Abstract
Objective
Blunt trauma patients may present with similar demographics and injury severity, yet differ with regard to survival. We hypothesized that this divergence was due to different trajectories of systemic inflammation, and utilized computational analyses to define these differences.
Design, Setting, and Patients
From a cohort of 493 victims of blunt trauma, we conducted a pairwise, retrospective, case-control study of patients who survived over 24h but ultimately died (non-survivors; n=19) and patients who, following ICU admission, went on to be discharged (survivors; n=19). Data on systemic inflammatory mediators assessed within the first 24h and over 7d were analyzed with computational modeling to infer dynamic networks of inflammation. A mouse model of trauma/hemorrhage was used to verify hypotheses derived from the clinical study.
Interventions
None in patients. Neutralizing anti-IL-17A antibody in mice.
Measurements and Main Results
Network density among inflammatory mediators in non-survivors increased in parallel with organ dysfunction scores over 7d, suggesting the presence of early, self-sustaining, pathological inflammation involving HMGB1, IL-23, and the Th17 pathway. Survivors demonstrated a pattern commensurate with a self-resolving, predominantly lymphoid response, including higher levels of the reparative cytokine IL-22. Mice subjected to trauma/hemorrhage exhibited reduced organ damage when treated with anti-IL-17A.
Conclusions
Variable type 17 immune responses are hallmarks of organ damage, survival, and mortality following blunt trauma, and suggest a lymphoid cell-based switch from self-resolving to self-sustaining inflammation.
Keywords: Trauma, Mortality, Inflammation, Mathematical Modeling, Immunology, Th17
Introduction
In the United States, injury is the foremost cause of death among people between the ages 1 and 44, the third leading cause of all deaths, and the single largest cause for years of life lost according to the Centers for Disease Control [1]. Most deaths occur in the first 24h because of uncontrollable hemorrhage or devastating head injuries, but later deaths often appear to be preceded by a set of secondary complications, nosocomial infections, and multiple organ failure without infectious etiology [2], and patients with seemingly identical injuries can have markedly divergent clinical trajectories.
We and others have hypothesized that differential initial conditions and dynamic trajectories of injury-induced systemic inflammation underlie some of the variability in clinical outcomes [2–4]. Mechanical injuries result in the production and release of damage associated molecular patterns (DAMPs) and downstream pro-and anti-inflammatory chemokines, cytokines, free radicals and cellular changes within the injured tissue, extending into the circulatory system, and affecting distant organs and tissues [2, 5]. Highly regulated inflammation leads to resolution, re-establishment of homeostasis, and tissue repair. However, a self-sustaining, seemingly irreversible inflammatory process can also be set in motion, driving a systemic, malignant pathobiology across multiple organ systems [6]. Despite progress correlating circulating inflammatory mediators with poor clinical trajectories and fatal outcomes [7–10], there are currently no validated inflammation biomarkers that predict mortality, nor FDA-approved drugs targeting trauma-induced inflammation [11].
In the present study, we hypothesized that due to heretofore unrecognized pre-existing differences, some trauma victims would respond to traumatic injury by elaborating a complex, dynamic inflammatory response which drives multiple organ dysfunction and leads to death, while otherwise quite similarly injured patients would exhibit a different response that does not lead to worsened organ dysfunction. We further hypothesized that these inflammatory responses could be characterized in the form of differential dynamic networks, in which the mediators would be represented as nodes and the interactions among them would be represented as edges connecting them. The blunt trauma setting is particularly advantageous for this type of dynamic analysis, as it affords the study an initiating time point from which the course of inflammation can be observed. Here, we report an early divergence between inflammatory networks that characterize survivors and non-survivors of blunt trauma in a manner which closely parallels their divergent trajectories of organ failure. Our analysis also implicates differential engagement of lymphocyte subsets in driving inflammatory stability versus mortality post-trauma.
Materials and Methods
Patient Enrollment and Study Design
We utilized a patient cohort of 493 blunt trauma victims reported previously [12–15]. From the 493 patients enrolled, we retrospectively performed a stringent, pairwise, case-control study with 1:1 matching of non-survivors to a sub-cohort of survivors according to age, sex, mechanism of injury, traumatized body region, injury severity, initial blood pressure, course of treatment, and incidence of nosocomial infection. Details of the stringent case identification are provided in Supplemental Digital Content – Text 1.
Experimental hemorrhagic shock/trauma (HS/T) and neutralization of IL-17A
Experimental procedures were carried out in accordance with all regulations regarding the care and use of experimental animals as published by the National Institutes of Health. A previously published experimental paradigm of combined hemorrhagic shock and trauma model (HS/T) [16] was utilized as described in Supplemental Digital Content – Text 2. Of note, these mouse studies were conducted in mice that are young relative to the human cohort. Plasma was used for quantification of IL-17A/F and alanine aminotransferase (ALT). At 6h following HS/T, a group of mice (n=6) received 100 µg of neutralizing monoclonal anti-IL-17 antibody (clone 17F3; Bio X Cell, West Lebanon, NH) diluted in PBS to a total volume of 100 µL via intraperitoneal injection 12h prior to HS/T and 100 µg at the time of resuscitation. A control group (n=6) received an injection of PBS in 100 µL 12h prior to HS/T and 100 µL at the time of resuscitation.
Analysis of Inflammation and Organ Damage Biomarkers in Human and Mouse Samples
A Luminex™ 100 IS analyzer (Luminex, Austin, TX) and Human Cytokine/Chemokine or Human Th17 MILLIPLEX™ Panel kit (Millipore Corporation, Billerica, MA) were used to measure plasma levels of interleukin (IL)-1β, IL-2, soluble IL-2 receptor-α (sIL-2Rα), IL-4, IL-5, IL-6, IL-7, IL-8 (CCL8), IL-10, IL-13, IL-15, IL-17, IL-21, IL-22, IL-23, interferon (IFN)-γ, IFN-γ inducible protein (IP)-10 (CXCL10), monokine induced by gamma interferon (MIG; CXCL9), macrophage inflammatory protein (MIP)-1α (CCL3), MIP-1β (CCL4), monocyte chemotactic protein (MCP)-1 (CCL2), granulocyte-macrophage colony stimulating factor (GM-CSF), Eotaxin (CCL11), and tumor necrosis factor alpha (TNF-α). High-mobility group protein B1 (HMGB1) measurement was performed using ELISA (Shino-Test Corp., Kanagawa, Japan, distributed by IBL international, Toronto, ON, Canada). NO2−/NO3 levels were measured by a Griess Reagent colorimetric assay (Cayman Chemical, Ann Arbor MI). To establish a baseline, inflammation biomarker data were obtained from 12 healthy volunteers (mean age: 46±2.1; 8 males and 4 females) with no history of recent trauma, infections, or existing co-morbidities. Mouse IL-17A/F was quantified by ELISA (R&D, Minneapolis, MN). Mouse ALT levels were assayed using a Dry-Chem Veterinary Chemistry Analyzer (HESKA, Loveland, CO; slides from Fujifilm Corporation, Asaka-shi Saitama, Japan).
Statistical Analysis
All data were analyzed using SigmaPlot™ 11 software (Systat Software, Inc., San Jose, CA). Statistical difference between survivors and non-survivors was determined by either Student’s t-test or Chi-square test as appropriate. Group-time interaction of plasma inflammatory mediators’ levels between survivors and non-survivors was determined by Two-Way Analysis of Variance (ANOVA) and quantified by area under the curve (AUC) using the mean values for each time point, then calculating non-survivors/survivors AUC fold change. Spearman’s rank correlation was calculated by using MATLAB™. P<0.05 was considered statistically significant for all analyses.
Computational Methods: DyBN and DyNA
We carried out Dynamic Bayesian Network (DyBN) and Dynamic Network Analysis (DyNA) inferences as previously described by our group [13, 14, 17, 18]. Further details on DyBN and DyNA are provided in Supplemental Digital Content – Text 3.
Results
Stringently-matched survivors and non-survivors diverge in clinical outcomes and inflammatory mediators
The stringent case identification resulted in a final sub-cohort of 19 non-survivors (mean age: 58.8±4.5; ISS: 23.3±2.1; GCS: 10.6±1.8), with a male to female ratio of 16:3. A highly-matched population of 19 survivors (mean age: 59.0±3.0, P=0.977; ISS = 23.4±2.0, P=0.912; GCS: 13.9±0.73, P=0.236), with an equivalent male to female ratio, served as a control sub-cohort (Supplemental Digital Content – Table 1). The sub-cohorts were essentially the same in composition and number across all 7d. An in depth comparison of survivor and non-survivor clinical outcomes is provided in Supplemental Digital Content – Figure 1 and Text 4, and the demographics of the overall 493 patients enrolled is provided in Supplemental Digital Content – Table 2.
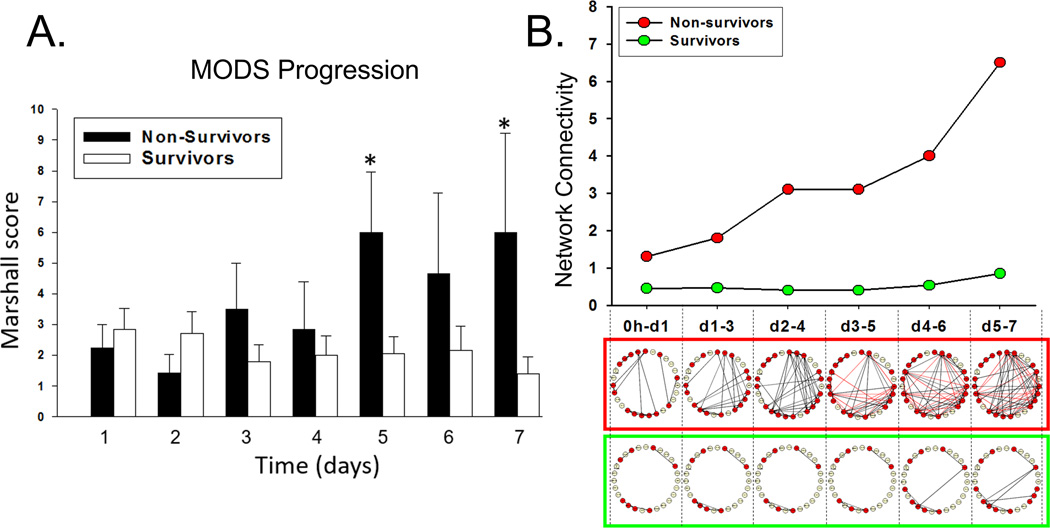
Despite this homogeneity between sub-cohorts, survivors and non-survivors experienced different degrees of organ dysfunction (Figure 1A). The survivor cohort exhibited a slight decrease in Marshall MOD score over 7d. The non-survivors exhibited later-onset and increasing multiple organ dysfunction, with statistically elevated and sustained multiple organ failure (MOD score ≥ 5) at approximately Day 5.
Figure 1. DyNA network complexity mimics Marshall MOD score trajectories of organ failure.
Trauma patients were recruited following IRB approval and informed consent. (A) A daily Marshall MOD score analysis for organ failure exhibited a slight decrease in Marshall MOD score over 7d in survivors. The non-survivors exhibited later-onset and increasing multiple organ dysfunction, reaching a state of sustained multiple organ failure (MOD score ≥ 5) by Day 5, significant (P<0.05) by Two-Way ANOVA over 7d. Plasma was obtained at multiple time points and analyzed for the presence of 27 inflammatory mediators in highly-matched sub-cohorts of survivors and non-survivors, followed by Dynamic Network Analysis (DyNA) as described in the Materials and Methods. (B) The time-evolution of networks in non-survivors (framed in red) and survivors (framed in green). In silico inference of inflammatory network complexity suggests a bifurcation of low-level versus self-amplifying inflammation in survivors and non-survivors, respectively. Notably, in silico-defined trajectories of inflammation mimic the clinical trajectories of organ dysfunction over 7d in both survivors and non-survivors.
Differntial networks of systemic inflammation implicate HMGB1, chemokines, and type 17 cell pathways
The divergent trajectories of organ dysfunction seen in survivors and non-survivors led us to hypothesize that these sub-cohorts would exhibit different time courses of systemic inflammation. Indeed, non-survivors exhibited significantly elevated circulating levels of HMGB1 (P<0.001), IL 8 (P=0.006), MIG (P=0.008), Eotaxin (P=0.023), and MIP-1β (P=0.049). In contrast, circulating IL-22 (P=0.028) was significantly higher in survivors vs. non-survivors (see Supplemental Digital Content - Table 3 for complete data). Our data (Supplemental Digital Content – Figure 2) support prior studies [9] pointing to HMGB1 as an early biomarker of trauma mortality. We further hypothesized that these inflammatory responses could be characterized in the form of differential dynamic networks, in which inflammatory mediators would be represented as nodes and the interactions among them would be represented as edges connecting them. Indeed, DyBN inference of all 27 mediators within the first 24h identified HMGB1 as a central node, with MIG and MCP 1 as output nodes, in both survivors and non-survivors (Supplemental Digital Content – Figure 3). DyBN analysis also suggested two central feedback nodes of control in non-survivors, HMGB1 and IL-23, leading us to hypothesize a feed-forward loop of interaction between HMGB1 and a type 17 polarizing cytokine within the first 24h.
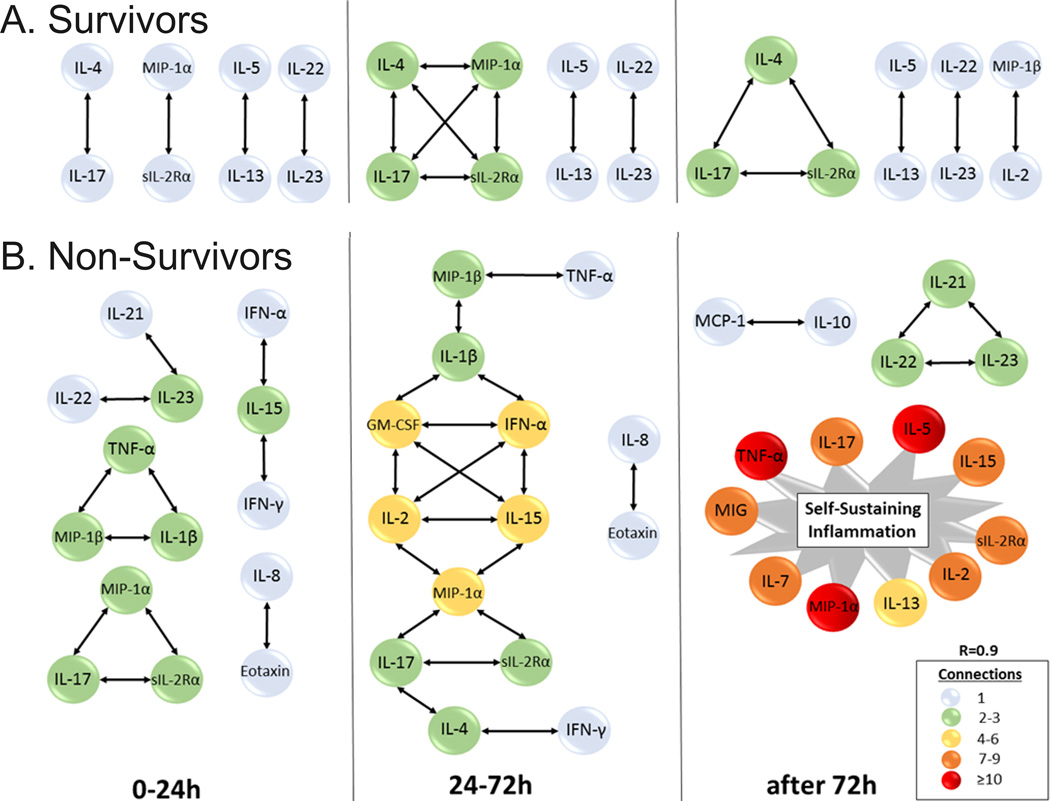
We next sought to determine, in a more granular fashion, the time-dependent evolution over 7 d of networks of systemic inflammation in these patients using DyNA (Figure 1B, Figure 2, and Supplemental Digital Content - Appendix A). One metric that can be used to assess the degree of interconnection is network complexity [18], which remained low and stable in survivors, while increasing progressively in non-survivors. Notably, network complexity indices closely paralleled the respective clinical trajectories of organ dysfunction (Figure 1B). Inferred dynamic networks in the survivors group predominantly involved cytokines associated with T cell activation (IL-4, IL-5, IL-13, IL-17A, IL-22, IL-23, and sIL-2Rα), discernible as early as 24h (Figure 2A). In survivors, DyNA suggested the early formation of relatively sparse networks of interaction involving predominantly T cell-related (lymphoid) mediators, which persisted over the 7d course. In contrast, the dynamic networks of non-survivors were characterized by interconnections among both innate immune mediators (eotaxin, IL-8, MIP-1α, MIP-1β, IL-1β, TNF-α, IFN-α, IFN-γ) and lymphoid mediators (IL-2, IL-4, IL-5, IL-7, IL-13, IL-17A, IL-21, IL-22, IL 23), starting early post-injury. These networks evolved into much larger, innate immune-dominant networks after 72h (Figure 2B). DyNA also inferred a nearly ubiquitous connection between IL-22 and IL-23 over 7d in both survivors and non-survivors. However, IL-21 was only discernable in the non-survivor networks. The 7d time courses for IL-21, IL-22, and IL-23 are provided in Supplemental Digital Content – Figure 4.
Figure 2. A well-balanced lymphoid network is associated with survival.
Trauma patients were recruited following IRB approval and informed consent. Plasma was obtained at multiple time points and analyzed for the presence of 27 inflammatory mediators in highly-matched sub-cohorts of survivors and non-survivors, followed by Dynamic Network Analysis (DyNA) as described in the Materials and Methods. (A) Inferred dynamic networks in the survivors group predominantly involved lymphoid-associated cytokines. Strongly connected networks of interaction between IL-17A, IL 4, and sIL-2Rα; IL-22 and IL-23; and between IL-5 and IL-13, were established early on and persisted over the 7d course in survivors. (B) Non-survivors were characterized by networks of both innate mediators and lymphoid mediators that evolved into much larger, innate-dominant networks by 72h. IL-17 in the figure refers to IL-17A. All original DyNA outputs are included in Appendix A.
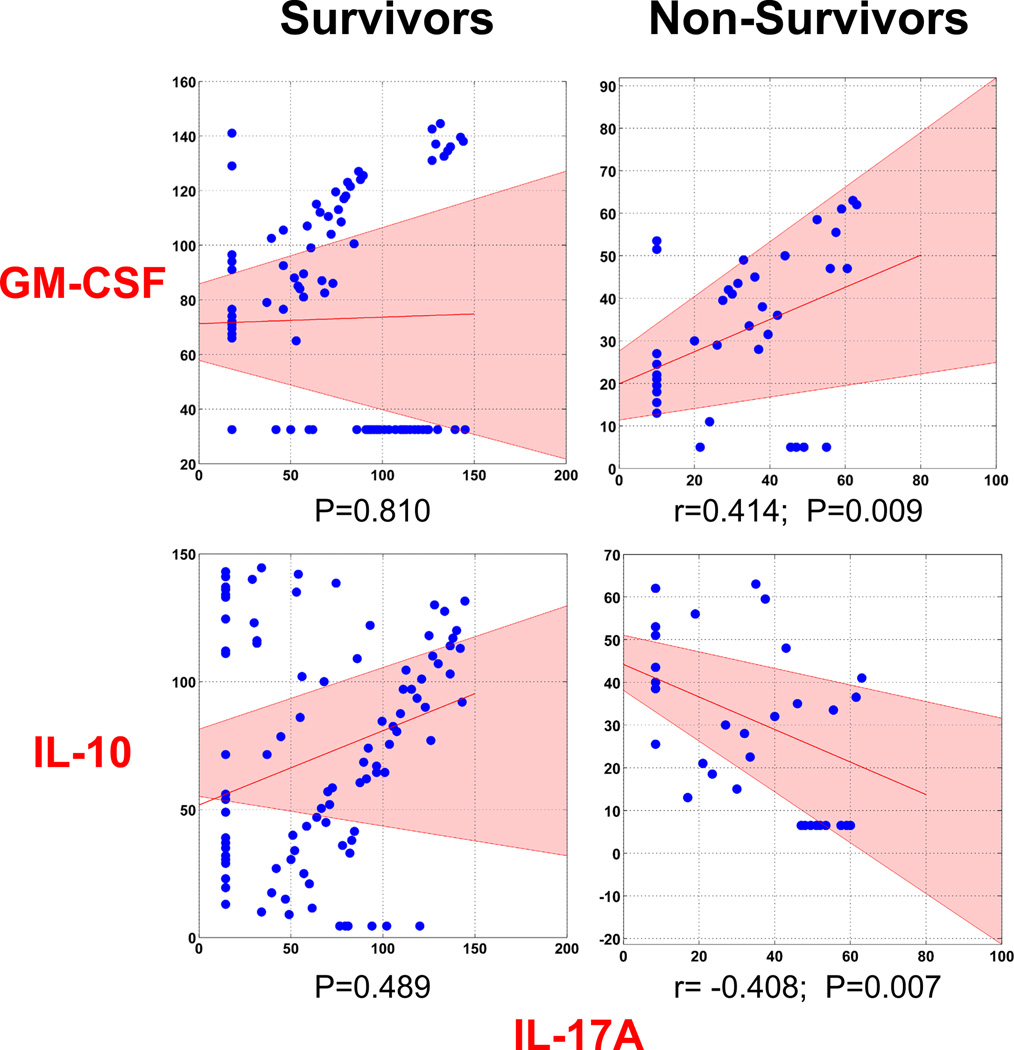
The DyBN-generated hypothesis associating HMGB1 with IL-23; the presence of IL-17A, IL-21, IL-22, and IL-23 in early time intervals in DyNA, and the known role of IL-23 in stabilizing Th17 cell populations [19] and group 3 innate lymphoid cells (ILC3) [20], led us explore the possibility of an early type 17 immune response to trauma. Kuchroo and co-workers have described a sub-population of type 17 cells known as pathogenic Th17 cells that, in addition to expressing IL-17A, upregulate GM-CSF and down-regulate IL-10 in a manner that is potentiated by IL-23 [21]. In line with this characterization, plasma IL-17A in non-survivors was correlated positively with circulating GM-CSF (r=0.414; P=0.009) and negatively with circulating IL-10 (r=−0.408, P=0.007), while IL-17A in survivors presented neither positive nor negative correlations with GM-CSF nor IL-10 (P=0.810 and P=0.489, respectively; Figure 3). The 7d time courses for IL-17A, GM-CSF, and IL-10 are also provided in Supplemental Digital Content – Figure 5.
Figure 3. A pathogenic Th17 phenotype is associated with non-survival.
Trauma patients were recruited following IRB approval and informed consent. Plasma was obtained at multiple time points and analyzed for the presence of 27 inflammatory mediators in highly-matched sub-cohorts of survivors and non-survivors, and the data were subjected to Bootstrapped Spearman’s rank correlation as described in the Materials and Methods. This analysis suggests a positive correlation between IL-17A and GM-CSF and a negative correlation between IL-17A and IL-10, consistent with a pathogenic Th17 phenotype. No significant correlation was found among these mediators in the survivors sub-cohort. The shaded area in pink represents the 95% confidence interval.
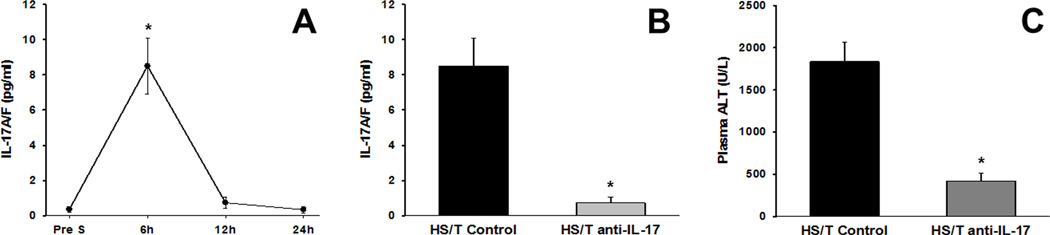
We next hypothesized that early activation of the type 17 cells could drive morbidity. In support of this hypothesis, mice subjected to experimental hemorrhagic shock/trauma (HS/T) had elevated levels of IL-17A (Figure 4A) at 6h post trauma, which declined subsequently. In support of a pathological role for IL-17, mice treated with neutralizing anti-IL-17 antibody exhibited reduced circulating IL-17A (Figure 4B) as well as a nearly 70% reduction in levels of alanine aminotransferase (a marker of organ dysfunction; Figure 4C).
Figure 4. Anti-IL-17 antibody reduces liver dysfunction in mice.
Mice (n=6 in all groups) were subjected to an experimental hemorrhagic shock and trauma model (HS/T) as described in the Materials and Methods. (A) Time course of circulating IL-17A/F in mice subjected to HS/T. *: P<0.05 vs. baseline which quickly diminished by 12h and 24h time points. (B and C) Neutralization of IL-17A/F by an anti-IL-17 monoclonal antibody at 6h resulted in significantly decreased plasma IL-17A/F when compared to the control group by Mann-Whitney Rank Sum test (panel B; P=0.002) and a marked decrease in alanine aminotransferase (ALT), a marker of liver dysfunction, when compared to the control group by t-test (panel C; P<0.001).
Discussion
We sought to utilize stringently-matched cohorts to reduce patient-to-patient variability combined with computational analytics to address the complexity of post-injury inflammation. By comparing highly matched cohorts of survivors and non-survivors of blunt trauma, we were able to define two disparate dynamic networks of systemic inflammation predictive of the unequivocal endpoints of post-traumatic outcomes – life and death. Several key observations were derived from this approach, some of which support earlier notions about how inflammation may drive outcomes post-trauma, and others that appear to set a new paradigm more in-common with chronic inflammatory conditions than with the acute inflammation typically associated with trauma.
The most evident insight is that injury severity alone does not appear to be the sole driver of mortality following blunt trauma. It is important to note that in the present study, patients presenting to the emergency department with a predicted survival of less than 24h were excluded from the study because most patients dying during this time period die of uncontrolled hemorrhage or devastating head injury [2]. Thus, we studied patients who survived the initial mechanical insult yet, due to underlying and undefined genetic, epigenetic, and pathophysiological reasons, went on to experience a higher degree of organ dysfunction and ultimately die. This suggests that the simplistic paradigm of increasing severity of injury leading ultimately to death may need to be replaced with one in which underlying and perhaps pre-existing inflammatory conditions contribute to the lethal outcome as much as, or more than, the magnitude of the injury alone. Notably, we calculated injury severity using the ISS scoring system, which is a mathematical aggregation of body region-specific injuries and a well-accepted method of stratifying the degree of injury for patients following trauma. Although this scoring system was designed to quantify the injury burden post-discharge, and has been improved subsequently to become more predictive of clinical outcomes of broad groups of trauma patients especially at the extremes of injury severity, it is incapable of correlating injury severity with individual outcomes [22, 23]. Also, it is possible that there were occult differences in injury severity between survivors and non-survivors.
A key insight from the in silico inference of dynamic complexity relates to the early bifurcation of well-regulated inflammatory processes in survivors versus an unregulated, self-sustaining systemic inflammatory response in those who die. Importantly, these dynamic inflammatory networks mimic the clinical trajectories of organ dysfunction over 7d in both survivors and non-survivors. Based on network analyses, this inflammatory bifurcation involved both well-appreciated innate immune pathways as well as lymphoid pathways more typically associated with chronic inflammation.
Within hours after the traumatic insult, we found supranormal levels of HMGB1 in both survivors and non-survivors. The early and important role of HMGB1 and similar prototypical DAMPs in response to trauma is well documented in the literature [9, 24], and this finding has served to support the putative role of DAMPS as activators and propagators of early innate immune pathways in which chemokines play a key role. Our dynamic Bayesian inferences in both survivors and non-survivors suggested that the chemokines MCP-1/CCL2 and MIG/CXCL9 are outputs downstream of HMGB1, validating the proposed sequence of events as well as work which places both MCP-1 and MIG as important drivers of post-traumatic inflammation [10, 14].
Our in silico analyses also unmasked a relationship between HMGB1 and a type 17 cell immune response as early as 24h among non-survivors (see Supplemental Digital Content – Text 5 for details on Type 17 cells). Over the past decade, IL-17 and IL-23 have been identified as important players in numerous chronic autoimmune inflammatory diseases [19, 25–27]. A link between HMGB1 and the IL-23-Th17 axis via toll-like receptor signaling was also established in experimental hemorrhagic shock [28]. However, to our knowledge, this is the first study to link DAMPs, chemokines, and the IL-23–Th17 immune axis with acute systemic inflammation following blunt trauma in humans. Furthermore, DyBN inference in the survivor cohort places IL-6 and IL-22, both of which are characteristic cytokines of type 17 immune responses, downstream of HMGB1 [29, 30]. The cytokine IL-22 was the only statistically significantly elevated mediator in the survivor cohort, and was ubiquitously present in DyBN and DyNA inflammatory networks. Innate-derived IL-22 has well-established roles in the protection and repair of epithelial tissue, particularly in the gut [31, 32]. Therefore, it is tempting to speculate that the higher IL-22 levels in survivors are mechanistically linked to resolution and survival. Along with IL-17A and GM-CSF, IL-22 is a signature cytokine of a type 17 immune response and is produced by both Th17 and type 3 innate lymphoid cells (ILC3) [19, 20]. While our data suggest evidence for both ILC and Th17 responses, we cannot yet conclusively determine the cellular source of IL-22 in human blunt trauma.
Considerable work on type 17 cell subsets has broadly divided Th17 cells into two categories: nonpathogenic Th17 cells that express both IL-17 and IL-10 [29], and highly inflammatory, pathogenic Th17 cells that upregulate GM-CSF and down-regulate host-protective IL-10 [21]. We found a strong correlation between IL-17A and GM-CSF and a strong negative correlation between IL-17A and IL-10 in non-survivors but not in survivors, leading us to hypothesize that pathogenic Th17 cells are associated with non-survival following trauma. In line with the characterization, early administration of an anti-IL-17 antibody in mice proved efficacious in reducing organ dysfunction in the context of experimental HS/T, though the fact that the mice are relatively young as compared to the human cohort is a limitation of that experiment.
Dynamic network analyses also elucidated that a core network comprised of IL-17A, IL 4, and sIL-2Rα is associated with survival, consistently present and sparsely connected with MIP-1α/CCL3 over the first 7d post-injury. A supplementary DyNA inference carried out on all 472 survivors from the overall 493 patient cohort also confirms the presence of a network involving connections among IL-17A, IL 4, and sIL-2Rα consistently after 72h (Supplemental Digital Content – Figure 6). We hypothesize that this is a relatively beneficial response network that reflects lymphocyte (Th17, Th2, and Treg) diversity, and a well-regulated, nonpathogenic Th17 response. This hypothesis is supported by considerable literature that cites the cross-regulatory effects of IL-4, IL-17, and sIL-2Rα on Th2, Th17, and Treg populations [33–36]. Indeed, a recent study reports that the transdifferentiation of Th17 cells to express Treg and Th2 regulatory phenotypes is critical for the resolution of inflammation [37].
MIP-1α /CCL3 is peripherally involved in the inflammatory networks of the matched sub-cohorts and consistently connected to the core IL-17A, IL-4, and sIL-2Rα in the supplementary analysis. A recent study cites the increased expression of CCL3 in a pathogenic Th17 mouse model of autoimmunity [38]. Considering the overall low mortality rate that characterizes civilian blunt trauma, there remains a need for larger, multi-center studies utilizing flow cytometry and analyses of key transcription factor expression [39–41] to validate any role for pathogenic Th17 cells or other cell types (e.g. innate lymphoid cells) [19, 20] in the pathophysiology associated with trauma mortality.
We therefore hypothesize the presence of a decisive “tipping point” [42] between an innate- versus lymphoid-dominant response that occurs between 24–72h after injury in non-survivors (Figure 5). Unlike the relatively stable, lymphoid-dominant inflammatory networks characteristic of survivors, the dynamic networks in non-survivors are characterized by sparsely connected innate and lymphoid mediators in the first 24h, which ultimately develop into highly-connected, self-sustaining networks by 72h. Notably, a recent study attributes three time-dependent transcriptional phases to the transition from naïve-like cells to Th17 cells following T cell receptor stimulation: induction (up to 4h); onset of phenotype and amplification (4–20h); and stabilization and IL-23 signaling (20–72h) [43]. In support of the latter study, we observed a predisposition to amplify a pathogenic or nonpathogenic Th17 phenotype (0–24h post-injury) along with a putative “tipping point” period (24–72h post-injury) in which the inflammatory response becomes either self-resolving or self-sustaining in nature. This period from 24–72h following trauma may actually determine the trajectory toward organ failure and nosocomial infection.
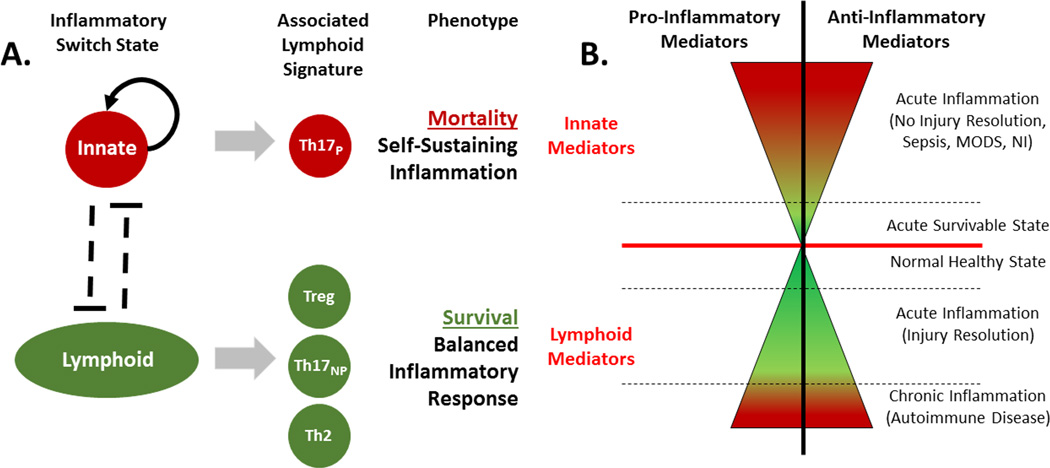
Figure 5. Inflammatory Switch State Hypothesis.
A hypothetical “inflammatory switch state”, involving innate and lymphoid mediators early on following trauma. In this paradigm, the magnitude of a pro- and anti-inflammatory response remains a critical aspect in determining clinical outcomes. (A) A well-balanced lymphoid signature following traumatic injury involves Treg, Th2, and nonpathogenic Th17 (Th17NP) cells. In contrast, the early activation of a pathogenic Th17 (Th17P) phenotype is triggered when the balance between lymphocytes is tipped leading to an uncontrolled inflammatory cascade – much like the balance of lymphocytes is tipped in those predisposed to develop chronic inflammatory diseases. (B) The severity of pro- and anti-inflammatory mediator involvement [represented horizontally in the x-axis] is either attenuated by a balanced lymphoid response or, conversely, exacerbated by a self-sustaining, predominantly innate-mediated response. Thus, this diagram represents the two major outcomes of these contrary inflammatory patterns: (1) a well-balanced lymphoid response leads to injury resolution and (2) a loss of lymphocyte regulation engages self-sustaining inflammation that impedes injury resolution.
Expanding on the existing paradigm of simultaneously increased activation of innate immune pathways and decreased activation of adaptive immune pathways following trauma [5], we hypothesize the presence of an “inflammatory switch state” involving innate and lymphoid mediators (Figure 5A). In this paradigm, the magnitudes of pro- and anti-inflammatory responses remain a critical aspect in determining clinical outcomes. However, we hypothesize that the magnitude of the inflammatory response is either attenuated by a balanced lymphoid response, or intensified by a self-sustaining, predominantly innate-mediated, response (Figure 5B). We predict that a well-balanced lymphoid signature involves nonpathogenic Th17 cells and an adequate IL-22 response to injury that is commensurate with survival. In contrast, the early activation of a pathogenic Th17 phenotype is triggered when the balance between lymphocytes is tipped, leading to progressive organ failure and death. We further hypothesize that a given individual may be somehow predisposed for an innate- or a lymphoid-dominant response upon traumatic insult, much like patients who have dysregulated Th17 responses are predisposed to develop chronic inflammatory disease – a predisposition that is likely controlled by a combination of many genetic and epigenetic factors, as well as age, sex, pre-existing diseases, microbiome composition, etc. This hypothesis requires extensive validation in further clinical and pre-clinical paradigms of traumatic injury, but holds the potential to drive a novel generation of diagnostic and therapeutic modalities.
Conclusions
Our data suggest that shortly after blunt trauma, and possibly due to pre-existing factors, some patients proceed towards a path of self-sustaining inflammation and associated organ dysfunction which culminates in death. Similarly injured patients respond to the injury with a lower-grade, sub-threshold inflammatory response associated with resolving organ dysfunction and eventual survival. This “inflammatory switch” may be triggered or regulated by variable type 17 immune responses.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIH grant P50-GM-53789
Dr. Vodovotz received support for article research from the National Institutes of Health (NIH) Dr. Abboud received support for article research from the NIH). Dr. Namas received support for article research from the NIH. Dr. Ramadan received support for article research from the NIH. His institution received funding from the NIH. Dr. Almahmoud received support for article research from the NIH. Dr. Abdul-Malak received support for article research from the NIH. Dr. Barclay received support for article research from the NIH. Dr. Yin received support for article research from the NIH. Dr. Zamora received support for article research from the NIH. Dr. Simmons received support for article research from the NIH. Dr. Billiar received support for article research from the NIH.
Footnotes
Copyright form disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Center for Disease Control and Prevention. Injury Prevention & Control: Data & Statistics (WISQARS™): Ten Leading Causes of Death and Injury [Google Scholar]
- 2.Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, Koenderman L, Kubes P, Lilford RJ. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384(9952):1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vodovotz Y, Billiar TR. In Silico Modeling: Methods and applications to trauma and sepsis. CritCare Med. 2013;41:2008–2014. doi: 10.1097/CCM.0b013e31829a6eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namas RA, Mi Q, Namas R, Almahmoud K, Zaaqoq AM, Abdul-Malak O, Azhar N, Day J, Abboud A, Zamora R, et al. Insights into the Role of Chemokines, Damage-Associated Molecular Patterns, and Lymphocyte-Derived Mediators from Computational Models of Trauma-Induced Inflammation. Antioxidants & redox signaling. 2015;23(17):1370–1387. doi: 10.1089/ars.2015.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. The Journal of experimental medicine. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 7.Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Brückner UB. IS interleukin 6 an early marker of injury severity following major trauma in humans? Archives of Surgery. 2000;135(3):291–295. doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 8.Namas R, Ghuma A, Torres A, Polanco P, Gomez H, Barclay D, Gordon L, Zenker S, Kim HK, Hermus L, et al. An adequately robust early TNF-alpha response is a hallmark of survival following trauma/hemorrhage. PloS one. 2009;4(12):e8406. doi: 10.1371/journal.pone.0008406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, Carles M, Howard M, Pittet JF. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Critical care (London, England) 2009;13(6):R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziraldo C, Vodovotz Y, Namas RA, Almahmoud K, Tapias V, Mi Q, Barclay D, Jefferson BS, Chen G, Billiar TR, et al. Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PloS one. 2013;8(12):e79804. doi: 10.1371/journal.pone.0079804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An G, Nieman G, Vodovotz Y. Toward computational identification of multiscale "tipping points" in acute inflammation and multiple organ failure. Annals of biomedical engineering. 2012;40(11):2414–2424. doi: 10.1007/s10439-012-0565-9. [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Namas RA, Almahmoud K, Zaaqoq A, Sarkar J, Barclay DA, Yin J, Ghuma A, Abboud A, Constantine G, et al. Trauma in silico: Individual-specific mathematical models and virtual clinical populations. Sci Transl Med. 2015;7(285):285–261. doi: 10.1126/scitranslmed.aaa3636. [DOI] [PubMed] [Google Scholar]
- 13.Namas RA, Vodovotz Y, Almahmoud K, Abdul-Malak O, Zaaqoq A, Namas R, Mi Q, Barclay D, Zuckerbraun B, Peitzman AB, et al. Temporal Patterns of Circulating Inflammation Biomarker Networks Differentiate Susceptibility to Nosocomial Infection Following Blunt Trauma in Humans. Annals of surgery. 2014 doi: 10.1097/SLA.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almahmoud K, Namas RA, Zaaqoq AM, Abdul-Malak O, Namas R, Zamora R, Sperry J, Billiar TR, Vodovotz Y. Prehospital Hypotension Is Associated With Altered Inflammation Dynamics and Worse Outcomes Following Blunt Trauma in Humans. Critical care medicine. 2015 doi: 10.1097/CCM.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 15.Zaaqoq AM, Namas R, Almahmoud K, Azhar N, Mi Q, Zamora R, Brienza DM, Billiar TR, Vodovotz Y. Inducible protein-10, a potential driver of neurally controlled interleukin-10 and morbidity in human blunt trauma. Critical care medicine. 2014;42(6):1487–1497. doi: 10.1097/CCM.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korff S, Loughran P, Cai C, Lee YS, Scott M, Billiar TR. Eritoran attenuates tissue damage and inflammation in hemorrhagic shock/trauma. J Surg Res. 2013;184(2):e17–e25. doi: 10.1016/j.jss.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azhar N, Ziraldo C, Barclay D, Rudnick D, Squires R, Vodovotz Y. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PloS one. 2013/;8:e78202. doi: 10.1371/journal.pone.0078202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, Bentley T, Billiar TR, Zamora R, Puyana JC, et al. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PloS one. 2011;6(5):e19424. doi: 10.1371/journal.pone.0019424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nature immunology. 2012;13(10):991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Ruden C, Woltmann A, Rose M, Wurm S, Ruger M, Hierholzer C, Buhren V. Outcome after severe multiple trauma: a retrospective analysis. Journal of trauma management & outcomes. 2013;7(1):4. doi: 10.1186/1752-2897-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawda MN, Hildebrand F, Pape HC, Giannoudis PV. Predicting outcome after multiple trauma: which scoring system? Injury. 2004;35(4):347–358. doi: 10.1016/S0020-1383(03)00140-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science (New York, NY) 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5(6):325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 27.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nature medicine. 2012;18(7):1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Yuan Y, Li Y, Zhang J, Xiao G, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol. 2009;182(1):572–580. doi: 10.4049/jimmunol.182.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature immunology. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 30.O'Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nature immunology. 2010;11(6):471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 31.Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9(4):229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 34.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-[beta]-induced Foxp3+ T cells and, together with TGF-[beta], generates IL-9+ IL-10+ Foxp3- effector T cells. Nature immunology. 2008;9(12):1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65(5 Pt 2):26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- 36.Guenova E, Skabytska Y, Hoetzenecker W, Weindl G, Sauer K, Tham M, Kim KW, Park JH, Seo JH, Ignatova D, et al. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(7):2163–2168. doi: 10.1073/pnas.1416922112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015 doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. The Journal of experimental medicine. 2014;211(1):89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27(5):786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature immunology. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An G, Nieman G, Vodovotz Y. Computational and systems biology in trauma and sepsis: Current state and future perspectives. Int J Burns Trauma. 2012;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 43.Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature. 2013;496(7446):461–468. doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.