Abstract
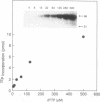
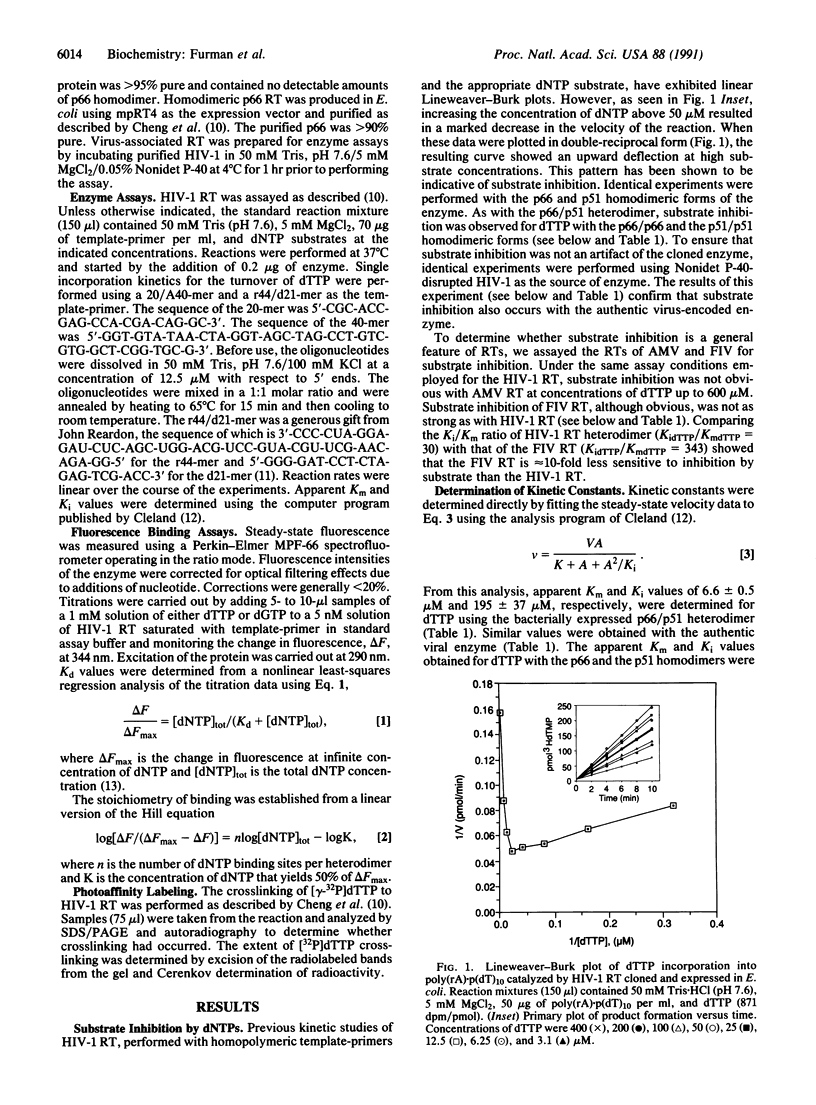
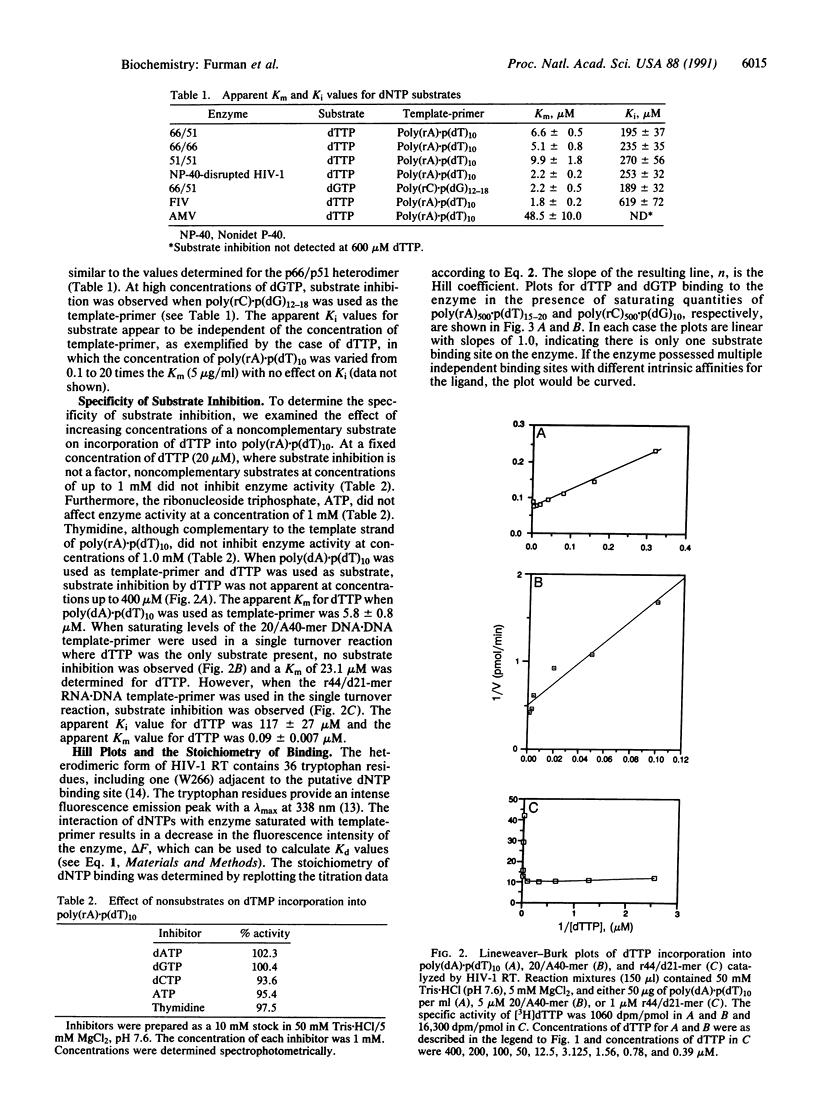
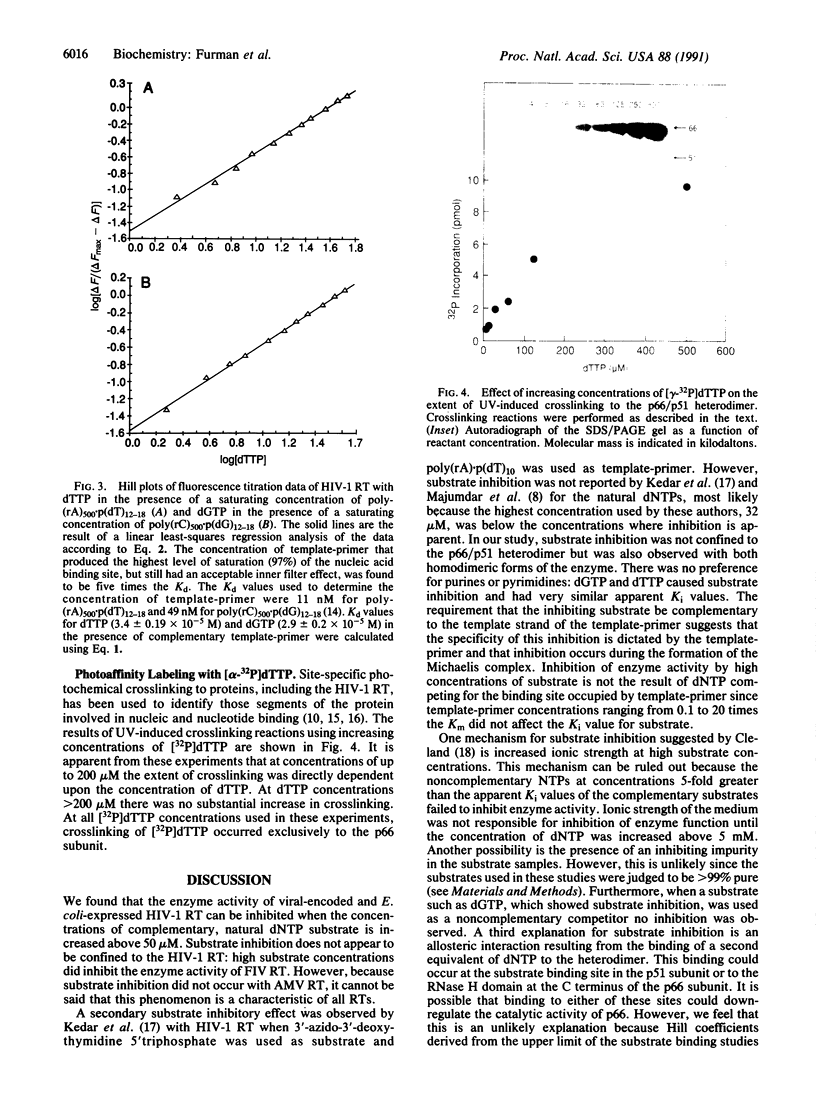
Substrate inhibition was observed with the heterodimeric (p66/p51) and the homodimeric (p66/p66, p51/p51) forms of human immunodeficiency virus type 1 reverse transcriptase (RNA-dependent DNA polymerase, EC 2.7.7.49). An apparent Ki value of 195 +/- 37 microM was determined for dTTP using the bacterial cloned and expressed heterodimer. Similar values were obtained with the homodimeric and the virus-encoded enzymes. When poly-(rC).p(dG)10 was used as template-primer, dGTP exhibited substrate inhibition with an apparent Ki value of 189 +/- 32 microM. Substrate inhibition was not observed with dTTP when DNA.DNA template-primers were used. Hill coefficients for substrate binding determined in the presence of saturating concentrations of template-primer were equal to 1.0, suggesting that substrate inhibition of the heterodimer is not the result of an allosteric mechanism involving the p51 subunit. Furthermore, UV crosslinking experiments with [gamma-32P]dTTP showed crosslinking only to the p66 subunit. Substrate inhibition was not as pronounced with other retroviral reverse transcriptases as it was with human immunodeficiency type 1 reverse transcriptase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A., Tirumalai R. S., Modak M. J. Substrate binding in human immunodeficiency virus reverse transcriptase. An analysis of pyridoxal 5'-phosphate sensitivity and identification of lysine 263 in the substrate-binding domain. J Biol Chem. 1989 May 25;264(15):8746–8752. [PubMed] [Google Scholar]
- Cheng N., Painter G. R., Furman P. A. Crosslinking of substrates occurs exclusively to the p66 subunit of heterodimeric HIV-1 reverse transcriptase. Biochem Biophys Res Commun. 1991 Jan 31;174(2):785–789. doi: 10.1016/0006-291x(91)91486-v. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Hansen J., Schulze T., Moelling K. RNase H activity associated with bacterially expressed reverse transcriptase of human T-cell lymphotropic virus III/lymphadenopathy-associated virus. J Biol Chem. 1987 Sep 15;262(26):12393–12396. [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedar P. S., Abbotts J., Kovács T., Lesiak K., Torrence P., Wilson S. H. Mechanism of HIV reverse transcriptase: enzyme-primer interaction as revealed through studies of a dNTP analogue, 3'-azido-dTTP. Biochemistry. 1990 Apr 17;29(15):3603–3611. doi: 10.1021/bi00467a003. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Larder B., Purifoy D., Powell K., Darby G. AIDS virus reverse transcriptase defined by high level expression in Escherichia coli. EMBO J. 1987 Oct;6(10):3133–3137. doi: 10.1002/j.1460-2075.1987.tb02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoote M. M., Coligan J. E., Folks T. M., Fauci A. S., Martin M. A., Venkatesan S. Structural characterization of reverse transcriptase and endonuclease polypeptides of the acquired immunodeficiency syndrome retrovirus. J Virol. 1986 Nov;60(2):771–775. doi: 10.1128/jvi.60.2.771-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar C., Abbotts J., Broder S., Wilson S. H. Studies on the mechanism of human immunodeficiency virus reverse transcriptase. Steady-state kinetics, processivity, and polynucleotide inhibition. J Biol Chem. 1988 Oct 25;263(30):15657–15665. [PubMed] [Google Scholar]
- Merluzzi V. J., Hargrave K. D., Labadia M., Grozinger K., Skoog M., Wu J. C., Shih C. K., Eckner K., Hattox S., Adams J. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990 Dec 7;250(4986):1411–1413. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Modak M. J., Gillerman-Cox E. Biochemistry of terminal deoxynucleotidyl transferase. Conditions for and characterization of ultraviolet light mediated substrate cross-linking to terminal deoxynucleotidyl transferase. J Biol Chem. 1982 Dec 25;257(24):15105–15109. [PubMed] [Google Scholar]
- Pauwels R., Andries K., Desmyter J., Schols D., Kukla M. J., Breslin H. J., Raeymaeckers A., Van Gelder J., Woestenborghs R., Heykants J. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990 Feb 1;343(6257):470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- Reardon J. E., Miller W. H. Human immunodeficiency virus reverse transcriptase. Substrate and inhibitor kinetics with thymidine 5'-triphosphate and 3'-azido-3'-deoxythymidine 5'-triphosphate. J Biol Chem. 1990 Nov 25;265(33):20302–20307. [PubMed] [Google Scholar]
- Tisdale M., Ertl P., Larder B. A., Purifoy D. J., Darby G., Powell K. L. Characterization of human immunodeficiency virus type 1 reverse transcriptase by using monoclonal antibodies: role of the C terminus in antibody reactivity and enzyme function. J Virol. 1988 Oct;62(10):3662–3667. doi: 10.1128/jvi.62.10.3662-3667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M., Larder B. A., Lowe D. M., Stammers D. K., Purifoy D. J., Ertl P., Bradley C., Kemp S., Darby G. K., Powell K. L. Structural characterization of HIV reverse transcriptase: a target for the design of specific virus inhibitors. J Antimicrob Chemother. 1989 Jan;23 (Suppl A):47–54. doi: 10.1093/jac/23.suppl_a.47. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]