Abstract
BACKGROUND
Despite good anatomic and functional outcomes, urogynecologic polypropylene meshes that are used to treat pelvic organ prolapse and stress urinary incontinence are associated with significant complications, most commonly mesh exposure and pain. Few studies have been performed that specifically focus on the host response to urogynecologic meshes. The macrophage has long been known to be the key cell type that mediates the foreign body response. Conceptually, macrophages that respond to a foreign body can be dichotomized broadly into M1 proinflammatory and M2 proremodeling subtypes. A prolonged M1 response is thought to result in chronic inflammation and the formation of foreign body giant cells with potential for ongoing tissue damage and destruction. Although a limited M2 predominant response is favorable for tissue integration and ingrowth, excessive M2 activity can lead to accelerated fibrillar matrix deposition and result in fibrosis and encapsulation of the mesh.
OBJECTIVE
The purpose of this study was to define and compare the macrophage response in patients who undergo mesh excision surgery for the indication of pain vs a mesh exposure.
STUDY DESIGN
Patients who were scheduled to undergo a surgical excision of mesh for pain or exposure at Magee-Womens Hospital were offered enrollment. Twenty-seven mesh-vagina complexes that were removed for the primary complaint of a mesh exposure (n = 15) vs pain in the absence of an exposure (n = 12) were compared with 30 full-thickness vaginal biopsy specimens from women who underwent benign gynecologic surgery without mesh. Macrophage M1 proinflammatory vs M2 proremodeling phenotypes were examined via immunofluorescent labeling for cell surface markers CD86 (M1) vs CD206 (M2) and M1 vs M2 cytokines via enzyme-linked immunosorbent assay. The amount of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) proteolytic enzymes were quantified by zymography and substrate degradation assays, as an indication of tissue matrix degradation. Statistics were performed with the use of 1-way analysis of variance with appropriate post hoc tests, t-tests, and Fisher’s Exact test.
RESULTS
Twenty-seven mesh-vaginal tissue complexes were excised from 27 different women with mesh complications: 15 incontinence mid urethral slings and 12 prolapse meshes. On histologic examination, macrophages surrounded each mesh fiber in both groups, with predominance of the M1 subtype. M1 and M2 cytokines/chemokines, MMP-9 (pro- and active), and MMP-2 (active) were increased significantly in mesh-vagina explants, as compared with vagina without mesh. Mesh explants that were removed for exposure had 88.4% higher pro-MMP-9 (P = .035) than those removed for pain. A positive correlation was observed between the profibrotic cytokine interleukin-10 and the percentage of M2 cells (r = 0.697; P = .037) in the pain group.
CONCLUSION
In women with complications, mesh induces a proinflammatory response that persists years after implantation. The increase in MMP-9 in mesh explants that were removed for exposure indicates degradation; the positive association between interleukin-10 and M2 macrophages in mesh explants that are removed for pain is consistent with fibrosis.
Keywords: cytokine, inflammatory response, macrophage phenotype, polypropylene mesh
Over the past decade, lightweight, wide-pore polypropylene mesh has been used increasingly in the repair of pelvic organ prolapse and stress urinary incontinence. Despite favorable anatomic and functional outcomes, mesh use has been associated with complications, most commonly mesh exposure through the vaginal epithelium and pain.1 Studies of similar meshes that are used in hernia repair have demonstrated that all polypropylene meshes induce a prolonged inflammatory response at the site of implantation.2,3 The magnitude and type of response is associated with the development of complications.3,4 Although it is tempting to extrapolate these findings to meshes that are applied to the vagina, data suggest that the host response at these 2 sites is distinct.5
The host response after the placement of any foreign material into the body has been well described. After the initial recruitment of neutrophils, macrophages become the primary immune cell to be involved in the clearance of debris and the initiation of the host response.6,7 Although this is an essential initial component of healing, the long-term presence of activated inflammatory cells, such as macrophages at the mesh tissue interface, can impact negatively the ability of the mesh to function as intended.
Macrophages have been classified as having diverse and plastic phenotypes along a continuum between M1 (classically activated; proinflammatory) and M2 (alternatively activated; remodeling, homeostatic) extremes.8–10 M1 macrophages are characterized by the secretion of reactive oxygen species and proinflammatory cytokines and chemokines and can be identified via the cell surface marker CD86. Persistence of M1 macrophages can lead to tissue damage and destruction. In contrast, M2 macrophages secrete growth factors and antiinflammatory immune modulators and can be identified by the cell surface marker CD206. M2 macrophages participate in the constructive healing and remodeling phase of the foreign body response and result in tissue deposition and in growth.8,10–12 However, an overzealous M2 response can also lead to excess tissue deposition and fibrosis.13 As such, macrophage polarization and plasticity play an important and determinant role in tissue remodeling after injury and the integration of biomaterials.13,14 Limited data exist about the macrophage response after implantation of urogynecologic meshes15,16 particularly in regard to implantation in the vagina.
The objective of the current study was to characterize the macrophage response that is present in patients who undergo mesh excision surgery and to define differences in this response according to the 2 most common complications: mesh exposure and pain.1 Mesh-tissue constructs from women who underwent mesh excision surgery for the indication of pain and exposure were compared with full-thickness vaginal biopsy specimens from women who underwent prolapse repairs with the use of morphologic, biochemical, and immunologic endpoints.
Materials and Methods
Patient acquisition
Patients who were scheduled to undergo surgical excision of mesh as part of a larger study (Magee Mesh Biorepository IRB# 10090194) were offered enrollment. For inclusion in the current study, mesh had to be removed from the anterior or apical compartment for the primary indication of exposure or pain. Mesh exposure was defined as at least 2 mm of mesh visible through the vaginal epithelium; pain was defined as mesh being removed for the primary complaint of pain (with palpation, ambulation, or intercourse) without evidence of exposure. Patients were excluded from the study if they had acute infection (fever, worsening pain, and pus in area of mesh) or erosion into the bowel or bladder. Patients were also excluded if they were unable to provide informed consent, were undergoing chronic immunosuppressive therapy, or had an autoimmune disorder. After consent was obtained, baseline demographic data that were abstracted from the electronic medical record included age, race/ethnicity, body mass index (BMI), gravidity, parity, hormone use, menopausal status, and smoking status (Table 1). Menopausal status was defined as premenopausal (regular menstrual periods within the last 12 months) and postmenopausal (no menstrual periods within the last 12 months). Hormone use was defined as current use of systemic estrogen with or without progesterone or vaginal estrogen for ≥ 3 months. Smoking was defined as current smoker (yes/no). Operative reports from the initial mesh surgery were reviewed, and the type of mesh was recorded.
TABLE 1.
Descriptive statistics of study population
| Variables | Mesh complications (n = 27) | Prolapse (n = 30) |
P value | |
|---|---|---|---|---|
| Mesh exposure (n = 15) |
Pain (n = 12) |
|||
| Mean age, y±SD | 56.1 ± 8.0 | 52.1 ± 9.7 | 52.9 ±9.2 | .527 |
| Mean body mass index, kg/m2± SD |
30.8 ± 5.9 | 27.3 ± 3.3 | 28.5 ± 4.0 | .158 |
| Median gravidity, n (25 %ile, 75 %ile) |
3.0 (2.0, 4.0) | 3.5 (2.0, 5.75) | 2.0 (2.0, 3.0) | .644 |
| Median parity, n (25 %ile, 75 %ile) |
2.0 (2.0, 3.0) | 3.0 (2.0, 5.0) | 2.0 (2.0, 3.0) | .899 |
| Mean time implanted, mo ± SD | 36.9 ± 30.3 | 30.9 ± 18.0 | NA | .527 |
| Menopausal status, n (%) | ||||
| Premenopausal | 2 (13) | 3 (25) | 12 (40) | |
| Postmenopausal | 13 (87) | 9 (75) | 18 (60) | .190 |
| Smoking, n (%) | ||||
| Nonsmoker | 12 (80) | 9 (75) | 27 (90) | |
| Smoker | 3 (20) | 3 (25) | 3 (10) | .713 |
| Race/ethnicity, n (%) | ||||
| White | 15 (100) | 12 (100) | 29 (97) | |
| Indian | 0 | 0 | 1 (3) | |
| Other | 0 | 0 | 0 | 1.000 |
| Hormonal usage, n (%) | ||||
| Yes | 7 (47) | 7 (58) | 7 (23) | |
| No | 8 (53) | 5 (42) | 23 (77) | .077 |
NA not applicable.
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
On the day of surgery, the excised mesh-tissue complex was placed in a sterile specimen container, immediately placed on ice, and sent for analysis. Samples from patients with mesh were age, BMI, and menopausal status–matched to full-thickness vaginal biopsy specimens that were obtained from the anterior vagina at the vaginal apex in mesh-naïve women with stage II or III prolapse with and without incontinence who underwent pelvic surgery, as described previously (IRB # 0412054). 17 This group of vaginal biopsy specimens was selected as a control for the present study because there is evidence that the vaginal biopsy specimens from women with prolapse and incontinence have increased matrix metalloproteinases (MMPs) relative to women with normal pelvic organ support. 18,19
Tissue extract acquisition and histologic preparation
Excised tissue-mesh complexes and nonmesh control tissues were extracted in high salt extraction buffer as described previously.20 Additional pieces of vagina-mesh-complexes and nonmesh control tissue were embedded into O.C.T. compound (Tissue-Tek; Sakura Finetek USA Inc, Torrance, CA), flash frozen in liquid nitrogen, sectioned (7 µm), and stored at −80°C.
Cytokine and chemokine determination
Quantification of cytokines interleukin (IL)-10, IL-4, tumor necrosis factor-α (TNF-α), IL-12p70, and IL-12p40p70 and chemokines CXCL10 and CCL17 was performed with the use of commercially available enzyme-linked immunosorbent assay kits (Life Technologies, Carlsbad, CA, and R&D Systems, Minneapolis, MN, respectively). All samples were run in duplicate or triplicate with the use of 40-µg total protein per sample per assay. A patient sample that had been characterized previously for analyte amounts served as an internal control.
Zymographic analyses
Samples that contained 30 µg total protein were analyzed in duplicate by substrate zymography according to manufacturer’s instructions (Novex; Life Technologies). For quantification of active and proenzyme forms of MMP-2, band density was measured with ImageJ software (National Institute of Health, Bethesda, MD), and measurements were normalized to an internal control.
MMP-9 activity assay
Endogenous MMP-9 activity was measured with a Fluorokine E Human Active MMP-9 kit (R&D Systems). For each experiment, 20 µg of protein from each sample was tested in duplicate. Fluorescence was read with a Spectramax M2 spectrophotometer (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 320 nm and an emission wavelength of 405 nm. Data were analyzed with the use of a 4-parameter regression curve (Masterplex ReaderFit; Miraibio, San Francisco, CA) and normalized to nanogram-active MMP-9 per milligram of protein in each sample.
Immunofluorescent labeling
Tissue sections were quadruple-labeled for pan-macrophage marker CD68, M1 macrophage marker CD86, M2 macrophage marker CD206, and nuclear marker 4’,6-diamidino-2-phenylindole, as described, 15 and imaged with a Nikon ECLIPSE 90i upright microscope (Nikon USA, Melville, NY). For meshvagina complexes, six ×200 images were acquired over 2 locations within the tissue. Three images were taken in an area of a mesh fiber (defined as a single fiber of polypropylene not immediately adjacent to other fibers), and 3 additional images were taken in the area of a mesh knot (defined as ≥3 single fibers immediately adjacent to each other). For control patients, six ×200 images were taken over 2 areas of each tissue. For each image, 2 trained technicians counted the number of total cells and the number of cells that coexpressed either CD68 and CD86 or CD68 and CD206 to define the M1 and M2 macrophage population, respectively. For each field the M2:M1 ratio was calculated, as described in Wolf et al,21 by the use of the formula (raw number of M2 macrophages+1)/(raw number of M1 macrophages +1) to avoid division by 0 in samples with no cells present.
Statistical analysis
Power analysis showed that 8 samples in each group were necessary to reach statistical significance for cytokines that used previously obtained IL-10 values in vaginal extracts from nonhuman primate with and without Gynemesh PS (Ethicon, Inc., Somerville, NJ) implanted via sacrocolpopexy.15 Statistical analysis was performed with SPSS software (version 21; IBM, Armonk, NY). For demographic data, a 1-way analysis of variance with Tukey post hoc testing, Kolmogorov-Smirnov tests, and Fisher’s Exact tests with significance level α = .05 were used. For the biochemical endpoints of mesh patients vs control patients and for the complication exposure vs pain, a 2-tailed Student’s independent samples t-test was performed. For histologic endpoints that compared areas of a mesh fiber with a mesh knot, a paired-sample t-test was used (α = .05); when we compared tissue removed for exposure vs pain, an independent-sample t-test was used (α = .05). Pearson’s correlations were used to correlate cytokines with histologic findings.
Results
Demographic data
Twenty-seven mesh-vaginal tissue complexes were excised from 27 women: 15 incontinence midurethral slings and 12 prolapse meshes. Four of the 27 meshes were implanted via abdominal sacrocolpopexy, and the remainder were inserted transvaginally. There were no differences in patient age, race/ethnicity, BMI, gravidity, parity, hormone use, menopausal status, smoking status, or duration of mesh implantation (all P > .05; Table 1 ). In addition, there were no differences in patient demographics or experimental endpoints when data were separated by prolapse meshes vs midurethral slings. The specific meshes that were excised are listed in Table 2. Mesh type was not found in 3 meshes that were removed for pain and 1 mesh that was removed for exposure. Meshes were explanted from 4.5–93 months after the index surgery.
TABLE 2.
Excised mesh brand and type categorized by mesh complications
| Mesh device | Removal because of exposure (n = 15) |
Removal because of pain (n = 12) |
|---|---|---|
| AMS Monarc TOTa | 1 | 0 |
| AMS Perigeea | 1 | 0 |
| Bard Ajust Single Incision Slingb | 0 | 1 |
| Bard Soft Meshb | 0 | 1 |
| Boston Scientific Lynx TVTc | 1 | 1 |
| Boston Scientific Obtryx TOTc | 1 | 0 |
| Boston Scientific Solyx mini slingc | 0 | 1 |
| Boston Scientific Upholdc | 1 | 0 |
| Caldera Desara Sling System for SUId | 1 | 0 |
| Coloplast Novasilke | 1 | 0 |
| Gynecare Prolift mesh kitf | 1 | 1 |
| Gynecare Gynemesh PSf | 4 | 0 |
| Gynecare TVT Securf | 1 | 2 |
| Gynecare TVTf | 1 | 1 |
| Gynecare TOTf | 0 | 1 |
| Original medical records not availableg | 1 | 3 |
American Medical Systems, Minnetonka, MN;
C.R. Bard, Inc., Murray Hill, NJ;
Boston Scientific Corporation, Marlborough, MA;
Caldera Medical, Inc., Agoura Hills, CA;
Coloplast Corporation, Minneapolis, MN;
Ethicon, Inc., Somerville, NJ;
Of the 27 patients who were enrolled in this study, mesh brand information could not be determined for 4 of the patients.
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
Biochemical endpoints
All M1 proinflammatory and M2 proremodeling cytokines and chemokines were increased in mesh explants as compared with nonmesh tissue (Table 3), which indicated a robust, active, and ongoing host response to polypropylene long after implantation. Examination of the proremodeling cytokines IL-4 and −10 showed a 1.18-fold increase (P = .011) and 1.45-fold increase (P = .016), respectively, in mesh-vagina explants vs controls. Proinflammatory cytokines TNF-α, IL-12p40p70, and IL-12p70 had a 2.13-fold increase (P < .001), a 2.19-fold increase (P < .001), and a 1.22-fold increase (P = .001), respectively, in mesh explants vs controls. The proinflammatory chemokine CXCL10 and the proremodeling chemokine CCL17 were increased 3.38-fold (P = .016) and 7.26-fold (P < .001) relative to controls, respectively. Comparison of the ratio of the M2 proremodeling cytokines (IL-10+IL-4) with the M1 proinflammatory cytokines (TNF-α+IL-12p70) revealed a decrease in mesh explants as compared with controls (P = .003), which indicated a shift towards a proinflammatory profile.
TABLE 3.
Distribution of biomarkers by study group
| Biomarkers | Mesh complications (exposure + pain) (n = 27), mean ± SD |
Prolapse control (n = 30), mean ± SD |
P value |
|---|---|---|---|
| Cytokines & chemokinesa | |||
| Interleukin-4 | 3.316 ± 0.860 | 2.800 ± 0.567 | .011 |
| Interleukin-10 | 17.738 ± 9.957 | 12.218 ± 5.932 | .016 |
| Tumor necrosis factor-α | 15.963 ± 6.392 | 7.487 ± 2.224 | .000 |
| Interleukin-12p40p70 | 28.519 ± 16.437 | 13.029 ± 7.227 | .000 |
| Interleukin-12p70 | 2.070 ± 0.471 | 1.691 ± 0.291 | .001 |
| CXCL10 | 62.534 ± 71.835 | 18.492 ± 61.065 | .016 |
| CCL17 | 33.940 ± 38.156 | 4.674 ± 3.681 | .000 |
| Proteases | |||
| Pro–matrix metalloproteinase2b | 1.192 ± 0.851 | 1.324 ± 0.971 | .601 |
| Active matrix metalloproteinase2b | 1.521 ± 1.636 | 0.733 ± 0.783 | .038 |
| Pro–matrix metalloproteinase-9c | 3.496 ± 2.643 | 1.226 ± 1.715 | .000 |
| M2/M1 cytokines & chemokines | |||
| (Interleukin-4+interleukin-10)/ (tumor necrosis factor-α + interleukin-12p70) |
1.209 ± 0.562 | 1.633 ± 0.486 | .003 |
| CCL17/CXCL10 | 0.852 ± 0.921 | 0.661 ± 0.597 | .364 |
Picograms per 40 µg protein;
Arbitrary units;
Nanograms per 15µg total protein.
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
Pro- and active MMP-9 were 2.85-fold increased (P < .0001) and 2.91-fold increased (P < .0001), respectively, in mesh explants as compared with controls. Although pro–MMP-2 was similar in complexes with and without mesh, active MMP-2 was 2.08-fold increased (P = .038).
Pro–MMP-9 was 1.88-fold higher with exposure than pain (P = .036). No statistical difference was observed for active MMP-9 (P = .067). Values for individual cytokines and chemokines and for levels of pro- and active MMP2 did not differ based on indication for mesh removal (Table 4).
TABLE 4.
Comparison of the profiles of cytokines, chemokines, and matrix metalloproteinase-2 in vaginal tissue excised for mesh exposure and pain
| Biomarkers | Mesh, mean ± SD | P value | |
|---|---|---|---|
| Exposure | Pain | ||
| Cytokines & chemokinesa | |||
| Interleukin-4 | 3.307 ± 0.973 | 3.328 ± 0.736 | .952 |
| Interleukin-10 | 19.323 ± 11.665 | 15.757 ± 7.308 | .342 |
| Tumor necrosis factor-α | 15.954 ± 5.011 | 15.976 ± 8.039 | .994 |
| Interleukin-12p40p70 | 31.739 ± 20.242 | 24.493 ± 9.235 | .231 |
| Interleukin-12p70 | 2.040 ± 0.564 | 2.108 ± 0.342 | .705 |
| CXCL10 | 71.384 ± 86.873 | 51.472 ± 48.495 | .459 |
| CCL17 | 38.445 ± 49.238 | 28.312 ± 17.132 | .467 |
| Proteases | |||
| Pro–matrix metalloproteinase-2b | 0.965 ± 1.018 | 1.420 ± 0.606 | .200 |
| Active matrix metalloproteinase-2b | 1.278 ± 0.887 | 1.764 ± 2.164 | .483 |
| Pro–matrix metalloproteinase-9c | 4.860 ± 3.464 | 2.586 ± 1.660 | .036 |
| Active matrix metalloproteinase-9c | 6.063 ± 4.674 | 3.578 ± 1.338 | .067 |
| M2/M1 cytokines & chemokines | |||
| (Interleukin-4+interleukin-10)/ (tumor necrosis factor-α +interleukin-12p70) |
1.266 ± 0.640 | 1.138 ± 0.462 | .551 |
| CCL17/CXCL10 | 0.864 ± 1.165 | 0.837 ± 0.529 | .937 |
Picograms per 40 µg protein;
Arbitrary units;
Nanograms per 15 µg total protein
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
Immunofluorescent labeling
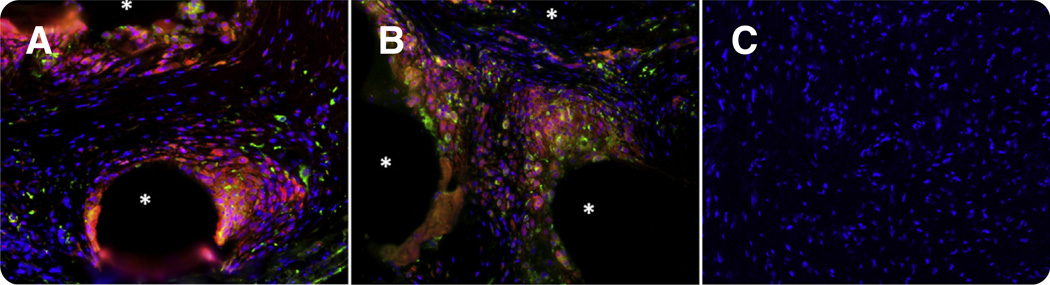
Mesh tissue complexes demonstrated a marked, but highly localized, foreign body response that was characterized by the presence of CD68+ cells (macrophages) that surrounded each mesh fiber. In areas where mesh fibers were in close proximity, the host response to neighboring fibers overlapped, which increased the magnitude of the response. Labeling with the M1-specific surface marker CD86 and the M2-specific marker CD206 demonstrated that control tissues contained few or no macrophages, as opposed to tissue-mesh complexes in which CD68+CD86+ proinflammatory M1 macrophages were concentrated around mesh fibers (Figure 1; Table 5). Mesh explants contained a higher number of total cells/ ×200 field when compared with controls (682.46 ± 142.61 cells vs 441.63 ± 126.13 cells; P < .001) and a lower ratio of M2:M1 macrophages (0.260 ± 0.161 cells vs 1.772 ± 1.919; P = .001), which supported an ongoing proinflammatory response.
FIGURE.
Immunofluorescent labeling of pan-macrophage marker CD68 (red), M1 proinflammatory marker CD86 (orange), M2 proremodeling macrophage marker CD206 (green), and 4’,6-diamidino-2-phenylindole (blue). A, A mesh-tissue section from a patient with an exposure who had been implanted with the AMS Perigee prolapse mesh (American Medical Systems, Minnetonka, MN) for 93 months; B, a mesh-tissue section from a patient with pain who had been implanted with the Gynecare TVT Secur (Ethion, Inc., Somerville, NJ) for 6 months; C, control tissue from patients without graft implantation. An asterisk indicates a predominance of proinflammatory M1 macrophages surround mesh fibers that is consistent with a prolonged immune response could be observed in both A and B; however, this response is limited to the area immediately adjacent to mesh fibers. Control tissue contained few or no macrophages as compared with mesh patient tissue. Original magnification ×200.
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
TABLE 5.
Macrophage phenotypes in vaginal tissue excised for mesh complications, compared with tissue derived from women with prolapse
| Variable | Mesh complications (n = 20), mean ± SD |
Prolapse (n = 24), mean ± SD |
Pvalue |
|---|---|---|---|
| Total nuclei | 682.46 ± 142.61 | 441.63 ±126.13 | .000 |
| M1 cell count | 197.95 ±97.15 | 0.889 ± 2.40 | .000 |
| % M1 cells | 28.06 ±11.03 | 0.181 ± 0.469 | .000 |
| M2 cell count | 41.90 ± 20.33 | 1.067 ± 2.034 | .000 |
| % M2 cells | 6.15 ± 2.80 | 0.313 ± 0.756 | .000 |
| M2/M1 ratio | 0.260 ± 0.161 | 1.772 ±1.919 | .001 |
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
In mesh explants, no differences in the number of macrophages that were present or the macrophage phenotype were observed when data were stratified based on indication for mesh removal (Table 6). However, when we compared areas that contained a mesh fiber vs a mesh knot in individual samples, the area of a mesh knot had a total cell density that was 1.23-fold increased (P = .037). Within the area of a mesh knot, the number of M1 macrophages was 2.00-fold increased (P = .003); the percentage of M1 macrophages (as a function of total cells) was 1.59-fold increased (P = .004); the number of M2 cells was 2.27-fold increased (P < .001), and the percentage of M2 macrophages was 1.77-fold increased (P = .002) as compared with a single mesh fiber (Table 7), which suggested that the host response was proportional to the amount of material in contact with the host. No significant difference was observed in the ratio of M2:M1 macrophages in the area of a mesh knot vs a mesh fiber. A positive correlation between IL-10 and the percentage of proremodeling/profibrotic M2 cells (r = 0.697; P = .037) in the pain group was observed that was not present in the exposure group. In the exposure group, a positive correlation was observed between the proinflammatory cytokine IL-12p40p70 and percentage of proinflammatory M1 macrophages (r = 0.584; P = .059), which did not reach statistical significance. This correlation was not observed in the pain group.
TABLE 6.
Comparison of macrophage phenotypic profile in vaginal tissue excised for mesh exposure and pain
| Variable | Exposure (n = 11), mean ± SD | Pain (n = 9), mean ± SD | P value |
|---|---|---|---|
| Total nuclei | 660.242 ± 130.892 | 709.615 ± 159.307 | .467 |
| M1 cell count | 167.008 ± 87.598 | 235.763 ± 99.497 | .124 |
| % M1 cells | 24.554 ± 10.669 | 32.344 ± 10.432 | .118 |
| M2 cell count | 40.296 ± 21.046 | 43.867 ± 20.494 | .706 |
| % M2 cells | 6.139 ± 3.157 | 6.164 ± 2.474 | .984 |
| M2/M1 ratio | 0.301 ± 0.206 | 0.210 ± 0.061 | .189 |
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
TABLE 7.
Comparison of macrophage phenotypic profile in areas of mesh knots and fibers
| Variable | Mesh knot (n = 13), mean ± SD |
Mesh fiber (n = 13), mean ± SD |
P value |
|---|---|---|---|
| Total nuclei | 659.87 ± 133.57 | 536.77 ± 168.30 | .037 |
| M1 cell count | 197.79 ± 97.02 | 98.79 ± 58.79 | .003 |
| % M1 cells | 28.89 ±11.63 | 18.13 ±9.88 | .004 |
| M2 cell count | 47.08 ± 20.20 | 20.72 ± 13.24 | <.0001 |
| % M2 cells | 7.17 ± 2.83 | 4.06 ± 2.45 | .002 |
| M2/M1 ratio | 0.303 ± 0.186 | 0.274 ±0.139 | .715 |
Nolfi et al. Vaginal macrophage profile in women with mesh complications. Am J Obstet Gynecol 2016.
Comment
A persistent foreign body response was observed in mesh-tissue complexes that were excised from women who required surgical excision of mesh months to years after mesh implantation. The host response was characterized by a predominance of macrophages with an increase in both proinflammatory and proremodeling cytokines/chemokines along with increased tissue degradation, as evidenced by increased MMP-2 and −9. Mesh-tissue complexes removed for mesh exposure had increased pro–MMP-9 that indicated a proinflammatory and tissue destruction–type response. In contrast, in mesh-tissue complexes that were removed for pain, the percentage of M2 macrophages (involved in tissue remodeling and fibrosis22–24) positively correlated with the amount of the antiinflammatory/profibrotic cytokine IL-10 consistent with tissue deposition and encapsulation.
The presence of macrophages, elevated cytokines, chemokines, and MMPs in tissue-mesh complexes that were excised from patients with exposure or pain suggests that polypropylene mesh elicits an ongoing host inflammatory response.7 Importantly, the presence of macrophages was limited to the area immediately surrounding the mesh fibers, with each fiber eliciting an independent reaction, the magnitude of which appeared to be proportional to the number of fibers in a given area. This points to the importance of maintaining meshes in a flat (as opposed to folded) configuration to minimize the amount of material per area and of choosing meshes in which the spaces between fibers (pores) are wide enough that the host response to 2 adjacent fibers does not overlap.25
In mesh-tissue complexes that were removed for exposure, pro–MMP-9 was increased compared with mesh that was removed for pain, which suggests a degenerative process. Studies of hernia meshes in animal models have shown that persistent inflammation with a prolonged release/activation of MMPs can lead to the degeneration of mesh implanted tissues and result in a deterioration in structural and mechanical integrity.26,27 In a nonhuman primate model, Gynemesh PS caused a decrease in key structural proteins (collagen and elastin) and increased MMP activity that led to thinning of the underlying and associated tissues and a deterioration of mechanical properties.20,28,29 Gynemesh PS has a highly unstable geometry when loaded that resulted in pore collapse and increasing stiffness of the product.30,31 Because virtually all meshes that are removed from women with complications have evidence of deformation and pore collapse,32–36 mesh exposure may represent a mechanical phenomenon in which altered mesh geometries result in increased mesh stiffness, which, in turn, leads to a degenerative response as a result of stress shielding and ongoing destructive inflammation.
Although the mechanism for the development of pain after mesh implantation is not clear, mesh deformation (contraction, retraction, or shrinkage) is observed frequently in meshes that are removed for pain.32,34,36–38 In normal wound healing, as inflammation resolves and remodeling begins, some amount of tissue contraction occurs with the formation of a scar.38 In the presence of a permanent foreign body, the implant is surrounded with a fibrotic capsule because it cannot be degraded. For hernia meshes, if the fibers are too close (<1 mm), the fibrotic response to neighboring fibers overlaps, or “bridges,” and results in “bridging fibrosis” or encapsulation of the mesh.25 Because myofibroblasts constitute the primary cellular component of the fibrotic capsule, when a mesh becomes encapsulated, the resulting contraction or “shrinkage” may place tension on adjacent tissues and result in pain. Indeed, mesh shrinkage (50–70%) has been described to occur after transvaginal insertion of prolapse meshes.32–37 In the present study, in meshes removed for pain, IL-10, which is a cytokine that, in increased amounts, has been associated with fibrosis,39–42 positively correlated with the percentage of M2 polarized macrophages (remodeling/fibrotic phenotype), which supports an ongoing remodeling/ fibrotic process that is involved in at least 1 mechanism that leads to pain.
A major limitation of the current study is that it was not possible to include a control group of mesh-tissue complexes that were obtained from women who underwent mesh implantation without a complication. As such, the current study does not assess the inflammatory response to prolapse mesh in women with a good outcome and focuses only on the inflammatory response in the setting of complications. Our strict inclusion criteria resulted in a limited sample size that limits the generalizability of the data, especially in regards to the impact of variables such as age, length of time of mesh implantation, and hormone use. All patients who were enrolled in the current study completed the Pelvic Pain Scale and a number of other validated questionnaires, the analysis of which is pending and will be the focus of a future article. Finally, it is our practice to remove as much mesh as possible in patients with a mesh complication; therefore, we cannot guarantee that the mesh that underwent analysis was at the exact site of the complication, regardless of whether it was removed for an exposure or pain.
In conclusion, the findings of the present study suggest that the 2 major mesh complications (exposure and pain) are associated with a marked proinflammatory response that persists years after mesh implantation. In addition, different mechanisms for mesh exposure and pain may be associated with differential macrophage activation. Future studies will focus on the specific risk factors that predispose to specific types of mesh complications.
Acknowledgments
Supported by National Institutes of Health awards R01 HD061811 (P.A.M) and K12HD043441 (B.N.B).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source had no involvement in the study design, collection of data, analysis of data, interpretation of data, writing of the report, or the decision to submit for publication.
Footnotes
P.A.M. and S.D.A. have cooperative research agreements with ACell, Columbia, MD; the other authors report no conflict of interest.
Presented at the American Urogynecologic Society (AUGS)/International Urogynecological Association (IUGA) Joint Scientific Meeting, Washington, DC, July 22-26, 2014, and the 36th Annual Meeting of American Urogynecologic Society PFD Week, Seattle, WA, October 13-17, 2015.
References
- 1.U.S. Food and Drug Administration. [Accessed May 24, 2016];UPDATE on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse: FDA Safety Communication. 2011 Jul 13; Available at: www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
- 2.Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429–435. doi: 10.1097/00042307-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Klinge U, Klosterhalfen B, Muller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999;165:665–673. doi: 10.1080/11024159950189726. [DOI] [PubMed] [Google Scholar]
- 4.Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12:63–69. doi: 10.1177/155335060501200109. [DOI] [PubMed] [Google Scholar]
- 5.Pierce LM, Rao A, Baumann SS, Glassberg JE, Kuehl TJ, Muir TW. Long-term histologic response to synthetic and biologic graft materials implanted in the vagina and abdomen of a rabbit model. Am J Obstet Gynecol. 2009;200:546.e1–546.e8. doi: 10.1016/j.ajog.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 7.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 10.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown BN, Badylak SF. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013;9:4948–4955. doi: 10.1016/j.actbio.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Gundra UM, Girgis NM, Ruckerl D, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123:e110–e122. doi: 10.1182/blood-2013-08-520619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: amacrophage-centered approach. Front Immunol. 2014;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown BN, Mani D, Nolfi AL, Liang R, Abramowitch S, Moalli PA. Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque. Am J Obstet Gynecol. 2015;213:668.e1–668.e10. doi: 10.1016/j.ajog.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill AJ, Unger CA, Solomon ER, Brainard JA, Barber MD. Histopathology of excised mid-urethral sling mesh. Int Urogynecol J. 2015;26:591–595. doi: 10.1007/s00192-014-2553-0. [DOI] [PubMed] [Google Scholar]
- 17.Zong W, Stein SE, Starcher B, Meyn LA, Moalli PA. Alteration of vaginal elastin metabolism in women with pelvic organ prolapse. Obstet Gynecol. 2010;115:953–961. doi: 10.1097/AOG.0b013e3181da7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:80–87. doi: 10.1007/s001920200020. [DOI] [PubMed] [Google Scholar]
- 19.Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106:953–963. doi: 10.1097/01.AOG.0000182584.15087.dd. [DOI] [PubMed] [Google Scholar]
- 20.Liang R, Zong W, Palcsey S, Abramowitch S, Moalli PA. Impact of prolapse meshes on the metabolism of vaginal extracellular matrix in rhesus macaque. Am J Obstet Gynecol. 2015;212:174.e1–174.e7. doi: 10.1016/j.ajog.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf MT, Dearth CL, Ranallo CA, et al. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials. 2014;35:6838–6849. doi: 10.1016/j.biomaterials.2014.04.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Bio. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venosa A, Malaviya R, Choi H, Gow AJ, Laskin JD, Laskin DL. Characterization of distinct macrophage subpopulations during nitrogen mustard-induced injury and fibrosis. Am J Respir Cell Mol Biol. 2016;54:439–446. doi: 10.1165/rcmb.2015-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW. Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res. 2012;176:423–429. doi: 10.1016/j.jss.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Jones JA, McNally AK, Chang DT, et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res. 2008;84:158–166. doi: 10.1002/jbm.a.31220. [DOI] [PubMed] [Google Scholar]
- 27.Klinge U, Junge K, Stumpf M, Ap AP, Klosterhalfen B. Functional and morphological evaluation of a low-weight, monofilament polypropylene mesh for hernia repair. J Biomed Mater Res. 2002;63:129–136. doi: 10.1002/jbm.10119. [DOI] [PubMed] [Google Scholar]
- 28.Feola A, Abramowitch S, Jallah Z, et al. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG. 2013;120:224–232. doi: 10.1111/1471-0528.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang R, Abramowitch S, Knight K, et al. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG. 2013;120:233–243. doi: 10.1111/1471-0528.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barone WR, Moalli PA, Abramowitch SD. Variable porosity of common prolapse mesh’s during uni-axial loading. Female Pelvic Med Reconstr Surg. 2013;19:S56–S57. [Google Scholar]
- 31.Otto J, Kaldenhoff E, Kirschner-Hermanns R, Muhl T, Klinge U. Elongation of textile pelvic floor implants under load is related to complete loss of effective porosity, thereby favoring incorporation in scar plates. J Biomed Mater Res. 2014;102:1079–1084. doi: 10.1002/jbm.a.34767. [DOI] [PubMed] [Google Scholar]
- 32.Caquant F, Collinet P, Debodinance P, et al. Safety of trans vaginal mesh procedure: retrospective study of 684 patients. J Obstet Gynaecol Res. 2008;34:449–456. doi: 10.1111/j.1447-0756.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 33.Rogowski A, Bienkowski P, Tosiak A, Jerzak M, Mierzejewski P, Baranowski W. Mesh retraction correlates with vaginal pain and over-active bladder symptoms after anterior vaginal mesh repair. Int Urogynecol J. 2013;24:2087–2092. doi: 10.1007/s00192-013-2131-x. [DOI] [PubMed] [Google Scholar]
- 34.Svabik K, Martan A, Masata J, El-Haddad R, Hubka P, Pavlikova M. Ultrasound appearances after mesh implantation: evidence of mesh contraction or folding? Int Urogynecol J. 2011;22:529–533. doi: 10.1007/s00192-010-1308-9. [DOI] [PubMed] [Google Scholar]
- 35.Tunn R, Picot A, Marschke J, Gauruder-Burmester A. Sonomorphological evaluation of polypropylene mesh implants after vaginal mesh repair in women with cystocele or rectocele. Ultrasound Obstet Gynecol. 2007;29:449–452. doi: 10.1002/uog.3962. [DOI] [PubMed] [Google Scholar]
- 36.Velemir L, Amblard J, Fatton B, Savary D, Jacquetin B. Transvaginal mesh repair of anterior and posterior vaginal wall prolapse: a clinical and ultrasonographic study. Ultrasound Obstet Gynecol. 2010;35:474–480. doi: 10.1002/uog.7485. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg VH, Steinberg M, Weiner Z, et al. Three-dimensional transperineal ultrasound for imaging mesh implants following sacrocolpopexy. Ultrasound Obstet Gynecol. 2014;43:459–465. doi: 10.1002/uog.13303. [DOI] [PubMed] [Google Scholar]
- 38.Marschke J, Hengst L, Schwertner-Tiepelmann N, Beilecke K, Tunn R. Transvaginal single-incision mesh reconstruction for recurrent or advanced anterior vaginal wall prolapse. Arch Gynecol Obstet. 2015;291:1081–1087. doi: 10.1007/s00404-014-3497-9. [DOI] [PubMed] [Google Scholar]
- 39.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 40.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 41.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 42.Sziksz E, Pap D, Lippai R, et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm. 2015;2015:764641. doi: 10.1155/2015/764641. [DOI] [PMC free article] [PubMed] [Google Scholar]