoperative liver dysfunction (eg, nonfunctional primary liver transplantation and liver failure after liver surgery).117 The HIRI rodent model is produced from 1-hour portal triad clamping followed by 24-hour reperfusion. Hepatocyte apoptosis is significantly increased in the HIRI group compared with sham-operated group, and this trend worsens at later time points (55-fold increased after 4-hour reperfusion and 200-fold increased after 24-hour reperfusion compared with sham).118 To date, the mechanism by which HIRI causes apoptosis and ultimately leads to liver dysfunction is not clear. Interaction among several types of liver cells, such as hepatocytes, Kupffer cells, and neutrophils contribute to the progression of HIRI.119 In terms of hepatocyte apoptosis during HIRI, several changes participate in this process, including the production of oxygen-free radicals, calcium overload, changes in mitochondrial permeability, and cytokine- and apoptosis-related gene expression.117,120,121 Ca2+ overload, anaerobic metabolism, acidosis, and oxidative stress collectively trigger hepatic apoptosis during HIRI. Intracellular Ca2+ overload activates Ca2+-dependent enzymes and ultimately leads to cell death.122 Oxidative stress causes mitochondrial dysfunction and lipid peroxidation, promoting apoptosis through induction of reactive molecules, such as ROS.123 Antioxidants, such as vitamin E derivatives, inhibit hepatocyte apoptosis in HIRI by inhibiting the production of oxygenic free radicals.124 In addition, changes in the metabolic pattern from aerobic to anaerobic inhibit the redox reaction inside hepatocytes and result in significant intercellular ATP depletion. A shortage of energy currency will lead to mitochondrial damage and microcirculation failure. In addition, enhanced anaerobic glycolysis decreases intercellular pH and leads to acidosis-related apoptosis.125
Both ischemic pre- and postconditioning protect hepatocytes from apoptosis after reperfusion.118 Pre- and postconditioning share some similar mechanisms of protection.117 Enhanced antioxidant capacity, activation of p38 pathway, and nitric oxide generation may participate in this protection.117,121
Hepatocyte Apoptosis and Drug-Induced Liver Injury (DILI)
Most drugs are metabolized by the liver and kidney; thus, the liver is susceptible to DILI. Different types of drugs can cause mild to severe liver injury. According to the data provided by Drug-Induced Liver Injury Network, the estimated global annual DILI incidence rate is 13.9–24.0 per million people.
The etiology of DILI differs among races and regions. According to a study that includes 24,112 cases in China, the leading drugs that cause DILI rank as follows: antituberculosis medicine (31.3%), complementary and alternative medicines (18.6%), anti-infectious drugs (9.7%), nonsteroidal anti-inflammatory drugs (NSAIDs, 7.6%), and antitumor drugs (4.7%).126 In a French population-based study, anti-infectious drugs (25%) are the primary cause of DILI, followed by psychotropic (22.5%) and hypolipidemic drugs (12.5%).127 In a Spanish population, NSAIDs (36.4%), analgesics (26.6%), and antibiotics (22.9%) are the top causes of DILI.128 On the other hand, DILI is likely to be more severe in patients who suffer from preexisting liver diseases, such as viral hepatitis and fatty liver diseases.129
Various types of drugs cause liver injury through diverse pathways. This characteristic of DILI exacerbates the difficulty of prevention and treatment. DILI is derived from three sources: 1) direct hepatocyte injury; 2) exacerbation of liver disease, especially hepatitis; and 3) a preexisting liver disease that alters the pharmacokinetics of drugs by typically prolonging the effective time of drugs, thus enhancing the toxicity.130 Through liver metabolism, drugs can elicit electrophilic products. These products will cause damage to the membrane of cell, mitochondria, and microsome via covalent binding.131
Most DILI is dose dependent. A daily dosage greater than 50 or 100 mg of oral medication is associated with a higher risk for DILI.132,133 In addition to the daily dosage, the “total dose of drug” should also be taken into consideration. Prolonged and continuous administration in the low “therapeutic” range may lead to severe liver damage.134 Dose-dependent liver injury may be due to the production of active metabolites, which exhaust cellular GSH and lead to lipid peroxidation and mitochondrial damage. ER stress is also believed to play a role in DILI.135 Here, we list three categories of drugs that are common causes of DILI and discuss their role in hepatocyte apoptosis.
Antituberculosis drugs
DILI is a common side effect of antituberculosis drugs (ATDs).136 Approximately 1%–18% of ATD-treated patients will develop DILI.129,137,138 Liver toxicity of first-line ATD, especially isoniazid, is mostly studied. Specifically, 5 mg/kg isoniazid per os daily for 9 months induces hepatitis at the overall rate of 1% and is age related.138 In vitro experiments indicate that 24-hour exposure to isoniazid dose dependently (13, 26, and 52 mM) caused cytotoxicity in HepG2 cells.139 In vivo data also indicate the likelihood to induce hepatotoxicity by oral administration of isoniazid and rifampicin (200 mg/kg daily) for 30 days in rats.140 Combined administration of isoniazid (50 mg/kg subcutaneously), rifampicin (250 mg/kg intragastrically), and pyrazinamide (45 mg/kg intragastrically) for 14 days in rats significantly induced hepatocyte apoptosis.141 The mechanism of ATD-induced DILI involves both the external apoptosis pathway mediated by the cell surface receptor Fas and the internal apoptotic pathway, which is p53-dependent. Simultaneously, the level of antiapoptotic Bcl-2 protein was reduced.141 Decreased intracellular antioxidases (eg, superoxide dismutase) and elevated ROS generation also play a role.139
Complementary and alternative medicines
Although herb medicine is not chemically synthesized, components, such as alkaloids, glycosides, toxic proteins, and metals may still cause liver toxicity.142 The clinical manifestation includes both acute and chronic toxicity. Although acute toxicity is typically caused by overdose or intravenous administration, chronic toxicity is more likely due to long-term usage-related accumulative toxicity.142 The property of an herb medicine is typically unique and complicated, thus making it difficult to determine the “typical mechanism” of its liver toxicity. Some drugs or their metabolites can cause directly injury to hepatocytes through toxic effects, whereas others initiate an immune response. Overdose is a common cause of herb medicine-induced liver toxicity. For example, 3–9 g of Gardenia is typically safe, whereas 30 g or more may induce liver injury.142 In fact, the degree of injury not only depends on the type and dose of drugs but also on the processing procedures. For example, Euphorbia kansui is used to treat edema, but it also shows liver toxicity at doses of 2.97 and 5.94 mg/mL to LO2 hepatocytes. Stir-baking with vinegar reduces its toxicity by reducing the levels of terpenoids and inhibiting the intrinsic apoptosis pathway.143
Antitumor drugs
Antitumor drugs are a large family of drugs that are involved in diverse molecular mechanisms. Among them, cytotoxic antitumor drugs are often reported to have liver toxicity. The side effects of antitumor drugs typically occur within 1–4 weeks from the beginning of chemotherapy. Alkylating agents such as Cytoxan; antimetabolites, such as methotrexate; DNA-damaging antibiotics, such as mitomycin; mitosis inhibitors, such as vindesine; and tyrosine kinase inhibitors (TKI), such as epithelial growth factor receptor TKI, induce DILI. Approximately 10% of patients developed DILI after docetaxel (mitosis inhibitor) treatment.144 ROS produced by antitumor drugs is an important mechanism to induce tumor cell death due to hepatocyte toxicity, especially in steatotic livers.145 Repeated use of 10 mg/mL nedaplatin causes significant apoptosis by reducing Bcl-2 expression, enhancing p53 expression, and increasing ROS production and mitochondria damage in cultured normal hepatic cell lines.146 Interestingly, when the antioxidant dihydromyricetin was administered together with nedaplatin, normal hepatocytes were protected from DILI, and the effect of nedaplatin on tumor cells was enhanced.146
Conclusion and Predictions
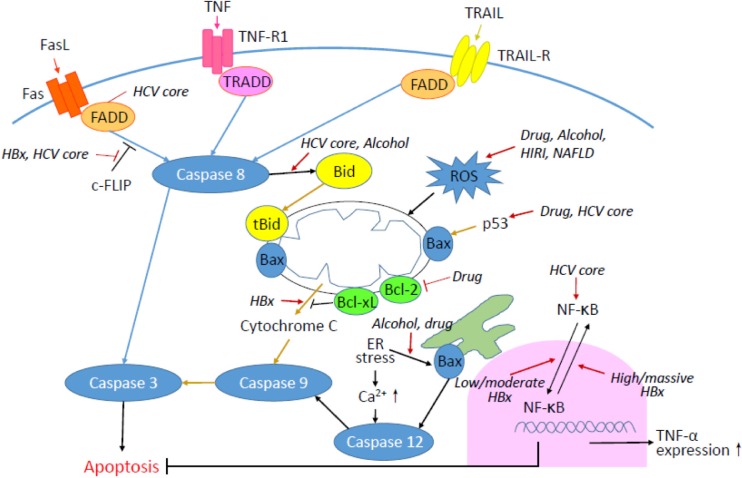
Hepatocyte injury is a complicated biological process that is mediated by various factors and may lead to hepatocyte apoptosis. In this review, we provide a general idea regarding apoptotic pathways in hepatocytes as well as unique features in particular liver diseases. The common apoptotic pathways in hepatocytes include fundamental extrinsic and intrinsic pathways, ER stress, and oxidative stress-mediated signaling. Four types of liver diseases accompanied by significant hepatocyte apoptosis are discussed here: viral hepatitis, steatotic liver, ischemia reperfusion injury, and drug-induced injury. Figure 1 summarizes the apoptotic pathways in hepatocytes, as well as the influence of viral and noninfectious factors in this process.
Figure 1.
The schematic diagram of apoptotic pathways in hepatocytes. Blue lines indicate extrinsic pathways, whereas light brown lines indicate intrinsic pathways. The influence of virus infection, alcohol, fat, ischemia reperfusion, and drug on hepatocyte apoptosis is also indicated by italics and red arrows.
Studies of the mechanism of hepatocyte apoptosis in different liver diseases are of great value for providing a better strategy to prevent and treat liver diseases. In the past decades, scientists have identified numerous apoptosis-related genes and signaling transduction pathways. However, some mysteries are still waiting to be unveiled. One of the most important questions is how to adjust the expression of apoptotic genes and pathways to prevent or alleviate liver diseases. Hepatic apoptosis intervention is a fascinating yet challenging journey to delay liver disease progression and reduce its morbidity. Several concerns need to be carefully investigated to accelerate both preclinical and clinical studies.
First, more accurate and dynamic methods of monitoring in vivo apoptosis must be explored. Gold stand, the classical diagnosis of liver damage, depends on a histological evaluation from biopsy samples. Most of our current knowledge about in vivo hepatocyte apoptosis is derived from this method. However, it is difficult to obtain dynamic data safely from invasive procedures, and sampling error is another major concern. Noninvasive evaluations, such as imaging techniques and serum biomarkers, are now widely used to estimate the level of liver injury. High-resolution imaging, specific biomarkers, and other new indicators that correlate with the onset, progression, and location of apoptosis must be explored.
Second, the switch among different modalities of cell death deserves intensive study. Based on our current understanding, liver injury includes several types of cell death: apoptosis, necroptosis, necrosis, and autophagic-dependent cell death. With methodological advancement, more death modalities may be identified. On the one hand, scientists should study the characteristics of different death modalities; on the other hand, understanding the switch mechanism among them and manipulating these switches are of great value to conquer the illness. For example, to eliminate intruding enemies, such as microorganisms and harmful metabolites, with minimal impact to hepatocytes, it is essential to understand the “on and off” mechanism of hepatocyte apoptosis and the transformation between apoptosis and other death modalities.
Third, findings from animal experiments must be cautiously evaluated and interpreted. The gap between animal models and clinical cases must be always kept in mind. Physiology and pathophysiology dissimilarities derived from metabolic enzymes, absorption profiles, and concomitant diseases all contribute to this gap. Furthermore, given that metabolic, genetic, and health largely influence the outcome of liver injury, precision medicine is very likely to be the future direction of hepatology. To accurately define the network controlling hepatocyte apoptosis, more time and work are needed.
Footnotes
ACADEMIC EDITOR: Garry M. Walsh, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 775 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by Research Center on Aging and Medicine, Fudan University (13dz2260700 to MJW) and Shanghai Pujiang Program (15PJ1400700 to MJW). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Author Contributions
Wrote the manuscript: LC, XBQ, and MJW. Revised the manuscript: WJZ, XOY, and MJW. All the authors approved the final version of manuscript.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 2.Martin SJ, Henry CM. Distinguishing between apoptosis, necrosis, necroptosis and other cell death modalities. Methods. 2013;61(2):87–89. doi: 10.1016/j.ymeth.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14(11):1631–1642. doi: 10.1080/15384101.2015.1038685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30(4):402–410. doi: 10.1055/s-0030-1267540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkmann X, Anstaett M, Hadem J, et al. Caspase activation is associated with spontaneous recovery from acute liver failure. Hepatology. 2008;47(5):1624–1633. doi: 10.1002/hep.22237. [DOI] [PubMed] [Google Scholar]
- 6.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39(2):273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 7.Roychowdhury S, McCullough RL, Sanz-Garcia CJ, et al. Receptor interacting protein 3 protects mice from high fat diet-induced liver injury. Hepatology. 2016 doi: 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin XM, Ding WX. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med. 2003;3(6):491–508. doi: 10.2174/1566524033479555. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Huang C, Wang Y, et al. Hepatitis B virus X protein sensitizes TRAIL-induced hepatocyte apoptosis by inhibiting the E3 ubiquitin ligase A20. PLoS One. 2015;10(5):e0127329. doi: 10.1371/journal.pone.0127329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corazza N, Kassahn D, Jakob S, Badmann A, Brunner T. TRAIL-induced apoptosis: between tumor therapy and immunopathology. Ann N Y Acad Sci. 2009;1171:50–58. doi: 10.1111/j.1749-6632.2009.04905.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Seong BL. Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 2003;22(9):2104–2116. doi: 10.1093/emboj/cdg210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battaglia V, Compagnone A, Bandino A, et al. Cobalt induces oxidative stress in isolated liver mitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int J Biochem Cell Biol. 2009;41(3):586–594. doi: 10.1016/j.biocel.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 14.Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5(2):201–212. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei Y, Xing D, Gao X, Liu L, Chen T. Real-time monitoring full length bid interacting with Bax during TNF-alpha-induced apoptosis. Apoptosis. 2007;12(9):1681–1690. doi: 10.1007/s10495-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 16.Imao M, Nagaki M, Imose M, Moriwaki H. Differential caspase-9-dependent signaling pathway between tumor necrosis factor receptor- and Fas-mediated hepatocyte apoptosis in mice. Liver Int. 2006;26(1):137–146. doi: 10.1111/j.1478-3231.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagaki M, Sugiyama A, Osawa Y, et al. Lethal hepatic apoptosis mediated by tumor necrosis factor receptor, unlike Fas-mediated apoptosis, requires hepatocyte sensitization in mice. J Hepatol. 1999;31(6):997–1005. doi: 10.1016/s0168-8278(99)80311-0. [DOI] [PubMed] [Google Scholar]
- 18.Nagaki M, Moriwaki H. Implication of cytokines: roles of tumor necrosis factor-alpha in liver injury. Hepatol Res. 2008;38(suppl 1):S19–S28. doi: 10.1111/j.1872-034X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 19.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 20.Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106(9):1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guicciardi ME, Bronk SF, Werneburg NW, Gores GJ. cFLIPL prevents TRAIL-induced apoptosis of hepatocellular carcinoma cells by inhibiting the lysosomal pathway of apoptosis. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1337–G1346. doi: 10.1152/ajpgi.00497.2006. [DOI] [PubMed] [Google Scholar]
- 22.Sun LN, Zhou DF, Zhou JY, Zhao CY, Zhen Z. Role of endoplasmic reticulum stress in alcoholic liver disease-related hepatocyte apoptosis. Zhonghua Gan Zang Bing Za Zhi. 2012;20(1):35–39. doi: 10.3760/cma.j.issn.1007-3418.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Petrasek J, Iracheta-Vellve A, Csak T, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110(41):16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11(4):372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 25.Egnatchik RA, Leamy AK, Noguchi Y, Shiota M, Young JD. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism. 2014;63(2):283–295. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petit PX, Goubern M, Diolez P, Susin SA, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426(1):111–116. doi: 10.1016/s0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 27.Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol. 2014;60(5):1063–1074. doi: 10.1016/j.jhep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22(2):263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawat S, Clippinger AJ, Bouchard MJ. Modulation of apoptotic signaling by the hepatitis B virus X protein. Viruses. 2012;4(11):2945–2972. doi: 10.3390/v4112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappaB activation. J Virol. 1999;73(6):4713–4720. doi: 10.1128/jvi.73.6.4713-4720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song CZ, Bai ZL, Song CC, Wang QW. Aggregate formation of hepatitis B virus X protein affects cell cycle and apoptosis. World J Gastroenterol. 2003;9(7):1521–1524. doi: 10.3748/wjg.v9.i7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo CY, Chou TY, Chen CM, Tsai YF, Hwang GY, Hwang TL. Hepatitis B virus X protein disrupts stress fiber formation and triggers apoptosis. Virus Res. 2013;175(1):20–29. doi: 10.1016/j.virusres.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi N, Mita E. Fas system and apoptosis in viral hepatitis. J Gastroenterol Hepatol. 1997;12(9–10):S223–S226. doi: 10.1111/j.1440-1746.1997.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe H, Wells F, Major ME. Clearance of hepatitis C in chimpanzees is associated with intrahepatic T-cell perforin expression during the late acute phase. J Viral Hepat. 2010;17(4):245–253. doi: 10.1111/j.1365-2893.2009.01172.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Chae DW, Kim SM, et al. Expression of FasL and perforin/granzyme B mRNA in chronic hepatitis B virus infection. J Viral Hepat. 2004;11(2):130–135. doi: 10.1046/j.1365-2893.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 37.Willberg CB, Ward SM, Clayton RF, et al. Protection of hepatocytes from cytotoxic T cell mediated killing by interferon-alpha. PLoS One. 2007;2(8):e791. doi: 10.1371/journal.pone.0000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YG, Liu SX, Liang XH, et al. Blockade of TRAIL pathway ameliorates HBV-induced hepatocyte apoptosis in an acute hepatitis model. Biochem Biophys Res Commun. 2007;352(2):329–334. doi: 10.1016/j.bbrc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Wen X, Huang C, Wei Y. Unraveling the complexity of hepatitis B virus: from molecular understanding to therapeutic strategy in 50 years. Int J Biochem Cell Biol. 2013;45(9):1987–1996. doi: 10.1016/j.biocel.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Chen HY, Tang NH, Li XJ, Zhang SJ, Chen ZX, Wang XZ. Transfection and expression of hepatitis B virus x gene and its effect on apoptosis in HL-7702 cells. World J Gastroenterol. 2004;10(7):959–964. doi: 10.3748/wjg.v10.i7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diao J, Khine AA, Sarangi F, et al. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J Biol Chem. 2001;276(11):8328–8340. doi: 10.1074/jbc.M006026200. [DOI] [PubMed] [Google Scholar]
- 42.Rawat S, Bouchard MJ. The hepatitis B virus (HBV) HBx protein activates AKT to simultaneously regulate HBV replication and hepatocyte survival. J Virol. 2015;89(2):999–1012. doi: 10.1128/JVI.02440-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barreiros AP, Sprinzl M, Rosset S, et al. EGF and HGF levels are increased during active HBV infection and enhance survival signaling through extracellular matrix interactions in primary human hepatocytes. Int J Cancer. 2009;124(1):120–129. doi: 10.1002/ijc.23921. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Zheng L, Lv G, Jin X, Sheng J. Hepatocytes treated with HBV X protein as cytotoxic effectors kill primary hepatocytes by TNF-alpha-related apoptosis-induced ligand-mediated mechanism. Intervirology. 2007;50(5):323–327. doi: 10.1159/000106463. [DOI] [PubMed] [Google Scholar]
- 45.Takada S, Shirakata Y, Kaneniwa N, Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18(50):6965–6973. doi: 10.1038/sj.onc.1203188. [DOI] [PubMed] [Google Scholar]
- 46.Chami M, Ferrari D, Nicotera P, Paterlini-Bréchot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278(34):31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- 47.Hu L, Chen L, Yang G, et al. HBx sensitizes cells to oxidative stress-induced apoptosis by accelerating the loss of Mcl-1 protein via caspase-3 cascade. Mol Cancer. 2011;10:43. doi: 10.1186/1476-4598-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka Y, Kanai F, Kawakami T, et al. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem Biophys Res Commun. 2004;318(2):461–469. doi: 10.1016/j.bbrc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 49.Pan J, Duan LX, Sun BS, Feitelson MA. Hepatitis B virus X protein protects against anti-Fas-mediated apoptosis in human liver cells by inducing NF-kappa B. J Gen Virol. 2001;82(pt 1):171–182. doi: 10.1099/0022-1317-82-1-171. [DOI] [PubMed] [Google Scholar]
- 50.Lee YI, Kang-Park S, Do SI, Lee YI. The hepatitis B virus-X protein activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2001;276(20):16969–16977. doi: 10.1074/jbc.M011263200. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, Shen Q, Gu B, Ren H, Zhang D. Effects of hepatitis B virus X gene on apoptosis and the activity of telomerase in HepG(2) cells. Zhonghua Gan Zang Bing Za Zhi. 2000;8(4):212–214. [PubMed] [Google Scholar]
- 52.Clippinger AJ, Gearhart TL, Bouchard MJ. Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-kappaB and the mitochondrial permeability transition pore. J Virol. 2009;83(10):4718–4731. doi: 10.1128/JVI.02590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen L, Zhang X, Hu D, et al. Hepatitis B virus X (HBx) play an anti-apoptosis role in hepatic progenitor cells by activating Wnt/beta-catenin pathway. Mol Cell Biochem. 2013;383(1–2):213–222. doi: 10.1007/s11010-013-1769-5. [DOI] [PubMed] [Google Scholar]
- 54.Zhai LL, Liu J, Xie YH. Dose-dependent modulation of cell apoptosis by hepatitis B virus X protein. J Microb Infect. 2011;6(2):6. [Google Scholar]
- 55.Knoll S, Fürst K, Thomas S, et al. Dissection of cell context-dependent interactions between HBx and p53 family members in regulation of apoptosis: a role for HBV-induced HCC. Cell Cycle. 2011;10(20):3554–3565. doi: 10.4161/cc.10.20.17856. [DOI] [PubMed] [Google Scholar]
- 56.Wei W, Huang W, Pan Y, Zhu F, Wu J. Functional switch of viral protein HBx on cell apoptosis, transformation, and tumorigenesis in association with oncoprotein Ras. Cancer Lett. 2006;244(1):119–128. doi: 10.1016/j.canlet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Caligiuri P, Cerruti R, Icardi G, Bruzzone B. Overview of hepatitis B virus mutations and their implications in the management of infection. World J Gastroenterol. 2016;22(1):145–154. doi: 10.3748/wjg.v22.i1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu R, Mok MT, Kang W, et al. Truncated HBx-dependent silencing of GAS2 promotes hepatocarcinogenesis through deregulation of cell cycle, senescence and p53-mediated apoptosis. J Pathol. 2015;237(1):38–49. doi: 10.1002/path.4554. [DOI] [PubMed] [Google Scholar]
- 59.Ebert G, Preston S, Allison C, et al. Cellular inhibitor of apoptosis proteins prevent clearance of hepatitis B virus. Proc Natl Acad Sci USA. 2015;112(18):5797–5802. doi: 10.1073/pnas.1502390112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jahan S, Ashfaq UA, Khaliq S, Samreen B, Afzal N. Dual behavior of HCV Core gene in regulation of apoptosis is important in progression of HCC. Infect Genet Evol. 2012;12(2):236–239. doi: 10.1016/j.meegid.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Brault C, Levy PL, Bartosch B. Hepatitis C virus-induced mitochondrial dysfunctions. Viruses. 2013;5(3):954–980. doi: 10.3390/v5030954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calabrese F, Pontisso P, Pettenazzo E, et al. Liver cell apoptosis in chronic hepatitis C correlates with histological but not biochemical activity or serum HCV-RNA levels. Hepatology. 2000;31(5):1153–1159. doi: 10.1053/he.2000.7123. [DOI] [PubMed] [Google Scholar]
- 63.Bantel H, Lügering A, Heidemann J, et al. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40(5):1078–1087. doi: 10.1002/hep.20411. [DOI] [PubMed] [Google Scholar]
- 64.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Qu A, Han X, Wang Y. HCV core protein represses the apoptosis and improves the autophagy of human hepatocytes. Int J Clin Exp Med. 2015;8(9):15787–15793. [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H, Ray R. Evasion of TNF-alpha-mediated apoptosis by hepatitis C virus. Methods Mol Biol. 2014;1155:125–132. doi: 10.1007/978-1-4939-0669-7_11. [DOI] [PubMed] [Google Scholar]
- 67.Machida K, Tsukiyama-Kohara K, Seike E, et al. Inhibition of cytochrome c release in Fas-mediated signaling pathway in transgenic mice induced to express hepatitis C viral proteins. J Biol Chem. 2001;276(15):12140–12146. doi: 10.1074/jbc.M010137200. [DOI] [PubMed] [Google Scholar]
- 68.Pavio N, Battaglia S, Boucreux D, et al. Hepatitis C virus core variants isolated from liver tumor but not from adjacent non-tumor tissue interact with Smad3 and inhibit the TGF-beta pathway. Oncogene. 2005;24(40):6119–6132. doi: 10.1038/sj.onc.1208749. [DOI] [PubMed] [Google Scholar]
- 69.Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007;13(36):4865–4872. doi: 10.3748/wjg.v13.i36.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seo YL, Heo S, Jang KL. Hepatitis C virus core protein overcomes H2O2-induced apoptosis by downregulating p14 expression via DNA methylation. J Gen Virol. 2015;96(pt 4):822–832. doi: 10.1099/vir.0.000032. [DOI] [PubMed] [Google Scholar]
- 71.Feng S, Li M, Zhang J, et al. Regulation of HepG2 cell apoptosis by hepatitis C virus (HCV) core protein via the sirt1-p53-bax pathway. Virus Genes. 2015;51(3):338–346. doi: 10.1007/s11262-015-1253-2. [DOI] [PubMed] [Google Scholar]
- 72.Chou AH, Tsai HF, Wu YY, et al. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174(4):2160–2166. doi: 10.4049/jimmunol.174.4.2160. [DOI] [PubMed] [Google Scholar]
- 73.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 74.Zhu N, Ware CF, Lai MM. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001;283(2):178–187. doi: 10.1006/viro.2001.0896. [DOI] [PubMed] [Google Scholar]
- 75.Schwer B, Ren S, Pietschmann T, et al. Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif. J Virol. 2004;78(15):7958–7968. doi: 10.1128/JVI.78.15.7958-7968.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122(2):366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 77.Ciccaglione AR, Marcantonio C, Tritarelli E, et al. The transmembrane domain of hepatitis C virus E1 glycoprotein induces cell death. Virus Res. 2004;104(1):1–9. doi: 10.1016/j.virusres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Lee SH, Kim YK, Kim CS, et al. E2 of hepatitis C virus inhibits apoptosis. J Immunol. 2005;175(12):8226–8235. doi: 10.4049/jimmunol.175.12.8226. [DOI] [PubMed] [Google Scholar]
- 79.Chiou HL, Hsieh YS, Hsieh MR, Chen TY. HCV E2 may induce apoptosis of Huh-7 cells via a mitochondrial-related caspase pathway. Biochem Biophys Res Commun. 2006;345(1):453–458. doi: 10.1016/j.bbrc.2006.04.118. [DOI] [PubMed] [Google Scholar]
- 80.Nomura-Takigawa Y, Nagano-Fujii M, Deng L, et al. Non-structural protein 4A of hepatitis C virus accumulates on mitochondria and renders the cells prone to undergoing mitochondria-mediated apoptosis. J Gen Virol. 2006;87(pt 7):1935–1945. doi: 10.1099/vir.0.81701-0. [DOI] [PubMed] [Google Scholar]
- 81.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(39):14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ribeiro PS, Cortez-Pinto H, Solá S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99(9):1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 83.Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18(1):157–163. doi: 10.1016/j.cld.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303(12):G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50(2):638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paolella G, Mandato C, Pierri L, Poeta M, Di Stasi M, Vajro P. Gut-liver axis and probiotics: their role in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(42):15518–15531. doi: 10.3748/wjg.v20.i42.15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xin J, Zeng D, Wang H, et al. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol. 2014;98(15):6817–6829. doi: 10.1007/s00253-014-5752-1. [DOI] [PubMed] [Google Scholar]
- 90.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34(2):248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 91.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83(6):519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 92.Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20(9):2136–2142. doi: 10.3748/wjg.v20.i9.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lívero FA, Stolf AM, Dreifuss AA, et al. The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact. 2014;217:19–27. doi: 10.1016/j.cbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 94.García-Ruiz C, Morales A, Ballesta A, Rodés J, Kaplowitz N, Fernández-Checa JC. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J Clin Invest. 1994;94(1):193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31(5):1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 96.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 97.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54(4):795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu LQ, Fan ZQ, Tang YF, Ke ZJ. The resveratrol attenuates ethanol-induced hepatocyte apoptosis via inhibiting ER-related caspase-12 activation and PDE activity in vitro. Alcohol Clin Exp Res. 2014;38(3):683–693. doi: 10.1111/acer.12311. [DOI] [PubMed] [Google Scholar]
- 99.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 100.Tatsukawa H, Fukaya Y, Frampton G, et al. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology. 2009;136(5):1783–95e10. doi: 10.1053/j.gastro.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cui R, Yan L, Luo Z, Guo X, Yan M. Blockade of store-operated calcium entry alleviates ethanol-induced hepatotoxicity via inhibiting apoptosis. Toxicol Appl Pharmacol. 2015;287(1):52–66. doi: 10.1016/j.taap.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 102.Smathers RL, Chiang DJ, McMullen MR, Feldstein AE, Roychowdhury S, Nagy LE. Soluble IgM links apoptosis to complement activation in early alcoholic liver disease in mice. Mol Immunol. 2016;72:9–18. doi: 10.1016/j.molimm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335(8705):1572–1573. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 104.Spahr L, Rubbia-Brandt L, Frossard JL, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37(4):448–455. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 105.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 106.McClain CJ, Hill DB, Song Z, et al. S-Adenosylmethionine, cytokines, and alcoholic liver disease. Alcohol. 2002;27(3):185–192. doi: 10.1016/s0741-8329(02)00224-0. [DOI] [PubMed] [Google Scholar]
- 107.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 108.Syn WK, Choi SS, Diehl AM. Apoptosis and cytokines in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):565–580. doi: 10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tamimi TI, Elgouhari HM, Alkhouri N, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol. 2011;54(6):1224–1229. doi: 10.1016/j.jhep.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Day CP, James OF. Steatohepatitis: a tale of two “hits”. Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 111.Zhang W, Kudo H, Kawai K, et al. Tumor necrosis factor-alpha accelerates apoptosis of steatotic hepatocytes from a murine model of non-alcoholic fatty liver disease. Biochem Biophys Res Commun. 2010;391(4):1731–1736. doi: 10.1016/j.bbrc.2009.12.144. [DOI] [PubMed] [Google Scholar]
- 112.Nomoto K, Tsuneyama K, Takahashi H, Murai Y, Takano Y. Cytoplasmic fine granular expression of 8-hydroxydeoxyguanosine reflects early mitochondrial oxidative DNA damage in nonalcoholic fatty liver disease. Appl Immunohistochem Mol Morphol. 2008;16(1):71–75. doi: 10.1097/PAI.0b013e31803156d5. [DOI] [PubMed] [Google Scholar]
- 113.Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16(11):1095–1105. doi: 10.1097/00042737-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 114.Sookoian S, Rosselli MS, Gemma C, et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1alpha promoter. Hepatology. 2010;52(6):1992–2000. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- 115.Cao J, Dai DL, Yao L, et al. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364(1–2):115–129. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y, Yang X, Shi H, Dong L, Bai J. Effect of alpha-linolenic acid on endoplasmic reticulum stress-mediated apoptosis of palmitic acid lipotoxicity in primary rat hepatocytes. Lipids Health Dis. 2011;10:122. doi: 10.1186/1476-511X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19(11):2036–2047. [PubMed] [Google Scholar]
- 118.Knudsen AR, Kannerup AS, Grønbæk H, et al. Quantitative histological assessment of hepatic ischemia-reperfusion injuries following ischemic pre- and post-conditioning in the rat liver. J Surg Res. 2013;180(1):e11–e20. doi: 10.1016/j.jss.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 119.Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J Gastroenterol. 2010;16(10):1285–1292. doi: 10.3748/wjg.v16.i10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuo KK, Wu BN, Chiu EY, et al. NO donor KMUP-1 improves hepatic ischemia-reperfusion and hypoxic cell injury by inhibiting oxidative stress and pro-inflammatory signaling. Int J Immunopathol Pharmacol. 2013;26(1):93–106. doi: 10.1177/039463201302600109. [DOI] [PubMed] [Google Scholar]
- 121.Guan LY, Fu PY, Li PD, et al. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6(7):122–128. doi: 10.4240/wjgs.v6.i7.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swift C, Garner JP. Non-operative management of liver trauma. J R Army Med Corps. 2012;158(2):85–95. doi: 10.1136/jramc-158-02-04. [DOI] [PubMed] [Google Scholar]
- 123.Jaeschke H. Reperfusion injury after warm ischemia or cold storage of the liver: role of apoptotic cell death. Transplant Proc. 2002;34(7):2656–2658. doi: 10.1016/s0041-1345(02)03464-4. [DOI] [PubMed] [Google Scholar]
- 124.Oishi K, Hagiwara S, Koga S, et al. The vitamin E derivative, EPC-K1, suppresses inflammation during hepatic ischemia-reperfusion injury and exerts hepatoprotective effects in rats. J Surg Res. 2012;176(1):164–170. doi: 10.1016/j.jss.2011.03.080. [DOI] [PubMed] [Google Scholar]
- 125.Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol. 2009;1(1):72–78. doi: 10.4254/wjh.v1.i1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou Y, Yang L, Liao Z, He X, Zhou Y, Guo H. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21,789 patients. Eur J Gastroenterol Hepatol. 2013;25(7):825–829. doi: 10.1097/MEG.0b013e32835f6889. [DOI] [PubMed] [Google Scholar]
- 127.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36(2):451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 128.Ibáñez L, Pérez E, Vidal X, Laporte JR, Grup d’Estudi Multicènteric d’Hepatotoxicitat Aguda de Barcelona (GEMHAB) Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol. 2002;37(5):592–600. doi: 10.1016/s0168-8278(02)00231-3. [DOI] [PubMed] [Google Scholar]
- 129.Fernández-Villar A, Sopeña B, Fernández-Villar J, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8(12):1499–1505. [PubMed] [Google Scholar]
- 130.Tarantino G, Di Minno MN, Capone D. Drug-induced liver injury: is it somehow foreseeable? World J Gastroenterol. 2009;15(23):2817–2833. doi: 10.3748/wjg.15.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wen B, Chen Y, Fitch WL. Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2009;37(7):1557–1562. doi: 10.1124/dmd.108.024851. [DOI] [PubMed] [Google Scholar]
- 132.Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58(1):388–396. doi: 10.1002/hep.26208. [DOI] [PubMed] [Google Scholar]
- 133.Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47(6):2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 134.Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C. Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases. Gastroenterology. 1991;100(6):1701–1709. doi: 10.1016/0016-5085(91)90672-8. [DOI] [PubMed] [Google Scholar]
- 135.Yang X, Shao H, Liu W, et al. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234(1):40–49. doi: 10.1016/j.toxlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saukkonen JJ, Cohn DL, Jasmer RM, et al. ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 137.Agal S, Baijal R, Pramanik S, et al. Monitoring and management of antituberculosis drug induced hepatotoxicity. J Gastroenterol Hepatol. 2005;20(11):1745–1752. doi: 10.1111/j.1440-1746.2005.04048.x. [DOI] [PubMed] [Google Scholar]
- 138.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999 and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 139.Bhadauria S, Mishra R, Kanchan R, et al. Isoniazid-induced apoptosis in HepG2 cells: generation of oxidative stress and Bcl-2 down-regulation. Toxicol Mech Methods. 2010;20(5):242–251. doi: 10.3109/15376511003793325. [DOI] [PubMed] [Google Scholar]
- 140.Santhosh S, Sini TK, Anandan R, Mathew PT. Effect of chitosan supplementation on antitubercular drugs-induced hepatotoxicity in rats. Toxicology. 2006;219(1–3):53–59. doi: 10.1016/j.tox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Bazhanova ED, Sukhanov DS, Teplyi DL. Pathways of apoptosis regulation in hepatocytes induced by first-line antitubercular drugs. Bull Exp Biol Med. 2015;158(5):650–653. doi: 10.1007/s10517-015-2828-6. [DOI] [PubMed] [Google Scholar]
- 142.Xu LM, Lin QX. Hepatic toxicity of Chinese herbal medicine. Zhonghua Gan Zang Bing Za Zhi. 2007;15(7):534–535. [PubMed] [Google Scholar]
- 143.Yan X, Zhang L, Guo J, et al. Processing of kansui roots stir-baked with vinegar reduces kansui-induced hepatocyte cytotoxicity by decreasing the contents of toxic terpenoids and regulating the cell apoptosis pathway. Molecules. 2014;19(6):7237–7254. doi: 10.3390/molecules19067237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Z, Liang X, Yu J, et al. Non-genetic risk factors and predicting efficacy for docetaxel—drug-induced liver injury among metastatic breast cancer patients. J Gastroenterol Hepatol. 2012;27(8):1348–1352. doi: 10.1111/j.1440-1746.2012.07131.x. [DOI] [PubMed] [Google Scholar]
- 145.Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105. doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang L, Zhang Q, Ren H, et al. Dihydromyricetin enhances the chemosensitivity of nedaplatin via regulation of the p53/Bcl-2 pathway in hepatocellular carcinoma cells. PLoS One. 2015;10(4):e0124994. doi: 10.1371/journal.pone.0124994. [DOI] [PMC free article] [PubMed] [Google Scholar]