Abstract
Previous findings have shown that individuals with autism spectrum disorder (ASD) evince greater intra-individual variability (IIV) in their sensory-evoked fMRI responses compared to typical control participants. We explore the robustness of this finding with a new sample of high-functioning adults with autism. Participants were presented with visual, somatosensory and auditory stimuli in the scanner whilst they completed a one-back task. While ASD and control participants were statistically indistinguishable with respect to behavioral responses, the new ASD group exhibited greater IIV relative to controls. We also show that the IIV was equivalent across hemispheres and remained stable over the duration of the experiment. This suggests that greater cortical IIV may be a replicable characteristic of sensory systems in autism.
Keywords: autism, fMRI, sensory-evoked, variability
Research on the neural basis of autism spectrum disorder (ASD) has begun to focus on widespread alterations in neural processing (Belmonte et al., 2004; Rubenstein and Merzenich, 2003). Because autism, as a disorder, is neurodevelopmental in origin, rather than a consequence of circumscribed brain injury or other focal disease, the expectation is that a deviation in neural development will affect brain circuits and neural processing quite widely, even beyond the recognized triad of symptoms (communication, language and repetitive behaviors) (DSM-IV). The prediction then is that the fundamental dynamics of neural responses may be affected potentially throughout cortex.
Here, we focus on the unreliability or inconsistency of cortical responses in individuals with autism. In principle, this greater intra-individual variability (IIV), also referred to as ‘wobble’ or ‘lability’, may be evident as variability in either behavioral performance (MacDonald et al., 2006) or in neural activity from one trial to the next (Rubenstein and Merzenich, 2003). The hypothesis that neural responses are unreliable in ASD is also compatible with the observation that individuals with autism may be over- or under-reactive to sensory stimulation and this may be highly variable even within individual (Cermak & Groza, 1998). Additionally, the comorbidity of epilepsy with autism might reflect similar mechanisms of neural instability (Tuchman and Rapin, 2002; Amiet et al., 2013). The claims of altered cortical dynamics in ASD have, however, been mostly speculative and only in the last few years has there been any empirical evidence that supports such claims.
One of the first studies to explicitly explore IIV in ASD was the re-analysis of data from a visual evoked potential (VEP) study (Milne et al., 2009; Milne, 2011), in which children/adolescents responded to the presence of an oddball image (a zebra) amongst a sequence of grating patch stimuli. This study reported several measures of variability across trials. First, they computed the degree of inter-trial phase consistency across the time-course, and second, they characterized various properties of the P1 waveform including variability of amplitude, magnitude, and latency. They found that intra-individual variability of P1 latency and P1 amplitude was greater in participants with ASD, and that inter-trial α-band (∼10 Hz) phase coherence was lower. In addition, there was a significant correlation between P1 peak amplitude variability and reaction time variability in detecting the presence of the oddball.
In our previous research, we used functional magnetic resonance imaging (fMRI) to assess variability in sensory and motor responses in individuals with autism and typical control participants (Dinstein et al., 2010; Dinstein et al., 2012). In one of these two studies (Dinstein et al., 2012), we measured responses evoked by visual, somatosensory and auditory stimuli. Although there were no differences in the mean amplitude of the fMRI responses in any of the sensory modalities between individuals with autism and controls, there was greater IIV, calculated as trial-to-trial variability, in the autism than control group and, concomitantly, weaker signal-to-noise ratios (SNRs). Greater IIV was also observed in visual cortex and motor cortex in the other of these two studies (Dinstein et al., 2010), in which participants were required to observe and execute hand movements. Together, these fMRI and EEG findings suggest that greater IIV might be a widespread alteration in cortical processing, and that greater variability in evoked activity from one trial to another might be an endophenotype of autism.
One possible mechanism for the inconsistent neural responses in autism is an imbalance in synaptic transmission. Genetic studies have identified abnormalities in areas of the genome associated with synaptic development, myelination and transmission (for example, see Glessner et al., 2009). Also, imbalances between neurotransmitters such as glutamate and GABA have been identified in genetic (DiCicco-Bloom et al., 2006; Polleux & Lauder, 2004) and cellular studies (Blatt & Fatemi, 2011; Fatemi et al., 2002), which could contribute to unstable synaptic activity. The IIV recorded at the neuronal level, however, is too small to be resolved by the MRI, which detects noise at the macro level. However, noise at the neuronal level may be correlated over time and across clusters of neurons. Therefore, if the noise affects groups of neurons, then the ensuing noise may be detected at the macro level too in the unreliability of the MRI response.
There are, however, a number of key questions that remain to be addressed. First, before we can claim with any confidence that IIV is characteristic of autism, it is critical that these initial findings of IIV in ASD be replicated and the robustness of IIV confirmed. There has been a recent move to replicate findings in autism (https://sfari.org/news-and-opinion/blog/2012/transparent-reports), especially in light of the findings of a recent study that found that 2 out of 13 well-known psychological manipulations failed to replicate (https://openscienceframework.org/project/WX7Ck/). Second, there are aspects of the fMRI IIV that have not been fully characterized, as yet. For example, our previous studies averaged the fMRI responses across the two hemispheres (Dinstein et al., 2012) with the aim of comparing the overall sensory-evoked responses between individuals with autism and controls. However, several studies have reported that hemispheric activation, structure, and/or functional connectivity differ between ASD and non-autistic individuals. For instance, reduced inter-hemispheric connectivity, implying less inter-hemispheric synchronization, has been noted in adults (Anderson et al., 2011) and toddlers (Dinstein et al., 2011) with ASD using resting state fMRI. There have also been several reports of anatomical differences between hemispheres in individuals with ASD versus controls, including larger right parieto-occipital regions (Hier et al., 1979), and smaller left planum temporale (Rojas et al., 2002). There are also functional hemispheric differences between ASD compared with controls such as decreased left insula activation under auditory stimulation (Anderson et al., 2010), and greater right than left hemisphere activity in prefrontal and parietal areas during an N-back task (Koshino et al., 2005). Understanding whether IIV manifests differently in the right and left hemispheres might have relevance for these structural and functional hemispheric differences.
Finally, a comprehensive examination of IIV requires that we understand its temporal dynamics. IIV can be generated by random trial-to-trial response changes or by a gradual change in response over time, for example, in cases of increasing fatigue throughout an experiment. Dissociating these two options is critical for better understanding the underlying cortical process generating increased IIV in ASD. The between- and within-block design we adopt in the current study enables us to explore how the IIV changes over time.
In this study, we replicated and extended the findings of greater stimulus-evoked IIV in sensory cortices in individuals with autism than in control participants (Dinstein et al., 2012). Although EEG has better temporal resolution, which would be of value here, there has already been one study that demonstrated IIV using EEG (Milne, 2011). We wished to determine whether fMRI, which has better spatial resolution, would reveal similar findings. We were specifically interested in examining primary visual, auditory and somatosensory cortices and MRI is well suited in this aspect. In the first section below, we show that a new group of individuals with ASD exhibit greater IIV compared to controls, as in the original report (Dinstein et al., 2012). The analyses were identical to those employed in the original study to permit the direct comparison between the ‘new autism group’ and the ‘original autism group’. In the second section, we demonstrate that in both the original and new autism groups, greater IIV, relative to controls, is stable across hemispheres and across the duration of the scan. Taken together, these data attest to the robustness of IIV findings and raise the challenge to understand the mechanism that gives rise to this ubiquitous and widespread alteration in cortical activity in autism.
Methods
Participants
Two groups of individuals with ASD, all of whom had IQ scores above 88 participated in this study. The first group (‘new autism’), 14 males (age range 19–44 years), all met the DSM-IV criteria for autism (full-scale IQ range 88–131). Diagnosis was confirmed with the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989) and Autism Diagnostic Interview (ADI) (Le Couteur et al., 1989; Lord et al., 1994) assessments carried out by expert clinicians at the Center For Excellence in Autism Research, at the University of Pittsburgh (see Table 1, Participants 1–14 for demographics). A study comparing the DSM-IV and the DSM-V criteria showed that the participants who met the criteria for autism under the DSM-IV also met the criteria for autism under the DSM-V (Mazefsky et al., 2013).
Table 1.
ADOS, ADI and IQ scores for individuals with autism. Participants 1–14 were the new autism group; participants 15–28 were the original autism group.
| Participant | Gender | Age (years) |
ADOS social |
ADOS communication |
ADOS stereotypical |
ADI social |
ADI communication |
ADI stereotypical |
Full scale IQ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | 8 | 2 | 1 | 20 | 11 | 3 | 129 |
| 2 | M | 23 | 6 | 4 | 1 | 21 | 18 | 8 | 123 |
| 3 | M | 41 | 6 | 4 | 2 | 24 | 18 | 10 | 96 |
| 4 | M | 19 | 7 | 3 | 3 | 22 | 15 | 5 | 96 |
| 5 | M | 33 | 5 | 3 | 3 | 26 | 18 | 12 | 131 |
| 6 | M | 42 | 6 | 6 | 1 | 32 | 22 | 10 | 129 |
| 7 | M | 45 | 6 | 3 | 4 | 15 | 8 | 10 | 106 |
| 8 | M | 22 | 11 | 5 | 3 | 20 | 15 | 3 | 107 |
| 9 | M | 29 | 6 | 3 | 1 | 15 | 12 | 2 | 116 |
| 10 | M | 31 | 7 | 4 | 2 | 25 | 9 | 8 | 117 |
| 11 | M | 30 | 10 | 6 | 2 | 23 | 17 | 6 | 128 |
| 12 | M | 22 | 13 | 6 | 1 | 23 | 13 | 4 | 88 |
| 13 | 32 | 9 | 4 | 0 | 18 | 18 | 9 | 92 | 32 |
| 14 | M | 44 | 9 | 4 | 3 | 24 | 19 | 5 | 108 |
| 15 | M | 33 | 7 | 5 | 1 | 19 | 16 | 8 | 103 |
| 16 | M | 21 | 8 | 4 | 2 | 21 | 17 | 6 | 123 |
| 17 | F | 19 | 7 | 5 | 3 | 27 | 20 | 6 | 107 |
| 18 | M | 20 | 8 | 5 | 1 | 27 | 22 | 5 | 124 |
| 19 | M | 21 | 9 | 5 | 1 | 22 | 15 | 5 | 108 |
| 20 | M | 27 | 6 | 2 | 3 | 20 | 16 | 7 | 104 |
| 21 | F | 31 | 10 | 6 | 3 | 15 | 9 | 6 | 121 |
| 22 | M | 22 | 6 | 5 | 6 | 19 | 11 | 4 | 127 |
| 23 | F | 31 | 7 | 2 | 4 | 10 | 8 | 6 | 123 |
| 24 | M | 39 | 7 | 4 | 1 | 21 | 16 | 8 | 116 |
| 25 | M | 24 | 13 | 6 | 3 | 10 | 16 | 3 | 118 |
| 26 | M | 30 | 13 | 5 | 3 | 20 | 13 | 3 | 134 |
| 27 | M | 24 | 10 | 5 | 2 | 20 | 12 | 4 | 95 |
| 28 | M | 27 | 9 | 4 | 3 | 20 | 17 | 7 | 100 |
| Means | |||||||||
| New autism | 31.1 | 7.2 | 4.1 | 2.3 | 20.9 | 14.6 | 6.8 | 112 | |
| Original autism |
26.4 | 8.6 | 4.5 | 2.6 | 19.4 | 14.9 | 5.6 | 114 | |
The second group (‘original autism’) included the participants who took part in the original study (Dinstein et al., 2012). Data from that study were reanalyzed and reported here. This original autism group was comprised of 2 females and 12 males (age-range 19–39; full-scale IQ range 95–134). They were also recruited through the Center For Excellence in Autism Research at the University of Pittsburgh, and met the same criteria for autism using ADOS and ADI measures (see Table 1, Participants 15–27).
The data from 14 age- and gender-matched control participants (age-range 20–40; full-scale IQ range 101–129), who were recruited from the general public and took part in the original study (Dinstein et al., 2012), were also used for this study. The controls were originally matched in age and gender to the original autism group. The new autism group was of a similar age and IQ range, but was not gender-matched. Two-tailed t-tests showed no significant difference between the new autism group, the original autism group or the control group on IQ (p>.607) or age (p>.051).
All participants had normal or corrected-to-normal vision. The Institutional Review Board at Carnegie Mellon University and the University of Pittsburgh approved this study, and all participants gave their informed consent. All participants were paid for their time.
Experimental design
The design was exactly the same as the design described in the original study (Dinstein et al., 2012). Participants took part in three sensory experiments measuring fMRI responses to visual, auditory and somatosensory stimuli. All three sensory experiments followed a rapid event-related design, which enabled measurement of response amplitude and variability.
Participants in both the present and the previous study were presented with 72 trials, 24 in each of three sensory modalities (auditory, somatosensory and visual). For each modality, the trial began with an adapter, followed by a test stimulus. The test stimulus was either identical to the adapter (the adapted condition), different from the adapter (the unadapted condition), or no test was presented (the no test condition).
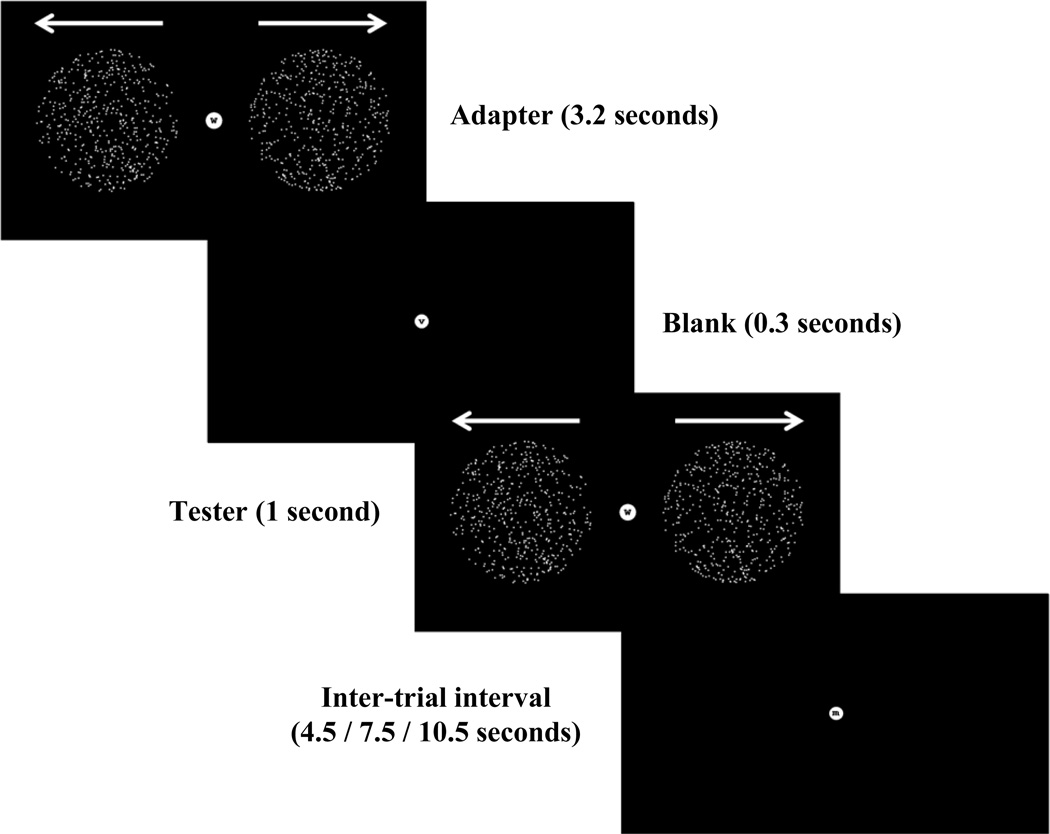
For the visual stimuli, the adapter stimulus consisted of two circular apertures six degrees in diameter, which appeared eight degrees on either side from the central fixation point. Each aperture contained 500 white dots that moved towards or away from fixation with 80% motion coherence. The test stimulus contained dots that either moved in the same direction as the test stimulus (adapted condition) or in the opposite direction (unadapted condition). No test stimulus was presented in the ‘no test’ condition. An example trial is shown in Figure 1.
Figure 1.
An example trial from the visual experiment. The adapter was shown for 3.2 seconds followed by a blank screen for 0.3 seconds, and the tested stimulus for 1 second. The inter-trial interval between trials were 4.5, 7.5 or 10.5 seconds in duration (in a randomized order). Auditory and somatosensory experiments had an identical structure.
For the somatosensory stimuli, the adapter stimulus comprised eleven air puffs that were directed to the back of the left hand. The test stimulus comprised three air puffs that were located at the same spatial location as the adapter stimulus (adapted condition) or at a different location 5 cm away from the location of the adapter stimulus (unadapted condition). Each air puff lasted 150ms followed by 150ms break. There was a 200ms delay between the adapter and the test stimuli.
For the auditory stimuli, the adapter stimulus was comprised of eleven pure tone beeps (either 400 or 600Hz) presented through headphones to both ears. The test stimulus contained three beeps of either the same tone as the adapter stimulus (adapted condition), or the other tone (unadapted condition), using the same timings as the somatosensory stimuli.
This study was conducted in six scans (two per sensory modality) with twelve trials of each condition (adapted, unadapted, and no-test) per scan. The six scans were interleaved and IIV analyses were performed for each condition separately, enabling assessment of IIV within each scan and across scans (test-retest reliability within individual).
During all six scans, participants were instructed to attend to a sequence of letters that appeared at fixation and press a button every time a letter repeated consecutively, thereby diverting attention away from the sensory stimuli. The letters, presented in lower case, were presented one at a time for a duration of 500 ms. Participants had 1 s to respond with their right index finger and received feedback such that, if they responded correctly, the fixation circle turned green, and if not, it turned red. Misses were not indicated. This design, in which the sensory stimulation was orthogonal (and irrelevant) to the task being performed was adopted to ensure that any sensory differences were not a function of differential attention or differential task performance.
Data Acquisition
The same 3T Siemens MRI scanner at the Carnegie Mellon University SIBR was used for both the present and previous study. Six functional (two per sensory modality) and one anatomical scan were acquired per participant. The scanner was equipped with a Siemens 12 channel birdcage head coil, which was used for RF transmit and receive. Functional images were acquired with a T2*-sensitive echo planar imaging pulse sequence (repetition time of 1,500 ms, echo time = 30 ms, flip angle = 75°, 24 slices, 3 × 3 × 3 mm voxels, field of view = 192 mm). Anatomical volumes were acquired with a T1-weighted 3D–MPRAGE pulse sequence (1 × 1 × 1 mm).
Data Analysis
Preprocessing
fMRI data were preprocessed using Brain Voyager and in-house code in Matlab (Mathworks, Natick, MA) as well as using the NeuroElf toolbox (http://neuroelf.net/, JW). Preprocessing included 3D motion correction, temporal high-pass filtering with a cutoff frequency of 6 cycles per run, spatial smoothing using a Gaussian kernel with 8 mm width at half height, alignment with the anatomical volume using trilinear interpolation, and transformation to the Talairach coordinate system (Talairach and Tournoux, 1988).
Scan segments and/or entire scans containing head movements in excess of 2 mm were removed such that approximately 10% of scans were excluded from data analysis. There was no significant difference between groups in the mean amount of residual head movement except in the visual experiment. See (Supplementary Materials Figure 1S) for details. All head motion estimates, however, were regressed out of the data so as to remove fMRI signal changes that were associated with head movements in each of the subjects.
Replication
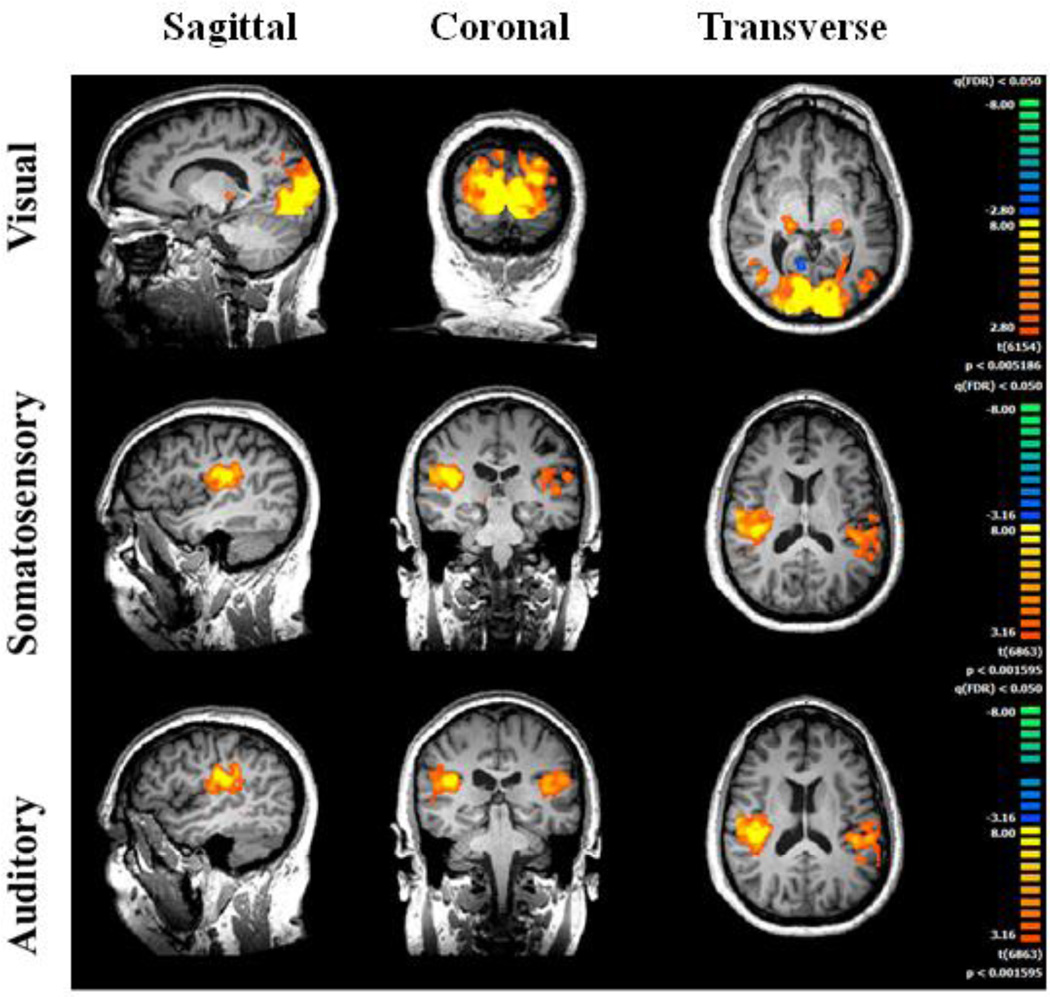
The first set of analyses was conducted using the same procedure as in the original study (Dinstein et al., 2012) to test whether the new autism group produced similar fMRI response profiles to those of the original autism group, and to allow for direct comparison with the previously published results. For each individual, in each hemisphere, for visual, auditory and somatosensory responses, regions of interest (ROIs) were created by identifying the 200 voxels with the most significant responses (activation) within the corresponding sensory cortex in each hemisphere (Figure 2).
Figure 2.
Statistical parameter maps of significant activation in the new autism group. (A) Visually-evoked activity, combined across adapted, unadapted and no test conditions. (B) Somatosensory. (C) Auditory. For similar maps for the original autism group and the control group, see Dinstein et al. (2012) Figure 1.
An epoch of the fMRI time series, for each voxel in the ROI, was then extracted from stimulus-onset to 15 s (8 time-points) after stimulus-onset. Response amplitudes were calculated, separately for each trial, by averaging the responses at time-points 4 and 5, which corresponded to the peak of the haemodynamic response. Response standard deviations were calculated by taking the average standard deviation of the fMRI responses to all 24 repetitions (12 presentations per scan) at time-points 3–6. Signal-to-noise ratios (SNRs) were calculated by dividing the response amplitudes by the response variances.
As this is a replication of a previous study (Dinstein et al., 2012), and we have a directional hypothesis, one-tailed t-tests were used to test for differences between the autism groups and the control group in their fMRI responses, separately for each sensory modality and separately for the response amplitudes, response standard deviations, and SNRs.
The responses from the no test condition were used for the main analysis due to the fact that the no test condition only contained one stimulus exposure and does not induce changes in adaptation dynamics. However, the responses between the new autism, the original autism and the control groups were similar for the adapted and unadapted conditions as well (see Supplementary Materials, Figure 3S).
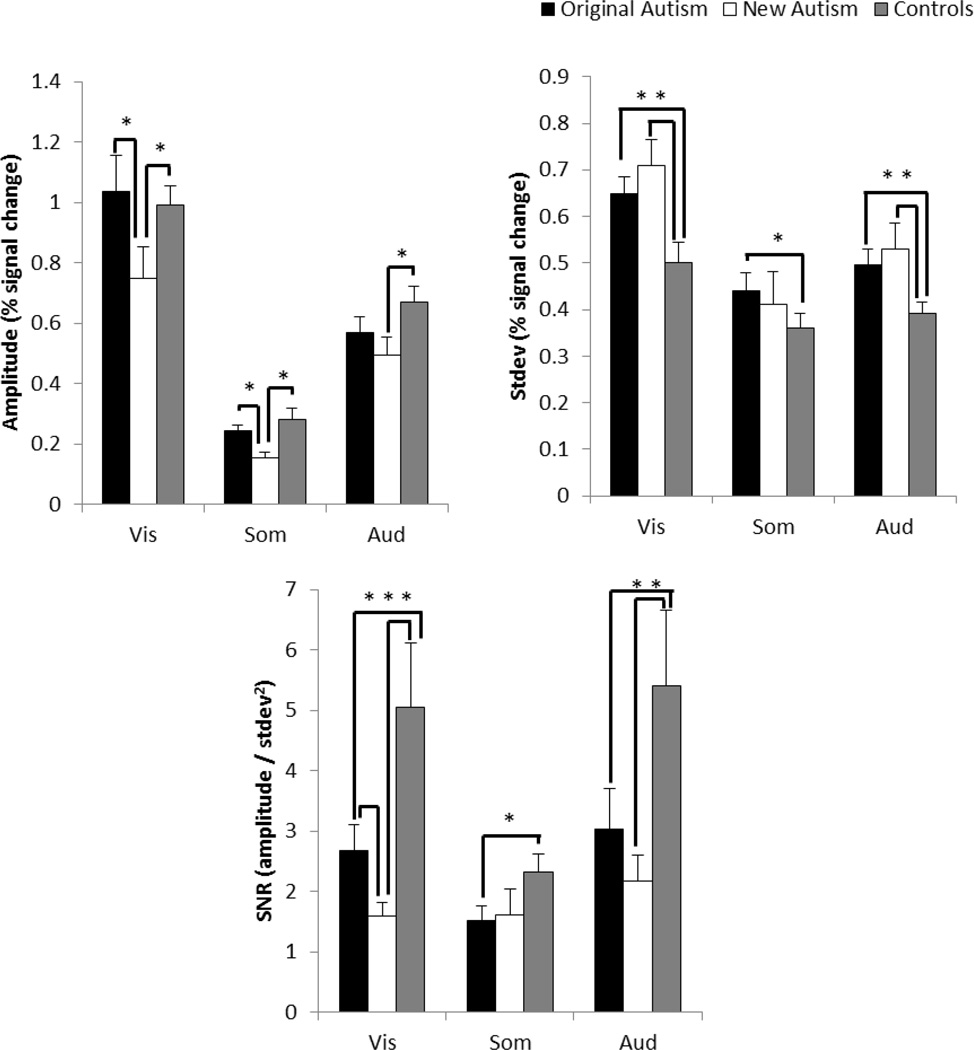
We also performed a complementary linear regression analysis using a general linear model (Figure 4). We used fMRI data from one scan to identify the relevant ROIs in each subject, and performed the response amplitude and variability analyses on the participants who had two scans to reduce bias due to ROI selection. The model contained a separate column/predictor for each trial using a canonical hemodynamic response function. This enabled us to estimate the response amplitude of each trial and compute trial-by-trial variability. Linear regression was used to fit the model to the data, and the amplitude, standard deviation and SNRs (amplitude/standard deviation2) were calculated across trials for each subject.
Figure 4.
Complementary linear regression analysis on the fMRI responses in the no test condition to visual, somatosensory and auditory stimuli for the original autism group, the new autism group and the control group. A) The amplitude of the fMRI responses. B) The standard deviation in the responses. C) SNRs (amplitude/standard deviation2). Error bars show one standard error. Asterisk indicates number of significant pair-wise comparisons (one-tailed p<.05).
Finally, we performed complementary randomization tests to assess the statistical significance of differences across groups. The participants’ data were randomly shuffled between the new autism group, the original autism group, and the control group (i.e., labels permuted) and differences in response amplitude, standard deviation, and SNR were computed for the randomly assigned groups. This was repeated 10,000 times, re-randomizing the labels each time, to provide null distributions of the differences across groups, according to the null hypothesis that there was no difference between groups. To be deemed statistically significant the actual difference between the correctly assigned groups had to exceed the 95th percentile of the null distribution (equivalent to a one tailed t-test, but without assuming that variables are normally distributed).
The t-tests analyzing the differences in the response amplitudes allowed for a direct comparison of the main results from the original study (Dinstein et al., 2012) and the current study. The linear regression analysis was included as it calculates the differences in mean and standard deviation in the fMRI responses across the entire response time-course and not just at the time-points (4–5) corresponding to the peak response. The regression analysis also takes into account the variable inter-trial intervals. The randomization test was likewise performed as a complementary analysis; it is a non-parametric test version of a t-test and so does not make any assumptions about the mean and standard deviation in responses being normally distributed.
Extension
To examine whether trial-by-trial variability of the fMRI responses changed over the course of the experiment or across the duration of a single run (i.e., potentially increased or even decreased), the standard deviation was calculated slightly differently. The method of data extraction was the same as the first section: the amplitude of the response was calculated by averaging the response at time-points 4 and 5, which correspond to the peak of the hemodynamic response. The standard deviation of the response, however, was calculated by taking the average response from the ROI at time-points 3–6 and taking the standard deviation separately for trials 1–4, trials 5–8 and trials 9–12 of each scan. This was computed separately for each participant. Averaging across four trials provided a measure of standard deviation in the fMRI responses, whilst keeping all the other variables (sensory modality, and condition – adapted, unadapted, and no-test – scanning session and repetition) as factors in the analysis.
A mixed ANOVA was used to determine the statistical significance of the results. Sensory modality (auditory, somatosensory and visual), hemisphere (left and right), scan (first or second scan of each sensory modality), condition (adapted, unadapted and no test), and standard deviation bin (i.e. the three trial-by-trial standard deviation values described above) served as within-subject variables, and group (autism compared to control group) served as a between-subjects variable. A second ANOVA was also performed with single trial amplitude (i.e. estimated response on each of the 12 trials) instead of standard deviation bin. This additional analysis enabled us to determine whether response amplitudes differed with respect to the other factors in the ANOVA. Violations in the assumption of sphericity in the analyses of variance were corrected using the Greenhouse Geisser adjustment by adjusting the degrees of freedom.
A separate ANOVA was used to analyze the SNRs. Due to the fact that the response standard deviations were calculated by binning the repetitions into sets of four trials, there was an unequal number of repetitions for the standard deviations (3 repetitions) compared to the response amplitudes (12 repetitions). Therefore, sensory modality (auditory, somatosensory and visual), hemisphere (left and right), scan (first or second scan in each sensory modality) and condition (adapted, unadapted and no test) served as within-subject variables, and group (autism compared to control group) served as a between-subjects variable.
Results
Replication
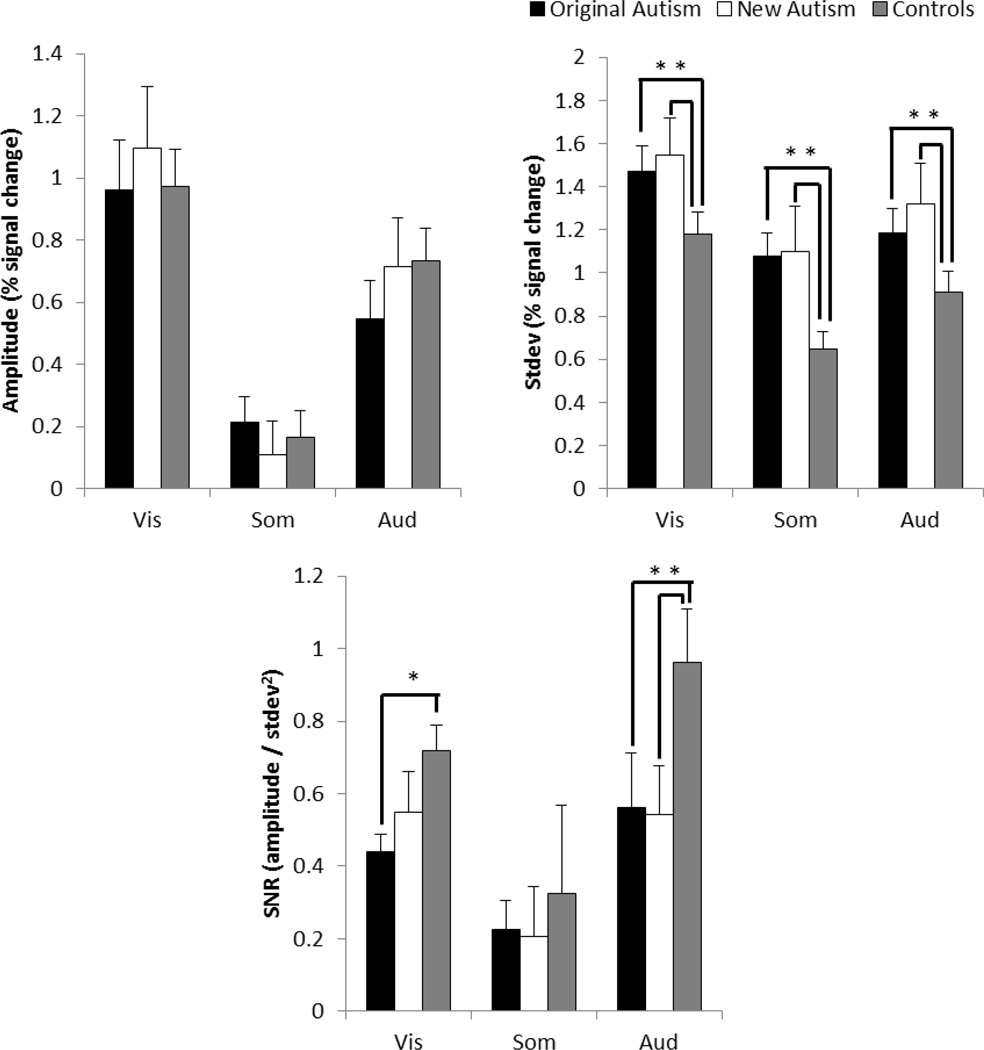
The signal-to-noise ratios (SNRs, response amplitude/response variance) of the stimulus-evoked fMRI responses were consistently lower in both autism groups compared to the control group (Figure 3C). The new autism group produced significantly lower SNRs compared to the control group for the visual (t(23)=3.10, p=.002) and auditory modalities (t(21)=2.38, p=.013), but not significantly so for the somatosensory modality (t(25)=1.39, p=.089), replicating our previously published result in a statistically independent sample. As reported previously, the original autism group produced significantly lower SNRs for all three sensory modalities compared to the control group (visual: t(24)=2.09, p=.024; somatosensory: t(25)=2.09, p=.024; auditory: t(24)=1.76, p=.046). There was no evidence for any differences in SNR between the new and original autism groups for the somatosensory (t(24)=0.18, p=.429) and auditory modalities (t(23)=0.99, p=.167), but the new autism group produced even lower SNRs compared to the original autism group for the visual modality (t(23)=2.23, p=.018).
Figure 3.
The fMRI responses for the original autism group, the new autism group and the control group for the visual, somatosensory and auditory responses. A) Mean response amplitudes. B) Standard deviations of the responses. C) Signal to noise ratios. Significant pair-wise comparisons are indicated with an asterisk (without correction for multiple comparisons); Bonferroni corrected comparisons are described in text.
The lower SNRs in the autism groups were partly due to smaller response amplitudes (in the new autism group; Figures 3A and 3B). The original autism group produced consistently greater response standard deviations than controls for all three sensory modalities (visual: t(24)=2.58, p=.008; somatosensory: t(25)=1.71, p=.050; auditory: t(24)=2.46, p=.011). The new autism group produced greater standard deviations compared to the control group for the visual (t(23)=3.07, p=.002) and auditory modalities (t(21)=2.31, p=.016), but not for the somatosensory (t(25)=0.67, p=.255). The new autism group did not significantly differ from the original autism group for visual (t(23)=0.96, p=.172), somatosensory (t(23)=0.54, p=.352), or auditory (t(24)=0.38, p=.298) response standard deviations.
The original autism group and the control group did not differ significantly in their response amplitudes (visual: t(24)=0.33, p=.374; somatosensory: t(25)=0.93, p=.180; auditory: (t(24)=1.35, p=.0582). There were significant differences in the response amplitudes between the new autism group and the control group (visual: t(23)=2.00, p=.029; somatosensory: t(25)=2.98, p=.003; auditory: t(21)=2.17, p=.021). There were also significant differences in response amplitudes between the new autism group and the original autism group for visual (t(23)=0.96, p=.044) and somatosensory modalities (t(24)=2.96, p=.001), but not for auditory modality (t(23)=0.98, p=.169). The same analyses were conducted on the data without correcting for motion and the results were the same (see Supplementary Materials, Figure 2S).
We also performed complementary randomization tests on the difference between the groups, and the results were similar, supporting the same conclusions. The new autism group produced significantly lower SNRs compared to the control group for the visual (p<.001) and auditory responses (p=.005), but only a trend for the somatosensory responses (p=.088). The new autism group also produced greater standard deviations compared to controls for the visual modality (visual: p=.002; somatosensory: p=.269; auditory: p=.015), and smaller response amplitudes compared to controls (visual: p=.031; somatosensory: p=.004; auditory: p=.021). The original autism group produced lower SNRs compared to the control group (visual: p=.014; somatosensory: p=.023; auditory: p=.034), greater response standard deviations compared to the control group (visual: p=.007, somatosensory: p=.045, auditory: p=.012), and no significant differences in response amplitudes compared to controls (visual: p=.379; somatosensory: p=.192; auditory: p=.099). The new autism group produced similar SNRs compared to the original autism group, except that the new autism group showed even weaker SNRs for the visual modality (visual: p=.009; somatosensory: p=.429; auditory: p=.191), similar standard deviations compared to original autism group (visual: p=.169; somatosensory: p=.366; auditory: p=.304), and smaller response amplitudes compared to the original autism group for the visual and somatosensory modalities (visual: p=.046; somatosensory: p=.004; auditory: p=.174).
The results were similar for all three adaptation conditions (adapted test, unadapted test, and no test) supporting the same conclusions (see Supplementary Materials, Figure 3S).
In addition, we performed a complementary linear regression analysis using a general linear model that contained a separate predictor for each trial. The results were similar and supported the same conclusions (Figure 4). Of specific importance, the greater standard deviation in fMRI responses in the new autism group compared to the control group is now significant in the all sensory modalities, and the response amplitudes are not significantly different across the sensory modalities between the three groups.
Due to the fact that the control group was matched to the original autism group rather than to the new autism group (although they did not differ on age or IQ), we redid the analyses with an age- and gender-matched subset of the controls and the new autism group (N=11). The results were similar to previous analyses (Supplementary Materials Figure 4S), with the new autism group producing weaker SNR than the age- and gender-matched controls.
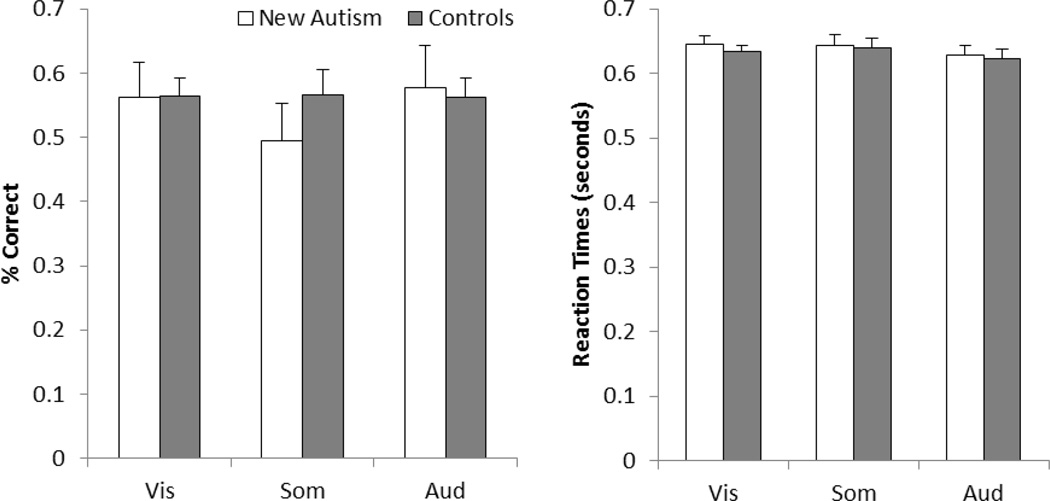
Behavioral performance in the one-back letter-detection task at fixation was analyzed to determine whether there was any indication that the smaller response amplitudes, larger IIV, and lower SNRs in the new autism group might have occurred because of fluctuations in attention or some other form of distraction (Figure 5). There were no significant differences, however, in performance accuracy (percent of targets correctly identified as being repeats) between the new autism group and the control group (Figure 5A; visual: t(23)=0.04, p=.486; somatosensory: t(25)=1.03, p=.157; auditory: t(21)=0.23, p=.412). Similarly, there were no significant differences in the reaction times (Figure 5B; visual: t(23)=0.74, p=.234; somatosensory: t(25)=0.13, p=.450; auditory: t(21)=0.22, p=.415). Together, this equivalence in performance suggests that the differences in fMRI responses between the new autism group and the controls are unlikely to be due to attentional, arousal, or motivational differences.
Figure 5.
Behavioral performance from the new autism group compared to the control group. (A) Accuracy, the percent of letter repeats that were correctly identified. (B) Reaction time. Error bars show one standard error.
To examine whether autism severity was related to the fMRI responses, correlations were conducted using the SNR in each sensory modality and the mean ADOS score of all the individuals with autism in this study. None of the correlations, however, were statistically significant. The correlations between the SNR for each sensory modality and IQ, or age, were also not significant.
Greater variability in cortical activity was evident only in the stimulus-evoked responses for the two autism groups, compared to controls (see Supplementary Materials, Figure 5S). To demonstrate this we repeated the analysis for each of the three sensory systems during sensory stimulation of another system. For example, we measured cortical activity (response amplitudes, response standard deviations, and SNRs) in auditory cortex, during visual and somatosensory stimulation. Likewise for visual cortex (auditory and somatosensory stimulation) and somatosensory cortex (visual and auditory stimulation). The results showed that there were no significant differences between groups in any of the sensory systems, for any of the three measures (response amplitudes, response standard deviations, SNRs).
It is unlikely that these results can be explained by trivial differences in non-neural sources of variability such as head motion or physiology. First, the group differences in SNR were unique to sensory-evoked responses in corresponding sensory cortical areas (see above and Supplementary Materials, Figure 5S). Second, although the new autism group moved slightly more on average than the original autism group and controls (see Supplementary Materials, Figures 1S), head motion was regressed out of the fMRI data before performing the analyses such that fMRI signal changes associated with head movements were removed from the analyzed data and were unlikely to generate differences across groups.
Interim Discussion
Both the new autism group and the original autism group showed significantly greater standard deviations in their fMRI responses compared to the control group, and this was evident in visual, somatosensory and auditory cortices. The lower SNRs in the original autism group compared to controls were predominantly a function of greater response standard deviations. The new autism group also produced lower SNRs compared to the control group although this depended, to some extent on which variant of the analysis was done (compare Figures 3, 4, 2S, 3S and 4S). A possible explanation for the differential magnitude in SNR across groups in the fMRI responses might be a result of the smaller response amplitudes in the new autism group. It is important to note that the aim of the analyses reported here was to directly compare the cortical profile of the two autism groups to one another, as well as to the control group. In summary, most of the results from our previous study (Dinstein et al., 2012) were replicated in a new group of individuals with autism. This replication supports the conclusion that greater cortical variability may be a fundamental neural characteristic of individuals with autism.
Extension
Having demonstrated that we can replicate the findings of greater variability of sensory-evoked activity in a second group of ASD participants, here, we pooled the new autism group (N=14) and the original autism group (N=14) together to examine the IIV more closely. In particular, we compared the IIV across the three sensory modalities, the two hemispheres, and over time (within and between scans) so as to uncover more detailed characteristics of the atypical cortical response profile.
There were significant differences between the visual, somatosensory and auditory response amplitudes and SNRs between the autism and control groups (see Supplementary Materials, Figure 6S). The different stimulus conditions (adapted, unadapted and no test) also evoked statistically significant group differences (see Supplementary Materials, Figure 6S). These served as checks that the stimuli induced changes in the fMRI responses.
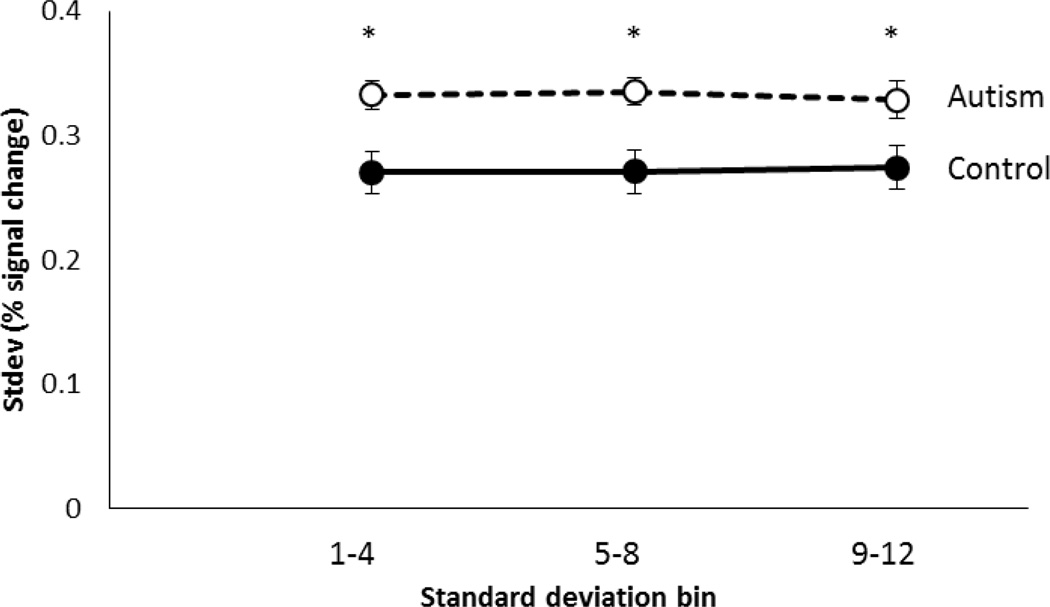
The combined (new and original) autism group exhibited greater standard deviations across trials compared to controls (F(1,40)=7.56, p=.009), replicating the group differences in the original study (Dinstein et al., 2012). There was no significant interaction between the autism group and controls in their response standard deviations in the first compared to the second block of scans (F(1,40)=3.59, p=.065), and there was no significant interaction between the two groups in their response standard deviations across time within scans (comparing the early standard deviation bin to the mid-session bin, to the late bin) (F(2,80)=0.74, p=.478). This suggests that the greater variability in fMRI responses in the autism group was consistent across the duration of the experiment, and there is no indication that the greater IIV is due to fatigue (Figure 6). There was also no significant interaction in response standard deviations between the two groups across the two hemispheres (F(1,40)=0.68, p=.415), again attesting to the widespread evidence of this group difference.
Figure 6.
Standard deviation of the fMRI responses in each of the three std bins (trials 1-4, 5-8, and 9-12 across the scans) for the autism (dotted line) and control (solid line) groups. Asterisks indicate significant differences between groups. Error bars show one standard error.
The fMRI response amplitudes were statistically indistinguishable between the combined autism group and the control group (F(1,40)=0.86, p=.358). There were no significant differences between the groups in response amplitudes across scans (F(1,40)=3.67, p=.063) across time within scans (F(11,440)=0.89, p=.416), or across hemispheres (F(1,40)=0.10, p=.755).
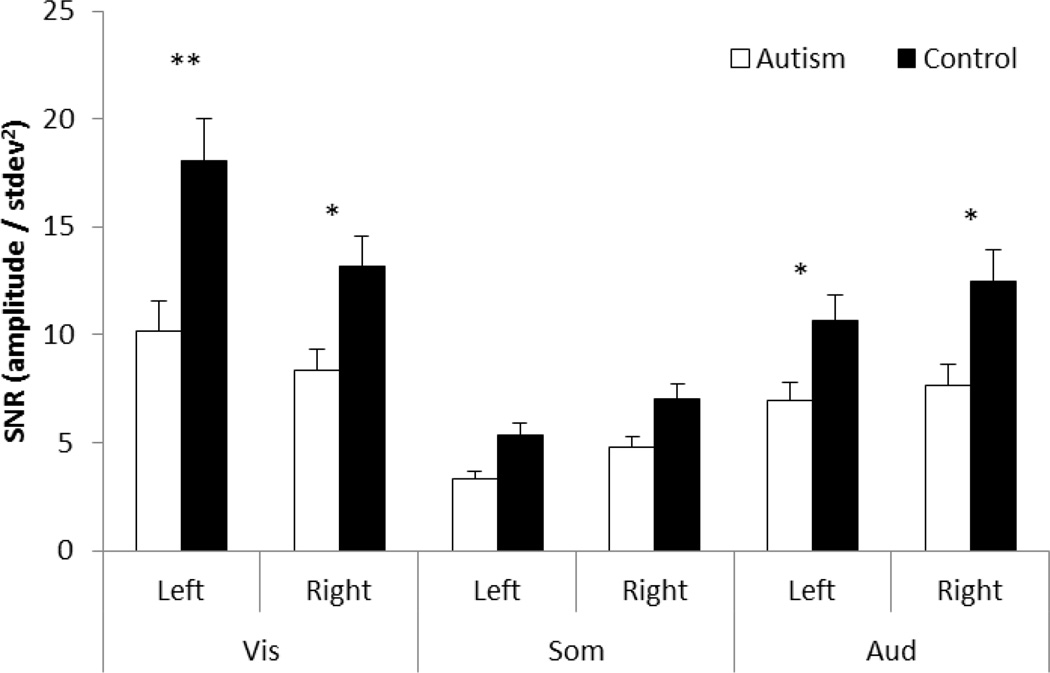
The final analysis adopted signal-to-noise ratio (SNR) as the dependent measure (Figure 7). We observed a main effect of group, with the control group producing stronger SNR compared to the autism group (F(2,40)=11.91, p=.001), again replicating the group differences in the original study (Dinstein et al., 2012). There were two significant interactions with group: a two-way interaction with sensory modality (F(2,80)=3.78, p=.027) and a three-way interaction between sensory modality, hemisphere and group (F(2,80)=5.11, p=.008). Tukey post-hoc tests showed that SNR in somatosensory responses were not significantly different between controls and individuals with autism. The three-way interaction was due to the controls producing a larger SNR in left hemisphere compared to right for visual responses only. Auditory and somatosensory responses were similar across hemispheres, and there was no significant difference between hemispheres in any of the sensory modalities for the autism group. In other words, the control group produced larger SNR in left hemisphere visual cortex than other sensory modalities (Figure 7).
Figure 7.
Signal-to-noise ratios (SNRs) of the fMRI responses for each sensory modality and hemisphere, shown separately for the control (black) and the combined (new and original) autism (white) groups. Asterisks indicate significant differences between groups, and double asterisk shows the result of the three-way interaction. Error bars show one standard error.
Discussion
Inter-individual variability is a well-known defining feature of ASD (hence the term ‘spectrum’ or ‘continuum’) and the heterogeneity in behavioral profile (for example Humphreys et al., 2007) is also accompanied by individually variable brain organization in autism (for example, Humphreys et al., 2008; Müller et al., 2003). There is, however, growing recognition that ASD is also characterized by greater behavioral and neural intra-individual variability (IIV) compared with that of non-ASD individuals. IIV reflects transient within-person changes and these fluctuations are evident in test-retest or trial-to-trial assessments. The focus of this study was on intra-individual variability of cortical activity, as measured by unreliable fMRI responses to repeated sensory stimuli in individuals with autism.
We began this investigation by using the same analytic approach as Dinstein et al. (2012) who characterized the IIV in a group of adults with ASD. We replicated the same pattern of IIV in a second group of individuals with ASD (‘the new autism group’) who, like the original autism group, produced greater standard deviation in fMRI responses, and weaker SNRs, compared to a group of non-autistic controls. This was true in all cases except in the somatosensory response in the first analysis examining at the variability in the peak of the fMRI response. These group differences in response amplitude might, therefore, reflect heterogeneity across the population of individuals with ASD with the new autism group evincing lower amplitude across the board. Notwithstanding the amplitude differences, the SNRs were smaller in both the original and new autism group, relative to the controls. This shows that even though the new autism group produced smaller fMRI response amplitudes, they produced equivalent (or perhaps even more) variability in their responses compared to the controls, which resulted in the small response amplitudes being swamped by the variability, resulting in weak SNR. The differences in cortical profile between the ASD and control participants (see Figure 5) could not be attributed to differences in attention or distraction as the behavioral responses did not differ significantly across the groups (and the sensory stimulation was orthogonal to the letter-repetition task so attention was not engaged on the visual, auditory or tactile stimuli). The key result, then, is that we were able to replicate the trial-to-trial within-individual variability in cortical dynamics in a second group of ASD participants and, together with the original finding, reveal the robustness of the sensory changes in the cortices of individuals with ASD.
To characterize the nature of the cortical changes further, we explored the IIV as a function of hemisphere and of temporal order. When these factors were included in the model, the combined (new and original) autism group still produced significantly greater variability in their fMRI responses and weaker SNRs, compared to controls, and there were no significant differences in the mean response amplitudes. Importantly, too, greater IIV in autism remained consistent over time. First, we observed that trial-to-trial variability in the fMRI responses was indistinguishable across the two separate scans. Second, the variability was also equivalent across the three bins of trials per scan. This suggests that the IIV is larger in ASD regardless of when it is estimated and that it does not decay (or increase) over the course of the study.
Taken together, these results suggest that sensory-evoked cortical responses in ASD are consistently more unreliable than in typical individuals and attest to the robustness of the greater IIV, regardless of the mean response amplitudes, across the brain, and over time.
The results of several other studies support the conclusion that cortical responses are inconsistent and unreliable in ASD. As mentioned earlier, a VEP study reported greater intra-individual variability of P1 latency and P1 amplitude in ASD, lower inter-trial α-band (∼10 Hz) phase coherence, and a significant correlation between P1 peak amplitude variability and reaction time (Milne, 2011). A magnetoencephalography (MEG) study of auditory-evoked responses reported weaker phase-locking in ASD compared to controls, which is compatible with unreliable responses in ASD (Gandal et al., 2010). And, in yet a further study, individuals with autism showed significantly greater intra-individual response time variability compared to both a group of typically developing (TD) matched control participants and a group of matched participants with ADHD (Geurts et al., 2008). The time scale of the variability in the haemodynamic response is much slower than the time scale of EEG and MEG measures, but taken together, these studies suggest that there is substantial within-subject variability at the neural and at the cortical level. We have also recently begun to explore the functional consequences of this cortical IIV and have observed greater trial-to-trial variability in ratings of roughness in individuals with autism compared to controls in a detailed psychophysical investigation (Haigh et al., accepted).
However, not all results appear compatible with the hypothesis that neural responses are unreliable in ASD. For example, a study using MEG to record responses during passive tactile stimulation of the thumb and index finger showed no differences in IIV in individuals with ASD versus typical controls (Coskun et al., 2009). A number of possible explanations may account for this apparent discrepancy, including the possibility that the heterogeneity of ASD population may have outweighed the intra-individual variability, or that the mean response and the type of stimulation (active/passive) may determine the presence of IIV. Indeed, above, we found that response amplitudes were consistently smaller for somatosensory stimulation compared to the visual- or auditory-evoked activity, and sensitivity for measuring differences in IIV might depend on the mean amplitude of the evoked responses: the amount of variability in fMRI responses from one trial to the next could have been limited by the responses being smaller in magnitude. We also found, both in the current study and the original study (Dinstein et al., 2012), that greater IIV was only evident in stimulus-evoked activity, not during resting state or in areas of cortex that were not strongly stimulus-driven (i.e., outside of the primary cortices stimulated).
Having established the robustness and replicability of the IIV profile in sensory cortices in ASD, the obvious question now concerns its causal basis. There was no statistically significant difference in behavioural responses between the new autism group and control group, suggesting that the variability in fMRI signal was not due to attentional differences. It is possible that dual attention is more variable in ASD than controls; however, Grubb et al. (2013) found no significant decrement in exogenous attention in the ASD group.
Given that we have no single comprehensive explanation for the increased IIV in autism, we suggest a number of possible accounts. One such account is that the IIV is due to imbalances at the neural level, perhaps resulting from an imbalance between neural excitation and inhibition (Jamain et al., 2008; Markam et al., 2007; Rubenstein & Merzenich, 2003; Vattikuti & Chow, 2010). The hypothesis is that there is excess excitation due to either increased glutamatergic activity, or reduced GABAergic signaling. The increased excitation in cortex in autism is consistent with the increased comorbidity with epilepsy (Levisohn, 2007; Rossi et al., 1995; Tuchman & Rapin, 2002), and the heightened sensory sensitivities, which are now recognized under the DSM-5 (Baron-Cohen et al., 2007; Gomot et al., 2002; Simmons et al., 2009).
A further possible etiology for greater IIV in ASD is dysfunctional modulation of select neurotransmitters. The catecholamine and acetylcholine systems can give rise to more neural noise (Bäckman et al., 2006) and greater IIV in response latencies can be systematically linked to diminished D2 receptor binding (MacDonald, et al., 2009) and changes in D1 receptor binding (MacDonald et al., 2012). The modulation of IIV by dopamine fits well with neurocomputational studies in which dopamine dysregulation is assumed to alter the signal-to-noise ratio of neural information processing, effectively impairing the neuron’s sensitivity to afferent signals, leading to noisier information (i.e., signal) processing and impaired cognitive functioning (Li et al., 2001; Lindenberger et al., 2011). Another potential neurotransmitter that has been linked to increased signal-to-noise ratios is oxytocin (Owen et al., 2013): individuals with autism show lower oxytocin levels compared to non-autistic individuals (Modahl et al., 1998; Wu et al., 2005), and oxytocin-related treatment has been suggested as a therapy for ASD (Kuehn, 2011; Modi & Young, 2012; Gordon et al., 2013).
Yet a further and final possibility we consider is that the greater IIV in autism reflects reduced cortical maturity and white matter refinement. Lower IIV is associated, in typical control individuals, with higher fractional anisotropy and lower overall diffusivity in white matter tracts throughout the brain, over the course of development (Tamnes et al., 2012). These findings support the proposition that, in control individuals, developmental reductions in IIV reflect maturation of white matter connectivity. Numerous studies of autism have shown abnormalities in white matter integrity (Barnea-Goraly et al., 2004; Thomas et al., 2011) and thus the greater IIV in ASD might be a reflection of these deviations in white matter development.
The atypical cortical profile with increased IIV may have direct consequences for some of the behavioral changes in ASD. For example, behavioral evidence has shown that individuals with ASD show atypical dynamics (slower rate of binocular rivalry alterations, longer mixed percept and increased likelihood to revert to the previously perceived object when exiting a mixed percept) (Robertson et al., 2013) and these findings are thought to mirror the push and pull of altered inhibitory and excitatory cortical dynamics which might serve as the basis of the increased IIV (although another study reported indistinguishable cortical dynamics in binocular rivalry with grating patch stimuli instead of object images; Said et al., 2012). As noted above, we have also documented increased IIV in roughness ratings in a group of adults with IIV. Further exploration is required to understand the relationship between the altered cortical profile and behavioral consequences, and how these relate to the structural differences recorded in individuals with autism.
The focus on inter-participant variability in studies of individuals with autism has largely overshadowed research on intra-individual variability. As previously noted (MacDonald et al., 2006), when intra-individual variability in performance is small, mean-level differences provide useful predictive information, but as intra-individual variability increases and represents systematic as opposed to random error, calculating mean performance from a single measurement in each individual can lead to flawed estimates of average group differences.
In conclusion, a robust and replicable signature of ASD is greater IIV in stimulus-evoked cortical activity. This greater variability in the sensory-evoked fMRI response in autism appears to be robust across different regions of cortex and across different time intervals. Greater sensory variability could be an endophenotype in autism but before concluding that this is the case, it remains to be determined whether this IIV profile is also evident in children with ASD and in those who are lower functioning than the participants of the present investigation.
Supplementary Material
Acknowledgments
The research described here was supported by a grant from the Simons Foundation Autism Research Initiative (177638) to DH and MB, and a NIH/NICHD grant (HD055748) to NJM. The authors thank Ryan Egan for helping with participant recruitment and fMRI testing. We also thank the staff at the Center for Excellence in Autism Research at the University of Pittsburgh for recruitment and assessment of participants.
Footnotes
Conflicts of interest
The authors declare that they have no conflict of interest pertaining to the data described here.
References
- American Psychiatric Association on DSM-IV. APATF. Diagnostic and statistical manual of mental disorders: DSM-IV. Amer Psychiatric Pub Inc; 1994. [Google Scholar]
- Amiet C, Gourfinkel-An I, Laurent C, Bodeau N, Génin B, Leguern E, Cohen D. Does epilepsy in multiplex autism pedigrees define a different subgroup in terms of clinical characteristics and genetic risk? Molecular Autism. 2013;4(1):47. doi: 10.1186/2040-2392-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, Abildskov T, Nielsen Ja, Cariello AN, Cooperrider JR, Bigler ED, Lainhart JE. Decreased Interhemispheric Functional Connectivity in Autism. Cereb. Cortex. 2011;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, Alexander AL, Bigler ED, Lainhart JE. Decreased Left Posterior Insular Activity during Auditory Language in Autism. Am. J. Neuroradiol. 2010;31:131–139. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and Abnormal Development of Brain Connectivity. J Neuro. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ, Fatemi SH. Alterations in GABAergic Biomarkers in the Autism Brain: Research Findings and Clinical Implications. Anat Rec. 2011;294(10):1646–1652. doi: 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein ML, Hoffman DD, Saidpour A. Parts of visual objects: an experimental test of the minima rule [Online] Perception. 1989;18:817–826. doi: 10.1068/p180817. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and Macroscopic Correlates of Minicolumnar Pathology in Autism. J. Child Neurol. 2002;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Cermak S, Groza V. Sensory Processing Problems in Post-Institutionalized Children: Implications for Social Work. Child Adolesc. Soc. Work J. 1998;15:5–37. [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, Castillo EM, Pearson DA, Loveland KA, Papanicolaou AC, Sheth BR. Increased response variability in autistic brains? Neuroreport. 2009;20:1543–1548. doi: 10.1097/WNR.0b013e32833246b5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. J. Autism Dev. Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The Developmental Neurobiology of Autism Spectrum Disorder. J. Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable Evoked Responses in Autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E. Disrupted Neural Synchronization in Toddlers with Autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal Movement Selectivity in Autism [Online] Neuron. 2010;66:461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Bio Psych. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biol. Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Grasman RPPP, Verté S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard M-H, Adrien J-L, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: Electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, Zagoory-Sharon O, Leckman JF, Feldman R, Pelphrey KA. Oxytocin enhances brain function in children with autism. Proc. Natl. Acad. Sci. 2013 doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Heeger DJ, Carrasco M. Exogenous spatial attention: evidence for intact functioning in adults with autism spectrum disorder. Journal of Vision. 2013;13(14) doi: 10.1167/13.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Hier D, LeMay M, Rosenberger P. Autism and unfavorable left-right asymmetries of the brain. J. Autism Dev. Disord. 1979;9:153–159. doi: 10.1007/BF01531531. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew N, Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Res. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45:685–695. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T. null, Linkage and association of the glutamate receptor 6 gene with autism. Mol. Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA. 2011;305:659–661. doi: 10.1001/jama.2011.117. [DOI] [PubMed] [Google Scholar]
- Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48:33–35. doi: 10.1111/j.1528-1167.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Sikström S. Aging cognition: from neuromodulation to representation. Trends Cogn. Sci. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what’s change got to do with it? Psychol. Aging. 2011;26:34. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Cervenka S, Farde L, Nyberg L, Bäckman L. Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia. 2009;47:2299–2304. doi: 10.1016/j.neuropsychologia.2009.01.016. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Karlsson S, Rieckmann A, Nyberg L, Bäckman L. Aging-Related Increases in Behavioral Variability: Relations to Losses of Dopamine D1 Receptors. J. Neurosci. 2012;32:8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, Markram K. The intense world syndrome--an alternative hypothesis for autism. Front. Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, McPartland JC, Gastgeb HZ, Minshew NJ. Brief report: Comparability of DSM-IV and DSM-5 ASD research samples. J. Autism Dev. Disord. 2013;43:1236–1242. doi: 10.1007/s10803-012-1665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent Component Analysis Reveals Atypical Electroencephalographic Activity During Visual Perception in Individuals with Autism. Biol. Psychiatry. 2009;65:22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front. Psychol. 2011;2:51. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green LA, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol. Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm. Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013 doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Slower Rate of Binocular Rivalry in Autism. J. Neurosci. 2013;33:16983–16991. doi: 10.1523/JNEUROSCI.0448-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neurosci. Lett. 2002;328:237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P. EEG features and epilepsy in patients with autism. Brain Dev. 1995;17:169–174. doi: 10.1016/0387-7604(95)00019-8. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell V, Oades R, Tannock R, Killeen P, Auerbach J, Johansen E, Sagvolden T. Response variability in Attention-Deficit/Hyperactivity Disorder: a neuronal and glial energetics hypothesis. Behav. Brain Funct. 2006;2:30. doi: 10.1186/1744-9081-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Egan RD, Minshew NJ, Behrmann M, Heeger DJ. Normal binocular rivalry in autism: implications for the excitation/inhibition imbalance hypothesis. Vision Res. 2013;77:59–66. doi: 10.1016/j.visres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Frontiers in Systems Neuroscience. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned Responses of Astrocytes and Their Influence on Hemodynamic Signals in the Visual Cortex. Sci. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JLR. Patterning and Plasticity of the Cerebral Cortex. Sci. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-Dimensional proportional system: an approach to cerebral imaging. New York: Thieme; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB. Becoming Consistent: Developmental Reductions in Intraindividual Variability in Reaction Time Are Related to White Matter Integrity. J. Neurosci. 2012;32:972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung K-J, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex. 2011;47:863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Vattikuti S, Chow CC. Biol. Psychiatry. Vol. 67. Elsevier Science; 2010. A computational model for cerebral cortical dysfunction in autism spectrum disorders; pp. 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive Association of the Oxytocin Receptor Gene (OXTR) with Autism in the Chinese Han Population. Biol. Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.