Abstract
Objective
To determine a) whether early electroencephalographic (EEG) background features were associated with survival and neurologic outcomes among children resuscitated from cardiac arrest and not treated with therapeutic hypothermia and b) if addition of EEG background to commonly used clinical criteria is more predictive of outcome than clinical criteria alone.
Design
Retrospective study.
Setting
Pediatric intensive care unit and Cardiac Intensive Care Units of a tertiary children’s hospital.
Patients
Patients resuscitated from in-hospital or out-of-hospital cardiac arrest who underwent clinically indicated EEG monitoring and were not treated with therapeutic hypothermia.
Interventions
None
Measurements and Main Results
One-hundred twenty-eight patients underwent EEG monitoring within one day of return of spontaneous circulation (ROSC). Background category was normal in 4 subjects (3%), slow-disorganized in 58 subjects (45%), discontinuous - burst-suppression in 24 subjects (19%) and attenuated-flat in 42 subjects (33%). Forty-six subjects (36%) had a reactive EEG. Twenty subjects (15%) had a seizure during EEG monitoring. Absence of reactivity (p<0.001) and seizures (p=0.04) were associated with worse EEG background category. After controlling for covariates, for each incrementally worse background score, the odds of death was 3.63 (95% CI: 2.18, 6.0, p<0.001) and the odds of unfavorable neurologic outcome was 4.38 (95% CI: 2.51, 7.17, p=0.001).
Conclusions
Worse EEG background early after resuscitation from both in-hospital and out-of-hospital cardiac arrest is associated with increased odds of death and unfavorable neurologic outcomes at hospital discharge. These EEG background patterns may be used in addition to clinical criteria to support prognostic decision making.
Keywords: EEG, Cardiac Arrest, Pediatric, Outcome, Seizure
Introduction
More than 10,000 children experience a cardiac arrest each year in the United States (1–4). Survival rates are 10–44%, and many survivors have unfavorable neurologic outcomes that impact quality of life (1–7). When children have initial return of spontaneous circulation (ROSC) post-cardiac arrest, families and clinicians desire useful prognostic information to guide appropriate care, yet neuroprognostication based on clinical findings in the first 24 hours is problematic. In addition, aggressive post-cardiac arrest supportive care and innovative interventions have been proposed to impact outcomes. Accurate stratification of severity of neurologic injury early after resuscitation is important for rigorous evaluation of post-cardiac arrest care interventions (8–13).
Continuous electroencephalography (cEEG) is recommended to monitor patients following cardiac arrest to identify non-convulsive seizures (14, 15). In heterogeneous cohorts of critically ill children, longer durations of electrographic seizure exposures are common and have been associated with worse outcomes (16–19). These data suggest that seizure identification and management might mitigate secondary brain injury and improve neurobehavioral outcomes. Furthermore, a number of small studies indicate EEG data may provide prognostic information which can help guide management decisions (20–28). Among neonates with hypoxic-ischemic brain injury, EEG data obtained using amplitude integrated EEG (aEEG) are associated with 18 month neurologic outcome and have been used to stratify severity of neurologic injury to guide treatment, but this type of brain injury severity stratification has not been evaluated in non-neonatal children (29, 30).
We had two aims in this study of consecutive children resuscitated from cardiac arrest managed without therapeutic hypothermia. First, we aimed to determine whether specific EEG features were predictive of short term outcome. We hypothesized that children with EEGs that were unreactive or abnormal (i.e., slow-disorganized, discontinuous-burst suppression, attenuated-flat) would have unfavorable short-term outcomes compared to those whose EEGs were reactive or normal. Second, we aimed to determine whether a prediction model based on commonly used clinical data by intensivists that also incorporated EEG data would have better predictive ability than a model using clinical data alone. We hypothesized that incorporation of EEG data would allow better prognostication.
Methods
We performed a retrospective study of infants and children treated in the Pediatric Intensive Care Unit (PICU) of a single tertiary care referral hospital between January 2010 and September 2013 who underwent clinically indicated cEEG after resuscitation from in-hospital cardiac arrest (IHCA) or out-of-hospital cardiac arrest (OHCA) and were not treated with therapeutic hypothermia. Our post cardiac arrest standard is to treat patient with controlled normothermia. This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board.
Consistent with recent guidelines (31) and consensus statements (14), clinical practice at our institution is to perform cEEG in all patients with encephalopathy following resuscitation from cardiac arrest to identify electrographic seizures. Encephalopathy post cardiac arrest was defined as any patient who was not at their neurologic baseline with our without continuous sedatives. Long-term monitoring was performed using a Grass-Telefactor video-EEG system. Twenty-one gold-over-silver scalp surface electrodes were positioned according to the international 10–20 system and affixed with collodion adhesive. EEG data were acquired on a portable bedside computer networked to the hospital’s EEG server. EEG monitoring is initiated urgently (24/7 coverage) with interpretation provided by the encephalography service and management by the PICU and Neurology Consultation services.
Clinical and cEEG data were collected by chart review using the institution’s electronic medical record system. Clinical data consisted of prospectively defined demographic variables, cardiac arrest characteristics, post-cardiac arrest care variables, EEG data and outcomes. EEG reports were reviewed by one electroencephalographer to convert the text reports into categorical data because large EEG files are not routinely stored after patient discharge. The initial EEG background within the first 12 hours of EEG initiation was categorized as normal, slow-disorganized, discontinuous-burst-suppression, or attenuated-flat. Because some clinical electroencephalographers use the terms “flat” on cEEG and attenuated interchangeably, we combined attenuated and flat into one category. Background EEG features of continuous, discontinuous, burst suppression and flat have previously been shown to have substantial agreement on a 30 minute EEG (32), but have not been evaluated on longer segments of continuous EEG monitoring. Reports were then reviewed for changes in background EEG features over the time the patient was monitored. Data were recorded as EEG background pattern change versus no change. If there was a change from the initial 12 hours of background to final documented background, then patients were classified as improved or worsened. The EEG was also scored regarding the presence or absence of reactivity during the first 12 hours during periods without seizures based on EEG response to bedside care or tactile stimulation. Finally, the entire EEG record was scored as no seizures, electrographic seizures, or electrographic status epilepticus.
Standard treatment post arrest in our ICU does not include the use of prophylactic anti-seizure medications. Both convulsive and non-convulsive seizures are treated with anti-seizure medications based on recommendations with our ICU neurology consult service, usually benzodiazepines, levetiracetam, phenytoin, and phenobarbital. Benzodiazepine infusions are often used to treat seizures or for sedation for post arrest care.
Outcomes were mortality and unfavorable neurologic outcome assessed at hospital discharge. Unfavorable neurologic outcome was defined as a Pediatric Cerebral Performance Category (PCPC) score of 3, 4, 5, 6 or a change from baseline ≥ 1 (9). The PCPC is a validated six-point scale categorizing degrees of functional impairment. PCPC categories are 1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma and vegetative state, and 6 = death (33). The pre-admission PCPC score was estimated based on information in the medical record provided by parents/guardians or prior medical visits included in the electronic medical record. The PCPC score at hospital discharge was determined by chart review.
Summary statistics are reported as medians and interquartile ranges (IQR) for continuous data and counts and proportions for categorical data. The association of each variable with mortality or neurologic outcome was examined using chi-square or Fisher’s exact tests for categorical variables and Wilcoxon’s rank-sum or Kruskal Wallis tests for continuous variables.
Background category was found to have a linear association with log odds of the outcome and therefore was evaluated as a continuous variable in the multivariable model. We present the Lowess curve in Figure 1. Chi-squared analysis and multivariable logistic regression were used to test the association between background category and outcomes. Variables with p<0.2 in univariable analysis were eligible for inclusion in the final logistic regression model. Doses of epinephrine and duration of CPR were a priori covariates included in the model because of their known associations with outcomes. If the introduction of a variable into the model did not change the association between background pattern and the outcome by at least 10%, then the variable was determined not to be a confounder and was removed from the model. Variables trialed in the model for death were seizure category, reactivity, benzodiazepine infusion, and initial rhythm. Variables trialed in the model for neurologic outcome were reactivity score, seizure category, arrest location, initial rhythm, and arrest cause. Epinephrine doses was collinear with duration of CPR and therefore only epinephrine doses was included in the model. Because only 43 patients had a documented serum lactate level within the first 6 hours of resuscitation, lactate level was not included in the regression model.
Figure 1.
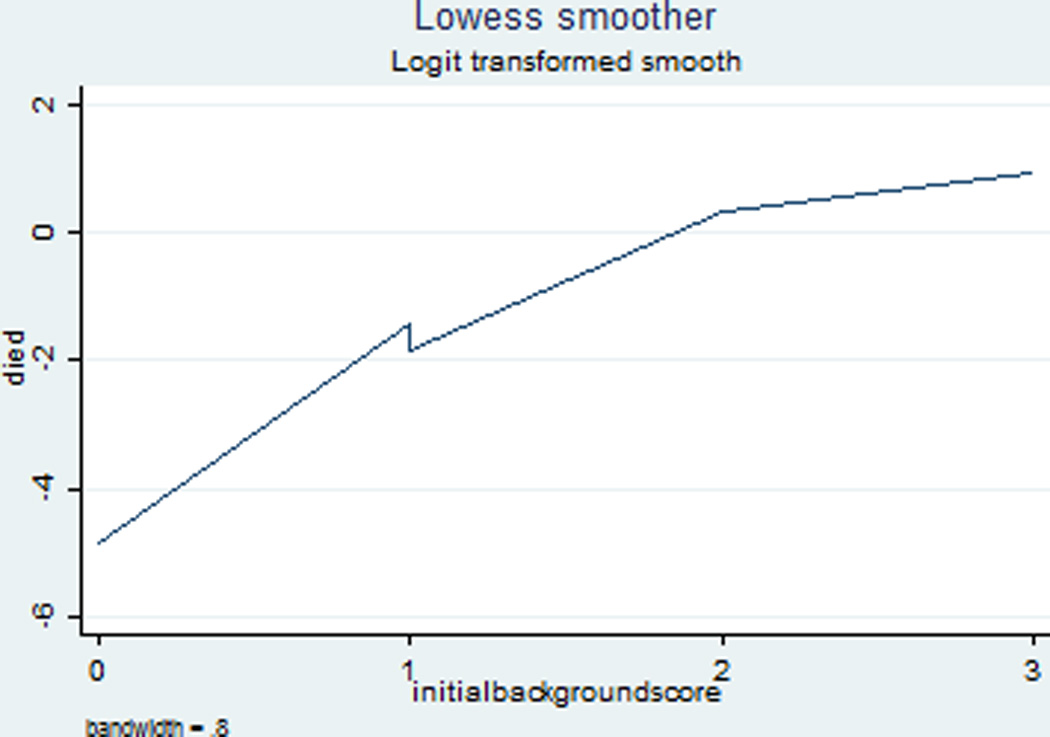
LOWESS curve showing the linear association between background category and log odds of mortality.
Finally, in a separate analysis, we performed a classification analysis to compare the c-statistics (area under the curve (AUC)) from the receiver operating characteristic curve (ROC) analyses to evaluate whether the prognostic impact of cardiac arrest variables commonly used by ICU clinicians (arrest location, number of doses of epinephrine administered, witnessed status and initial rhythm) for outcomes was improved with the addition of EEG background category. We report the c-statistic for each model as well as the comparison of the c-statistics for these models to determine if the c-statistics differed statistically between the models (34). These models are reported in a separate Table. All statistics were performed on Stata 13.0 (College Station, TX).
Results
One-hundred twenty-eight children were evaluated. Seventy eight patients (60%) had an IHCA. The median age was 2.6 [0.4, 10.7] years. Fifty-two (40%) subjects were male. The median PICU length of stay was 14 [5, 37] days and the median hospital length of stay was 20 [6, 49] days. Subject characteristics are provided in Table 1. Baseline PCPC scores were normal in 85 (66%), mild disability in 19 (15%), moderate disability in 7 (5%), severe disability in 6 (4%).
Table 1.
Clinical Characteristics by EEG background Category (N=128). Data presented as N (%) or median (IQR). CPR: cardiopulmonary resuscitation, EEG: electroencephalogram; IQR: interquartile range; PEA: pulseless electrical activity; SIDS: sudden infant death syndrome; VF: ventricular fibrillation; VT: ventricular tachycardia
| Variable | Total N= 128 |
EEG Background Category | ||||
|---|---|---|---|---|---|---|
| Normal N=4 |
Slow – Disorganized N=58 |
Discontinuous – Burst Suppression N=24 |
Attenuated N=42 |
p-value | ||
| Age (years) | 2.6 (0.4, 10.7) | 9 (2.7, 14.7) | 2.4 (0.7, 11.8) | 1 (0, 9.9) | 2.5 (0.3, 9.7) | 0.11 |
| Male | 52 (40) | 2 (50) | 25 (43) | 9 (37) | 16 (38) | 0.91 |
| Pre-existing Condition | 91 (70) | 2 (50) | 42 (72) | 18 (75) | 28 (67) | 0.66 |
| Arrest Location | ||||||
| In-Hospital | 78 (60) | 0 (0) | 43 (74) | 14 (58) | 21 (50) | 0.004 |
| Witnessed | 104 (82) | 2 (50) | 52 (91) | 19 (79) | 31 (74) | 0.03 |
| Bystander CPR (n=50) | 32 (26) | 4 (100) | 11 (19) | 6 (25) | 12 (28) | 0.30 |
| Duration of CPR (min) | 12 (5, 30) | 2.5 (1, 4) | 8.5 (4, 20) | 20 (6, 34) | 14 (6, 38) | 0.03 |
| Initial Rhythm | 0.002 | |||||
| PEA/Asystole | 55 (43) | 0 (0) | 19 (33) | 11 (46) | 25 (60) | |
| Bradycardia | 26 (20) | 0 (0) | 18 (31) | 4 (17) | 4 (9) | |
| VF/VT | 12 (9) | 0 (0) | 6 (10) | 5 (21) | 1 (2) | |
| Unknown | 35 (27) | 4 (100) | 15 (26) | 4 (17) | 12(29) | |
| Arrest Cause | 0.03 | |||||
| SIDS | 6 (5) | 0 (0) | 0 (0) | 0 (0) | 6 (14) | |
| Drowning | 8 (6) | 0 (0) | 5 (9) | 2 (8) | 1 (2) | |
| Shock | 58 (45) | 0 (0) | 29 (50) | 12 (50) | 16 (38) | |
| Respiratory Failure | 26 (20) | 1 (25) | 13 (22) | 5 (21) | 7 (16) | |
| Trauma | 16 (12) | 0 (0) | 6 (10) | 3 (13) | 7 (16) | |
| Other | 15 (12) | 3 (75) | 5 (9) | 2 (8) | 5 (12) | |
| Epinephrine Doses | 0.002 | |||||
| 0 | 21 (16) | 4(100) | 11 (19) | 1 (4) | 5 (12) | |
| 1 | 25 (20) | 0 (0) | 14 (24) | 6 (25) | 5 (12) | |
| 2 | 21 (16) | 0 (0) | 9 (16) | 7 (29) | 5 (12) | |
| >=3 | 58 (45) | 0 (0) | 21 (36) | 10 (42) | 27 (64) | |
| Unknown | 3 (2) | 0 (0) | 3 (5) | 0 (0) | 0 (0) | |
| Initial lactate (n=43) | 6.2 (3.7, 12.2) | 2.6 (1.7, 3.4) | 4.5 (2.5, 9.1) | 5.6 (4.6, 13.1) | 10.4 (5.0, 12.4) | 0.04 |
| Lowest pH first 6 hours after arrest |
7.25 (7.11, 7.35) | 7.35 (7.23, 7.4) | 7.26 (7.18, 7.37) | 7.32 (7.21,7.39) | 7.15 (7.01, 7.28) | 0.003 |
| Benzodiazepine infusion | 84 (66) | 2 (50) | 44 (76) | 12 (50) | 26 (62) | 0.09 |
| EEG Reactivity Present | 46 (36) | 4 (100) | 34 (59) | 7 (29) | 1 (2) | <0.001 |
| Electrographic Seizures | 0.04 | |||||
| None | 108 (84) | 4 (100) | 53 (91) | 16 (67) | 35 (83) | |
| Seizure(s) | 4 (3) | 0 (0) | 1 (2) | 3 (13) | 0 (0) | |
| Status Epilepticus | 16 (12) | 0 (0) | 4 (7) | 5 (20) | 7 (17) | |
All EEG recordings were initiated within one day of ROSC, and 65% were initiated on the same day as ROSC. The median cEEG duration was 2 [1, 3] days. EEG characteristics are provided in Table 1. The EEG background category was normal in 4 subjects (3%), slow-disorganized in 58 subjects (45%), discontinuous - burst-suppression in 24 subjects (19%) and attenuated-flat in 42 subjects (33%).
Twenty one patients (16%) had a change from their initial EEG background: 7 with an initial slow-disorganized, 11 discontinuous-burst-suppression and 2 attenuated-flat. All patients with a normal initial EEG background had no change. Of the 7 patients with an initial slow-disorganized background, 5 improved to normal; 4 survived with a favorable neurologic outcome and 1 survived with an unfavorable neurologic outcome. Two of the 7 patients had worsening of their background: 1 to a discontinuous - burst-suppression pattern and 1 to attenuated-flat; both survived with unfavorable neurologic outcome. Of the 11 patients who had an initial discontinuous - burst-suppression background, 7 improved to slow-disorganized; one died, 4 survived with a favorable neurologic outcome and 2 survived with an unfavorable neurologic outcome. Four of the eleven worsened, became attenuated- flat and all four died. Of the three patients who were initially attenuated-flat, all three improved to slow-disorganized; two died and one survived with an unfavorable neurologic outcome.
Forty-six subjects (36%) had a reactive EEG. One-hundred and eight subjects (84%) did not have an electrographic seizure while 20 subjects (16%) had a seizure. Of the 20 subjects with a seizure, 16 (80%) had status epilepticus. Subjects who seized were more likely to have a non-reactive EEG than those who did not seize: 2/20 (10%) vs 44/108 (41%), p =0.01.
During the study 17 patients received phenytoin, 27 received phenobarbital, 22 received levetiracetam, 1 received valproate and 1 received lacosamide. All 4 patients with seizures and 15/16 were with status epilepticus treated with at least one anti-seizure medication and 1 patient with seizures and 15/16 with status epilepticus were maintained on a benzodiazepine infusion. Eight patients had pre-existing epilepsy and 5 were treated with anti-seizure medications in the post arrest period. Only 1 of them had seizures during this time. Table 1 summarizes clinical characteristics by EEG background categories. Longer duration of CPR, unwitnessed arrests, OHCA location, cause of arrest, and administration of more doses of epinephrine were associated with a worse initial EEG background patterns. Subjects with worse EEG background patterns had higher initial lactate measurements and lower pH measurements within the first 6 hours of ROSC.
Similar univariable analyses were performed for clinical characteristics by EEG reactivity (Supplemental Table 2). A reactive EEG was associated with a shorter duration of CPR, less asystole/pulseless electrical activity (PEA) as a first documented rhythm, fewer administered epinephrine doses, cause of arrest, a lower post-ROSC lactate and higher post-ROSC pH. Seizures or status epilepticus were not associated with any arrest variable. Patients who were in status epilepticus received more benzodiazepine infusion.
Fifty-four patients (42%) died. Mortality was due to irreversible cessation of neurologic function (36%), withdrawal of technological support due to severe neurologic injury (26%), and withdrawal of technological support due to refractory respiratory or cardiovascular failure (20%). None of these patients had an active Do Not Attempt Resuscitation order at the time of the initial cardiac arrest. Of those subjects who survived, 55/75 (74%) had a favorable neurologic outcome. Five patients had a favorable outcome despite an initial attenuated-flat EEG background. Two of these five patients had severe neurologic dysfunction pre-arrest, three were treated with benzodiazepine infusions and three required CPR < 7 minutes. One had pre-existing head trauma and a brief bradycardic arrest. None had post arrest seizures.
Univariable analyses of clinical and EEG variables and outcomes (mortality and neurologic outcome) are shown in Table 3. Worse EEG background categories (p<0.001) and absence of EEG reactivity (p<0.001) were associated with mortality and unfavorable neurologic outcome. While seizure status was not associated with mortality, it was associated with unfavorable discharge neurologic outcome.
Table 3.
Clinical and EEG Characteristics and Outcome (n=128). Data presented as N (%) or median (IQR). CPR: cardiopulmonary resuscitation, EEG: electroencephalogram; IQR: interquartile range; PEA: pulseless electrical activity; SIDS: sudden infant death syndrome; VF: ventricular fibrillation; VT: ventricular tachycardia
| Variable | Survival Outcome | Neurologic Outcome | ||||
|---|---|---|---|---|---|---|
| Dead N=54 |
Alive N=74 |
P-value | Unfavorable N=73 |
Favorable N=55 |
p-value | |
| Age (years) | 2.4(0.3, 11) | 2.4 (0.5, 10.5) | 0.9 | 2.2 (0.3, 9.3) | 2.8 (0.5, 12.9) | 0.53 |
| Male | 24 (44) | 28 (37) | 0.47 | 29 (39) | 23 (40) | 0.86 |
| Pre-existing Condition | 39 (72) | 51 (69) | 0.7 | 51 (69) | 39 (71) | 1.00 |
| Arrest Location | 0.9 | 0.06 | ||||
| In-Hospital | 32 (59) | 46 (62) | 34 (47) | 16 (29) | ||
| Witnessed | 41 (77) | 63 (85) | 0.35 | 54 (75) | 50 (91) | 0.03 |
| Bystander CPR (n=50) | 12 (55) | 20 (71) | 0.25 | 20 (59) | 12 (75) | 0.35 |
| Duration of CPR (min) | 17.5 (6.5, 44) | 8 (4, 20) | 0.005 | 14 (6, 30) | 7.5 (4, 27) | 0.11 |
| Initial Rhythm | 0.01 | 0.02 | ||||
| PEA/Asystole | 32 (59) | 23 (31) | 39 (53) | 16 (29) | ||
| Bradycardia | 8 (15) | 18 (24) | 11 (15) | 15 (27) | ||
| VF/VT | 3 (6) | 9 (12) | 4 (5) | 8 (15) | ||
| Unknown | 11 (20) | 24 (33) | 19 (26) | 16 (29) | ||
| Epinephrine Doses | 0.02 | 0.035 | ||||
| 0 | 3 (6) | 18 (24) | 7 (10) | 14 (25) | ||
| 1 | 10 (19) | 15 (20) | 14 (19) | 11 (20) | ||
| 2 | 9 (17) | 12 (16) | 13 (18) | 8 (15) | ||
| ≥3 | 31 (57) | 27 (36) | 37 (51) | 21 (38) | ||
| Unknown | 1 (2) | 2 (3) | 2 (3) | 1 (2) | ||
| Arrest Cause | 0.23 | 0.17 | ||||
| SIDS | 5 (9) | 1 (1) | 6 (8) | 0 (0) | ||
| Drowning | 2 (4) | 6 (8) | 7 (10) | 1 (2) | ||
| Shock | 25 (46) | 32 (43) | 29 (39) | 28 (51) | ||
| Respiratory Failure | 10 (19) | 16 (21) | 13 (18) | 13 (23) | ||
| Trauma | 8 (15) | 8 (11) | 11 (15) | 5 (9) | ||
| Other | 4 (7) | 11 (15) | 7 10) | 8 (15) | ||
| Initial lactate (n=43) | 12 (7, 13.6) | 4.6 (2.8, 8.5) | <0.001 | 10(4.2, 13.3) | 4.9 (2.5, 9) | 0.02 |
| Lowest pH first 6 hours after arrest |
7.2 (7.02. 7.32) | 7.28 (7.2, 7.37) | 0.005 | 7.21 (7.1, 7.3) | 7.31 (7.21, 7.38) |
0.001 |
| Benzodiazepine infusion | 31 (57) | 53 (72) | 0.13 | 48 (66) | 36 (65) | 1.00 |
| EEG Background Category | <0.001 | <0.001 | ||||
| Normal | 0(0) | 4 (5) | 0(0) | 4 (7) | ||
| Slow-Disorganized | 10 (19) | 48 (65) | 19 (26) | 39 (71) | ||
| Discontinuous – Burst Suppression |
14 (26) | 10 (14) | 17 (23) | 7 (13) | ||
| Attenuated | 30 (56) | 12 (16) | 37 (51) | 5 (9) | ||
| EEG Reactivity Present | 10 (19) | 36 (49) | <0.001 | 17 (23) | 29 (53) | 0.001 |
| Electrographic Seizures | 0.12 | 0.008 | ||||
| None | 45 (83) | 63 (85) | 58 (80) | 50 (91) | ||
| Seizure(s) | 0 (0) | 4 (5) | 1 (1) | 3 (5) | ||
| Status Epilepticus | 9 (17) | 7 (10) | 14 (19) | 2 (4) | ||
After controlling for covariates, for each incrementally worse EEG background score, the odds of death was 3.1 (95% CI: 1.84, 5.22, p<0.001) and the odds of unfavorable neurologic outcome was 4.24 (95% CI: 2.51, 7.17, p=0.001) (Table 4).
Table 4.
Multivariable logistic regression for mortality and poor neurologic outcome by Decline in one EEG Background Category. All variables analyzed in the final model are represented in the table below. CI: confidence interval; CPR: cardiopulmonary resuscitation, EEG: electroencephalogram; OR: odds ratio
| Mortality | OR (95% CI) | p-value |
| Decline by One EEG Background Category |
3.63 (2.18, 6.0) | <0.001 |
| Doses of Epinephrine | ||
| 0 | Reference | |
| 1 | 4.81 (0.9, 25.9) | 0.07 |
| 2 | 4.36 (0.79, 23.9) | 0.09 |
| >=3 | 4.89 (1.07, 22.2) | 0.04 |
| Unknown | 8.6 (0.50, 148.7) | 0.14 |
| Unfavorable Neurologic Outcome | OR (95% CI) | p-value |
| Decline by One EEG Background Category |
4.38 (2.51, 7.17) | 0.001 |
| Doses of Epinephrine | ||
| 0 | Reference | |
| 1 | 2.36 (0.55, 10.1) | 0.25 |
| 2 | 2.43 (0.53, 11.1) | 0.25 |
| >=3 | 1.78 (0.47, 6.7) | 0.39 |
| Unknown | 8.2 (0.56, 119.2) | 0.12 |
Between IHCA and OHCA survivors, there was no difference in seizure prevalence: 10/78(13%) vs 10/50 (20%); p=0.3 or presence of a reactive EEG: 30/78 (38%) vs 16/59 (32%); p=0.57. Subjects who experienced an IHCA were more likely to have a slow and disorganized background category (55% vs 30%, p= 0.003). There was no difference in survival to discharge between subjects experiencing an IHCA versus an OHCA (59% vs 56%, p = 0.86); however, more IHCA survivors had a favorable neurologic outcome (50% vs 32%, p = 0.04).
We performed a separate classification analysis for mortality based on clinical variables used by clinicians to determine outcome specifically including IHCA vs. OHCA location, initial rhythm, number of epinephrine doses received and witnessed status. The c-statistic for death was 0.70. The full regression models are presented in Table 5. When we added EEG background category to the ROC model, the c-statistic for death significantly improved to 0.82 (p=0.004) (Figure 1). When examining neurologic outcome using the same variables, we found that the same model without EEG background score had an AUC of 0.73 and was improved with the addition of EEG background category to an AUC of 0.85 (p=0.004) (Figure 2).
Table 5.
Classification analysis of clinical cardiac arrest variables used by ICU clinicians to predict outcome compared to those variables with the addition of EEG background. Multivariable logistic regression models for mortality and unfavorable neurologic outcome with and without EEG Background Category including AUC for each model. All variables analyzed in the final model are represented in the table below. CI: confidence interval; CPR: cardiopulmonary resuscitation, EEG: electroencephalogram; OR: odds ratio
| Mortality | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Out of Hospital Arrest | 0.97 (0.34, 2.7) | 0.96 | 0.68 (0.2, 2.3) | 0.53 |
| Initial rhythm | ||||
| VF/VT | Reference | Reference | ||
| Bradycardia | 1.42 (0.28, 7.3) | 0.67 | 1.89 (0.31, 11.6) | 0.49 |
| PEA/Asystole | 3.3 (0.75, 14.1) | 0.12 | 2.7 (0.54, 13.3) | 0.23 |
| Unknown | 1.6 (0.33, 7.9) | 0.56 | 1.1 (0.19, 6.5) | 0.90 |
| Number of epinephrine doses |
||||
| 0 | Reference | Reference | ||
| 1 | 3.5 (0.73, 17.7) | 0.11 | 4.3 (0.73, 16) | 0.11 |
| 2 | 4.2 (0.87. 20.4) | 0.07 | 4.5 (0.73, 28.0) | 0.11 |
| >=3 | 5.5 (1.3, 23.3) | 0.02 | 3.9 (0.77, 19.7) | 0.1 |
| Witnessed | 0.52 (0.14, 1.9) | 0.31 | 0.61 (0.2, 2.3) | 0.5 |
| Decline by one EEG background category |
3.7 (2.1, 6.6) | <0.001 | ||
| AUC (c-statistic) | 0.70 | 0.82 | ||
|
Unfavorable Neurologic Outcome |
OR (95% CI) | p-value | OR (95% CI) | p-value |
| Out of Hospital Arrest | 2.0 (0.71, 5.9) | 0.19 | 2.0 (0.58, 7.4) | 0.27 |
| Initial rhythm | ||||
| VF/VT | Reference | Reference | ||
| Bradycardia | 2.4 (0.5, 11.7) | 0.8 | 4.1 (0.68, 25) | 0.13 |
| PEA/Asystole | 4.7 (1.1, 20.2) | 0.04 | 4.8 (0.9, 15.0) | 0.06 |
| Unknown | 2.7 (0.58, 12.3) | 0.2 | 3.1 (0.5, 18.6) | 0.22 |
| Number of epinephrine doses |
||||
| 0 | Reference | Reference | ||
| 1 | 3.7 (0.9, 15.4) | 0.07 | 3.9 (0.73, 20.5) | 0.11 |
| 2 | 3.9 (0.9, 16.1) | 0.05 | 3.0 (0.60, 15.5) | 0.18 |
| >=3 | 4.3 (1.2, 15.3) | 0.3 | 2.3 (0.52, 10.3) | 0.27 |
| Witnessed | 0.4 (0.09, 1.5) | 0.18 | 0.34 (0.06, 1.9) | 0.23 |
| Decline by one EEG background category |
4.3 (2.4, 7.6) | <0.001 | ||
| AUC (c-statistic) | 0.73 | 0.85 | ||
Figure 2.
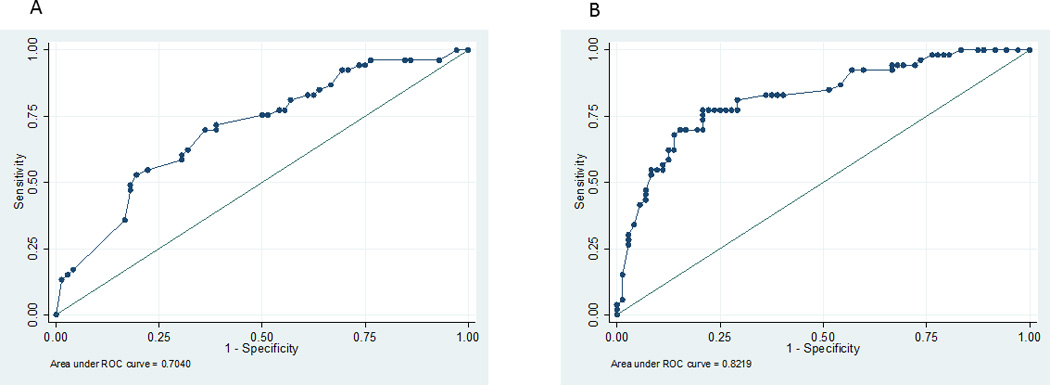
Receiver Operating Characteristic Curves for multivariable model association of mortality with clinical variables: arrest location, initial rhythm, number of epinephrine doses, witnessed status (1A) and the same model with the addition of early EEG background category (1B).
Discussion
This retrospective study of children resuscitated from cardiac arrest who underwent continuous EEG evaluation showed that worse EEG background categories are associated with progressively higher odds of death and unfavorable neurologic outcome at hospital discharge. Furthermore, addition of EEG background data to clinical arrest characteristics modestly improved the model’s ability to predict mortality and neurologic outcome. Interestingly, seizures were present in only 16% of these patients. Status epilepticus was associated with unfavorable neurologic outcome.
Importantly, no patient with a normal background had an unfavorable outcome, but 5 patients with an initial attenuated-flat background and 7 with an initial discontinuous- burst suppression background had a favorable outcome. Therefore, while worse EEG background was associated with worse outcomes, EEG background alone was not a reliable predictor of outcome.
Our study included a large cohort of consecutive patients with IHCA and OHCA not managed with therapeutic hypothermia who underwent continuous EEG monitoring. We evaluated EEG background patterns as progressively worse categories of severity (continuous, slow-disorganized, discontinuous – burst suppression, and attenuated-flat). The odds of death for patients increased with each progressively worse EEG background category. Thus, a subject with a slow-disorganized pattern had 3.1 times higher odds of mortality than a patient with a normal EEG pattern, whereas a patient with a discontinuous – burst suppression background pattern had a 9.6 higher odds of death than a patient with a normal background pattern.
Several cardiac arrest clinical characteristics were associated with worse EEG background categories. OHCA subjects had worse EEG background patterns such as discontinuous – burst-suppression and attenuation. This is consistent with OHCA patients who often have more unwitnessed arrests and longer no-flow times prior to the initiation of CPR which may lead to more severe brain injury. Worse EEG background patterns occurred in patients who received longer durations of CPR, received more intra-arrest doses of epinephrine, had higher rates of asystole and PEA, had higher serum lactate levels and had lower serum pH levels. These data support that these markers of longer no-flow and low-flow durations as well more severe end organ ischemic injury were associated with more severe neurologic injury.
Because clinician prognostication is often multifactorial, including cardiac arrest characteristics, clinical examination signs, neuroimaging, and EEG data, we performed a separate classification analysis evaluating prediction models using key arrest characteristics with outcome (including arrest location, initial rhythm, witnessed status and number of doses of epinephrine) with models that additively incorporated EEG data. For prediction of mortality there was a clinically modest statistically significant increase in the AUC from 0.70 (without EEG background category) to 0.82 (with EEG background category). For the prediction of unfavorable neurologic outcome there was also a clinically modest statistically significant increase in the AUC 0.73 (without EEG background category) to 0.85 (with background EEG category). These data indicate that incorporation of EEG data into prediction models may yield more accurate mortality and discharge neurologic outcome predictions.
The rate of electrographic seizures in our study was approximately 16% (20/129), and 16/20 subjects (80%) with seizures had electrographic status epilepticus. These data differ from our previous study evaluating a small cohort of children treated with a standardized therapeutic hypothermia protocol and continuous EEG monitoring (15). In that study, the rate of electrographic seizures was 47% (9/19) of which 67% (6/19) developed electrographic status epilepticus. It is unlikely that hypothermia caused these seizures, but more likely the lower prevalence in this current study reflects the more inclusive standardized cEEG monitoring program for all encephalopathic cardiac arrest patients, thus removing selection bias. Furthermore, it is possible that standardization and advances in post-resuscitation care may have decreased secondary neurologic insults and thus decreased the incidence of seizures. Importantly, electrographic status epilepticus occurred in the majority of seizing patients. Status epilepticus was associated with worse short-term neurologic outcome, but seizures and status epilepticus were not independently associated with mortality.
A previous study of 200 critically ill children managed in our PICU showed that both short-term and long-term outcomes were worse in the presence of electrographic status epilepticus but not electrographic seizures (16, 17, 35). A study by Payne et al indicated that increasing seizure burden was associated with worse short-term neurologic outcomes (19). It remains uncertain whether electrographic seizure identification and management improves outcomes, but based on the outcome data discussed above, recent consensus statements recommend EEG monitoring to identify and manage electrographic status epilepticus (14, 31).
In this study EEG reactivity was highly associated with EEG background category. Notably, there was a decrease in rates of EEG reactivity with worse EEG background categories. Furthermore, absence of reactivity was associated with both mortality and worse neurologic outcome. Similarly, lack of electrographic reactivity to stimulation predicts poor prognosis in adults treated with therapeutic hypothermia (36–39). Of note, in one of the largest series involving 111 adults treated with therapeutic hypothermia after cardiac arrest, an unreactive EEG background was a more sensitive predictor of neurologic recovery than clinical signs such as motor responsiveness (37) Furthermore, in adults treated with therapeutic hypothermia after cardiac arrest, bispectral index monitoring scores of zero (reflecting a low amplitude, featureless, unreactive EEG) predict unfavorable outcome or death (40–42). However, it is unclear whether this factor is best used alone as a predictor or combined with other EEG features such as voltage suppression, burst suppression patterns, or generalized epileptiform discharges in a composite “malignant EEG” categorization (36, 39).
We did not specifically evaluate the impact of evolving background EEG patterns over time on outcome. While our primary aim was to evaluate the predictive value of early background EEG, 21 patients did have a change in background over time. Of patients who had an initially normal background (n=4) or improvement to a normal background (n=5), all but 1 survived with a favorable neurologic outcome. Some patients with initial burst suppression who had improvement in their background to slow-disorganized had favorable outcomes, while patients who started off as attenuated-flat and improved all had unfavorable outcomes.
Following neonatal hypoxic ischemic encephalopathy, a normal EEG background at 6 hours was highly predictive of normal outcomes (43). However, many neonates had improvement in their background, therefore by 48 hours a normal EEG was less predictive of a normal outcome, while a persistent abnormal EEG background at 48 hours was highly predictive of unfavorable outcomes. Our cohort differs from this neonatal cohort in that mechanisms of injury are different and timing of injury is known, but the time to the first EEG background recording was variable. Interestingly, less than 20% of our patients had a change in background over time, a substantial difference when compared to the neonatal cohort in whom the background was much more likely to change over time. Our study was further limited by the retrospective methodology and therefore non-standardized timing of EEG, as well as the less robust neurological outcome assessments.
The EEG background scoring system we utilized is simple and contained only four categories. Though patients in this study underwent EEG monitoring, the EEG background categories used could be obtained in a routine EEG, making this method accessible to centers without the capacity for continuous EEG monitoring.
Previous studies suggest that clinical, laboratory, imaging, and neurophysiologic data may be useful prognostically, but none have perfect positive or negative predictive value (44). Among adults resuscitated from cardiac arrest, several EEG classification systems have been developed (36, 39, 45, 46) and an American Academy of Neurology report has concluded that diffuse voltage suppression under 20 microvolts, burst-suppression, and generalized periodic complexes are strongly but not invariably associated with poor outcome (47). Furthermore, neonatal data supports the use of EEG background patterns determined on aEEG within the first 6 hours after delivery to stratify severity of brain injury for both long term outcome assessment and management (30, 48, 49).
Fewer data are available in children (20, 21). In a small study of 35 children managed with therapeutic hypothermia after cardiac arrest, we demonstrated that those with backgrounds scored as unreactive, discontinuous-burst suppression, or lack of discernable cerebral activity were associated with unfavorable outcomes, both during hypothermia and after return to normothermia (20). However, in that study we evaluated only children managed with therapeutic hypothermia and did not account for clinical cardiac arrest characteristics in assessing outcome.
This study has several limitations. First, we measured only short-term outcome using mortality and a simple outcome assessment tool (PCPC), and these outcomes may not reflect long-term neurodevelopmental status. Studies utilizing longer-term and more detailed neurodevelopmental outcome assessments are needed. Second, all data were collected by chart review. The initial PCPCs were assessed retrospectively using data from medical records provided by the children's parents/guardians and discharge PCPCs were assigned by chart review using available information. EEG data were obtained by review of EEG reports and not by re-review of EEG tracings, potentially resulting in low inter-rater reliability due to a large number of EEG readers. The categories we used are broad and commonly applied by pediatric electroencephalographers, however, inter-rater reliability of these background categories and EEG reactivity derived from clinical reports is unknown. Despite the “noise” of inter-rater reliability issues inherent with this observational design, it is remarkable that progressively worse background EEG patterns were robustly associated with progressively worse outcomes. Third, we aimed to create a simple interpretation system using only EEG features available from a standard (i.e., 20–30 minute) EEG recording. However, this strategy may have led us to discount important EEG features that might have improved prediction. Finally, EEG results were known to the clinical teams providing care which may have influenced decisions to withdraw technological support based on their perceptions of the association between EEG background abnormalities and the high likelihood of a poor outcome. To minimize the impact of this problem, unfavorable outcome was defined as a PCPC of 3, 4, 5, 6 or a change in PCPC ≥ 1 and not only death (PCPC 6) so individual decisions to withdraw technological support would be less likely to influence outcome categorization. Whether a family chose to withdraw support or continue support of a child with severe neurologic injury, the child’s outcome would have been scored as unfavorable.
Conclusions
Worse EEG background, specifically slow-disorganized, discontinuous-burst suppression, and attenuated-flat, early after resuscitation from both IHCA and OHCA are associated with increased odds of death and unfavorable neurologic outcomes. These EEG background patterns may be used in addition to clinical criteria to support prognostic decision making and appropriate risk stratification.
Supplementary Material
Figure 3.
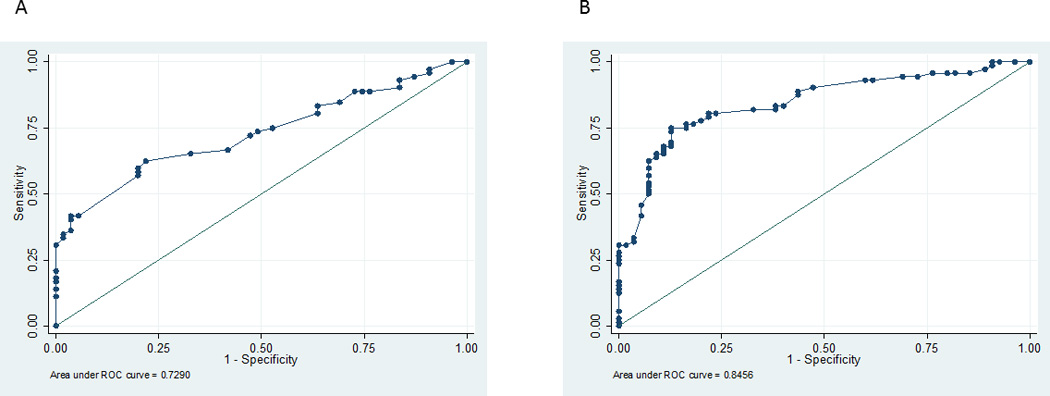
Receiver Operating Characteristic Curves for multivariable model association of unfavorable neurologic outcome with clinical variables: arrest location, initial rhythm, number of epinephrine doses, witnessed status (2A) and the same model with the addition of early EEG background category (2B).
Acknowledgments
Funding
Dr. Topjian is funded by NIH grants K23NS075363.
Dr. Abend is funded by NIH Grant K23NS076550.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from get with the guidelines-resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. Jama. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015 doi: 10.1007/s00134-015-3789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zellem L, Utens EM, Legerstee JS, et al. Cardiac Arrest in Children: Long-Term Health Status and Health-Related Quality of Life. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 8.Topjian AA, Lin R, Morris MC, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:479–490. doi: 10.1097/PCC.0b013e318198bdb5. [DOI] [PubMed] [Google Scholar]
- 9.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42:1518–1523. doi: 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topjian AA, Clark AE, Casper TC, et al. Early lactate elevations following resuscitation from pediatric cardiac arrest are associated with increased mortality*. Pediatr Crit Care Med. 2013;14:e380–e387. doi: 10.1097/PCC.0b013e3182976402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starling RM, Shekdar K, Licht D, et al. Early Head CT Findings Are Associated With Outcomes After Pediatric Out-of-Hospital Cardiac Arrest. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink EL, Berger RP, Clark RS, et al. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Crit Care Med. 2014;42:664–674. doi: 10.1097/01.ccm.0000435668.53188.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, Part I: Indications. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013;31:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81:383–391. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler S, Topjian AA, Guterrez-Colina AM, et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocritical Care. 2011;14:37–43. doi: 10.1007/s12028-010-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishisaki A, Sullivan J, 3rd, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med. 2007;8:10–17. doi: 10.1097/01.pcc.0000256621.63135.4b. [DOI] [PubMed] [Google Scholar]
- 22.Pampiglione G, Harden A. Resuscitation after cardiocirculatory arrest. Prognostic evaluation of early electroencephalographic findings. Lancet. 1968;1:1261–1265. doi: 10.1016/s0140-6736(68)92287-3. [DOI] [PubMed] [Google Scholar]
- 23.Tasker RC, Boyd S, Harden A, et al. Monitoring in non-traumatic coma. Part II: Electroencephalography. Arch Dis Child. 1988;63:895–899. doi: 10.1136/adc.63.8.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheliout-Heraut F, Sale-Franque F, Hubert P, et al. Cerebral anoxia in near-drowning of children. The prognostic value of EEG. Neurophysiol Clin. 1991;21:121–132. doi: 10.1016/s0987-7053(05)80066-8. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandrannair R, Sharma R, Weiss SK, et al. Reactive EEG patterns in pediatric coma. Pediatr Neurol. 2005;33:345–349. doi: 10.1016/j.pediatrneurol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Mandel R, Martinot A, Delepoulle F, et al. Prediction of outcome after hypoxic-ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr. 2002;141:45–50. doi: 10.1067/mpd.2002.125005. [DOI] [PubMed] [Google Scholar]
- 27.Pampiglione G, Chaloner J, Harden A, et al. Transitory ischemia/anoxia in young children and the prediction of quality of survival. Ann N Y Acad Sci. 1978;315:281–292. doi: 10.1111/j.1749-6632.1978.tb50346.x. [DOI] [PubMed] [Google Scholar]
- 28.Evans BM, Bartlett JR. Prediction of outcome in severe head injury based on recognition of sleep related activity in the polygraphic electroencephalogram. J Neurol Neurosurg Psychiatry. 1995;59:17–25. doi: 10.1136/jnnp.59.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toet MC, Hellstrom-Westas L, Groenendaal F, et al. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81:F19–F23. doi: 10.1136/fn.81.1.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 31.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 32.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol. 2011;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 34.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 35.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy & Behavior. doi: 10.1016/j.yebeh.2015.03.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roest A, van Bets B, Jorens PG, et al. The prognostic value of the EEG in postanoxic coma. Neurocrit Care. 2009;10:318–325. doi: 10.1007/s12028-008-9178-4. [DOI] [PubMed] [Google Scholar]
- 37.Rossetti AO, Oddo M, Logroscino G, et al. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 38.Rossetti AO, Oddo M, Liaudet L, et al. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72:744–749. doi: 10.1212/01.wnl.0000343006.60851.62. [DOI] [PubMed] [Google Scholar]
- 39.Thenayan EA, Savard M, Sharpe MD, et al. Electroencephalogram for prognosis after cardiac arrest. J Crit Care. 2010;25:300–304. doi: 10.1016/j.jcrc.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 40.Leary M, Fried DA, Gaieski DF, et al. Neurologic prognostication and bispectral index monitoring after resuscitation from cardiac arrest. Resuscitation. 2010;81:1133–1137. doi: 10.1016/j.resuscitation.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Stammet P, Werer C, Mertens L, et al. Bispectral index (BIS) helps predicting bad neurological outcome in comatose survivors after cardiac arrest and induced therapeutic hypothermia. Resuscitation. 2009;80:437–442. doi: 10.1016/j.resuscitation.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Seder DB, Fraser GL, Robbins T, et al. The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med. 2010;36:281–288. doi: 10.1007/s00134-009-1691-1. [DOI] [PubMed] [Google Scholar]
- 43.Murray DM, Boylan GB, Ryan CA, et al. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459–e467. doi: 10.1542/peds.2008-2190. [DOI] [PubMed] [Google Scholar]
- 44.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–39. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 45.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol. 1988;5:161–174. doi: 10.1097/00004691-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Young GB, McLachlan RS, Kreeft JH, et al. An electroencephalographic classification for coma. Can J Neurol Sci. 1997;24:320–325. doi: 10.1017/s0317167100032996. [DOI] [PubMed] [Google Scholar]
- 47.Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 48.al Naqeeb N, Edwards AD, Cowan FM, et al. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103:1263–1271. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- 49.Shalak LF, Laptook AR, Velaphi SC, et al. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111:351–357. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


