Abstract
Background
The porcine nodule worm Oesophagostomum dentatum is a strongylid class V nematode rather closely related to the model organism Caenorhabditis elegans. However, in contrast to the non-parasitic C. elegans, the parasitic O. dentatum is an obligate sexual organism, which makes both a gender and developmental glycomic comparison possible.
Methods
Different enzymatic and chemical methods were used to release N-glycans from male and female O. dentatum as well as from L3 and L4 larvae. Glycans were analysed by MALDI-TOF MS after either 2D-HPLC (normal then reversed phase) or fused core RP-HPLC.
Results
Whereas the L3 N-glycome was simpler and more dominated by phosphorylcholine-modified structures, the male and female worms express a wide range of core fucosylated N-glycans with up to three fucose residues. Seemingly, simple methylated paucimannosidic structures can be considered ‘male’, while methylation of fucosylated glycans was more pronounced in females. On the other hand, while many of the fucosylated paucimannosidic glycans are identical with examples from other nematode species, but simpler than the tetrafucosylated glycans of C. elegans, there is a wide range of phosphorylcholine-modified glycans with extended HexNAc2-4PC2-4 motifs not observed in our previous studies on other nematodes.
Conclusion
The interspecies tendency of class V nematodes to share most, but not all, N-glycans applies also to O. dentatum; furthermore, we establish, for the first time in a parasitic nematode, that glycomes vary upon development and sexual differentiation.
General significance
Unusual methylated, core fucosylated and phosphorylcholine-containing N-glycans vary between stages and genders in a parasitic nematode.
Keywords: glycomics, mass spectrometry, HPLC, Nematoda, Strongylida
1. Introduction
Nematode parasites are widespread not only as tropical diseases of humans, livestock and crop plants, but also occur in temperate zones and, even in the earlier 20th century, nematodal gut infections were still very common in European and North American populations. Indeed, as the human immune system co-evolved with chronic nematode infections, the absence of worms may contribute to the imbalance associated with the growing numbers of patients with allergies or autoimmunity in developed countries [1–3]. Oesophagostomum dentatum is an intestinal parasite of pigs, but some related species also cause soil-transmitted nodular disease in humans, e.g., in parts of western and eastern Africa [4–6]. It is also a model for other strongylid species (e.g., hookworms) that infect humans as well as a wide range of socioeconomically important animals.
All nematodes have a lifecycle including four larval stages; in parasitic species, infection occurs frequently by ingestion of a particular larval stage and commonly results in accumulation of adults in the intestine. In the case of O. dentatum, it is the L3 larvae, which are ingested and then develop via the L4 stage, in nodules in the wall of the large intestine, into male and female adults which reside in the colon; this contrasts to the non-parasitic model Caenorhabditis elegans in which the L4 larvae primarily develop into hermaphrodites whereas males represent a very low proportion of the population and females are completely absent. Therefore, due to its dioecious nature (i.e., having two sexes) and the recent sequencing of its genome [7], O. dentatum represents a model to investigate both developmental and gender-specific aspects of gene and protein expression in nematodes; there is also evidence to suggest alterations in glycoconjugates during its lifecycle as judged by lectin binding [8].
Glycans on proteins and lipids on cell surfaces or in secretions often have important roles in cell-cell and host-pathogen interactions; in the case of nematode parasites, there is evidence to suggest that glycoconjugates are important immunomodulatory factors, which aid parasite survival by altering the Th1/Th2 balance of the host immune system. The best-documented example is probably the excretory-secretory glycoprotein ES-62 from Acanthocheilonema viteae. This protein, which carries phosphorylcholine modifications on its N-glycans, has been shown to have anti-inflammatory activity probably due to its interaction with Toll-like receptor 4 (TLR4) [9]. In the case of other parasites such as Trichuris suis, interactions of nematode glycans with host carbohydrate-binding proteins (including lectins such as the mannose receptor and DC-SIGN) have been described [10], which may in part explain the immunomodulatory potential of these species. O. dentatum is probably no exception, as heat-stable components (potentially carbohydrate) of extracts of this parasite suppress allergic responses in a mouse model [11].
In the present study, considering the unique possibility to isolate larvae and adults in reasonable quantities from pigs, we have performed a comprehensive N-glycomic analysis of various stages and both genders of O. dentatum. By using selective N-glycan release as well as 1D- or 2D-HPLC approaches, rather significant shifts in the structures expressed by not only adults as compared to larvae, but also males as compared to females, were observed. Furthermore, although many of the approximately 100 glycans are familiar from other nematodes, a number of novel structures were detected, which is another indication of phylum- and species-specific aspects of nematode glycosylation.
2. Experimental Procedures
2.1. Isolation of nematodes
Oesophagostomum dentatum (OD-Hann strain) was maintained in pigs; third-stage larvae (L3) were harvested from standard coproculture by micro-agar gel migration and exsheathed using hypochlorite as described [12]; fourth-stage larvae (L4) were derived from in vitro culture in the presence of pig serum after 14 days and separated from arrested L3 as described [13]. Adults were recovered from the large intestines of patently infected pigs by agar-gel migration [14] and separated into males and females visually. Batches of larvae or adults were washed 2-3 times in large quantities (50-150 ml) of 0.9% NaCl at 38°C, left for sedimentation, and after removal of the supernatant pellets of larvae/adults were snap frozen in liquid nitrogen and kept at -80°C until use.
2.2. Enzymatic release of N-glycans
After homogenisation followed by proteolysis with pepsin, glycopeptides were enriched by cation-exchange chromatography and gel filtration; N-glycans were then released using peptide:N-glycosidase F (PNGase F; Roche) as previously described [15], with a subsequent digestion of remaining glycopeptides using peptide:N-glycosidase A (PNGase A; Roche), or with PNGase A alone. N-glycans were prepared at least twice from each stage/gender from independent biological samples. Alternatively, samples were subject to hydrazinolysis as described below (see also the Scheme in the Supplement).
2.3. Hydrazinolysis of adult nematode glycopeptides
Male and female adults were homogenised and subject to proteolysis with thermolysin. After cation exchange and gel filtration chromatography, glycopeptides were transferred into a glass reaction tube and dried overnight prior to adding 500 µL of anhydrous hydrazine (prepared from monohydrate hydrazine; Sigma-Aldrich) and incubated at 100 °C for 5 h. Unreacted anhydrous hydrazine was removed by centrifugal evaporation. Samples were cooled to 0 °C and were re-N-acetylated by the addition of 100 mM sodium bicarbonate solution (200 μl) and acetic anhydride (21 μl) and incubated at 0 °C for 60 min. The samples were then acidified by addition of 5% TFA (600 μl) to the samples and incubated at 4°C for 60 min in order to liberate the reducing end of the glycans. Thereafter, the N-glycans were isolated from remaining (glyco)peptides by passage through a Dowex 50W×8 column (10 mL) and washing with 2% acetic acid (30 mL). Finally, the N-glycans were purified by solid-phase extraction first on non-porous graphitized carbon (elution with 40% acetonitrile with 0.1% TFA) and then on Lichroprep C18 (elution with water) prior to pyridylamination.
2.4. HPLC purification of N-glycans
Separation of PA-labelled glycans was carried out on a Shimadzu HPLC system equipped with a fluorescence detector (RF 10 AXL). In case of 1D-HPLC, an RP amide column (Ascentis Express, Supelco) was used with 100 mM ammonium acetate, pH 4.0 (buffer A) and 30% (v/v) methanol (buffer B) and a gradient of buffer B up to 35% over 34 minutes was applied at a flow rate of 0.8 ml/min as follows: 0-4 min, 0% B; 4-14 min, 0-5% B; 14-24 min, 5-15% B; 24-34 min, 15-35% B; 34-35 min, return to starting conditions [16]. For 2D-HPLC, normal-phase HPLC (Takara Palpak type N column) was performed with an inverse gradient of acetonitrile (gradient of 0.825% acetic acid/triethylamine, pH 7.3, in 74.5% acetonitrile up to 1.5% acetic acid/triethylamine, pH 7.3, in 50% acetonitrile from 5 to 40 minutes; 1 ml/min) prior to refractionation on Hypersil C18 (gradient of 1% methanol per minute using 0.1 M ammonium acetate, pH 4, as buffer; 1.5 ml/min) [15]. Glycans were detected by fluorescence with excitation/emission wavelengths of 320/400 or 310/380 nm. NP- and RP-HPLC columns were calibrated daily in terms of glucose units using a pyridylaminated dextran hydrolysate; the order of elution of the standards on the RP-amide column was confirmed by MALDI-TOF MS of collected calibrant fractions.
2.5. MALDI TOF MS analysis
The N-glycomes of the different stages or genders as well as all collected RP-HPLC peaks, were profiled by MALDI-TOF MS (Autoflex Speed, Bruker Daltonics, Germany; FlexControl 3.4 software) in positive ion mode using 6-aza-2-thiothymine (ATT) as matrix; MS/MS to confirm the composition of all proposed structures was performed by laser-induced dissociation (precursor ion selector was generally set to ±0.6%). The detector voltage was generally set at 1977 V for MS and 2133 V for MS/MS; 1000-5000 shots from different regions of the sample spots were summed. Spectra were processed with the manufacturer’s software (Bruker Flexanalysis 3.3.80) using the SNAP algorithm with a signal/noise threshold of 6 for MS (unsmoothed) and 3 for MS/MS (four-times smoothed). In total approximately 4000 MS and MS/MS spectra were manually interpreted on the basis of the mass and fragmentation pattern. At least five MS/MS fragment ions were used to aid definition of each of the structures.
2.6. Structural elucidation using exoglycosidases and chemical treatment
The following exoglycosidases were employed: recombinant β-galactosidase from Aspergillus niger (prepared in-house) [17], bovine kidney α-L-fucosidase (Sigma-Aldrich), recombinant Apis mellifera FDL β1,2-N-acetylglucosaminidase (prepared in-house; specific for the non-reducing terminal GlcNAc on the α1,3-arm) [18], Streptomyces plicatus β1,3/4/6-specific hexosaminidase (chitinase; New England Biolabs) [19], recombinant C. elegans HEX-4 β-N-acetylgalactosaminidase (prepared in-house) [18], jack bean α-mannosidase (Sigma-Aldrich) and recombinant Xanthomonas manihotis α1,2/3- and α1,6-specific mannosidases (New England Biolabs) [20]. In general, a 1-2 µl aliquot of a HPLC fraction was incubated with 0.2 µl exoglycosidase and 0.5 µl 100 mM ammonium acetate solution, pH 5.0, overnight at 37 °C (except for incubations with FDL which were performed for only 3 hours or with HEX-4 which were at pH 6.5). For removal of phosphodiesters or α1,3-linked fucose [21, 22], glycan samples were dried in a Speed-Vac and then incubated with 3 µl of 48% (w/v) hydrofluoric acid (HF) on ice for 24 hours prior to drying again. Chemically or enzymatically treated glycans were reanalysed by MALDI-TOF MS and MS/MS without further purification. Interpretations of the initial MS and MS/MS data before and after digest, under consideration of the RP-HPLC elution times, were applied to resolve isomeric structures.
3. Results
3.1. Overall comparisons of O. dentatum larval and adult N-glycomes
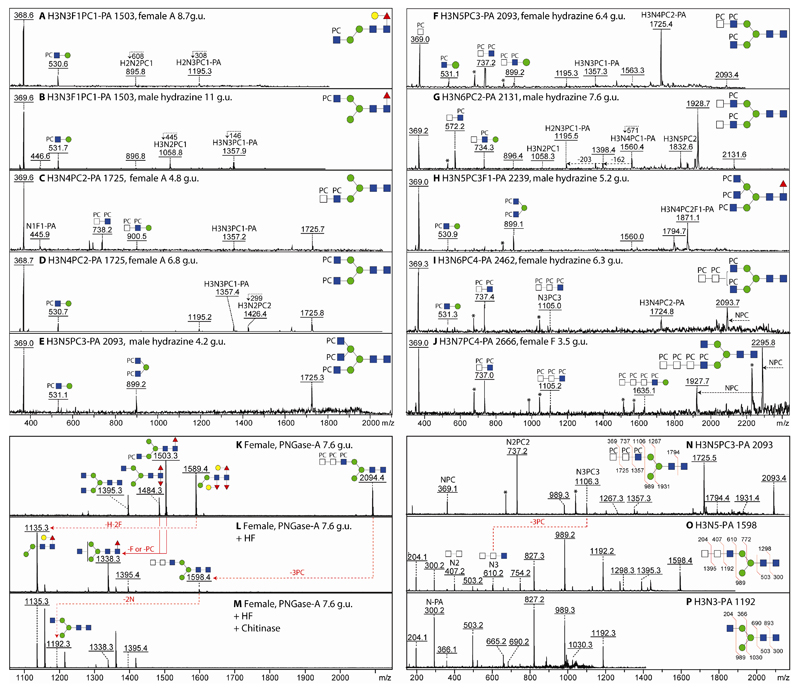
An initial N-glycomic comparison of larvae (L3 and L4) and adults (male and female) was made by examining the profiles of N-glycans released by PNGase A alone followed by pyridylamination (to introduce a fluorescent label), MALDI-TOF MS and NP-HPLC. While all samples show the presence of pauci- and oligomannosidic glycans typical of invertebrates (Hex3HexNAc2Fuc0-2 and Hex5-9HexNAc2; Supplementary Figure 1), there were further glycans with m/z values reminiscent of those of other nematodes. A first round of MS/MS indicated that m/z 1357, 1503, 1725 and 1871 in the L3 sample were probably modified with phosphorylcholine as judged by the presence of fragments of m/z 369 (HexNAc1PC1). In the L4 and particularly both adult samples, glycans of m/z 1265 and 1427 were obvious in the overall profiles and were predicted to be trifucosylated glycans (Hex2-3HexNAc2Fuc3). The impression was therefore that there was a higher amount of modification by phosphorylcholine, but a lower degree of multiple fucosylation in the L3 larvae as compared to the adults; furthermore, there were obvious differences in the relative intensities of glycans between the male and female as well as larval samples.
The further analysis was by 2D-HPLC (i.e., serial NP- and RP-HPLC on Palpak type N followed by Hypersil C18), which enables resolution of glycans by both size and isomeric status, thereby reducing complexity of single fractions. This procedure enabled calculation of relative amounts of structural isomers based on fluorescence intensity of the individual fractions. All RP-HPLC fractions from the 2D-HPLC runs were subject to MS and MS/MS; furthermore, most were treated with glycosidases or with hydrofluoric acid in order to verify the structural assignments. A selection of the resulting data is discussed below in order to illustrate the different N-glycan classes.
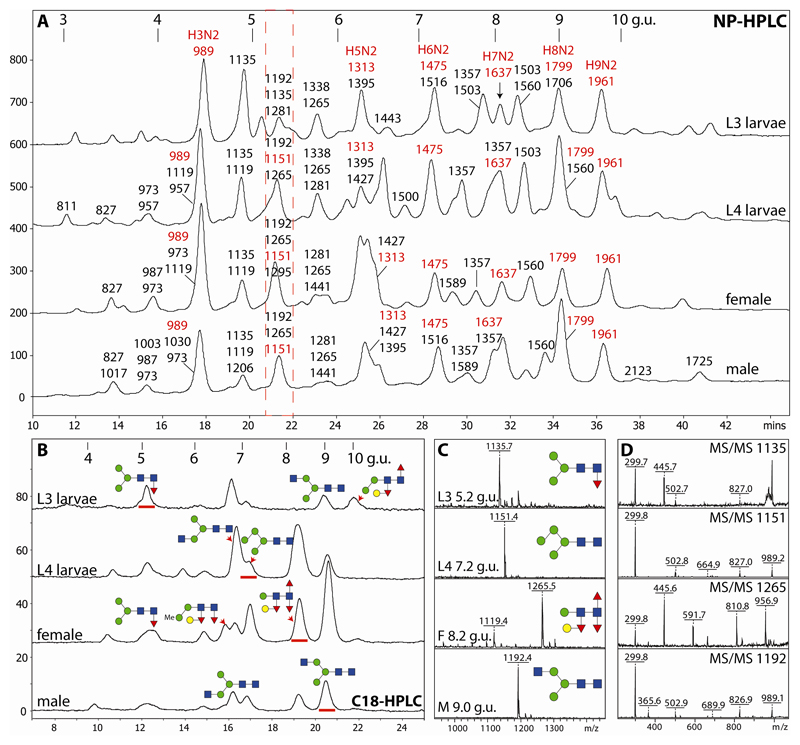
The initial NP-HPLC chromatograms and the subsequent off-line RP-HPLC-MALDI-TOF-MS analyses yielded further indications of N-glycomic variations between the samples (Figure 1 and Supplementary Table). Although there is a correlation between NP-HPLC retention time and mass, some modifications result in ‘aberrant’ elution; for instance, methylated glycans eluted earlier than expected for their mass (e.g., Hex3HexNAc3Me1-2, m/z 1003 and 1017, eluted earlier than the ‘parent’ Hex3HexNAc2, m/z 989; see ‘male’ NP-HPLC chromatogram), whereas phosphorylcholine-modified forms were more strongly retained (e.g., Hex3HexNAc3PC1, m/z 1357, almost co-eluted with Hex7HexNAc2, m/z 1637, which is a shift of over 2 g.u. as compared to the parent Hex3HexNAc3, m/z 1192). The position of the core modifications also appears to have an effect (e.g., core α1,6-fucosylation resulting in slightly earlier NP-HPLC elution than proximal core α1,3-fucosylation).
Figure 1. Two-dimensional HPLC of O. dentatum N-glycans.
(A) Pyridylaminated PNGase A-released N-glycans from larvae and adults were separated on a Palpak NP-HPLC column and each collected fraction was subjected to RP-HPLC on Hypersil C18 followed by MALDI-TOF MS; the major observed m/z values are indicated together with (in red) abbreviated compositions of mannosidic glycans (HxN2). (B) RP-HPLC analyses of the 5.2 g.u. NP-HPLC fractions (red dashed box in panel A) indicate differences in the co-eluting ‘size-fractionated’ glycans. (C and D) MALDI-TOF MS/MS of four selected glycans (as [M+H]+) of differing RP-HPLC retention times (underlined with red in panel B) isolated from larvae and adults; the proposed structures are shown using the Symbol Nomenclature for Glycans. NP- and RP-HPLC columns were externally calibrated daily in terms of glucose units (g.u.) with, in both cases, the smallest glucose oligomers eluting first as proven by MALDI-TOF MS of collected calibrant fractions (the g.u. on the C18 column differ from those for the RP amide column used for 1D-HPLC); fluorescence intensities are given in mV.
3.2. Comparison of N-glycan release methods for male and female adults
The analyses of the adult N-glycomes were repeated using a different fractionation approach, which aided separation of distinct structures without the need for relatively time-intensive 2D-HPLC. Thereby, a first pool of glycans was released in each case using PNGase F and the remaining glycopeptides were then incubated with PNGase A. After individually labelling both pools (see also the Scheme in the Supplement), the N-glycans were separated on a fused-core RP-amide column under conditions used recently for other nematode N-glycomes [16, 23]. The fused-core column was chosen for the 1D-HPLC approach, instead of the classical C18, due to its better resolution of isomers and its ability to isolate phosphorylcholine-containing glycans without resorting to high methanol concentrations. As only one HPLC step was performed, the potential for losing low abundance glycans is reduced; thus, in total, more structures were detected in each fraction. Therefore, quantification is less reliable as it depends not just on fluorescence intensity, but also on the ionisation in the MALDI-TOF MS instrument; thus only qualitative estimates of N-glycan abundance were made. As we have used the RP-amide column with Pristionchus, Haemonchus and Trichuris [16, 23, 24], we could retrospectively annotate some of the N-glycans of O. dentatum, whereas ‘unknown’ glycans as well as other selected structures were subject to chemical and/or enzymatic treatments.
As only PNGase A can remove N-glycans with core α1,3-fucose, there were differences in the MALDI-TOF MS and RP-amide HPLC profile as compared to the PNGase F-released pools (Figures 2 and 3) with trifucosylated glycans being solely present in the PNGase A pool and glycans with multiple phosphorylcholine residues being more pronounced in the PNGase F pool; this also applies to the larval L4 glycans (see Supplementary Figure 2). Hydrazinolysis on the other hand appeared to result in some partial demethylation of phosphorylcholine and a reduction in the amounts of core α1,3-fucosylated species, but did not reveal more complex core-modified structures (i.e., glycans with a hexose modification of core α1,3-fucose detected by others upon hydrazine-release of C. elegans N-glycans [25] and confirmed by our own unpublished observations) and so supported the conclusion that enzymatic release of N-glycans from O. dentatum is sufficient to assess the N-glycomic potential of male and female worms (see Supplementary Figure 3). In addition to N-glycans, some O-glycans including glycosaminoglycan cores (to be published at a later date) were released by hydrazine under the conditions used.
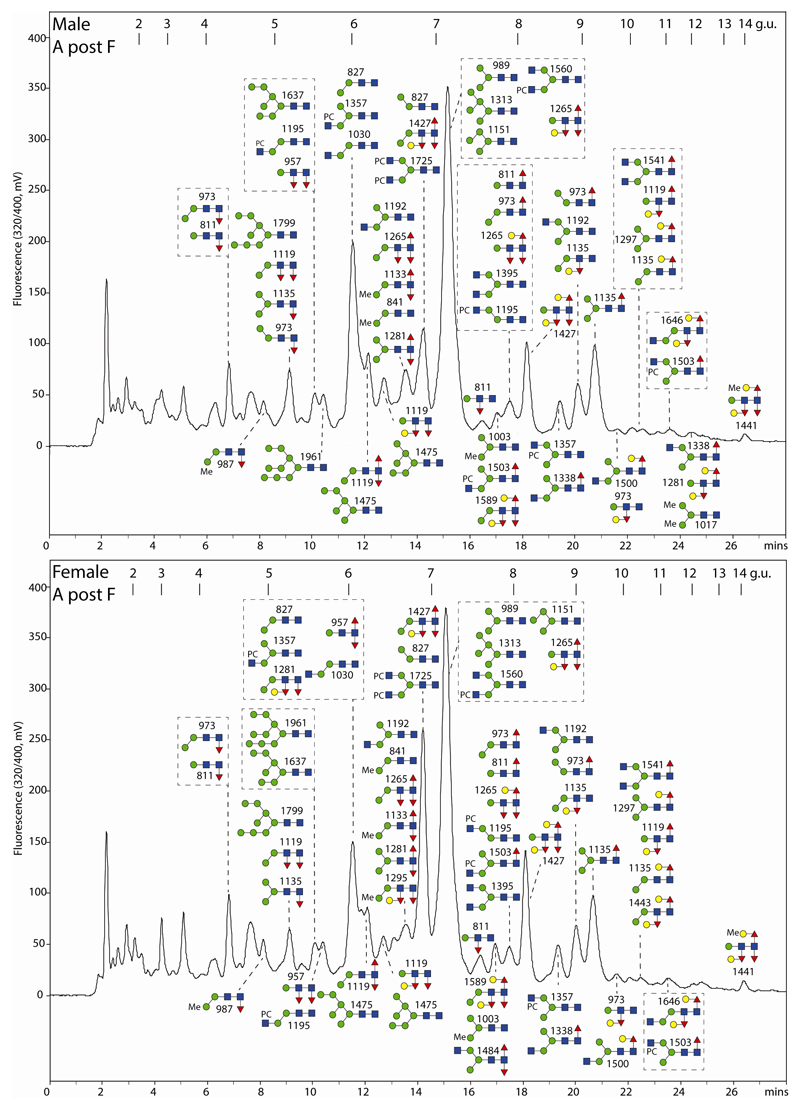
Figure 2. RP-amide HPLC of PNGase F-released O. dentatum N-glycans.
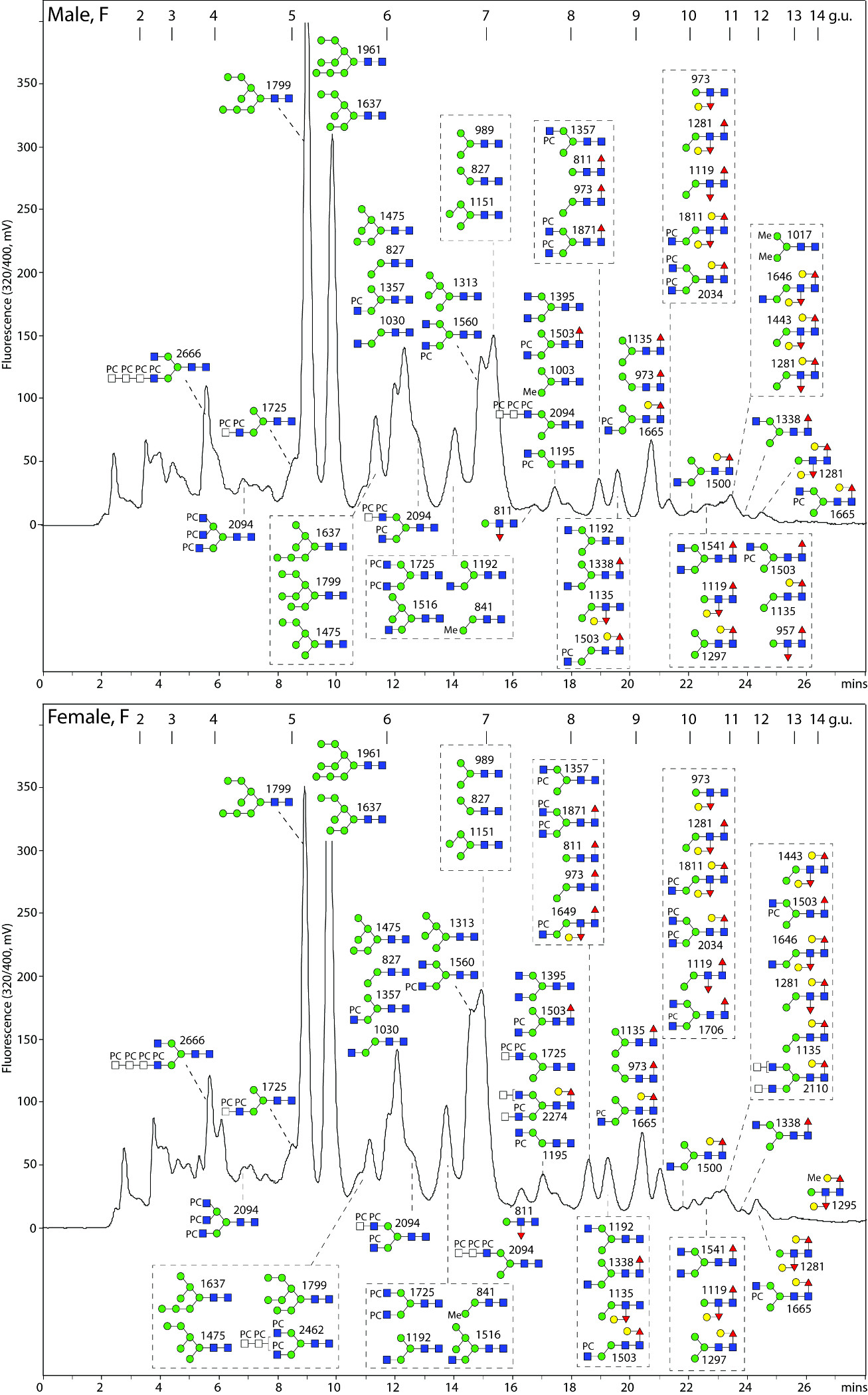
Pyridylaminated PNGase F-released N-glycans from male and female adults are shown annotated with the proposed structures (as confirmed by MS/MS and digestion data; see Figures 4-7 and the Supplementary Table); calibration is in terms of glucose units (g.u.). The PNGase F-released glycomes are dominated by oligomannosidic, α1,6-fucosylated, GalFuc-modified (late eluting) as well as phosphorylcholine-modified glycans.
Figure 3. RP-amide HPLC of PNGase A-released O. dentatum N-glycans.
Pyridylaminated PNGase A-released N-glycans (after separation from the PNGase F-released glycans) from male and female adults are shown annotated with the proposed structures (see Figures 4-7 and the Supplementary Table); calibration is in terms of glucose units (g.u.). There are a number of multi-fucosylated glycans as well as small, early-eluting α1,3-fucosylated structures, not present in the PNGase F-released pools.
3.3. Oligomannosidic N-glycans
The two most dominant glycans in the PNGase F-released male, female and L4 glycomes were Man8/9GlcNAc2, while in the PNGase A digests those residual oligomannosidic glycans present were primarily Man3-5GlcNAc2; the peak containing Man3-5GlcNAc2 was also dominant in the chromatograms of hydrazine-released glycans (see Figures 2 and 3 and Supplementary Figures 2 and 3). As such structures are well characterised in other organisms and relationship of the isomeric status with retention time in terms of glucose units using the same column and MS/MS is established for nematode and fungal oligomannosidic N-glycans [16, 26], the structural conclusions are summarised in the Supplementary Table. Overall, the oligomannosidic glycans account for between 27 and 52% of the N-glycomes (lowest in female and highest in L3 larvae).
3.4. Core fucosylated N-glycans
On the RP-amide column, the later-eluting PNGase F-released glycans as well as the majority of the PNGase A-released glycans were predicted, on the basis of MS and MS/MS data, to contain fucose. Signature core fragments of m/z 446 and 592 (HexNAc1Fuc1-2-PA) were very common as well as their hexosylated versions such as m/z 608 or 754 fragments (Hex1HexNAc1Fuc1-2-PA) which are familiar in the context of β1,4-galactosylation of the core α1,6-fucose as found in a number of invertebrates. While N-glycans with up to two fucose residues are found in the PNGase F digest (with either m/z 446 or 608 core fragments), the PNGase A digest contained structures with up to three fucoses with m/z 446, 592, 608 and 754 fragments (for example MS/MS spectra, see Figure 4). Thereby, as PNGase F cannot release core α1,3-fucosylated glycans, the second fucose in that pool was supposed, due to the similar elution times as especially found in C. elegans fut-1;fut-8 deletion mutant and wild-type strains [27], to be α1,3-linked on the distal (second core) GlcNAc in addition to the classical α1,6-fucose on the proximal GlcNAc (i.e., the reducing terminus). On the other hand, the trifucosylated PNGase A-released glycans were supposed to carry α1,3-fucose on both the distal and proximal GlcNAc residues in addition to the core α1,6-fucose. The different fucose modifications influence the behaviour on RP-HPLC columns. In general, α1,3-fucosylation of the proximal GlcNAc results in early retention on RP-HPLC; this effect is counteracted when the glycan is also α1,6-fucosylated [27]. Comparisons of O. dentatum elution times to those of proven structures from Pristionchus and Haemonchus were made [16, 23], whereas an analysis of an O. dentatum late-eluting fraction (containing glycans of m/z 1119, 1281, 1297, 1443, 1503, 1646) has been previously reported [28].
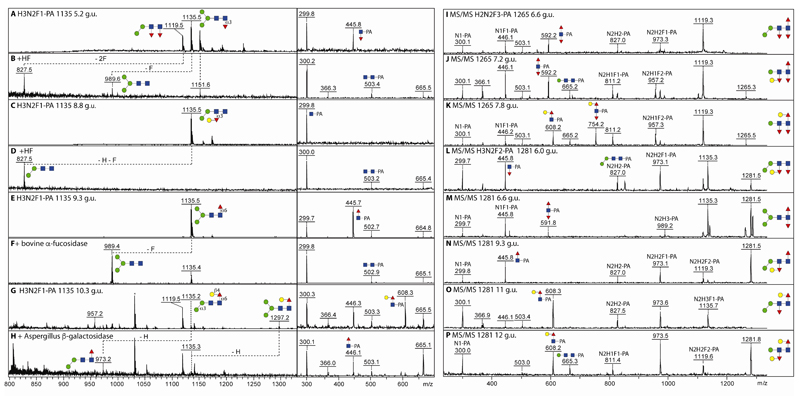
Figure 4. Analysis of fucosylated O. dentatum N-glycans by MALDI-TOF MS.
(A-H) Four isomers of Hex3HexNAc2Fuc1-PA (m/z 1135; PNGase A-released female pyridylaminated N-glycans) separated by RP-amide HPLC (with the indicated elution times in terms of glucose units) were analysed by MALDI-TOF MS ([M+H]+) and MS/MS before and after hydrofluoric acid, bovine α-fucosidase or Aspergillus β-galactosidase treatment; the losses of fucose or hexose are indicated (-F or -H). (I-K) MALDI-TOF MS/MS of RP-amide separated isomers of Hex2HexNAc2Fuc3-PA (m/z 1265) and Hex3HexNAc2Fuc2-PA (m/z 1281; L-P). Key fragments and proposed structures are annotated with a standard symbolic nomenclature or with compositions in terms of fucose (F), hexose (H) and N-acetylhexosamine (N). The presence of m/z 665 or 827 fragments correlates with either one or two mannose residues, whereas the m/z 592, 608 and 754 fragment ions are diagnostic for the modifications of the 2-aminopyridine-tagged reducing terminus (Fuc1-2Gal0-1GlcNAc1-PA).
In order to prove the assignments of fucosylated structures, digests with β-galactosidase, α-fucosidase and hydrofluoric acid were performed in combination with MS and MS/MS. Furthermore, to determine the isomeric status in terms of any terminal mannose residues, a specific α1,2/3-mannosidase was employed. Losses of 146 (Fuc), 162 (Hex), 308 (HexFuc) and 454 (Fuc+HexFuc) as well as relevant changes in the MS/MS spectra were used to resolve the individual structures; these data are summarised in the Supplementary Table, but some examples are shown in Figure 4.
In the case of Hex3HexNAc2Fuc1 (m/z 1135), a total of four isomers could be separated by HPLC with the proposed order of elution (proximal core α1,3-fucose, distal galactosylated α1,3-fucose, core α1,6-fucose and galactosylated α1,6-fucose) being familiar from glycomic studies on other nematodes [16, 23, 27]. Whereas hydrofluoric acid removed core α1,3-fucose or hexosylated distal fucose with an alteration in MS/MS obvious for the 5.2 g.u. glycan but not the 8.8 g.u. isomer (Figure 4 A-D), bovine α-fucosidase or Aspergillus β1,4-preferring galactosidase resulted in shifts in m/z and in the fragmentation spectra for the 9.3 and 10.3 g.u. species (Figure 4 E-H). Further combinations with galactosylation of either the proximal α1,6- or distal α1,3-fucose are obvious as deduced from the MS/MS data for the three isomers of m/z 1265 (Hex2HexNAc2Fuc3; Figure 4 I-K) and five isomers of m/z 1281 (Hex3HexNAc2Fuc2; Figure 4 L-P) with signature core fragments of m/z 300, 446, 592, 608 and 754 (Hex0-1HexNAc1Fuc0-2-PA). It should be noted that the distal modification is poorly seen in MS/MS (at best a very minor ‘rearrangement’ fragment ion is observed), whereas the proximal core α1,3-fucose is somewhat more labile than the α1,6-fucose, which means that the ratio of the m/z 300 to 446 ion intensities is itself an indication for the type of core fucosylation; the assumptions though are also verified by other methods (digest results and elution time; see also the Supplementary Table).
One of the most dominant masses in the male and female overall PNGase A-released MS profiles is m/z 1427 (Hex3HexNAc2Fuc3), but this mass occurs in two HPLC fractions indicative for isomeric forms (6.8 or 8.0 g.u; Figure 5A and E). While MS/MS of the former results in fragment ions of m/z 446 and 592 (HexNAc1Fuc1-2-PA), for the latter m/z 608 and 754 (Hex1HexNAc1Fuc1-2-PA) were observed; this pattern of elution and fragmentation was reminiscent of structures from P. pacificus and H. contortus with one or two hexosylated fucose residues respectively [16, 23]. In order to verify the structural assumption, α1,2/3-mannosidase, β-galactosidase and hydrofluoric acid treatments were performed. While in both cases, hydrofluoric acid removed two fucose and one hexose residue and resulted in changes in the core fragmentation patterns (Figure 5D and H), α1,3-mannosidase only removed a hexose from the 6.8 g.u. structure (Figure 5C), whereas β-galactosidase removed one residue from the 8.0 g.u. structure resulting in loss of the m/z 608 and 754 fragment ions (Figure 5I). Thus, the 8.0 g.u. structure carries galactosylated fucose α1,6-linked to the proximal GlcNAc and α1,3-linked to the distal GlcNAc, whereas the 6.8 g.u. form has only the distal galactosylated fucose modification; based on GC-MS and digestion data of this feature in C. elegans, a Galα1,2Fucα1,3 substitution of the distal GlcNAc is assumed [29]. These two glycans are major adult structures, also showing a gender bias, with 17% estimated combined abundance in female worms as opposed to 8% in males; see Supplementary Table). The most complex trifucosylated glycan (Hex4HexNAc2Fuc3; m/z 1589) is a ‘cross’ between the two forms of m/z 1427 as it possesses both an α1,3-mannose and a proximal GalFuc modification. As in C. elegans, no glycan with a distal core modification also contained an α1,6-mannose residue; on the other hand, no elongation of the proximal modification beyond Galβ1,4Fuc was observed in O. dentatum.
Figure 5. MALDI-TOF MS analysis of two Hex3HexNAc2Fuc3 isomers.
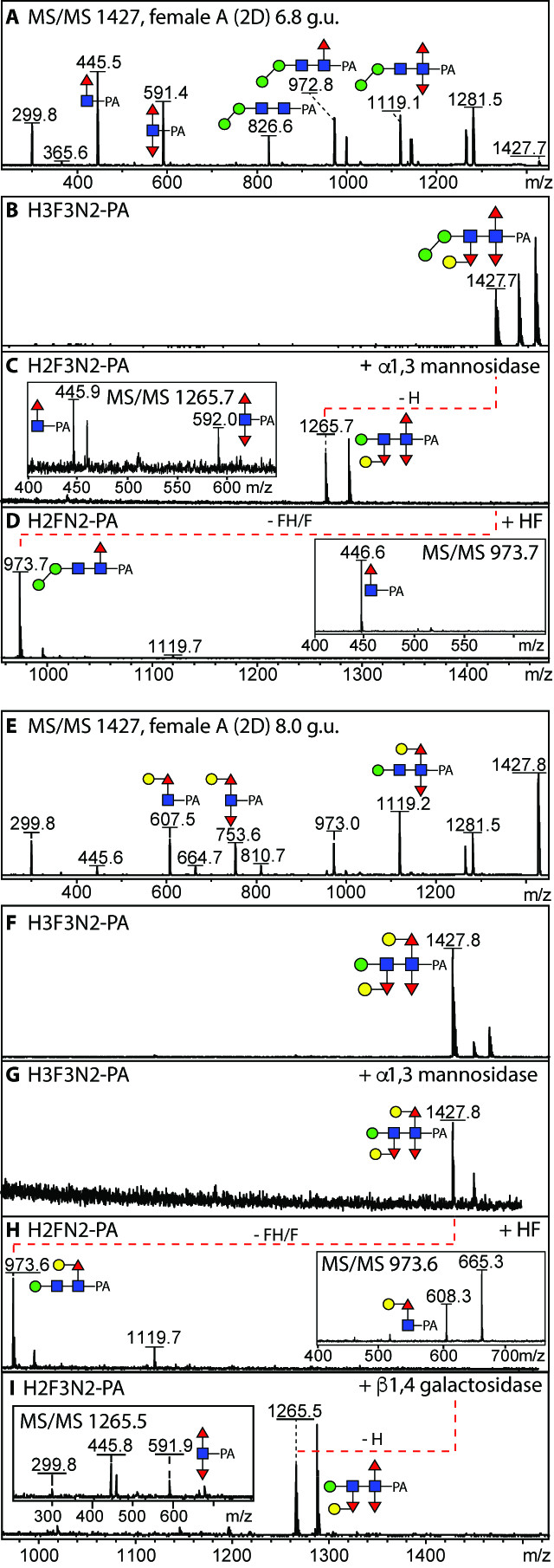
(A and E) MS/MS of 2D-HPLC separated isomers of Hex3HexNAc2Fuc3 (m/z 1427 as [M+H]+; 6.8 and 8.0 g.u. from the PNGase A-released female glycome) with key fragments symbolically annotated; this is the most dominant multiply-fucosylated composition in the O. dentatum N-glycome. (B-D and F-I) MALDI-TOF MS before and after incubation with either Xanthomonas α1,2/3-mannosidase, hydrofluoric acid or Aspergillus β-galactosidase; the effect of these treatments is indicated with the dashed lines and alterations in MS/MS spectra (insets). The first isomer (B) corresponds to the sixth most dominant N-glycan in Haemonchus, whereas the second (F) is a minor structure in Pristionchus.
3.5. Methylated N-glycans including female and male-specific structures
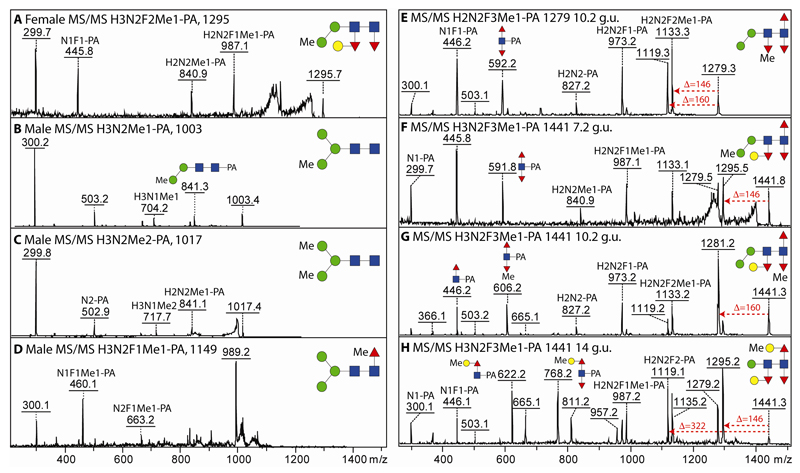
A thorough examination of the 2D-HPLC fractions revealed that some glycans were detected in females, but not in males and vice versa. Specifically, in the 2D-analysis, glycans of m/z 1003 and 1017 were present in males (Hex3HexNAc2Me1-2; 1.3% abundance) and a glycan of m/z 1295 in females (Hex3HexNAc2Fuc2Me1; 0.6% abundance); only trace amounts of these glycans were found in the opposite gender in the 1D-analysis. In these cases, MS/MS (Figure 6 A-C) and/or mannosidase resistance indicated that the methyl groups were associated with α-mannose residues; the third hexose of the m/z 1295 glycan is attached to the distal α1,3-fucose residue as judged by its removal along with both fucoses upon hydrofluoric acid treatment, while only one mannose could be removed by jack bean α-mannosidase from the monomethylated m/z 1003 structure (see Supplementary Table). Methylation of mannose is also proposed for minor glycans of m/z 841, 987, 1133 and 1206 (Hex2-3HexNAc2-3Fuc0-2Me1) detected in female and male glycomes.
Figure 6. MALDI-TOF MS/MS analysis of methylated N-glycans including gender-specific structures.
(A-D) MS and MS/MS of 2D-HPLC-separated PNGase A-released glycans specifically detected in either female (A) or male (B-D) N-glycomes. (E-H) MS/MS of selected RP-amide-separated methylated female PNGase A-released glycans highlighting the different methylation positions (on fucose, mannose or galactose) on one isomer of Hex2HexNAc2Fuc3Me1-PA and three isomers of Hex3HexNAc2Fuc3Me1-PA; the latter isomer (H) is also found in the male glycome. Example losses of fucose (146), methylfucose (160) or methylgalactose together with fucose (322) are shown. Relevant supporting digestion data (hydrofluoric acid or α-mannosidase) are summarised in the Supplementary Table.
However, there were other positions for methylation, as also α1,3-linked fucose and β1,4-galactose could also be methylated, especially in the female (3.3% as compared to 1.1% in the male). This is highlighted by a comparison of the MS/MS data for one isomer of m/z 1279 and three isomers of m/z 1441 (Hex3HexNAc2Fuc3Me1). In the first example, serial loss of fucose and methylfucose (or vice versa; Δm/z of 306) followed by a further loss of fucose verified the composition of Hex2HexNAc2Fuc3Me1; however, the methyl group is not on either proximal core fucose as judged by the fragment of m/z 592, but can be proposed to be on the distal core fucose for which only the ‘losses’ are detectable in MS/MS (Figure 6E). For the three HPLC-separated isomers of Hex3HexNAc2Fuc3Me1 (m/z 1441) the different core fragments of m/z 592 (HexNAc1Fuc2-PA), 606 (HexNAc1Fuc2Me1-PA) and 622/768 (HexNAc1Fuc1-2Hex1Me1-PA) as well as losses of 146, 160 or 322 were indicative of, respectively, a methylation of a non-core position, of a core fucose or of a galactose attached to core fucose (Figure 6 F-H), while an m/z 841 fragment is associated with methylation of an α-mannose. Hydrofluoric acid digestions of these structures with losses of 160 Da in the two cases where a methylated 3-linked fucose is proposed, supported the conclusions (Supplementary Table). One low abundance glycan in the male had a retention time, mass and fragmentation pattern (Hex3HexNAc2Fuc1Me1; m/z 1149) indicative of methylation of the core α1,6-fucose as in Pristionchus (fragment of m/z 460; Figure 6D). Obviously methylated structures are adult-specific as they were not detected in either larval stage.
3.6. Phosphorylcholine-modified N-glycans
A number of glycans were predicted to have modifications of 165 Da, which is indicative of the presence of phosphorylcholine, a phosphodiester occurring on the N-glycans of nematodes, cestodes and even a mollusc with proven sensitivity towards hydrofluoric acid [16, 23, 30, 31]. On the RP-amide column, modification with phosphorylcholine in general reduces the retention time (as compared to the parent structure), an effect even more pronounced for glycans with multiple phosphorylcholine residues [16]; this contrasts to the increased retention on a classical C18 column. The modification of GlcNAc residues with phosphorylcholine correlates with an intense signature MS/MS fragment of m/z 369 (HexNAc1PC1; see examples in Figure 7); other related fragments (m/z 531, 572 and 734) correspond to Hex0-1HexNAc1-2PC1 elements and are indicative of whether or not the phosphodiester is associated with a HexNAc2 moiety; further HexNAcPC motifs are indicated by fragments of m/z 737, 899, 1105 and 1635 (see below). In the case of m/z 1105 (HexNAc3PC3), this means that three HexNAc residues in series are each modified with phosphorylcholine, whereas the m/z 899 fragment (Hex1HexNAc2PC2) is compatible, in the absence of a HexNAc2PC2 fragment (m/z 737), with a disubstituted mannose carrying two HexNAc residues, as previously found in P. pacificus on a triantennary N-glycan [16]. As the ionisation properties of phosphorylcholine dominate the MS/MS spectra, the ‘backbone’ of these zwitterionic glycans can be judged only in part by the ‘losses’ of core GlcNAc1Fuc0-1-PA (Δm/z 299 or 445); otherwise hydrofluoric acid treatment to remove the phosphodiester can reveal the underlying parent structure, but the intensity of the relevant ions is then much reduced. In total, between 7 and 15% of the N-glycans are estimated to be modified with phosphorylcholine (lowest in the female adults and highest in L3 larvae).
Figure 7. Analysis of PC-containing O. dentatum N-glycans.
(A-J) MALDI-TOF MS/MS spectra of selected phosphorylcholine-modified N-glycans (as [M+H]+) separated by RP-amide HPLC. (K-P) MALDI-TOF MS and MS/MS of a phosphorylcholine-containing N-glycan present in the 7.6 g.u. fraction before and after treatment with hydrofluoric acid and chitinase; due to components in the enzyme preparation, sodiated adducts dominate, but only the protonated forms are annotated. In all panels, key fragments are annotated either symbolically or with abbreviations with fragment ions at m/z 369 being a signature for HexNAc1PC1, whereas m/z 737, 1105 and 1635 fragments are indicative of phosphorylcholine modifying two, three or four HexNAc residues in series; ions 59 mass units lower than a fragment containing multiple PC moieties are marked with an asterisk and could be due to loss of N(Me)3 from the choline moiety, whereas ambiguity in terms of the antennal configuration of the m/z 2462 structure is indicated by a bracket. The example data are of PNGase A-, F- or hydrazine-released glycans from female or male adults eluting from the RP-amide HPLC column at the indicated glucose units.
Up to four phosphorylcholine groups were present on the detected N-glycans with compositions ranging from Hex3HexNAc3Fuc0-1PC1 (m/z 1357 and 1503) through to Hex3HexNAc6-7PC4 (m/z 2462 and 2666), whereby in many cases the same masses were detected in multiple HPLC fractions. In part, the different elution times could be correlated to the fragmentation pattern or by reference to previous studies. For instance, there were three isomers of Hex3HexNAc3Fuc1PC1 (m/z 1503) distinguishable by either combined loss of a hexose and a fucose or only a fucose (for MS/MS of the glycans of 8.7 and 11 g.u., see Figure 7A and B). Based, respectively, on β-galactosidase digestion and elution time, it could be further concluded that the 8.7 g.u. form carries a galactosylated core fucose in addition to the phosphorylcholine-modified GlcNAc on the ‘lower’ α1,3-arm, whereas the 11 g.u. glycan has the zwitterionic modification on the ‘upper’ α1,6-arm. On the other hand, the two isomers of Hex3HexNAc4PC2 (m/z 1725) differed in terms of the presence of m/z 531 or 737 fragments (amongst others; Figure 7C and D). While the 6.8 g.u. isomer is concluded to be a typical biantennary glycan with one HexNAc1PC1 motif on each arm, akin to the structures seen in H. contortus and P. pacificus, the 4.8 g.u. form is concluded to carry a HexNAc2 motif on the lower arm with two phosphorylcholine groups. Other than the 8.7 g.u. isomer of m/z 1503, a number of other glycans carried both GalFuc and phosphorylcholine modifications (m/z 1649, 1665, 1811, 2034 and 2274; see Figure 2 and Supplementary Table).
The fragmentation patterns of the tri- and tetra-modified zwitterionic N-glycans were generally more complex to interpret. There were various elution positions of Hex3HexNAc5PC3 (m/z 2093) and the form eluting at 4.2 g.u. had an MS/MS spectrum more-or-less identical to the triantennary tri-modified glycan from P. pacificus as supported by the presence of m/z 531 and 899 ions (Figure 7E); on the other hand, MS/MS of the isomers eluting at 6.4 and 7.6 g.u. resulted in fragment ions at m/z 369, 737 and 899 or 1105 which are suggestive of two or three HexNAc1PC1 motifs in series (Figure 7F and N). The structural conclusions for the 7.6 g.u. glycan were verified by first releasing the phosphorylcholine using hydrofluoric acid (with the appearance of m/z 407 and 610 HexNAc2-3 B-fragment ions) and then treating with chitinase which removed two HexNAc residues (Figure 7K-P).
The fragments of m/z 531, 572, 734 or 737, 899, 1105 and 1635 indicated different combinations or juxtapositions of hexose, HexNAc and phosphorylcholine in the cases of the larger phosphorylcholine-modified glycans of m/z 2131, 2239, 2462 and 2666 (Figure 7G-J). Thus, it is concluded that glycans antennae with up to four HexNAc residues in series each modified with a phosphorylcholine residue can be detected in intact form in the glycomes of O. dentatum. As treatment with the specific HEX-4 N-acetylgalactosaminidase was generally negative, it can be assumed (but not specifically proven) that ‘GlcNAc-only’ HexNAc2-4 motifs underlie the antennal modifications.
4. Discussion
4.1. N-Glycomic analyses of O. dentatum
This study represents a comprehensive analysis of the N-glycome of Oesophagostomum dentatum; in total, four different pools were obtained for both male and female worms: (i) a ‘complete’ PNGase A-released N-glycome, (ii) a PNGase F-released partial N-glycome (lacking the core α1,3-fucosylated glycans), (iii) a PNGase A-released (post PNGase F) partial N-glycome (enriched in core α1,3-fucosylated glycans) and (iv) a hydrazine-released pool (see also the Scheme in the Supplement). Additionally, the N-glycomes of L3 and L4 larvae were analysed. Different chromatographic procedures (serial normal and reversed-phase HPLC or ‘one-dimensional’ HPLC on a fused core reversed phase column with calibration in terms of glucose units) in addition to MS/MS before and after hydrofluoric acid or glycosidase treatments were employed.
Our high resolution ‘multi-dimensional’ approach allowed identification of some one hundred structures of differing abundance in larvae and adults and extends a previous study in which we showed the presence of both proximal and distal galactosylated fucose (‘GalFuc’) residues in this and other nematode species [28]. Trifucosylation of the core of nematode N-glycans was first observed in H. contortus [32] and, thereafter, in C. elegans [25] and P. pacificus [16]. Interestingly, we detected neither Lewis-type fucosylation of antennae, as previously found in H. contortus and T. suis [23, 24], nor bisecting galactose or extensions of proximal GalFuc, as found in C. elegans [29]. Considering the absence of antennal fucosylation and that, other than the standard oligomannosidic and agalactosyl-bianntennary structures, all N-glycans from O. dentatum are unlike those of the host, there is no evidence for mimickry of host glycans as proposed for some parasites and other pathogens [33, 34].
Considering the ‘non-core’ motifs, extended phosphorylcholine-modified antennae were observed, which are shorter than the chito-oligomeric antennae concluded to occur in Onchocerca volvulus [35]. In that case, permethylated glycans with up to fifteen HexNAc residues were detected after hydrofluoric acid treatment which would strip any Lewis-type and phosphorylcholine modifications [35], whereas in an affinity-purified Trichinella spiralis sample the maximum appeared to be ten HexNAc residues with two core GlcNAc residues and up to four LacdiNAc motifs [36]; in the case of analyses with perdeuteroacetylated glycans, Hex3HexNAc5-6Fuc1PC1 are the largest nematode structures detected [35, 36]. Therefore, the Hex3HexNAc7PC4 glycan (m/z 2666) found in O. dentatum is probably the largest ‘native’ structure found to date in a nematode, which exceeds the Hex3HexNAc5Fuc1PC3 glycan (m/z 2240) found when applying a similar offline LC-MALDI approach to P. pacificus [16]. Furthermore, the combination of proximal and/or distal ‘GalFuc’ on the core and phosphorylcholine on the antenna (glycans of m/z 1503, 1649, 1665, 1811, 2034 and 2274; see the Supplementary Table and Figure 7A) has also previously not been described.
Methylation of glycans is a feature of some, but not all, nematodes. Thereby, mannose and/or fucose residues have been found in methylated form on N- and/or O-glycans of P. pacificus, C. elegans, Toxocara spp. and Heligmosomoides polygyrus [16, 27, 37, 38]; in O. dentatum, the galactose of the proximal GalFuc epitope can also be methylated. While methyl groups are ‘foreign’ to mammals, they are also known on the N-glycans of some molluscs [31, 39, 40] and of Planaria [41]. Such residues present an analytical challenge as they are generally resulting in resistance to digestion with known glycosidases, but differences corresponding to the mass of a methylated hexose (Δm/z 176) must also be carefully assessed to rule out the presence instead of glucuronic acid; however, glucuronylation does result in obvious signals in the negative ion mode, while methylation tends to cause late retention on reversed-phase HPLC columns.
4.2. Developmental glycomic alterations
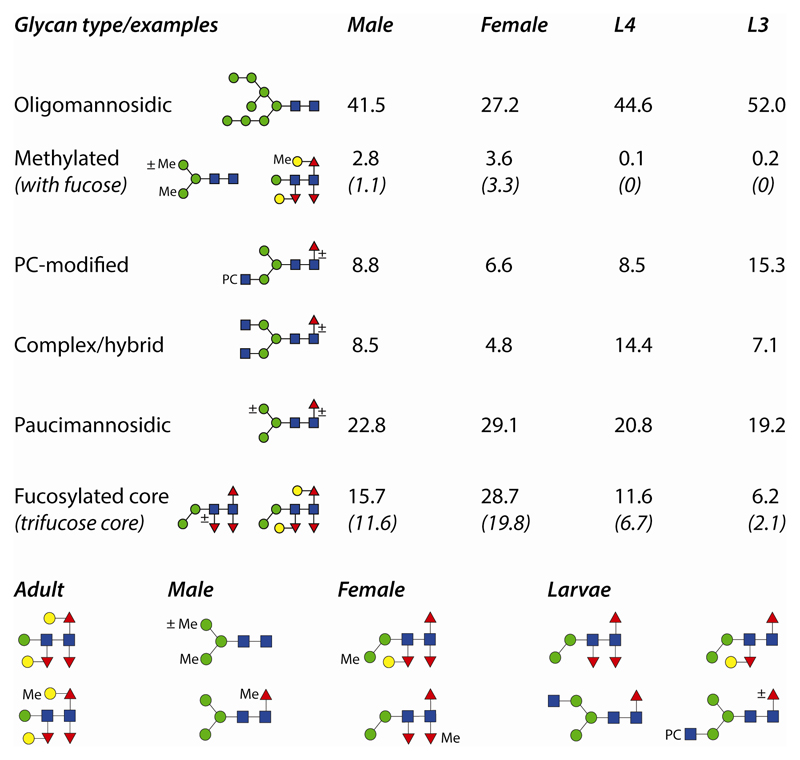
The basic trend was that the larval N-glycomes were simpler than the adult glycomes in terms of the complexity and number of detected glycan structures. Based on the fluorescent intensities of 2D-HPLC fractions (one preparation each), it was judged that the presence of oligomannosidic and phosphorylcholine-modified glycans was reduced by half (when comparing L3 larvae and female adults), that methylation increased from being absent in larvae to approximately 3% in both male and female adults and that core fucosylation also increased from 6% in L3 larvae to 15% in males and 28% in females (see also Figure 8 for a summary). These approximate quantifications from the 2D-HPLC analyses of PNGase A-released N-glycans were qualitatively borne out by the results of the other preparations. As noted above, exact quantification based on MALDI-TOF MS analyses of RP-amide fractions each containing a mixture of glycans would be inaccurate due to the varying ionisation potentials of different N-glycan classes.
Figure 8. Summary of occurrence and example structures of O. dentatum N-glycans.
Percentage occurrence of categories of N-glycans, together with example structures, are shown; the percentages for male and female adults as well as L3 and L4 larvae are based on the fluorescence intensity of 2D-HPLC fractions and indicate glycomic trends during development and differentiation. For the subcategories ‘methylated with fucose’ and ‘trifucose core’, the percentages in brackets show further distinctions in the gender and stage-specific glycomes. Also shown are examples of glycans with a specific or rather biased expression in either adults (regardless of gender), males, females or larvae.
In comparison to other studies on nematode glycomics, this is the first to examine differences between male and female adults; although this comparison is not possible in C. elegans (the populations tend to be over 99% hermaphrodite with far less than 1% male), parasitic worms tend to be dioecious. Nevertheless, other than Schistosoma mansoni [42], previous glycomic analyses on parasitic helminths, including our own, have only referred to ‘adults’ or to specific larval stages. However, rarely have comparisons been made within a single study and so conclusions are difficult, but it would appear that phosphorylcholine is present on N-glycans of L1 larvae (muscle stage) of T. spiralis, but is absent from the adults [43], whereas distal core fucosylation is probably lacking from L3 larvae of H. contortus [44]: the trend to a lower degree of phosphorylcholine modification and more core trifucosylation does hold for O. dentatum adults and, furthermore, methylation also significantly increases.
It has been suggested that the PNGase F-released N-glycomes of C. elegans L1 and dauer larvae are the most complex of six stages [45], but previous studies certainly underestimated the high heterogeneity of the wild-type adult glycome. For S. mansoni, which is a trematode and so expresses different N-glycans, reduced complexity (including loss of core xylose) and switch from Lewis-type to LacdiNAc antennae during development has been concluded [42]; however, differences in expression of fucosylated and non-fucosylated forms of LacNAc as opposed to LacdiNAc antennae between female and male S. mansoni have also been reported [46]. In the case of humans, sex- and age-dependent differences in IgG glycosylation have been observed [47], but the documented examples of ‘gender-specific’ glycosylation are rather of cell-type-specific processing of specific glycoproteins such as glycodelin or CD52 [48, 49]. In O. dentatum it appears that certain methylated structures are more-or-less male or female specific, whereas the female glycome is shifted to more distal core fucosylation and galactosylation of core fucose. Due to a lack of tools to locate or enrich methylated and certain core-modified glycans, it remains uncertain as to whether such differences are due to gonadal tissues or are more general in the organism.
4.3. Predictions of glycogenomic capacity and biological relevance
In order to generate this range of glycans, it would be expected that various N-acetylglucosaminyltransferases, α1,3- and α1,6-fucosyltransferases (at least homologues of C. elegans FUT-1, FUT-6 and FUT-8), phosphorylcholinyltransferases, sugar-modifying methyltransferases, galactosyltransferases (including a homologue of C. elegans core fucose-modifying GALT-1), Golgi α-mannosidases and β-hexosaminidase(s) are encoded by the O. dentatum genome. Indeed, searches of the partially annotated unplaced genomic scaffold sequences suggest that N-acetylglucosaminyltransferases I (potentially only one as compared to the three in C. elegans), II and V as well as multiple potential fucosyltransferases are encoded by the O. dentatum genome and so the occurrence of triantennary and fucosylated glycans is explicable. On the other hand, it may be that O. dentatum has fewer α1,2-fucosyltransferases and β1,4-galactosyltransferases than C. elegans due to the lack of bisecting β1,4-galactose and terminal α1,2-fucose residues on its N-glycans; the genomic basis for methylation or phosphorylcholination of nematode glycans is still unknown.
The overall impression from the glycomic data is that processing by Golgi glycosidases and transferases is higher in adults than in larvae, although there may be some reduction in transfer of phosphorylcholine residues after the L3 stage, multiply-fucosylation of cores and methylation are more pronounced in the adults. This may well have some biological relevance as it is the infective L3 stage and which traverses the intestinal mucosa to form nodules; after moulting in the nodules, the L4 larvae emerge and migrate back to the intestine. Certainly, phosphorylcholine modifications are considered to be immunomodulatory and, as the L3 larvae constitute a critical stage in the interaction with the host, it may be advantageous for this stage of the parasite to be covered in, or to secrete, glycoproteins modified by phosphorylcholine. Later, the core fucosylated and methylated N-glycans could be either targets of IgE [50] or be ‘resistant’ to digestion by glycosidases produced by the host or by intestinal bacteria; indeed, many invertebrates (including nematodes and molluscs) express methylated glycans which may serve a double function of being markers of ‘self’ as well as being resistant to catabolism by other organisms. It is also possible that the ‘investment’ in a higher degree of glycan processing (which requires a higher usage of activated transferase donors) is more ‘worthwhile’ for adults than for the shorter-lived larvae. It is not clear whether glycomic changes are governed at the transcriptional, post-transcriptional, translational or metabolic levels, but certainly a variety of other genes in O. dentatum have already been shown to display transcriptional alterations during development or upon sexual differentiation [7]. Analysis of the developmental and gender-specific N-glycomes of O. dentatum is thereby a starting point for further studies to determine how post-translational modifications of proteins have a role in the ecological and immunological aspects of host-parasite interactions.
Supplementary Data
A supplementary Scheme shows the overall workflows, Supplementary Figures the overall MALDI-TOF MS profiles (S1), RP-amide chromatograms for L4 larvae N-glycomes (S2) and RP-chromatograms of hydrazine-released N-glycans (S3) and a Supplementary Table summarises the data including key fragments before and after chemical/enzymatic treatments.
Highlights.
Differential release and HPLC separation of 100 N-glycans from a parasitic nematode
MS/MS and digestions demonstrate a number of novel N-glycan structures
Glycan processing increases between larval and adult stages
Adult-specific methylation and core modifications vary between male and female worms
Acknowledgments
This work was funded by a grant from the Austrian Science Fund (FWF) to K.P. (grant P21946); J.V. is supported by the GlycoPar Marie-Curie Initial Training Network (European Union; PITN-GA-2013-608295). The authors thank Karin Hummel for performing some of the initial mass spectrometric analyses, Martin Dragosits for recombinant enzymes and Iain Wilson for help with preparation of the manuscript.
References
- [1].Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- [3].Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- [4].Polderman AM, Krepel HP, Baeta S, Blotkamp J, Gigase P. Oesophagostomiasis, a common infection of man in northern Togo and Ghana. Am J Trop Med Hyg. 1991;44:336–344. doi: 10.4269/ajtmh.1991.44.336. [DOI] [PubMed] [Google Scholar]
- [5].Polderman AM, Blotkamp J. Oesophagostomum infections in humans. Parasitol Today. 1995;11:451–456. doi: 10.1016/0169-4758(95)80058-1. [DOI] [PubMed] [Google Scholar]
- [6].Ghai RR, Chapman CA, Omeja PA, Davies TJ, Goldberg TL. Nodule worm infection in humans and wild primates in Uganda: cryptic species in a newly identified region of human transmission. PLoS Negl Trop Dis. 2014;8:e2641. doi: 10.1371/journal.pntd.0002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tyagi R, Joachim A, Ruttkowski B, Rosa BA, Martin JC, Hallsworth-Pepin K, Zhang X, Ozersky P, Wilson RK, Ranganathan S, Sternberg PW, et al. Cracking the nodule worm code advances knowledge of parasite biology and biotechnology to tackle major diseases of livestock. Biotechnol Adv. 2015;33:980–991. doi: 10.1016/j.biotechadv.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Joachim A, Ruttkowski B, Daugschies A. Characterisation of stage-specific proteins of Oesophagostomum dentatum by preparative isoelectric focusing and lectin blotting. Parasitol Int. 2001;50:41–45. doi: 10.1016/s1383-5769(00)00070-2. [DOI] [PubMed] [Google Scholar]
- [9].Pineda MA, Lumb F, Harnett MM, Harnett W. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. Mol Biochem Parasitol. 2014;194:1–8. doi: 10.1016/j.molbiopara.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [10].Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, Cummings RD, Kraal G, van Die I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. 2013;43:191–200. doi: 10.1016/j.ijpara.2012.10.021. [DOI] [PubMed] [Google Scholar]
- [11].Schabussova I, Ul-Haq O, Hoflehner E, Akgun J, Wagner A, Loupal G, Joachim A, Ruttkowski B, Maizels RM, Wiedermann U. Oesophagostomum dentatum extract modulates T cell-dependent immune responses to bystander antigens and prevents the development of allergy in mice. PLoS One. 2013;8:e67544. doi: 10.1371/journal.pone.0067544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Daugschies A, Watzel C. In vitro development of histotropic larvae of Oesophagostomum dentatum under various conditions of cultivation. Parasitol Res. 1999;85:158–161. doi: 10.1007/s004360050527. [DOI] [PubMed] [Google Scholar]
- [13].Joachim A, Ruttkowski B, Daugschies A. Changes in antigen and glycoprotein patterns during the development of Oesophagostomum dentatum. Int J Parasitol. 1998;28:1853–1860. doi: 10.1016/s0020-7519(98)00163-5. [DOI] [PubMed] [Google Scholar]
- [14].Slotved HC, Barnes EH, Bjørn H, Christensen CM, Eriksen L, Roepstorff A, Nansen P. Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet Parasitol. 1996;63:237–245. doi: 10.1016/0304-4017(95)00916-7. [DOI] [PubMed] [Google Scholar]
- [15].Paschinger K, Hykollari A, Razzazi-Fazeli E, Greenwell P, Leitsch D, Walochnik J, Wilson IBH. The N-glycans of Trichomonas vaginalis contain variable core and antennal modifications. Glycobiology. 2012;22:300–313. doi: 10.1093/glycob/cwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yan S, Wilson IBH, Paschinger K. Comparison of RP-HPLC modes to analyse the N-glycome of the free-living nematode Pristionchus pacificus. Electrophoresis. 2015;36:1314–1329. doi: 10.1002/elps.201400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dragosits M, Pflugl S, Kurz S, Razzazi-Fazeli E, Wilson IBH, Rendić D. Recombinant Aspergillus β-galactosidases as a robust glycomic and biotechnological tool. Appl Microbiol Biotechnol. 2014;98:3553–3567. doi: 10.1007/s00253-013-5192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dragosits M, Yan S, Razzazi-Fazeli E, Wilson IBH, Rendić D. Enzymatic properties and subtle differences in the substrate specificity of phylogenetically distinct invertebrate N-glycan processing hexosaminidases. Glycobiology. 2015;25:448–464. doi: 10.1093/glycob/cwu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robbins PW, Overbye K, Albright C, Benfield B, Pero J. Cloning and high-level expression of chitinase-encoding gene of Streptomyces plicatus. Gene. 1992;111:69–76. doi: 10.1016/0378-1119(92)90604-n. [DOI] [PubMed] [Google Scholar]
- [20].Wong-Madden ST, Landry D. Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology. 1995;5:19–28. doi: 10.1093/glycob/5.1.19. [DOI] [PubMed] [Google Scholar]
- [21].Schneider P, Ferguson MAJ. Microscale analysis of glycosylphosphatidylinositol structures. Methods Enzymol. 1995;250:614–630. doi: 10.1016/0076-6879(95)50100-2. [DOI] [PubMed] [Google Scholar]
- [22].Haslam SM, Coles GC, Morris HR, Dell A. Structural characterisation of the N-glycans of Dictyocaulus viviparus: discovery of the Lewisx structure in a nematode. Glycobiology. 2000;10:223–229. doi: 10.1093/glycob/10.2.223. [DOI] [PubMed] [Google Scholar]
- [23].Paschinger K, Wilson IBH. Two types of galactosylated fucose motifs are present on N-glycans of Haemonchus contortus. Glycobiology. 2015;25:585–590. doi: 10.1093/glycob/cwv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wilson IBH, Paschinger K. Sweet secrets of a therapeutic worm: Mass spectrometric N-glycomic analysis of Trichuris suis. Anal Bioanal Chem. 2016;408:461–471. doi: 10.1007/s00216-015-9154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hanneman AJ, Rosa JC, Ashline D, Reinhold V. Isomer and glycomer complexities of core GlcNAcs in Caenorhabditis elegans. Glycobiology. 2006;16:874–890. doi: 10.1093/glycob/cwl011. [DOI] [PubMed] [Google Scholar]
- [26].Hykollari A, Eckmair B, Voglmeir J, Jin C, Yan S, Vanbeselaere J, Razzazi-Fazeli E, Wilson IBH, Paschinger K. More than just oligomannose: An N-glycomic comparison of Penicillium species. Mol Cell Proteomics. 2016;15:73–92. doi: 10.1074/mcp.M115.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yan S, Jin C, Wilson IBH, Paschinger K. Comparisons of Caenorhabditis fucosyltransferase mutants reveal a multiplicity of isomeric N-glycan structures. J Proteome Res. 2015;14:5291–5305. doi: 10.1021/acs.jproteome.5b00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yan S, Bleuler-Martinez S, PlazaGutierrez DF, Künzler M, Aebi M, Joachim A, Razzazi-Fazeli E, Jantsch V, Geyer R, Wilson IBH, Paschinger K. Galactosylated fucose epitopes in nematodes: increased expression in a Caenorhabditis mutant associated with altered lectin sensitivity and occurrence in parasitic species. J Biol Chem. 2012;287:28276–28290. doi: 10.1074/jbc.M112.353128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yan S, Brecker L, Jin C, Titz A, Dragosits M, Karlsson N, Jantsch V, Wilson IBH, Paschinger K. Bisecting galactose as a feature of N-glycans of wild-type and mutant Caenorhabditis elegans. Mol Cell Proteomics. 2015;14:2111–2125. doi: 10.1074/mcp.M115.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Paschinger K, Gonzalez-Sapienza GG, Wilson IBH. Mass spectrometric analysis of the immunodominant glycan epitope of Echinococcus granulosus antigen Ag5. Int J Parasitol. 2012;42:279–285. doi: 10.1016/j.ijpara.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eckmair B, Jin C, Abed-Navandi D, Paschinger K. Multi-step fractionation and mass spectrometry reveals zwitterionic and anionic modifications of the N- and O-glycans of a marine snail. Mol Cell Proteomics. 2016;15:573–597. doi: 10.1074/mcp.M115.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Haslam SM, Coles GC, Munn EA, Smith TS, Smith HF, Morris HR, Dell A. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J Biol Chem. 1996;271:30561–30570. doi: 10.1074/jbc.271.48.30561. [DOI] [PubMed] [Google Scholar]
- [33].Yoshino TP, Wu XJ, Gonzalez LA, Hokke CH. Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp Parasitol. 2013;133:28–36. doi: 10.1016/j.exppara.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haslam SM, Houston KM, Harnett W, Reason AJ, Morris HR, Dell A. Structural studies of N-glycans of filarial parasites. Conservation of phosphorylcholine-substituted glycans among species and discovery of novel chito-oligomers. J Biol Chem. 1999;274:20953–20960. doi: 10.1074/jbc.274.30.20953. [DOI] [PubMed] [Google Scholar]
- [36].Morelle W, Haslam SM, Olivier V, Appleton JA, Morris HR, Dell A. Phosphorylcholine-containing N-glycans of Trichinella spiralis: identification of multiantennary lacdiNAc structures. Glycobiology. 2000;10:941–950. doi: 10.1093/glycob/10.9.941. [DOI] [PubMed] [Google Scholar]
- [37].Khoo K-H, Maizels RM, Page AP, Taylor GW, Rendell NB, Dell A. Characterisation of nematode glycoproteins: the major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology. 1991;1:163–171. doi: 10.1093/glycob/1.2.163. [DOI] [PubMed] [Google Scholar]
- [38].Hewitson JP, Nguyen DL, van Diepen A, Smit CH, Koeleman CA, McSorley HJ, Murray J, Maizels RM, Hokke CH. Novel O-linked methylated glycan antigens decorate secreted immunodominant glycoproteins from the intestinal nematode Heligmosomoides polygyrus. Int J Parasitol. 2016;46:157–170. doi: 10.1016/j.ijpara.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kurz S, Jin C, Hykollari A, Gregorich D, Giomarelli B, Vasta GR, Wilson IBH, Paschinger K. Haemocytes and plasma of the eastern oyster (Crassostrea virginica) display a diverse repertoire of sulphated and blood group A-modified N-glycans. J Biol Chem. 2013;288:24410–24428. doi: 10.1074/jbc.M113.478933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JFG, Wood EJ. Primary structure of a low-molecular-mass N-linked oligosaccharide from hemocyanin of Lymnaea stagnalis 3-O-methyl-D-mannose as a constituent of the xylose-containing core structure in an animal glycoprotein. Eur J Biochem. 1986;160:621–625. doi: 10.1111/j.1432-1033.1986.tb10083.x. [DOI] [PubMed] [Google Scholar]
- [41].Paschinger K, Razzazi-Fazeli E, Furukawa K, Wilson IBH. Presence of galactosylated core fucose on N-glycans in the planaria Dugesia japonica. J Mass Spectrom. 2011;46:561–567. doi: 10.1002/jms.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smit CH, van Diepen A, Nguyen DL, Wuhrer M, Hoffmann KF, Deelder AM, Hokke CH. Glycomic Analysis of Life Stages of the Human Parasite Schistosoma mansoni Reveals Developmental Expression Profiles of Functional and Antigenic Glycan Motifs. Mol Cell Proteomics. 2015;14:1750–1769. doi: 10.1074/mcp.M115.048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morelle W, Haslam SM, Morris HR, Dell A. Characterization of the N-linked glycans of adult Trichinella spiralis. Mol Biochem Parasitol. 2000;109:171–177. doi: 10.1016/s0166-6851(00)00241-3. [DOI] [PubMed] [Google Scholar]
- [44].Haslam SM, Coles GC, Reason AJ, Morris HR, Dell A. The novel core fucosylation of Haemonchus contortus N-glycans is stage specific. Mol Biochem Parasitol. 1998;93:143–147. doi: 10.1016/s0166-6851(98)00020-6. [DOI] [PubMed] [Google Scholar]
- [45].Cipollo JF, Awad A, Costello CE, Hirschberg CB. N-glycans of Caenorhabditis elegans are specific to developmental stages. J Biol Chem. 2005;280:26063–26072. doi: 10.1074/jbc.M503828200. [DOI] [PubMed] [Google Scholar]
- [46].Wuhrer M, Koeleman CA, Fitzpatrick JM, Hoffmann KF, Deelder AM, Hokke CH. Gender-specific expression of complex-type N-glycans in schistosomes. Glycobiology. 2006;16:991–1006. doi: 10.1093/glycob/cwl020. [DOI] [PubMed] [Google Scholar]
- [47].Krištić J, Vučković F, Menni C, Klarić L, Keser T, Beceheli I, Pučić-Baković M, Novokmet M, Mangino M, Thaqi K, Rudan P, et al. Glycans are a novel biomarker of chronological and biological ages. J Gerontol A Biol Sci Med Sci. 2014;69:779–789. doi: 10.1093/gerona/glt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Morris HR, Dell A, Easton RL, Panico M, Koistinen H, Koistinen R, Oehninger S, Patankar MS, Seppala M, Clark GF. Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J Biol Chem. 1996;271:32159–32167. doi: 10.1074/jbc.271.50.32159. [DOI] [PubMed] [Google Scholar]
- [49].Schröter S, Derr P, Conradt HS, Nimtz M, Hale G, Kirchhoff C. Male-specific modification of human CD52. J Biol Chem. 1999;274:29862–29873. doi: 10.1074/jbc.274.42.29862. [DOI] [PubMed] [Google Scholar]
- [50].van Die I, Gomord V, Kooyman FNJ, van der Berg TK, Cummings RD, Vervelde L. Core α 1→ 3-fucose is a common modification of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.