Abstract
Objective: The purpose of this study was to measure the number of repeat computed tomography (CT) scans performed across an established health information exchange (HIE) in New York City. The long-term objective is to build an HIE-based duplicate CT alerting system to reduce potentially avoidable duplicate CTs.
Methods: This retrospective cohort analysis was based on HIE CT study records performed between March 2009 and July 2012. The number of CTs performed, the total number of patients receiving CTs, and the hospital locations where CTs were performed for each unique patient were calculated. Using a previously described process established by one of the authors, hospital-specific proprietary CT codes were mapped to the Logical Observation Identifiers Names and Codes (LOINC®) standard terminology for inter-site comparison. The number of locations where there was a repeated CT performed with the same LOINC code was then calculated for each unique patient.
Results: There were 717 231 CTs performed on 349 321 patients. Of these patients, 339 821 had all of their imaging studies performed at a single location, accounting for 668 938 CTs. Of these, 9500 patients had 48 293 CTs performed at more than one location. Of these, 6284 patients had 24 978 CTs with the same LOINC code performed at multiple locations. The median time between studies with the same LOINC code was 232 days (range of 0 to 1227); however, 1327 were performed within 7 days and 5000 within 30 days.
Conclusions: A small proportion (3%) of our cohort had CTs performed at more than one location, however this represents a large number of scans (48 293). A noteworthy portion of these CTs (51.7%) shared the same LOINC code and may represent potentially avoidable studies, especially those done within a short time frame. This represents an addressable issue, and future HIE-based alerts could be utilized to reduce potentially avoidable CT scans.
Keywords: health information exchange, computed tomography, crossover, avoidable, electronic health records
Background
The advent of health information exchanges (HIEs) has facilitated the sharing of clinical data across different provider organizations in a way not previously possible. With increased use of electronic health records (EHRs), it is hoped that HIEs will enhance the American healthcare infrastructure, enabling physicians to access previously unavailable patient information to support clinical decision-making.
As patients often seek care across multiple institutions, their health records may become fragmented.1–3 Lack of access to previous records may lead to providers ordering repeat examinations, some of which may be potentially unnecessary. Interoperability through HIE is expected to reduce cost,4–10 aid in transitions of care,11 reduce hospitalizations,9,12,13 and improve patient safety and quality of care.4,5,9,14,15 One expected benefit of HIE is the reduction of repeat testing when patients seek care in multiple clinical settings.7,14,16 Some studies have already demonstrated evidence that HIEs may reduce unnecessary treatments.9,17,18 As such, the federal government has taken a vested interest in promoting EHRs and interoperability,14,19–21 with the Meaningful Use requirements promoting HIE adoption,22,23 and provider organizations demanding the integration of HIE.24,25
Investigations have shown that imaging studies are particularly costly, and comprise some of the most frequently performed diagnostic tests.26,27 There is additional concern for the increased utilization of CTs and the potential health effects of ionizing radiation associated with their use.28,29 While the health effects of ionizing radiation are dependent upon multiple factors,30 growth in computed tomography (CT) usage may be associated with an increased cumulative radiation exposure to patients.29 Higher cumulative doses of radiation from CTs have been associated with a risk of cancer in children.31 Predictive models have estimated risk to other patient groups,28,32 with even higher risks associated with patients who receive multiple CTs.33
Given the potential harms associated with CT scans, clinical decision support systems and computerized provider order entry alerts have already been utilized to reduce unnecessary imaging studies within single institutions.34–36 However, less is known about the potentially avoidable repeat imaging studies that occur across otherwise unaffiliated institutions in an HIE.
Although some repeat CTs may be ordered intentionally, others may be performed simply because prior results are unavailable or unidentified.37 As radiology reports are among the most commonly distributed results in established HIE systems,38,39 there is much speculation and some evidence that established HIEs could reduce repeat imaging.7,10,14 Reductions in cost, increased patient safety, and improved productivity from avoiding unnecessary repeat imaging are estimated to result in national savings in the many millions to possibly billions of dollars.6,14,40
Patient “crossover” occurs when patients visit more than one institution for care,1,41,42 and when repeat CTs are performed across two or more institutions; we refer to these as “crossover CTs.” Although the ultimate goal is to reduce potentially avoidable crossover CTs (CT scans which hold the potential to be unnecessary when performed a second time at a second location), there are still multiple obstacles to overcome before the full potential of HIE is reached, and a quantifiable reduction in these CTs is possible. Primarily, the magnitude of the problem, how many crossover CTs are performed on the same patient at different institutions in an HIE, is still unknown.
Objective
The purpose of this study was to demonstrate the existence of crossover CT scans within an HIE. We define crossover CT scans as those imaging studies performed on the same patient at two or more institutions. We hypothesize that some of these crossover CT scans are potentially avoidable, and that those crossover CTs that share the same Logical Observation Identifiers Names and Codes (LOINC) and were performed within short time frames are more likely to be avoidable. By demonstrating the existence of crossover CT scans in an HIE, we further define the scope of the problem, estimate national rates of crossover CTs, and hope to support future work toward an HIE-based duplicate CT alerting system to notify providers of previous studies at time of order-entry and reduce the number of potentially avoidable CT scans.
Methods
Setting
De-identified data were retrospectively examined from imaging studies performed between March 2009 and July 2012 and were provided by The New York Clinical Information Exchange (NYCLIX), an HIE that included hospitals in New York, NY. NYCLIX has since merged to form a larger HIE called Healthix.43,44 The HIE does not include data on dosimetry or indications for studies, and because both the HIE and the New York State Department of Health policies preclude the use of identifiable HIE data for research, and full reports of imaging results often contain protected health information, these were also not available.
These data were organized in a Structured Query Language (Server 2012) database and SAS® Version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for analysis.
Preparation of Data
Identifiers were removed or hashed in accordance with the aforementioned de-identification policy and duplicate records were removed from the database. We defined duplicate events as those with the same master patient index (MPI) ID, site ID, accession number, and exam date and time. The MPI ID is an HIE-level identifier that links unique patients across the entire HIE. The site ID identifies each unique location within the HIE. The accession number is an institution-level identifier that pertains to unique CTs that were performed at each location. A second stage of data cleaning was performed as some duplicate studies remained, due to different elements of the same CT sharing an accession number. The second stage of de- duplication was based on the unique proprietary name assigned by each institution to a specific type of CT, as well as the MPI ID, site ID, exam date, and exam time.
The final data set was limited to five hospital systems that participated in NYCLIX and performed CTs in Manhattan, and which had already been mapped in our previous work.45 These included four large academic tertiary care facilities and three small academic hospitals with residency programs. Inpatient discharges for these institutions ranged from 18 611 to 58 725 patients in 2012.46 All hospital systems participating in NYCLIX that were not primarily based in Manhattan were excluded.
Mapping of Exams
Imaging studies, which had originally been organized by each individual hospital’s Picture Archiving and Communication Systems, were mapped to the LOINC® Standard terminology. This universal coding system allows for electronic transmission and reporting of laboratory and clinical information across systems, and allowed us to measure CTs across locations.47 Mapping was done via a previously described process that had a 90% accuracy when mapping 99% of CTs performed.45 The process consisted of examining the names of each institution’s CT description and finding a corresponding LOINC code, in part by using the Regenstrief LOINC Mapping Assistant and in part by manual review of the LOINC code database.48–50 Given the variability between local CT names and corresponding LOINC codes, multiple codes were sometimes mapped in order to ensure each study was accounted for in the data set. Up to five LOINC codes were mapped to each local CT name, with the first being the most granular. The least granular code may correspond to the body region within which the study of interest took place. For instance: a study labeled “CT Sella turcica w/o Contrast” could be mapped to the first LOINC code 30591-2 “Pituitary and Sella turcica CT WO contrast.” However, the additional codes 36932-2 “Pituitary and Sella turcica CT” and 24725-4 “Head CT” were included as less granular codes. In some instances, studies could not be mapped to any existing LOINC code due to either ambiguity in the description of the study or from a lack of available appropriate LOINC codes and were excluded. A more detailed description of the mapping process for this dataset is described in Beitia, 2013.45
Framework
In designing our approach we adapted the conceptual framework published by the American College of Radiology’s Harvey L. Neiman Health Policy Institute, which defines different classes of repeat imaging into four categories.51
For the purposes of our adaptation of the American College of Radiology’s framework we focused solely on CT imaging. Follow-up CTs were defined as any repeat CT acquired for monitoring of interval change in acute (e.g., head trauma) or chronic conditions (eg, lung mass). Unrelated CTs are any CT that is acquired for a new condition (eg, in a patient who has a CT of the abdomen and pelvis with contrast to rule out appendicitis, and then several weeks later falls off of a ladder and gets the same CT done to assess for acute trauma). Supplementary CTs include any repeat CT that is performed to gain more information for a given condition (eg, a non-contrast head CT to rule-out hemorrhagic stroke, and then a contrast head CT to assess an intracranial mass not suspected before the first CT). Finally, some portion of repeat CTs are duplicate CTs, which are studies performed because prior results were not immediately available, or because the ordering provider was not aware that prior imaging existed.
Although all forms of repeat CTs can be classified into the above four categories, our dataset lacked the clinical detail (ie, indication) necessary to categorize them. Nonetheless, this framework provides a useful conceptual model to our studies findings. We presume that while many of these scans were follow-up, unrelated or supplementary, some proportions are likely potentially avoidable duplicate CTs. We hypothesize that those crossover CTs sharing the same LOINC code are more likely to represent duplicate CT scans and therefore are more likely to be potentially avoidable studies.
The study protocol was deemed “not human research” by the Mount Sinai Institutional Review Board and therefore did not require formal review and approval.
Analysis
The number of CTs performed at each of the five locations was quantified from the dataset. The total number of patients who received CTs was calculated, as was the total number of locations where any kind of CT was performed for each unique patient. Finally, the total number of locations where there was a CT performed with the same LOINC code was calculated for each unique patient. As discussed in the Methods section under mapping process, location-specific CT scans were mapped to up to five LOINC codes. When CTs across locations had at least one of these five LOINC codes in common (defaulting to the most granular), they were considered a match, and defined as sharing the same LOINC code.
The results were then analyzed to measure the number of studies performed on (1) all patients with at least one CT performed (“all CT patients”), and the subsets of patients who (2) had CTs at only one location (“single location CT patients”) or (3) had CTs at multiple locations (“total CT crossover patients”), and (4) the subset of total CT crossover patients who had at least one CT performed with the same LOINC codes at more than one location (“CT crossover patients with the same LOINC code”).
Similarly, the total number of CT scans performed was quantified for all CT patients (“all CTs”), single-location CT patients (“single-location CTs”), total CT crossover patients (“total crossover CTs”), and for the subset of total CT crossover patients with the same LOINC code (“crossover CTs with the same LOINC code”).
Descriptive statistics including total number of patients, total number of CTs, mean number of CTs per patient, and total number of visits where a CT was performed were calculated. Each of these values was totaled for all patients, single location patients, total CT crossover patients, and CT crossover patients with the same LOINC code.
Given the goals of the study, no comparison was made between single–location and multi-location repeat exams, as our interest was specific to patients that visited multiple locations within the HIE.
To identify the greatest number of times a crossover CT with the same LOINC code had been performed on a CT crossover patient with the same LOINC code, we quantified the number independent locations (up to the maximum of the five) that an individual patient had visited and received the same CT.
To study the trends in transition of care between locations, a correlation matrix was developed for those crossover CTs with the same LOINC code performed on CT crossover patients with the same LOINC code. When a crossover CT with the same LOINC code was identified, the pair of locations at which those exams were performed was tallied in the matrix. For example, when a CT was performed at location 1 and then again at location 2, a single count was added to the first cell of the matrix. Sums of each column represent all crossover CTs with the same LOINC code performed at least once at that location.
To examine the most commonly performed studies in out dataset, frequencies of the LOINC codes associated with the most granular matches of our total number of crossover CTs with the same LOINC code were calculated.
Finally, descriptive statistics were calculated for the time between studies for all crossover CTs with the same LOINC code and the patients on which they were performed using intervals of 3, 7, 30, 60, and 90 days.
Results were organized in Microsoft Excel 2011 for review (Microsoft, Redmond, WA, USA).
Results
The analysis yielded 719 520 CTs performed on 350 156 patients. A very small percentage of CT’s could not be mapped due to lack of appropriate LOINC code, representing 2289 (0.31% of 719 520) scans performed on 835 (0.23% of 350 156) patients. This left 717 231 (99.7% of 719 520) CTs performed on 349 321 (99.8% of 350 156) patients.
Of the 349 321 patients included in the study, 339 821 patients (97.3% of 349 321) had all of their imaging studies performed at a single location, accounting for 668 938 CTs (93.3% of 717 231) over the course of 359 311 hospital visits. The mean number of CTs per single location patient was 1.97 (SD = 2.01) over this 41-month period.
The remaining 9500 patients (2.7% of 349 321) had CTs performed at more than one location. These patients accounted for 48 293 total crossover CTs (6.7% of all 717 231 CTs) over the course of 19 490 hospital visits. The mean number of CTs per total CT crossover patient was 5.08 (SD = 4.01).
Of these 9500 total CT crossover patients, there were 6284 CT crossover patients with the same LOINC code (66.1% of 9500) who received 24 978 crossover CTs with the same LOINC code, representing 51.7% (24 978 of 48 291) of total crossover CTs. These CTs were performed over the course of 12 896 hospital visits, with a mean 3.18 (SD = 2.29) CTs per patient.
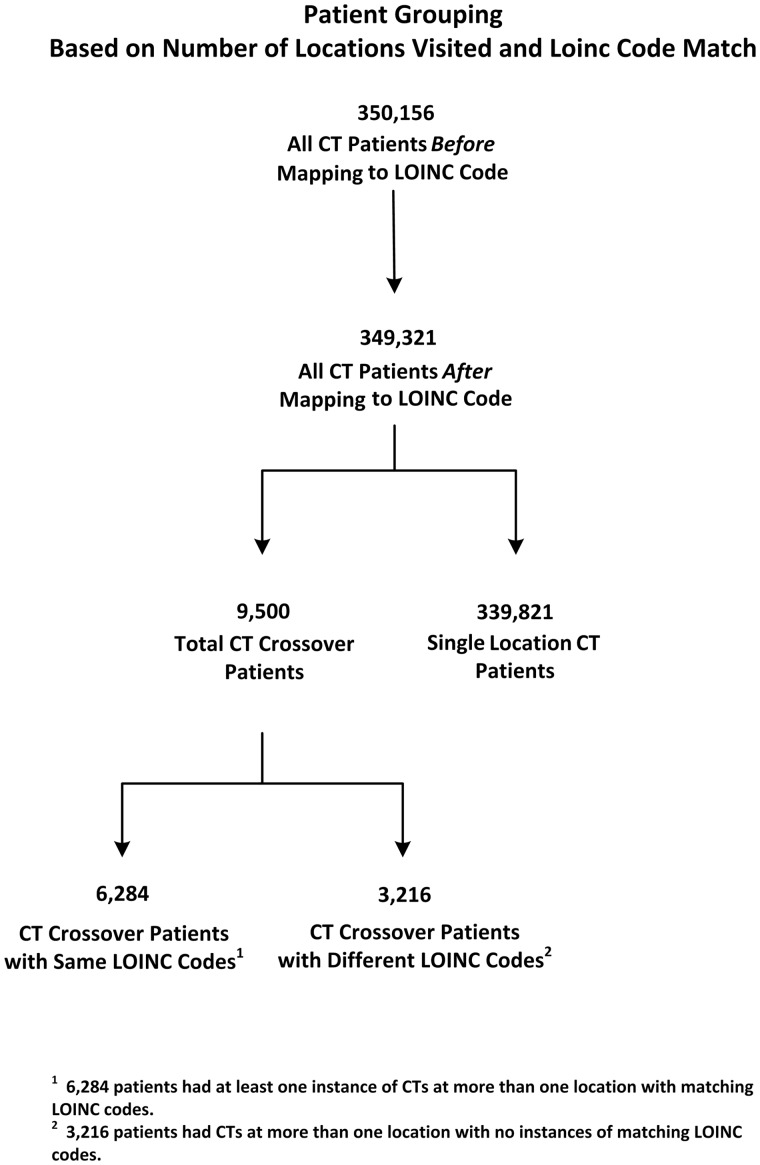
A flow chart demonstrating patient grouping can be found in Figure 1. Descriptive statistics for all groups can be found in Table 1.
Figure 1.
Flow diagram representing the total number of patients before and after the mapping process as well as the number of patients in each subgroup including (1) Single Location CT patients, (2) Total CT crossover patients, and the (3) subgroups of CT crossover patients with the Same and Different LOINC codes.
Table 1.
The Number of Patients and Respective Number of CTs and Visits When a CT was Performed for (1) all CT patients, (2) single-location CT patients, (3) total CT crossover patients, and (4) CT crossover patients with the same LOINC code
| Patient Group | Total Pts | Total CTs | Mean CTs per Pt (SD) |
|---|---|---|---|
| All Patients | 349 321 | 717 231 | 2.05 (2.15) |
| Single Location CT Patients | 339 821 | 668 938 | 1.97 (2.01) |
| Total CT Crossover Patients | 9500 | 48 293 | 5.08 (4.01) |
| CT Crossover Patients with the same LOINC code | 6284 | 24 978 | 3.97 (3.35) |
Of the 6284 CT crossover patients with the same LOINC code, there were 6006 (95.6%) patients that had the same CT performed at two locations that participated in the HIE. Of the remaining 278 (4.4% of 6284) patients, 236 (3.8%), and 34 (0.54%) had the same CTs performed at three and four locations, respectively, while 8 (0.13%) CT crossover patient with the same LOINC code had the same CT performed at all five locations.
Seventy-four LOINC codes accounted for the 24 978 crossover CTs with the same LOINC code. The most commonly performed CT was LOINC code 30799-1 (Head CT WO contrast) with 11 856 (47.5% of the 24 978) exams. All LOINC codes that correspond to crossover CTs with the same LOINC code and their frequencies can be found in Table 2.
Table 2.
The Descriptions, Codes, and Frequencies of the 74 LOINC codes That Made Up the 24 978 Crossover CTs With the Same LOINC Code
| CT description | LOINC | Frequency | CT description | LOINC | Frequency |
|---|---|---|---|---|---|
| Head CT WO contrast | 30799-1 | 11 856 | Facial bones and Sinuses Narrative CT | 24696-7 | 10 |
| Chest CT | 24627-2 | 2902 | Spine Thoracic CT | 24978-9 | 10 |
| Abdomen and Pelvis CT W contrast IV | 36813-4 | 2442 | Neck CT WO contrast | 36514-8 | 10 |
| Abdomen and Pelvis CT | 44115-4 | 1788 | Lower extremity - bilateral CT W contrast IV | 50755-8 | 10 |
| Chest CT WO contrast | 29252-4 | 1579 | Head CT W and WO contrast IV | 24726-2 | 9 |
| Abdomen and Pelvis CT WO contrast | 36952-0 | 1043 | Upper extremity Narrative CT | 35981-0 | 9 |
| Chest CT W contrast IV | 24628-0 | 776 | Narrative PET whole body | 44139-4 | 9 |
| Head CT | 24725-4 | 690 | Heart CT | 58744-4 | 7 |
| Spine Cervical CT WO contrast | 30592-0 | 403 | Spine Lumbar CT W contrast IV | 24964-9 | 6 |
| Abdomen CT | 41806-1 | 203 | Abdomen CT WO contrast | 36424-0 | 6 |
| Chest vessels CT angiogram W and WO contrast IV | 30804-9 | 191 | Lower extremity – left CT WO contrast | 36452-1 | 6 |
| Spine Lumbar CT WO contrast | 30620-9 | 106 | Neck CT W and WO contrast IV | 30586-2 | 5 |
| Neck CT W contrast IV | 36235-0 | 79 | Temporal bone CT WO contrast | 36866-2 | 5 |
| Lower extremity CT | 35971-1 | 72 | Unspecified body region CT | 25045-6 | 4 |
| Abdomen and Pelvis CT W and WO contrast IV | 42274-1 | 72 | Sinuses CT | 30588-8 | 4 |
| Neck CT | 36051-1 | 62 | Lower extremity vessels CT angiogram W and WO contrast IV | 30807-2 | 4 |
| Chest vessels Narrative CT angiogram W contrast IV | 36266-5 | 52 | Lower extremity – bilateral CT | 35973-7 | 4 |
| Pelvis CT W contrast IV | 24866-6 | 47 | Lower extremity – left CT W contrast IV | 36164-2 | 4 |
| Pelvis CT | 24865-8 | 40 | Lower extremity – right CT WO contrast | 36454-7 | 4 |
| Spine Lumbar CT | 24963-1 | 39 | Lower extremity Narrative CT WO contrast | 30625-8 | 3 |
| Spine Thoracic CT WO contrast | 30597-9 | 37 | Pulmonary artery Narrative CT angiogram W contrast IV | 36147-7 | 3 |
| Abdomen CT W and WO contrast IV | 36267-3 | 37 | Lower extremity – right CT W contrast IV | 36166-7 | 3 |
| Head vessels CT angiogram W and WO contrast IV | 30593-8 | 28 | Upper extremity – right CT W contrast IV | 36170-9 | 3 |
| Pelvis CT WO contrast | 30615-9 | 24 | Temporal bone CT | 36773-0 | 3 |
| Head to thigh Narrative PET | 58741-0 | 24 | Heart CT for scoring | 36934-8 | 3 |
| Abdominal vessels CT angiogram W and WO contrast IV | 30805-6 | 23 | Head CT W contrast IV | 24727-0 | 2 |
| Chest CT W and WO contrast IV | 30598-7 | 22 | Spine Cervical CT W contrast IV | 24933-4 | 2 |
| Facial bones and Maxilla CT WO contrast | 30802-3 | 21 | Spine Thoracic CT W contrast IV | 24979-7 | 2 |
| CT Guidance for biopsy of Lung | 24823-7 | 20 | Lower extremity Narrative CT W contrast IV | 30624-1 | 2 |
| Spine Cervical CT | 24932-6 | 20 | Maxilla CT | 36050-3 | 2 |
| Sinuses CT WO contrast | 36529-6 | 20 | Spine Lumbar CT W and WO contrast IV | 36402-6 | 2 |
| Narrative CT whole body | 46305-9 | 20 | Upper extremity – left CT WO contrast | 36457-0 | 2 |
| Abdomen CT W contrast | 30599-5 | 17 | Upper extremity – right CT WO contrast | 36458-8 | 2 |
| Neck vessels CT angiogram W and WO contrast IV | 30594-6 | 14 | Mandible CT WO contrast | 36512-2 | 2 |
| Chest CT high resolution WO contrast | 37441-3 | 14 | Head vessels and Neck vessels Narrative CT angiogram W contrast IV | 37498-3 | 2 |
| Facial bones and Maxilla CT | 41808-7 | 14 | Chest and Abdomen CT W and WO contrast IV | 42277-4 | 2 |
| Pelvis vessels CT angiogram W and WO contrast IV | 30623-3 | 13 | Orbit CT WO contrast | 46331-5 | 2 |
| Total | 24 978 | ||||
For the 6284 crossover CT patients with the same LOINC code, there was a mean time of 312.46 days (SD = 279.94 days) and a median of 232 days (range: 0–1227) between the initial CT and the second CT performed on patients at another location. When restricted to a 3-day period, there were 241 patients who had the same CT performed at two or more locations, with a mean of 2.29 (SD = 0.76) CTs and a mean time between studies of 1.8 days (SD = 0.99 days). Further analysis of seven, 30 60 and 90-day intervals, as well as the entire study period are demonstrated in Table 3.
Table 3.
CT Crossover Patients With the Same LOINC Code With Descriptive Statistics for Time Between CTs Performed Within 3, 7, 30, 60, 90, and >90 Days
| Time, days | No. of Pts | No. of CTs | Mean CTs per Pt (SD) | Range CTs (median) | Mean no. of days (SD) | Range days (median) |
|---|---|---|---|---|---|---|
| 3 | 241 | 553 | 2.29 (0.76) | 2–7 (2) | 1.8 (0.99) | 0–3 (2) |
| 7 | 510 | 1327 | 2.6 (1.34) | 2–11 (2) | 3.97 (2.13) | 0–7 (4) |
| 30 | 1344 | 5000 | 3.72 (3.29) | 2–25 (2) | 14.33 (8.42) | 0–30 (14) |
| 60 | 1981 | 8136 | 4.11 (4.0) | 2–40 (2) | 25.96 (16.91) | 0–60 (23) |
| 90 | 2400 | 10 400 | 4.33 (4.46) | 2–49 (2) | 36.63 (25.5) | 0–90 (32) |
| >90 | 6284 | 24 978 | 3.97 (3.35) | 2–59 (3) | 312.46 (279.94) | 0–1227 (232) |
Values are cumulative and include prior time intervals.
The correlation matrix in Table 4 demonstrates all crossover CTs with the same LOINC code. Each location is depicted as a row or column, and the cell demonstrates the number of CTs shared between each pair of locations. For example: 6712 CTs were performed that share the same LOINC code at both locations 1 and 2, while 223 crossover CTs with the same LOINC code were performed at both locations 4 and 5.
Table 4.
Correlation Matrix Demonstrating the Crossover of the Number of Crossover CTs With the Same LOINC Code at Each Pair of Institutions
| Location | |||||
|---|---|---|---|---|---|
| 1 | 1 | ||||
| 2 | 6712 | 2 | |||
| 3 | 4467 | 3882 | 3 | ||
| 4 | 2305 | 1327 | 1215 | 4 | |
| 5 | 2552 | 879 | 1416 | 223 | Total |
| Total | 16 036 | 6088 | 2631 | 223 | 24 978 |
Each tally represents a CT performed at the two sites represented in the row and column. The totals of each column represent the number of crossover CTs with the same LOINC code performed at least once at that location.
Discussion
Approximately 3% of our study population had CT scans performed at more than one location, which accounted for 48 293 total crossover CTs. Two-thirds (66.2%) of these total CT crossover patients had a repeat CT with the same LOINC code performed at two or more locations resulting in 24 978 Crossover CTs with the Same LOINC code (3.5% of all CTs). It is likely that given the number of crossover CTs performed, some of these repeat studies may have been potentially avoidable duplicates.
The vast majority of our study population (93%) had CTs performed only at a single location; however, patients who underwent imaging at two or more locations (CT crossover patients) received 2.6 more CTs on average compared with individuals being imaged at only one location. We discovered that crossover CTs with the Same LOINC code represent only 3.5% of the total number of CTs performed in our dataset, yet they represent a large absolute number of imaging studies (24 978) and 52% of the crossover CTs performed.
In the United States during 2012 there were approximately 85 million CT scans performed.52,53 Although our sample likely over- represents large academic medical centers when compared to CT scans ordered nationwide, if we extrapolate our findings, nearly six million of these CTs have the potential to be crossover studies and nearly three million could be the exact same study based on a LOINC code match. A 2011 study found the average cost of a head CT in the United States to be $510, while an abdominal CT was $584 and a pelvis was $522.54 If even only a small percentage of these three million presumed duplicate CT scans performed annually could be avoided with an HIE-based alerting system, the savings could be substantial.
Because our study only included five institutions in the New York metropolitan area, and the HIE has now grown to over 50 institutions, we expect that our current approach underestimates the true number of crossover and potentially avoidable duplicate CT scans. Additionally, we defined same CT scans across two or more locations as those that shared at least one common LOINC code, and in the mapping process each location-specific proprietary CT code was mapped to as many as five LOINC codes with varying levels of granularity. It is possible that CTs performed on the same anatomical region but on two separate areas of interest (ie, CT Liver and CT Kidneys) did not map to a common LOINC code as part of the mapping process, but still represent potentially avoidable duplicate studies given the appropriate clinical scenario.
The median time between CTs in our study was 232 days, which suggests that the majority of studies were clinically relevant and independently warranted, based on the considerable time between CTs. However, there were a noteworthy number of scans performed within a shorter time frame, which we believe to be more likely to represent avoidable CTs. For example, 510 CT crossover patients with the same LOINC code had a total of 1327 crossover CTs with the same LOINC code, with a mean of 2.6 CTs per patient performed at different locations within 7 days. Given the short interval of time between exams, it is possible that some of these CTs could be avoidable duplicates. Further investigation might explore determination of the optimal amount of time between repeat studies where the second or subsequent exam is likely to be potentially avoidable.
Additionally, we discovered that the vast majority (47.5%) of crossover CTs with the same LOINC code are non-contrast studies of the head. While we did not explore if this frequency is proportional to the entire cohort, it does suggest that there are specific CTs that may comprise of a disproportionate number of potentially avoidable duplicate studies. Future research could determine which specific LOINC codes are most predictive of unnecessary studies, and guide interventions to where they are most effectual.
Six thousand seven hundred twelve of the crossover CTs with the same LOINC code (27%) were performed between two locations (first CT at location 2, second at location 1) with an additional 4467 (18%) CTs performed first at location 3 and then a location 1 (Table 3). Given the limitations of our study we are unable to determine why a disproportionate number of secondary crossover CTs with the same LOINC code were performed at location 1, but further research could focus on location-specific utilization of HIE information, studies of the migration of patients between locations in the HIE, and barriers to access to clinical information provided by the HIE at this specific location.
Overall, it is known that existent HIEs are drastically underutilized.8,18 Even in the scenario of the unfamiliar patient, where HIE was expected to play a key role,55 studies have shown that clinicians take advantage of HIEs less frequently than expected.56,57 Lack of training, lack of workflow integration and optimization, and other usability issues in fast-paced healthcare environments are just some of the reasons cited for this lack of adoption and usage of HIE.56,58,59 Other authors have suggested that regardless of availability, the risk of missing a clinically significant diagnosis by not repeating a study justifies the cost of the duplicate test.40 However, it may be insufficient to simply require the utilization of HIE,56 or to hold those providers who order avoidable studies accountable.40 Instead, it has been proposed that the direct integration of HIE into EHRs, while inherently challenging, could facilitate the ease of use of already available systems.56
Ultimately, improved interoperability offers an opportunity to create a HIE-empowered duplicate CT alerting system. It is possible that a system could be built by mapping all CT names and codes across all locations in an HIE to LOINC, and implementing alerting algorithms based on certain rules (eg, time between studies, anatomical proximity of subsequent exams, etc.). Alerts could then be presented at the point of provider order entry, prior to actual placement of an order, thereby offering the provider an opportunity to review prior results and consider an alternative diagnostic approach if appropriate. Future research might focus on measurement of mapping reliability to develop best practices, and development of a simple semantic anatomical ontology to assess when similar studies (such as those with anatomical proximity, eg, CT head and CT sinuses) are being performed at different provider organizations. Additionally, future studies should include mapping to the forthcoming combined LOINC/RadLex terminology, work that is funded under a contract from the National Institute of Biomedical Imaging and Bioengineering.60,61
Limitations
Because our analysis was limited to the information available in the HIE, which included only de-identified data in compliance with both Healthix and New York State policies, the analysis was limited in several respects. First, no information regarding the indication for studies was available, so it was not possible to determine if repeated studies were being performed for a new indication (ie, unrelated CTs). Second, no information on dosimetry was available, so actual radiation exposure was not calculated. Third, no additional patient characteristics were available, so further analysis to determine factors that lead to crossover CTs was not possible. Fourth, we presume that potentially avoidable scans are more likely to occur in shorter time frames. Therefore, the 5000 CTs performed within 30 days may contain the most potentially avoidable duplicate studies, but further research would be necessary to elicit the timeframes that contain the most avoidable CTs. Fifth, because there is no semantic anatomical ontology in LOINC that we could leverage, we were unable to determine which CTs might be similar (e.g., CT abdomen at one location with IV contrast, followed by a CT abdomen at another location without IV contrast), and we were only able to study exact LOINC code matches. Finally, this study was based on an existing dataset that contained CT data from only five location. Healthix now contains more than 50 locations that perform CTs. It is reasonable to assume that the greater the number of locations in a dataset available for this type of analysis, the greater the number of crossover occurrences that will be detected, and the more accurate the measurement of CT crossover. This dataset likely underestimates the true degree of crossover in the New York City region, and may undervalue the potential impact of a repeat CT alerting system.
Conclusions
HIEs are expected to play key roles in reducing repeat testing, including minimizing the number of CT scans performed on patients in the United States. This study shows that although only a relatively small proportion of patients received repeat CTs at more than one location, the absolute number of patients, and their disproportionately large number of repeat CTs, defines an addressable problem. A HIE-based repeat CT alerting system could play a role in health care by enabling novel ways to reduce unnecessary testing.
ACKNOWLEDGEMENTS
The authors would like to thank HEALTHIX, the regional health information organization of New York for providing the data used in the study. The authors would also like to thank the Department of Emergency Medicine at Mount Sinai for institutional support.
Contributors
Study conception and design were provided by all authors, acquisition of data was provided by J.S.S. and T.L., analysis was provided by T.L. and C.D., interpretation of data was provided by all authors, and study supervision was provided by J.S.S. Drafting of the manuscript was provided by BHS with critical revisions for important intellectual content and final approval of the version to be published provided by all authors. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
J.S.S., A.O.B., and B.N.D. were supported during revisions of this manuscript by the National Library of Medicine of the National Institutes of Health under Award Number R01LM012196. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests
None.
REFERENCES
- 1. Laborde DV, Griffin JA, Smalley HK, Keskinocak P, Mathew G. A framework for assessing patient crossover and health information exchange value. J Am Med Inform Assoc. 2011;18(5):698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourgeois FC, Olson KL, Mandl KD. Patients treated at multiple acute health care facilities: quantifying information fragmentation. Arch Int Med. 2010;170(22): 1989–1995. [DOI] [PubMed] [Google Scholar]
- 3. Finnell JT, Overhage JM, Grannis S. All health care is not local: an evaluation of the distribution of Emergency Department care delivered in Indiana. AMIA. Annual Symposium Proceedings/AMIA Symposium. AMIA Symposium. 2011;2011:409–416. [PMC free article] [PubMed] [Google Scholar]
- 4. Overhage JM, Evans L, Marchibroda J. Communities' readiness for health information exchange: the National Landscape in 2004. J Am Med Inform Assoc. 2005;12(2): 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hillestad R, Bigelow J, Bower A, et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Aff. 2005;24(5):1103–1117. [DOI] [PubMed] [Google Scholar]
- 6. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff. 2005;(Suppl Web Exclusives):W5-10-W5-18 PubMed PMID: 15659453. [DOI] [PubMed] [Google Scholar]
- 7. Lammers EJ, Adler-Milstein J, Kocher KE. Does health information exchange reduce redundant imaging? Evidence from emergency departments. Medical Care. 2014;52(3):227–234. [DOI] [PubMed] [Google Scholar]
- 8. Rudin RS, Motala A, Goldzweig CL, Shekelle PG. Usage and effect of health information exchange: a systematic review. Ann Int Med. 2014;161(11): 803–811. [DOI] [PubMed] [Google Scholar]
- 9. Frisse ME, Johnson KB, Nian H, et al. The financial impact of health information exchange on emergency department care. J Am Med Inform Assoc. 2012;19(3): 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bailey JE, Pope RA, Elliott EC, Wan JY, Waters TM, Frisse ME. Health information exchange reduces repeated diagnostic imaging for back pain. Ann Emerg Med. 2013;62(1):16–24. [DOI] [PubMed] [Google Scholar]
- 11. Campion TR, Jr, Vest JR, Ancker JS, Kaushal R, Investigators H. Patient encounters and care transitions in one community supported by automated query-based health information exchange. AMIA… Annual Symposium Proceedings/AMIA Symposium. AMIA Symposium. 2013;2013:175–184 [PMC free article] [PubMed] [Google Scholar]
- 12. Vest JR, Kern LM, Campion TR, Jr, Silver MD, Kaushal R. Association between use of a health information exchange system and hospital admissions. Appl Clin Inform. 2014;5(1):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vest JR, Kern LM, Silver MD, Kaushal R, investigators H. The potential for community-based health information exchange systems to reduce hospital readmissions. J Am Med Inform Assoc. 2015;22(2):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bailey JE, Wan JY, Mabry LM, et al. Does health information exchange reduce unnecessary neuroimaging and improve quality of headache care in the emergency department? J General Int Med. 2013;28(2):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carr CM, Gilman CS, Krywko DM, Moore HE, Walker BJ, Saef SH. Observational study and estimate of cost savings from use of a health information exchange in an academic emergency department. J Emerg Med. 2014;46(2):250–256. [DOI] [PubMed] [Google Scholar]
- 16. Vest JR, Kaushal R, Silver MD, Hentel K, Kern LM. Health information exchange and the frequency of repeat medical imaging. Am J Managed Care 2014;20(11 Spec No. 17):eSP16–eSP24. [PubMed] [Google Scholar]
- 17. Overhage JM, Dexter PR, Perkins SM, et al. A randomized, controlled trial of clinical information shared from another institution. Ann Emerg Med. 2002;39(1):14–23. [DOI] [PubMed] [Google Scholar]
- 18. Johnson KB, Unertl KM, Chen Q, et al. Health information exchange usage in emergency departments and clinics: the who, what, and why. J Am Med Inform Assoc. 2011;18(5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. New Engl J Med. 2010;363(6):501–504. [DOI] [PubMed] [Google Scholar]
- 20. Viola A. Information exchange receives a big push in meaningful use, stage 2: new requirements encourage further integration with EHRs. J AHIMA. 2012;83(6):64–65. [PubMed] [Google Scholar]
- 21. Office of the National Coordinator for Health Information Technology DoH, Human S. Health information technology: standards, implementation specifications, and certification criteria for electronic health record technology, 2014 edition; revisions to the permanent certification program for health information technology. Final rule. Federal Register. 2012;77(171): 54163–54292. [PubMed] [Google Scholar]
- 22. HealthIT.gov. HITECH Programs & Advisory Committees: State Health Information Exchange. Secondary HITECH Programs & Advisory Committees: State Health Information Exchange. 2011. http://www.healthit.gov/policy-researchers-implementers/state-health-information-exchange. Accessed 20 August 2015.
- 23. Yeager VA, Walker D, Cole E, Mora AM, Diana ML. Factors related to health information exchange participation and use. J Med Sys. 2014;38(8):78. [DOI] [PubMed] [Google Scholar]
- 24. Shapiro JS, Crowley D, Hoxhaj S, et al. Health information exchange in emergency medicine. Ann Emerg Med. 2016;67(2):216–226. [DOI] [PubMed] [Google Scholar]
- 25. Group AHAsAIA. Achieving Interoperability that Supports Care Transformation 7/9/15. http://www.aha.org/content/15/1507-iagreport.pdf. Accessed 20 August 2015.
- 26. Hillman BJ, Goldsmith JC. The uncritical use of high-tech medical imaging. New Engl J Med. 2010;363(1):4–6. [DOI] [PubMed] [Google Scholar]
- 27. Iglehart JK. Health insurers and medical-imaging policy–a work in progress. New Engl J Med. 2009;360(10):1030–1037. [DOI] [PubMed] [Google Scholar]
- 28. Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232(3):735–738. [DOI] [PubMed] [Google Scholar]
- 29. Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. New Engl J Med. 2007;357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 30. National Research Council (U.S.). Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 31. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Int Med. 2009;169(22):2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175–184. [DOI] [PubMed] [Google Scholar]
- 34. Raja AS, Ip IK, Prevedello LM, et al. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology. 2012;262(2):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ip IK, Raja AS, Gupta A, Andruchow J, Sodickson A, Khorasani R. Impact of clinical decision support on head computed tomography use in patients with mild traumatic brain injury in the ED. Am J Emerg Med. 2015;33(3):320–325. [DOI] [PubMed] [Google Scholar]
- 36. O'Connor SD, Sodickson AD, Ip IK, et al. Journal club: Requiring clinical justification to override repeat imaging decision support: impact on CT use. Am J Roentgenol. 2014;203(5):W482–W490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaplan D. A new way to manage radiology utilization could help limit costs. Managed Healthcare Executive. 2006. http://managedhealthcareexecutive.modernmedicine.com/managed-healthcare-executive/content/new-way-manage-radiology-utilization-could-help-limit-costs. Accessed 20 August 2015. [Google Scholar]
- 38. Furukawa MF, Patel V, Charles D, Swain M, Mostashari F. Hospital electronic health information exchange grew substantially in 2008-12. Health Aff. 2013;32(8):1346–1354. [DOI] [PubMed] [Google Scholar]
- 39. Campion TR, Jr., Edwards AM, Johnson SB, Kaushal R, investigators H. Health information exchange system usage patterns in three communities: practice sites, users, patients, and data. Int J Med Inform. 2013;82(9):810–820. [DOI] [PubMed] [Google Scholar]
- 40. Frisse ME, Holmes RL. Estimated financial savings associated with health information exchange and ambulatory care referral. J Biomed Inform. 2007;40(6 Suppl):S27–S32. [DOI] [PubMed] [Google Scholar]
- 41. Grinspan ZM, Abramson EL, Banerjee S, Kern LM, Kaushal R, Shapiro JS. Potential value of health information exchange for people with epilepsy: crossover patterns and missing clinical data. AMIA… Annual Symposium Proceedings/AMIA Symposium. AMIA Symposium. 2013;2013:527–536. [PMC free article] [PubMed] [Google Scholar]
- 42. Kho AN, Lemmon L, Commiskey M, Wilson SJ, McDonald CJ. Use of a regional health information exchange to detect crossover of patients with MRSA between urban hospitals. J Am Med Inform Assoc. 2008;15(2):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Healthix: A Tale of Two RHIOs. Secondary Healthix: A Tale of Two RHIOs. http://hosted.verticalresponse.com/930243/4649b22240/286907 401/089 35c2d85/. Accessed August 20 2015.
- 44. Healthix, Inc. and the Brooklyn Health Information Exchange (BHIX) Announce Plans to Merge. Secondary Healthix, Inc. and the Brooklyn Health Information Exchange (BHIX) Announce Plans to Merge August 18 2014. http://www.marketwatch.com/story/healthix-inc-and-the-brooklyn-health-information-exchange -bhix-announce-their-merger-effective-december-1-2013-12-03. Accessed August 20 2015.
- 45. Beitia AO, Kuperman G, Delman BN, Shapiro JS. Assessing the performance of LOINC(R) and RadLex for coverage of CT scans across three sites in a health information exchange. AMIA… Annual Symposium Proceedings/AMIA Symposium. AMIA Symposium. 2013;2013:94–102. [PMC free article] [PubMed] [Google Scholar]
- 46. Statewide Planning and Research Cooperative System (SPARCS). Secondary Statewide Planning and Research Cooperative System (SPARCS). http://www.health.ny.gov/statistics/sparcs/. Accessed 15 August 2015. [Google Scholar]
- 47. Abhyankar S, Demner-Fushman D, McDonald CJ. Standardizing clinical laboratory data for secondary use. J Biomed Inform. 2012;45(4): 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eidemuller M, Holmberg E, Jacob P, Lundell M, Karlsson P. Breast cancer risk among Swedish hemangioma patients and possible consequences of radiation-induced genomic instability. Mutat Res. 2009;669(1-2):48–55. [DOI] [PubMed] [Google Scholar]
- 49. Huff SM, Rocha RA, McDonald CJ, et al. Development of the Logical Observation Identifier Names and Codes (LOINC) vocabulary. J Am Med Inform Assoc. 1998;5(3): 276–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fiszman M, Shin D, Sneiderman CA, Jin H, Rindflesch TC. A Knowledge Intensive Approach to Mapping Clinical Narrative to LOINC. AMIA… Annual Symposium Proceedings/AMIA Symposium. AMIA Symposium. 2010;2010: 227–231. [PMC free article] [PubMed] [Google Scholar]
- 51. Kassing P., DR The Neiman Report: Repeat Medical Imaging: A Classification System for Meaningful Policy Analysis and Research: Harvey L. Neiman Health Policy Institute, American College of Radiology (ACR), 2013 http://www.acr.org/∼/media/ACR/Documents/PDF/Research/Brief%2002/Policy BriefHPI012013.pdf. Accessed 20 August 2015. [Google Scholar]
- 52. Brenner DJ. Minimising medically unwarranted computed tomography scans. Ann ICRP. 2012;41(3-4):161–169. [DOI] [PubMed] [Google Scholar]
- 53. Ventures IM. 2012CT Market Outlook Report. International Marketing Ventures. Des Plaines, IL; 2012. [Google Scholar]
- 54. Plans IFoH. 2011 Comparative Price Report: Medical and Hospital Fees by Country. London: IFHP; 2011. [Google Scholar]
- 55. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426–432. [DOI] [PubMed] [Google Scholar]
- 56. Vest JR, Zhao H, Jasperson J, Gamm LD, Ohsfeldt RL. Factors motivating and affecting health information exchange usage. J Am Med Inform Assoc. 2011;18 (2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vest JR, Jasperson S, Zhao H, Gamm LD, Ohsfeldt RL. Use of a health information exchange system in the emergency care of children. BMC Med Inform Decis Mak. 2011;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gadd CS, Ho YX, Cala CM, et al. User perspectives on the usability of a regional health information exchange. J Am Med Inform Assoc. 2011;18(5):711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Genes N, Shapiro J, Vaidya S, Kuperman G. Adoption of health information exchange by emergency physicians at three urban academic medical centers. Appl Clin Inform. 2011;2(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. America RSoN. RadLex Playbook.
- 61. Bioengineering NIoBIa. RSNA and Regenstrief Institute Launch Effort to Unify Radiology Procedure Naming. 2013. https://www2.rsna.org/timssnet/media/pressreleases/pr_target.cfm?ID=721. Accessed 20 August 2015. [Google Scholar]