Abstract
Orange Carotenoid Protein (OCP) plays a unique role in protecting many cyanobacteria from light-induced damage. The active form of OCP is directly involved in energy dissipation by binding to the phycobilisome (PBS), the major light-harvesting complex in cyanobacteria. There are two structural modules in OCP, an N-terminal domain (NTD) and a C-terminal domain (CTD), which play different functional roles during the OCP-PBS quenching cycle. Because of the quasi-stable nature of active OCP, structural analysis of active OCP has been lacking compared to its inactive form. In this report, partial proteolysis was used to generate two structural domains, NTD and CTD, from active OCP. We used multiple native mass spectrometry (MS) based approaches to interrogate the structural features of the NTD and the CTD. Collisional activation and ion mobility analysis indicated that the NTD releases its bound carotenoid without forming any intermediates and the CTD is resistant to unfolding upon collisional energy ramping. The unfolding intermediates observed in inactive intact OCP suggest that it is the N-terminal extension and the NTD-CTD loop that lead to the observed unfolding intermediates. These combined approaches extend the knowledge of OCP photo-activation and structural features of OCP functional domains. Combining native MS, ion mobility and collisional activation promises to be a sensitive new approach for studies of photosynthetic protein-pigment complexes.
Graphical abstract
1. Introduction
Cyanobacterial photosynthesis contributes dramatically to the global carbon and nitrogen cycle [1–3]. In cyanobacteria, solar energy is mostly captured by the phycobilisome (PBS), a light-harvesting antenna complex that is anchored to the stromal side of the thylakoid membrane. The energy is then transferred to membrane-embedded reaction centers Photosystems I and II (PSI, and PSII) where photochemical reactions take place [4–7]. Regulation of energy transfer between the antenna and reaction centers is extremely important for energy allocation to the two photosystems and cellular adaptation as well as to changing light conditions in the environment. Under strong light conditions, many cyanobacteria exhibit a self-protection mechanism called non-photochemical quenching (NPQ), a process in which extra energy collected by the PBS is dissipated as heat [8, 9]. The orange carotenoid protein (OCP) acts as a sensor and practitioner in the NPQ regulatory process. OCP is in its inactive orange form under low-light or dark conditions. Under strong-light conditions, however, inactive OCP can be activated to its red active form and consequently is recruited to bind to the PBS. The carotenoid molecule intercepts energy from the PBS and prevents over-energization of photosystems, especially PSII where toxic singlet oxygen species are inevitably produced by PSII photochemistry [10].
OCP photo-activation has been intensely studied [11–16]. Although high-resolution structural models for inactive intact OCP and truncated active OCP N-terminal domain (NTD) expressed in E. coli have been reported [17, 18], detailed information about the photo-induced conformational changes and the carotenoid-protein interactions are still limited for the intact active OCP. One challenge is the quasi-stable feature of active OCP that tends to relax to its inactive form, making currently available analytical characterization extremely difficult. It was observed, however, that the NTD alone could bind the carotenoid in its red form and is conformationally stable and functionally effective in PBS energy quenching [19].
Partial digestion or limited proteolysis experiments have been used in many structural biology studies [20, 21]. In a typical limited proteolysis experiment, proteins are digested by proteases under native conditions. The enzyme cleavage sites exposed on the protein surface or in flexible regions are available for enzymatic cleavage, while those sites buried inside the interior of a protein are not accessible for enzyme attack. The partial digestion results in large protein fragments that represent intact domains or stable structural modules of a protein. When partial digestion analysis is combined with mass spectrometry (MS), a rapid and sensitive tool, a wealth of structural information can be obtained [22–25].
Other sensitive MS-based approaches in protein characterization have already been employed in studies of OCP photo-activation [17, 26–28]. For example, we and others have analyzed the global conformational changes of OCP upon photo-activation by using MS-based protein footprinting [26, 28]. Native MS is a relatively new approach to characterize protein structure under conditions in which the native protein conformation is maintained in the gas phase for MS analysis [29–34].
Many mass spectrometers are equipped with Ion Mobility (IM) analysis [32, 35], which may have applications in systems such as OCP. In brief, the protein-ion travel time through the drift tube under an applied electric field against the carrier buffer gas is a function of the protein ion’s mass-to-charge ratio, as well as its size and shape (conformation). Based on the traveling time (Drift Time, DT) recorded in IM analysis, the collisional cross section (CCS), which is a measure of ion size and conformation, can be determined [36, 37]. The combination of native MS with IM has become a fast-growing research field in the last decade to determine protein conformational information [38].
Here we report an extension of our previous native MS studies on intact OCP [27]. We analyzed OCP functional domains (NTD and CTD) from partial digestion of active OCP by using IM and collisional unfolding [39] under native MS conditions. Two OCP functional domains were compared with inactive intact OCP. Information on the conformation and carotenoid interaction of each functional module were elucidated.
2. Material and methods
2.1 Chemical and reagents
HPLC grade water and ammonium acetate were purchased from Sigma Aldrich (Sigma-Aldrich, St. Louis, MO). LC-MS grade water with 0.1% formic acid, acetonitrile with 0.1% formic acid, and formic acid were purchased from Fisher Scientifics (Pittsburgh, PA).
2.2 OCP protein purification and Partial digestion
The growth and the OCP isolation were described elsewhere [10, 27]. The OCP partial digestion was carried out as previously described [19].
2.3 LC-MS analysis
The partially digested OCP sample was analyzed by a Waters Synapt G2 mass spectrometer coupled with Waters nanoAquity UPLC system (Waters Corporation, Milford, MA). The sample was loaded into a custom-packed capillary column (Polymer Resin Phase, 1000 Å, 5 μm, 5 cm x 100 μm, Sepax Technologies, Newark, DE), and the proteins were trapped and desalted by 85% Solvent A (Water with 0.1% Formic acid) for 15 min prior to the elution. The sample was eluted by increasing solvent B (Acetonitrile with 0.1% Formic acid) from 15 to 85% over 25 min at a flow rate of 0.7 uL/min. The eluted sample was directly introduced into the mass spectrometer through a nano electrospray source. The mass spectrometer was operated under the resolution mode (‘V’ optics for TOF analyzer, TOF resolution is 20,000 FWHM at m/z 956) with mass range from 50 to 1995 m/z. The spray voltage as 2.8 kV; cone voltage was 30 V and extraction voltage as 4 V. The trap region collisional energies was 6 V. The data were manually analyzed by using MassLynx (Waters Corporation, Milford, MA) and Massign software to assign the molecular weight [40].
2.4 Native MS and Ion Mobility Analysis
The partially digested OCP sample was washed by 200 mM ammonium acetate solution (pH 6.8) in a 3 kDa molecular weight cut off filter (Vivspin, Goettingen, Germany). The original buffer and salts were removed during 10–15 cycles of washing. The OCP sample was introduced into the mass spectrometer source by using off-line nano-electrospray tips. The native MS and ion mobility experiments were conducted in the Waters Synapt G2 mass spectrometer. The mass spectrometer was operated in the “sensitive mode” (‘V’ optics for TOF analyzer, TOF resolution is 10,000 FWHM) with a mass range from 100 to 10000 m/z. The backing pressure (pressure read out from the first pirani gauge at the source region) was adjusted to 5–6 mBar for transferring intact protein ions. The collision voltage for transfer region was 20 V. The collision voltage for trap region was adjusted from 5 to 200 V to unfold the protein ions [41, 42]. For ion-mobility experiments, the gas flow rate was 35 mL/min, the IMS wave height was 20 V, and the IMS wave velocity was 500 m/s. The data were output from MassLynx and plotted by Origin (Origin Lab Corporation, Northampton, MA). The intact OCP sample was also analyzed by native MS and ion-mobility experiments. The ion-mobility experiment was calibrated to give cross sections with standard proteins based on published protocols [36, 43]. The drift time information from the native MS ion-mobility experiment was converted into collisional cross section [36, 43].
3. Results and discussion
3.1 Partial digestion and analysis of OCP
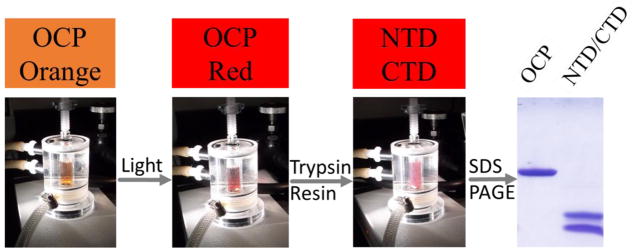
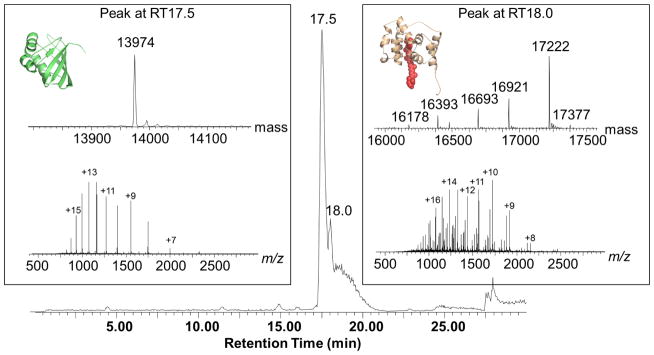
When we performed SDS-PAGE analysis of limited digestion OCP, we found two major polypeptide fragments with apparent molecular weights of 14 and 17 kDa respectively (Figure 1), consistent with a previous report [19]. We then analyzed the partially digested OCP samples by regular LC-MS and found large peptides covering the NTD and CTD. Because regular LC-MS is run under denaturing conditions, the non-covalent interactions between the protein and carotenoid were lost, as no peptide-carotenoid complex could be detected in the LC-MS experiment. The OCP sequence was analyzed for in-silico trypsin digestion. We assigned peaks from the LC-MS experiment to corresponding digestion products based on the molecular weight. There are two major groups of digestion products observed in LC-MS experiments (peaks were assigned to N-terminal (at retention time 18 mins) and C-terminal digestion product/fragments (at retention time 17.5 mins) separately (Figure 2)). We also observed that each group includes a series of peptide fragments. For example, in the first group of peaks (retention time), the major species has molecular weight 13,974 Da and was assigned to the C-terminal fragment (from amino acid residue 186 to 310; theoretical MW of 13974.03 Da). The second group of peaks contains multiple, co-eluting species in the molecular weight range from 16.1 to 17.3 kDa. They were assigned to N-terminal fragments. The major peak of 17,222 Da is the N-terminal fragment from amino acid residue 10–170, the theoretical MW is 17222.8 Da. Our data showed the detailed, heterogeneous partial digestion peptide patterns that otherwise cannot be detected by traditional SDS-PAGE.
Figure 1.
Partial proteolysis of photoactivated Red OCP under low temperature (4 C) by trypsin.
Figure 2.
The LC-MS chromatogram of partially digested OCP, when analyzed by MS, reveals two groups of peaks. The mass spectra for each group (bottom) and the deconvoluted mass spectra (top) are displayed in the two boxes.
We conducted partial digestion under light irradiation (active form of OCP or “red OCP”). The most exposed cleavage sites are between residues 161–196, which includes the loop region between the NTD and the CTD of OCP. The LC-MS experiment identified two major fragments (N- and C- terminal fragments) from the cleavage at R10, K170, R185, and K310, which confirmed previous published results [19].
3.2 Native MS of OCP partially digested sample
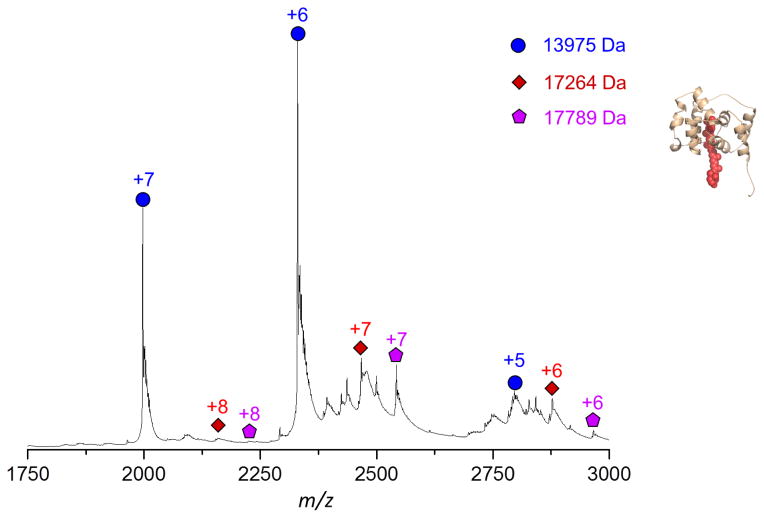
In the native MS experiment, the partially digested OCP sample was subjected to desalting and was buffer exchanged to ammonium acetate solution and then was directly introduced into the mass spectrometer. Multiple charged OCP fragments were observed in the region from 1800 to 3500 m/z. Both regular LC-MS and native MS experiments produced multiple charged protein signals. As shown in Figure 3, the charge states and distributions of protein ions are different under native MS than under regular ESI MS [29, 44], i.e., the distribution is three charge-states wide for native MS (from +6 to +8), while it extended to more than five charge-states wide for regular ESI MS (LC-MS) (from +8 to +17). In regular ESI MS, the denatured OCP is fully unfolded, and all positively charged residues are available for carrying charges. In contrast, the non-covalent interactions are well preserved in native MS, the higher order structure of OCP fragments are conserved, and only positively charged residues on the surface of the fragments are available for carrying charges.
Figure 3.
The native MS of partial digested OCP. The N-terminal domain fragments are labeled in red and purple, and the C-terminal domain fragment in blue.
In native MS, the measured MW of the NTD was different from that of the denatured one in regular ESI MS, indicating that it still retained the carotenoid. The mass shifts induced by carotenoid binding were considered in the peak assignment. Two sets of species were clearly observed in the native MS: one is the NTD and the other is the CTD (Figure 3). The measured MW in the native MS experiment confirmed that only the NTD contains carotenoid [19]. Native MS provides a platform under which protein complexes are analyzed under conditions comparable to the native state, i.e., non-covalent interactions among complexes are preserved [30, 31]. If the CTD strongly interacts with the NTD or the carotenoid in the active form of OCP, native MS may be able to detect a complex containing two domains or a CTD plus carotenoid. Failure to detect the complex containing two domains in OCP partial digestion samples suggests that the non-covalent interactions inside of the CTD and carotenoid have changed upon photo-activation.
Photo-activation induces significant conformational changes of OCP and results in dramatic rearrangements of interactions between protein and the carotenoid [19, 26, 45, 46]. In the active OCP (red form), the CTD no longer holds half of the carotenoid as the inactive OCP does, and the interface between NTD and CTD becomes detached and more solvent accessible [26, 28], making the loop region more available for protease action. Two individual domains, represented by NTD and CTD, are stable in their native state without the loop connection. Because the NTD contains the carotenoid and is red in color, it is capable of interacting with the PBS and initiating NPQ [19]. The CTD loses the interaction with carotenoid and is detached from the NTD in active OCP. Simultaneous analysis of the NTD and CTD in their native states is a sensitive approach, and opens the door to ion mobility (IM) measurements to elucidate structural information for both NTD and CTD.
3.3 Ion mobility analysis of OCP partial digested sample
We then submitted NTD and CTD to native MS with IM. The inactive OCP was analyzed for comparison. The IM analysis of active red OCP, however, is still a challenge for the current approach. It should be noted that the unstable nature of active red OCP and incomplete conversion of the inactive form to the active form of OCP make native IM analysis of active OCP extremely difficult. For inactive OCP, we saw two species in native MS (Figure S-1), and based on their measured MWs, the two species are monomeric and dimeric forms of OCP respectively. The measured drift times (DT) of protein standards calibrate the drift tube to permit a correlation between measured DT and CCS [36, 43]. The measured CCSs of monomer and dimer OCP are 2382–2619 Å2 and 4172–4456 Å2 (at the leading and trailing 10% peak heights), respectively. The CCSs can also be calculated using data from the OCP crystal structures [17, 18, 47]. The calculated CCS ranges from 2216 to 2372 Å2 for OCP monomer and from 3618 to 3785 Å2 for dimeric OCP (from a total of eight models from PDB, Table S-1). We observed that the CCS of monomeric OCP determined by using IM has similar values (within 10% for the mean value) to those of crystal structures, whereas the OCP dimer has relatively larger CCS values than those of the crystal structures (>15% for the mean value). This may suggest that the OCP dimer observed in native MS has a somewhat different conformation than the OCP dimer in the crystal structure. We extended the IM analysis to partially digested OCP samples. The CCSs for the major NTD are 1479–1849 Å2. The CCSs for the CTD are 1266–1462 Å2. The calculated CCSs for OCP NTD crystal structure from E. coli are 1293–1335 Å2 [17]. The discrepancy of our observation and the calculated CCS from the crystal structure results from the fact that the NTD used in our research contains 161 amino acids (AA), while the crystal structure (PDB ID: 4XB4) contains 146 AA without the polyhistidine tag. These results demonstrate the robustness of IM as a powerful biophysical method to characterize protein shape and conformation.
The function of the NTD and CTD are determined by their higher order structures. IM analysis suggested that both NTD and CTD have different interactions with carotenoid and that the partial digestion locks in their active state without unfolding each module. Both the NTD and CTD are independent, functional units without the loop connection. In additional to the determination of the absolute CCS value for each species, we further applied collisional activation in IM analysis to monitor the structural response of the NTD and CTD during protein unfolding, so as to observe different unfolding processes.
3.4 Unfolding of NTD and CTD
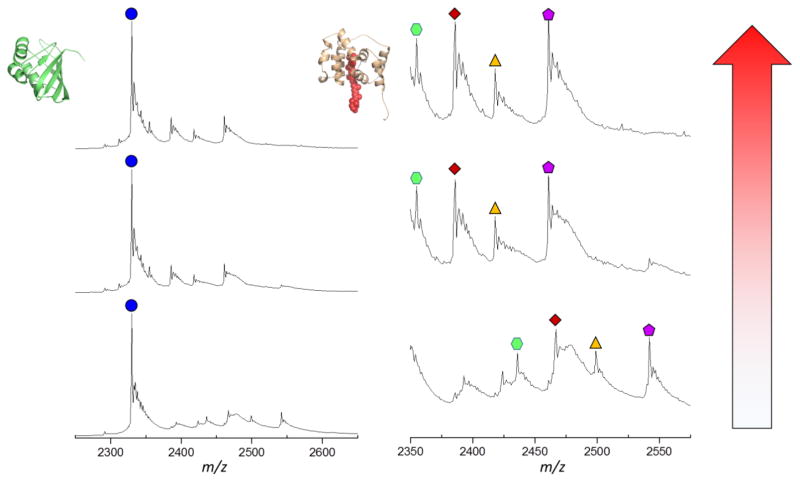
In native MS, protein ions can be interrogated by different dissociation methods. The most common approach is collisional activated dissociation (CAD). In top-down MS experiments, CAD induces the backbone cleavages of denatured protein and provides primary (sequence) structure information [48, 49]. For ions introduced by native MS, collisional activation mostly causes single subunit protein unfolding or protein complex dissociation. Because CAD breaks the non-covalent interactions in proteins, protein unfolding in the gas phase is usually accompanied by the release of ligands or cofactors of a protein [50]. Collision-induced carotenoid release from the OCP protein complex provides useful information about the protein-carotenoid interactions [27]. The collisional energy required for releasing a cofactor is related to the strength of the protein-cofactor interactions that are characteristic of a protein’s higher order structure. For intact OCP and the OCP NTD from partial digestion, we were able to detect the release of the carotenoid from OCP by increasing the collision energy (Figure 4) [27].
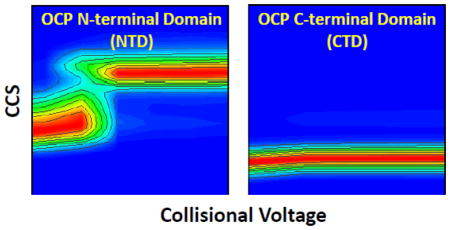
Figure 4.
Native MS and collisional activation of partial digested OCP. The left column refers to the C-terminal domain fragment; the right column to the N-terminal domain fragment.
The combination of native MS and IM is sensitive enough to monitor protein conformational changes and compare relative propensities to change [51, 52]. By following the gas phase unfolding pattern of protein ions, the unfolding “heat maps” [41] are a function of protein higher order structure and protein-ligand binding state [39, 42, 53–55]. Both intact OCP and NTD undergo gas phase unfolding to increase their CCS, whereas CTD remarkably undergoes no significant unfolding (see Figure 5 for maps of intact OCP monomer, dimer, OCP NTD and CTD).
Figure 5.
The collisional unfolding heat map for intact OCP and partially digested OCP. (A) N-terminal domain fragment from partially digested OCP. (B) C-terminal domain fragment from partially digested OCP. (C) Intact OCP monomer. (D) Intact OCP dimer.
OCP crystal structures show that both intact OCP monomer and dimer have the same interaction with carotenoids [18]. The OCP monomer and dimer, however, have different unfolding pathways in the gas phase. Dimer OCP is more resistant to collisional activation than OCP monomer. OCP monomer starts to unfold at 40 V collision voltage, whereas OCP dimer stays unchanged until 60 V. Thus, dimerization of OCP increases its conformational stability. This finds confirmation in the observation that more collisional energy is required for the OCP dimer to release the bound carotenoid than monomer [27]. IM also reveals that there are fewer unfolding intermediates for the OCP dimer than monomer, suggestive that dimerization further limits the structural flexibility of OCP. The “heat map” of intact OCP provides extra information about its conformation and demonstrates the capability to study the conformation of OCP functional domain fragments.
The carotenoid is embedded in the NTD and significant conformational changes (as measured by CCS) are required to release the carotenoid during collisional activation. We observed that there is no intermediate state for NTD unfolding, while intermediate states were observed for intact OCP monomer during collisional activation. For intact OCP monomer (inactive), NTD-CTD and CTD-carotenoid interactions need to be altered to release the bound carotenoid. The conformational change of NTD during collisional activation is only restricted by the NTD-carotenoid interaction. It could be the reason why there is no intermediate state observed during the NTD unfolding. Without the loop connecting the NTD and CTD, fragments from both domains are stabilized as their modular state [19]. On the contrary, there is no significant conformational change observed for the OCP CTD. This suggests that the OCP CTD adopts a stable conformation after it loses the interactions with the NTD and the carotenoid during photo-activation.
4. Conclusions
Because high-resolution structural models of intact active OCP are not yet available, various MS-based biophysical approaches, including protein footprinting [26, 28], have been employed to generate structural information. Native MS and IM sensitively monitor OCP functional domains. We found that both NTD and CTD are independent functional modules that adopt stable conformations that are different from those in the intact inactive OCP. The NTD, which harbors a carotenoid, is the module that binds the PBS during NPQ. The reversibility of intact OCP upon photo-activation, however, is missing after partial digestion. This indicates that the CTD and the loop linking the NTD and CTD are crucial for the conversion of active OCP to inactive OCP. However, the direct IM analysis of active intact OCP is still a challenge for the current approach. The ultimate goal of the current study is to stabilize the active intact OCP and to make it available for direct IM analysis. Our current native MS and IM studies lay the ground for further investigation of the interaction between NTD and CTD in the active OCP (red form) by MS based protein footprinting and cross-linking.
Supplementary Material
Highlights.
We used multiple native mass spectrometry (MS) based approaches to interrogate the structural features of the NTD and the CTD of OCP.
The CTD is resistant to unfolding upon collisional energy ramping.
These combined approaches extend the knowledge of OCP photo-activation and structural features of OCP functional domains.
Acknowledgments
This research is supported by grant DE-FG02-07ER15902 from the Photosynthetic Systems Program of the US Department of Energy, Office of Science, Basic Energy Sciences. HL and all cell growth and sample preparation were supported by the Photosynthetic Systems grant. HZ, YL and native and ion mobility mass spectrometry were supported by the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the DOE, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC 0001035. REB and MLG are PIs of PARC under the EFRC grant. MLG is also PI for National Mass Spectrometry Resource supported by National Institute of General Medical Science Grant Number: 8 P41 GM103422.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angermayr SA, Hellingwerf KJ, Lindblad P, de Mattos MJ. Energy biotechnology with cyanobacteria. Curr Opin Biotechnol. 2009;20:257–63. doi: 10.1016/j.copbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Berla BM, Saha R, Immethun CM, Maranas CD, Moon TS, Pakrasi HB. Synthetic biology of cyanobacteria: unique challenges and opportunities. Front Microbiol. 2013;4:246. doi: 10.3389/fmicb.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartzman D, Caldeira K, Pavlov A. Cyanobacterial emergence at 2.8 gya and greenhouse feedbacks. Astrobiology. 2008;8:187–203. doi: 10.1089/ast.2006.0074. [DOI] [PubMed] [Google Scholar]
- 4.Mullineaux CW. Phycobilisome-reaction centre interaction in cyanobacteria. Photosynth Res. 2008;95:175–82. doi: 10.1007/s11120-007-9249-y. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach G, Schubert F, Kana R. Cryo-imaging of photosystems and phycobilisomes in Anabaena sp. PCC 7120 cells. Journal of photochemistry and photobiology B, Biology. 2015 doi: 10.1016/j.jphotobiol.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Liu X, Li Y, Liu CC, Yang F, Zhao J, Sui SF. Structural organization of an intact phycobilisome and its association with photosystem II. Cell research. 2015;25:726–37. doi: 10.1038/cr.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chukhutsina V, Bersanini L, Aro EM, van Amerongen H. Cyanobacterial Light-Harvesting Phycobilisomes Uncouple From Photosystem I During Dark-To-Light Transitions. Scientific reports. 2015;5:14193. doi: 10.1038/srep14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirilovsky D, Kerfeld CA. The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim Biophys Acta. 2012;1817:158–66. doi: 10.1016/j.bbabio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Rakhimberdieva MG, Stadnichuk IN, Elanskaya IV, Karapetyan NV. Carotenoid-induced quenching of the phycobilisome fluorescence in photosystem II-deficient mutant of Synechocystis sp. FEBS Lett. 2004;574:85–8. doi: 10.1016/j.febslet.2004.07.087. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A, Punginelli C, Gall A, Bonetti C, Alexandre M, Routaboul JM, Kerfeld CA, van Grondelle R, Robert B, Kennis JT, Kirilovsky D. A photoactive carotenoid protein acting as light intensity sensor. Proc Natl Acad Sci U S A. 2008;105:12075–80. doi: 10.1073/pnas.0804636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadnichuk IN, Yanyushin MF, Zharmukhamedov SK, Maksimov EG, Muronets EM, Pashchenko VZ. Quenching of phycobilisome fluorescence by orange carotenoid protein. Dokl Biochem Biophys. 2011;439:167–70. doi: 10.1134/S1607672911040053. [DOI] [PubMed] [Google Scholar]
- 12.Tian L, Gwizdala M, van Stokkum IH, Koehorst RB, Kirilovsky D, van Amerongen H. Picosecond kinetics of light harvesting and photoprotective quenching in wild-type and mutant phycobilisomes isolated from the cyanobacterium Synechocystis PCC 6803. Biophys J. 2012;102:1692–700. doi: 10.1016/j.bpj.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berera R, Gwizdala M, van Stokkum IH, Kirilovsky D, van Grondelle R. Excited states of the inactive and active forms of the orange carotenoid protein. J Phys Chem B. 2013;117:9121–8. doi: 10.1021/jp307420p. [DOI] [PubMed] [Google Scholar]
- 14.De Re E, Schlau-Cohen GS, Leverenz RL, Huxter VM, Oliver TA, Mathies RA, Fleming GR. Insights into the structural changes occurring upon photoconversion in the orange carotenoid protein from broadband two-dimensional electronic spectroscopy. J Phys Chem B. 2014;118:5382–9. doi: 10.1021/jp502120h. [DOI] [PubMed] [Google Scholar]
- 15.Niedzwiedzki DM, Liu H, Blankenship RE. Excited state properties of 3′-hydroxyechinenone in solvents and in the orange carotenoid protein from Synechocystis sp. PCC 6803. J Phys Chem B. 2014;118:6141–9. doi: 10.1021/jp5041794. [DOI] [PubMed] [Google Scholar]
- 16.Sedoud A, Lopez-Igual R, Ur Rehman A, Wilson A, Perreau F, Boulay C, Vass I, Krieger-Liszkay A, Kirilovsky D. The Cyanobacterial Photoactive Orange Carotenoid Protein Is an Excellent Singlet Oxygen Quencher. Plant Cell. 2014;26:1781–1791. doi: 10.1105/tpc.114.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leverenz RL, Sutter M, Wilson A, Gupta S, Thurotte A, Bourcier de Carbon C, Petzold CJ, Ralston C, Perreau F, Kirilovsky D, Kerfeld CA. PHOTOSYNTHESIS. A 12 A carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science. 2015;348:1463–6. doi: 10.1126/science.aaa7234. [DOI] [PubMed] [Google Scholar]
- 18.Wilson A, Kinney JN, Zwart PH, Punginelli C, D’Haene S, Perreau F, Klein MG, Kirilovsky D, Kerfeld CA. Structural determinants underlying photoprotection in the photoactive orange carotenoid protein of cyanobacteria. J Biol Chem. 2010;285:18364–75. doi: 10.1074/jbc.M110.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leverenz RL, Jallet D, Li MD, Mathies RA, Kirilovsky D, Kerfeld CA. Structural and functional modularity of the orange carotenoid protein: distinct roles for the N- and C-terminal domains in cyanobacterial photoprotection. Plant Cell. 2014;26:426–37. doi: 10.1105/tpc.113.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard SJ. The structural aspects of limited proteolysis of native proteins. Biochim Biophys Acta. 1998;1382:191–206. doi: 10.1016/s0167-4838(97)00175-1. [DOI] [PubMed] [Google Scholar]
- 21.Columbus L. Post-expression strategies for structural investigations of membrane proteins. Curr Opin Struct Biol. 2015;32:131–8. doi: 10.1016/j.sbi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen SL. Domain elucidation by mass spectrometry. Structure. 1996;4:1013–6. doi: 10.1016/s0969-2126(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 23.Suh MJ, Pourshahian S, Limbach PA. Developing limited proteolysis and mass spectrometry for the characterization of ribosome topography. J Am Soc Mass Spectrom. 2007;18:1304–17. doi: 10.1016/j.jasms.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gheyi T, Rodgers L, Romero R, Sauder JM, Burley SK. Mass spectrometry guided in situ proteolysis to obtain crystals for X-ray structure determination. J Am Soc Mass Spectrom. 2010;21:1795–801. doi: 10.1016/j.jasms.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajabi K, Reuther J, Deuerling E, Radford SE, Ashcroft AE. A comparison of the folding characteristics of free and ribosome-tethered polypeptide chains using limited proteolysis and mass spectrometry. Protein Sci. 2015;24:1282–91. doi: 10.1002/pro.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Zhang H, King j D, Wolf NR, Prado M, Gross ML, Blankenship RE. Mass spectrometry footprinting reveals the structural rearrangements of cyanobacterial orange carotenoid protein upon light activation. Biochim Biophys Acta. 2014;1837:1955–63. doi: 10.1016/j.bbabio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Liu H, Niedzwiedzki DM, Prado M, Jiang J, Gross ML, Blankenship RE. Molecular mechanism of photoactivation and structural location of the cyanobacterial orange carotenoid protein. Biochemistry. 2014;53:13–9. doi: 10.1021/bi401539w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Guttman M, Leverenz RL, Zhumadilova K, Pawlowski EG, Petzold CJ, Lee KK, Ralston CY, Kerfeld CA. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc Natl Acad Sci U S A. 2015;112:E5567–74. doi: 10.1073/pnas.1512240112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Heuvel RH, Heck AJ. Native protein mass spectrometry: from intact oligomers to functional machineries. Curr Opin Chem Biol. 2004;8:519–26. doi: 10.1016/j.cbpa.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Ruotolo BT, Robinson CV. Aspects of native proteins are retained in vacuum. Curr Opin Chem Biol. 2006;10:402–8. doi: 10.1016/j.cbpa.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Benesch JL, Ruotolo BT, Simmons DA, Robinson CV. Protein complexes in the gas phase: technology for structural genomics and proteomics. Chem Rev. 2007;107:3544–67. doi: 10.1021/cr068289b. [DOI] [PubMed] [Google Scholar]
- 32.Rajabi K, Ashcroft AE, Radford SE. Mass spectrometric methods to analyze the structural organization of macromolecular complexes. Methods. 2015;89:13–21. doi: 10.1016/j.ymeth.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Wen J, Zhang H, Gross ML, Blankenship RE. Native electrospray mass spectrometry reveals the nature and stoichiometry of pigments in the FMO photosynthetic antenna protein. Biochemistry. 2011;50:3502–11. doi: 10.1021/bi200239k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Cui W, Gross ML, Blankenship RE. Native mass spectrometry of photosynthetic pigment-protein complexes. FEBS Lett. 2013;587:1012–20. doi: 10.1016/j.febslet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer MM, Donohoe GC, Valentine SJ. Advances in ion mobility-mass spectrometry instrumentation and techniques for characterizing structural heterogeneity. Analyst. 2015;140:6782–98. doi: 10.1039/c5an00922g. [DOI] [PubMed] [Google Scholar]
- 36.Ruotolo BT, Benesch JL, Sandercock AM, Hyung SJ, Robinson CV. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protoc. 2008;3:1139–52. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 37.Uetrecht C, Rose RJ, van Duijn E, Lorenzen K, Heck AJ. Ion mobility mass spectrometry of proteins and protein assemblies. Chem Soc Rev. 2010;39:1633–55. doi: 10.1039/b914002f. [DOI] [PubMed] [Google Scholar]
- 38.Benesch JL, Ruotolo BT. Mass spectrometry: come of age for structural and dynamical biology. Curr Opin Struct Biol. 2011;21:641–9. doi: 10.1016/j.sbi.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu S, Ruotolo BT. Collisional unfolding of multiprotein complexes reveals cooperative stabilization upon ligand binding. Protein Sci. 2015;24:1272–81. doi: 10.1002/pro.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgner N, Robinson CV. Massign: an assignment strategy for maximizing information from the mass spectra of heterogeneous protein assemblies. Anal Chem. 2012;84:2939–48. doi: 10.1021/ac300056a. [DOI] [PubMed] [Google Scholar]
- 41.Hyung SJ, Robinson CV, Ruotolo BT. Gas-phase unfolding and disassembly reveals stability differences in ligand-bound multiprotein complexes. Chem Biol. 2009;16:382–90. doi: 10.1016/j.chembiol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Eschweiler JD, Rabuck-Gibbons JN, Tian Y, Ruotolo BT. CIUSuite: A Quantitative Analysis Package for Collision Induced Unfolding Measurements of Gas-Phase Protein Ions. Anal Chem. 2015 doi: 10.1021/acs.analchem.5b03292. [DOI] [PubMed] [Google Scholar]
- 43.Michaelevski I, Kirshenbaum N, Sharon M. T-wave ion mobility-mass spectrometry: basic experimental procedures for protein complex analysis. J Vis Exp. 2010 doi: 10.3791/1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashcroft AE. Recent developments in electrospray ionisation mass spectrometry: noncovalently bound protein complexes. Nat Prod Rep. 2005;22:452–64. doi: 10.1039/b417724j. [DOI] [PubMed] [Google Scholar]
- 45.Thurotte A, Igual RL, Wilson A, Comolet L, Bourcier de Carbon C, Xiao F, Kirilovsky D. Regulation of Orange Carotenoid Protein Activity in Cyanobacterial Photoprotection. Plant physiology. 2015;169:737–47. doi: 10.1104/pp.15.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maksimov EG, Shirshin EA, Sluchanko NN, Zlenko DV, Parshina EY, Tsoraev GV, Klementiev KE, Budylin GS, Schmitt FJ, Friedrich T, Fadeev VV, Paschenko VZ, Rubin AB. The Signaling State of Orange Carotenoid Protein. Biophys J. 2015;109:595–607. doi: 10.1016/j.bpj.2015.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marklund EG, Degiacomi MT, Robinson CV, Baldwin AJ, Benesch JL. Collision cross sections for structural proteomics. Structure. 2015;23:791–9. doi: 10.1016/j.str.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Cui W, Rohrs HW, Gross ML. Top-down mass spectrometry: recent developments, applications and perspectives. Analyst. 2011;136:3854–64. doi: 10.1039/c1an15286f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan CM, Souda P, Bassilian S, Ujwal R, Zhang J, Abramson J, Ping P, Durazo A, Bowie JU, Hasan SS, Baniulis D, Cramer WA, Faull KF, Whitelegge JP. Post-translational modifications of integral membrane proteins resolved by top-down Fourier transform mass spectrometry with collisionally activated dissociation. Mol Cell Proteomics. 2010;9:791–803. doi: 10.1074/mcp.M900516-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharon M, Robinson CV. Peeling back the layers of complexity. Curr Opin Struct Biol. 2011;21:619–21. doi: 10.1016/j.sbi.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Konijnenberg A, Butterer A, Sobott F. Native ion mobility-mass spectrometry and related methods in structural biology. Biochim Biophys Acta. 2013;1834:1239–56. doi: 10.1016/j.bbapap.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Zhong Y, Hyung SJ, Ruotolo BT. Ion mobility-mass spectrometry for structural proteomics. Expert Rev Proteomics. 2012;9:47–58. doi: 10.1586/epr.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han L, Ruotolo BT. Ion Mobility-Mass Spectrometry Differentiates Protein Quaternary Structures Formed in Solution and in Electrospray Droplets. Anal Chem. 2015;87:6808–13. doi: 10.1021/acs.analchem.5b01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landreh M, Liko I, Uzdavinys P, Coincon M, Hopper JT, Drew D, Robinson CV. Controlling release, unfolding and dissociation of membrane protein complexes in the gas phase through collisional cooling. Chem Commun (Camb) 2015;51:15582–4. doi: 10.1039/c5cc07045g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian Y, Han L, Buckner AC, Ruotolo BT. Collision Induced Unfolding of Intact Antibodies: Rapid Characterization of Disulfide Bonding Patterns, Glycosylation, and Structures. Anal Chem. 2015 doi: 10.1021/acs.analchem.5b03291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.