Abstract
The transcription factor deltaFosB (ΔFosB) is induced in the nucleus accumbens (NAc) by repeated exposure to drugs of abuse and natural rewards. Less is known about its role in other brain areas. Here, we compared the effects of mating versus cocaine history on induction of ΔFosB in the medial preoptic area (MPOA), an integral site for reproductive behavior, and in the NAc. ΔFosB immunoreactivity (ir) was increased in the MPOA of previously naïve and experienced male rats that mated the day before euthanasia, compared to unmated controls and experienced males with recent mating abstinence. Western immunoblots confirmed that the 35–37-kDa isoform of ΔFosB was increased more in recently mated males. Conversely, previous plus recent cocaine did not increase ΔFosB-ir in the MPOA, despite an increase in the NAc. Next, a viral vector expressing ΔFosB, its dominant negative antagonist ΔJunD, or green fluorescent protein (GFP) control, were microinjected bilaterally into the MPOA. ΔFosB overexpression impaired copulation and promoted female-directed aggression, compared to ΔJunD and control males. These data suggest that ΔFosB in the mPOA is expressed in an experience-dependent manner and affects systems that coordinate mating and aggression.
Keywords: sexual behavior, mating, cocaine, deltaFosB, medial preoptic area
Natural rewards, such as mating and feeding, reinforce behaviors that are adaptive for survival and reproductive success. In contrast, drugs of abuse subvert natural reward systems and dysregulate behavior (Wise & Koob, 2014). In rodents, sexual experience enhances copulatory ability and motivation (reviewed in Hull & Dominguez, 2015) but can also enhance drug rewards (Pitchers et al., 2013). However, it remains unclear whether drugs of abuse and natural rewards share common neural systems and transcriptional mediators that underlie long-lasting neurobiological and behavioral alterations. Studies suggest that the natural reward- and drug-mediated effects on plasticity are similar in canonical reward regions such as the striatum (Olsen, 2011; Pitchers et al., 2013), but it is unknown whether similarities occur in regions that regulate natural reward behaviors such as mating.
One mediator of interest is deltaFosB (ΔFosB), a highly stable truncated splice variant of FosB, a member of the Fos family of transcription factors that are transiently expressed in response to acute stimulation (reviewed in Nestler, 2008, 2015; Ruffle, 2014). Unlike other Fos family transcription factors, ΔFosB has unique properties that allow it to accumulate in striatal regions, notably the nucleus accumbens (NAc; Nestler, 2015), in response to chronic drugs of abuse or repeated natural rewards, including palatable foods, mating, and voluntary wheel running (Pitchers et al., 2010, 2013; Teegarden, Nestler, & Bale, 2008; Wallace et al., 2008; Werme et al., 2002). Further, overexpression of ΔFosB in the NAc in rodents facilitates mating on the first sexual experience (Hedges, Chakravarty, Nestler, & Meisel, 2009; Pitchers et al., 2010; Wallace et al., 2008), whereas overexpression of its dominant negative, ΔJunD, inhibits sexual reward (Been, Hedges, Vialou, Nestler, & Meisel, 2013; Pitchers et al., 2010).
While it is well established that ΔFosB accumulates in striatal brain regions and mediates aspects of natural and drug rewards, less is known about its possible roles elsewhere in the brain and how its induction compares between natural and drug rewards. Given that the medial preoptic area (MPOA) is critical for reproductive behaviors, including male mating (reviewed in Hull & Dominguez, 2015), as opposed to the more general motivational functions of the mesolimbic tract, and the NAc in particular, we examined the induction and function of ΔFosB in the MPOA for male sexual behavior and in response to cocaine and in comparison to the response to cocaine in the NAc. Further, sexual experience enhances copulatory ability and motivation putatively through a number of experience-induced changes within the MPOA (reviewed in Hull & Dominguez, 2015). The transcription factors that regulate these changes are not known, though sexually experienced males have greater copulation-induced expression of the transcription factor c-Fos in the MPOA, compared to sexually naïve males (Lumley & Hull, 1999). The contributions of c-Fos and other members of the Fos family of transcription factors to experience-induced sexual enhancement are not known.
We hypothesized that copulation would increase expression of ΔFosB in the MPOA and that overexpression of ΔFosB would increase copulatory efficiency and motivation. In order to compare effects in the MPOA with those in the NAc, we tested copulation- or drug-induced expression in both areas. First, immunohistochemistry and Western blotting were used to examine the expression of mating- or cocaine-induced ΔFosB in the MPOA and NAc following varying schedules of mating or cocaine exposure. Then, viral-mediated gene transfer in the MPOA was utilized to determine whether up- or down-regulation of ΔFosB functionally alters aspects of mating or social behavior.
Materials and Method
Animals
Male Long Evans/Blue Spruce rats (250–275 g, Harlan, Indianapolis, IN) were individually housed in large plastic cages in climate-controlled rooms on a 14:10-hr light–dark cycle, with lights off at 1100 and on at 2100. Food and water were available ad libitum. All procedures were in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the Florida State University.
Experiment 1 Design: Effects of Mating History on ΔFosB-ir and Protein
Twenty-four animals were randomly divided into four groups: naïve no-sex (N) received no sexual experience; naïve sex (NS) received one mating session 18–24 hr prior to euthanasia; experienced no-sex (ENS) received 14 thrice-weekly mating sessions, with an abstinence period of 3 days prior to euthanasia; and experienced sex (ES) received 15 thrice-weekly mating sessions, with the final session occurring 18–24 hr prior to euthanasia. Tissue sections from the NAc and MPOA were immunoprocessed and quantified using an ABC-peroxidase-DAB reaction for FosB/ΔFosB. In other animals, total protein was extracted from tissue punches from MPOA and NAc and quantified using the Western immunoblot technique.
Copulation Testing
Stimulus females were brought into behavioral estrus, and receptivity was confirmed with a stud male before females were used for testing. Copulation testing took place in the male’s home cage under dim red light and lasted a total of 30 min from the first intromission or for 30 min total if the male failed to intromit. Data were recorded as previously described (see McHenry, Bell, Parrish, & Hull, 2012).
Immunohistochemistry
Eighteen to 24 hr after the final mating or control handling session, animals were deeply anesthetized with sodium pentobarbital (65 mg/kg), transcardially perfused, and immunoprocessed as previously described (Muschamp, Dominguez, Sato, Shen, & Hull, 2007). Sections were incubated overnight (17 hr) at 4 °C in blocking solution containing primary antibody for rabbit anti-FosB/ΔFosB (1:1500, SC-48, Santa Cruz Biotechnology, CA). This antibody is raised against the N-terminal region shared by the FosB and ΔFosB proteins and does not cross-react with c-Fos (Dobrazanski et al., 1991; Perrotti et al., 2004). Because stimulus-induced FosB peaks after 6 hr and is degraded by 18 hr, while the ΔFosB isoform remains stably expressed, all cells labeled with the pan-FosB antibody were considered to reflect ΔFosB (Carle et al., 2007; Perrotti et al., 2004). The following day, sections were incubated in biotin-conjugated goat antirabbit IgG secondary antibody (1:500, Vector Laboratories, CA), and then amplified in PBS with avidin-biotin-peroxidase for 90 min (1:50, Vectastain ABC Elite kit; Vector Laboratories, CA) and immunoreactivity was visualized with diaminobenzidine (0.02%, Sigma, St. Louis, MO) enhanced with 2% nickel sulfate.
Microscopy
Bright field microscopy utilizing the ImagePro Plus 7.0 program (Media Cybernetics) on an Olympus BX60 light microscope was used to quantify the number of ΔFosB-ir cells and verify GFP-ir cells. Two sections from the anterior, medial, and posterior regions of the MPOA and NAc were counted from each subject by an experimenter blind to the treatment group. If no differences were found spanning the anterior to posterior region of interest, counts were averaged across sections. All counts were converted into cell density (cells/mm2) for comparison purposes.
Western Immunoblot
Total protein was extracted from 2.0 mm tissue punches of total MPOA and NAc. Tissue samples were homogenized in extraction buffer, and supernatant was extracted according to methods of Lee (2007). Protein estimates were calculated from this supernatant using spectrophotometry (PowerWaveX; BioTek, MD). These protein estimates were used as a standard for loading volume (35 μg of protein each) onto 12% polyacrylamide gels for separation by SDS electrophoresis (Lee, 2007). Western immunoblots were processed as previously described (see McHenry et al., 2012). The membranes were incubated overnight at 4 °C in blocking solution and FosB rabbit polyclonal antibody (1:1k, 5G4 Mab #2251, Cell Signaling, MA) and the next day for 90 min in horseradish peroxidase (HRP)-conjugated goat antirabbit IgG secondary antibody (1:500, SC-2030, Santa Cruz Biotechnology, CA). The membrane was assessed for HRP-conjugated chemiluminescence (ECL; Amersham Biosciences, NJ) by exposing the membrane to Kodak BioMax film. The membrane was then stripped and labeled against mouse anti-β-actin monoclonal antibody (1:1k, Sigma-Aldrich, MO) as a loading control. The developed films were scanned into a computer, and band density was quantified using the NIH ImageJ program (Version 1.33U, NIH).
Experiment 2 Design: Effects of Cocaine History on ΔFosB-ir and Protein
Sixteen animals were randomly and evenly divided into four groups and treated with a schedule identical to Experiment 1, except that cocaine was administered in place of mating. Specifically, saline controls (S) received 15 thrice-weekly injections of saline (1 ml/kg 0.9% sodium chloride i.p.); saline cocaine (SC) animals received 14 thrice-weekly injections of 0.9% saline with a single final injection of cocaine (15 mg/kg cocaine hydrochloride in saline i.p.); cocaine saline (conditional stimulus (CS)) animals received 14 thrice-weekly injections of cocaine and a final injection of saline; and cocaine cocaine (CC) animals received 15 thrice-weekly injections of cocaine. In all cases, the final injection was administered 18–24 hr prior to euthanasia. Tissue sections from the MPOA and NAc were immunoprocessed for ΔFosB-ir and quantified using the procedure outlined in Experiment 1. Additional animals received the same treatments, but punches from their brains were taken as described in Experiment 1. Western immunoblots from the MPOA and NAc were processed and quantified as described in Experiment 1.
Experiment 3 Design: Effects of ΔFosB Manipulation in the MPOA on Mating
Seventy-five animals were randomly divided into three groups. Subjects received bilateral intracranial microinjections of adeno-associated viral vectors (AAV) encoding green fluorescent protein (GFP, n = 18), wild-type ΔFosB and GFP (ΔFosB, n = 18), or ΔJunD and GFP (ΔJunD, n = 18). ΔFosB heterodimerizes with JunD to form an AP-1; thus the truncated ΔJunD, which lacks a DNA binding domain, is a dominant negative antagonist of ΔFosB-mediated transcription (Jorissen et al., 2007; Nestler, 2008; Winstanley et al., 2007). Animals were tested for sexual behavior on three occasions, each 1 week apart, beginning 5 weeks after microinjections of AAV vectors to ensure ample time for transfection and stable viral expression. Three tests were chosen because our previous work has revealed increases in number of ejaculations between the first and second tests, and on some comparisons, between the second and third tests (Gil, Bhatt, Picotte, & Hull, 2013).
Viral-Mediated Gene Transfer
Animals were anesthetized with isoflurane (2.5–3.5%) and received intracranial microinjections of AAV. Vectors were bilaterally microinjected into the MPOA using a Hamilton syringe to a volume of 0.5 μl/side over 10 min (stereotaxic coordinates: AP + .20 mm; ML ± 0.5 mm from bregma; DV, −8.5 mm from skull surface; adapted from Pellegrino, Pellegrino, and Cushman (1979). Buprenex (1.5 mg/0.1 ml) was administered subcutaneously following all surgical procedures to alleviate postoperative discomfort, and animals were tested 5 weeks later.
Verification of AAV Transfection
Eighteen to 24 hr following ejaculation on the final mating test, rats were anesthetized, and brain tissue was prepared, extracted, and immunoprocessed to verify successful placement and transfection using GFP as a marker of transfection. All immunoprocessing procedures for GFP immunofluorescence were the same as those described for single peroxidase labeling except for incubation steps. Instead, sections were incubated in a blocking solution consisting of 2% Normal Donkey Serum (Vector Laboratories, CA) in PBS + 0.4% Triton-X 100 for 1 hr, followed by a 17 hr room-temperature incubation in blocking solution containing monoclonal GFP primary antibody (1:1500; rabbit anti-GFP antibody, Molecular Probes, NY). The following day, sections were incubated in fluorescence-conjugated donkey antirabbit IgG Alexa Fluor 488 secondary antibody (1:500, Invitrogen, Molecular Probes, NY) for 30 min in darkness. Only animals with correct placement were considered in statistical analyses of behavior.
Copulation Measurements
The following behavioral measures were recorded and calculated: mount and intromission latencies (ML and IL), time from the introduction of the female to the first mount or intromission; ejaculation latency (EL), time from the first intromission to the subsequent ejaculation; total frequencies of mounts (MFT), intro-missions (IFT), and ejaculations (EFT), and total intromissions divided by mounts plus intromissions for the entire test (copulatory efficiency).
Aggression Assessment
In certain cases, female-directed aggression was unexpectedly observed during the AAV mating test. Males were classified as either aggressive or nonaggressive for nonparametric testing. Specifically, a male was considered nonaggressive if there were no physical attacks directed at the female and only normal mating behavior was observed. Conversely, a male was considered aggressive if he physically attacked (including biting) a female at any point during the test.
Statistical Analysis
All statistical analyses were performed with SPSS (Version 18.0). For Experiments 1 and 2, one-way analyses of variance (ANOVA) were used in conjunction with Tukey’s HSD post hoc analyses to probe for group differences in ΔFosB-ir density. For Experiment 3, two-way ANOVAs were used in conjunction with Tukey’s HSD post hoc analyses to probe for group differences in all copulatory measures. Independent Student’s t tests (excluding ΔFosB males, because fewer than 3 animals in that group copulated to ejaculation) were used to compare ΔJunD- and GFP-treated animals for ejaculation latency. In addition, chi square tests were used to compare the fraction of animals in each group that displayed female-directed aggression. For all statistical analyses, p < .05 was considered statistically significant, and p < .10 was considered a trend.
Results
Effects of Mating Experience on ΔFosB Expression in the MPOA and NAc
Mating and cocaine experience resulted in different patterns of ΔFosB-ir in the total MPOA and NAc. Mating the day before euthanasia increased ΔFosB-ir in the total MPOA (Figure 1C, F(3,23) = 10.339, p < .001. NS and ES males had significantly more ΔFosB-ir compared to both N controls (p = .001) and ENS males (p = .002).
Figure 1.
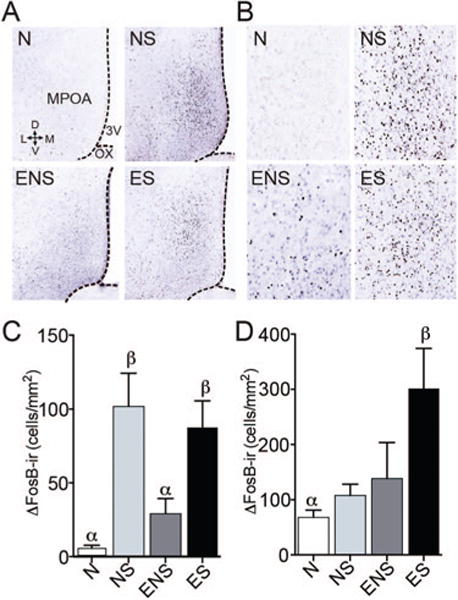
Mating experience significantly affected the amount of ΔFosB-ir in the MPOA and NAc. (A) Representative coronal sections from the MPOA of males that were sexually naïve (N), previously naïve but with sex on the day before euthanasia (NS), sexually experienced but with no sex on the day before euthanasia (ENS), and sexually experienced with sex the day before euthanasia (ES). Sections show immunoreactivity for ΔFosB. (B) Magnified representation of ΔFosB-ir in the MPOA. (C) The mean numbers of ΔFosB-ir neurons in the MPOA of males in the above four groups. Both NS and ES males had significantly more ΔFosB-ir compared to N controls. (D) In the NAc core, ES males expressed more ΔFosB-ir than naïve males. Bars designated with the same letter are not different from each other, but are different from those with the other letter. See the online article for the color version of this figure.
In addition, group differences were observed in the NAc core (Figure 1D, F(3,17) = 3.627, p = .034), with ES males expressing more ΔFosB-ir than naïve males (p = .032). No other group differences were observed in the NAc core, and no mating-induced differences were observed in the NAc shell.
Western immunoblot analysis from MPOA tissue punches showed a similar pattern of ΔFosB-ir (Figure 2C, F(3,27) = 9.396, p < .001). NS and ES males had significantly more 35–37 kDa ΔFosB-ir than N controls (p = .001, p = .007, respectively) and ENS animals (p = .009 for both). Group differences were also observed in tissue punches from the NAc (Figure 2D, F(3,12) = 4.436, p = .036); only ES males expressed more 35–37 kDa ΔFosB-ir than N controls (p = .048). No differences were observed in levels of full-length FosB (~50 kDa).
Figure 2.
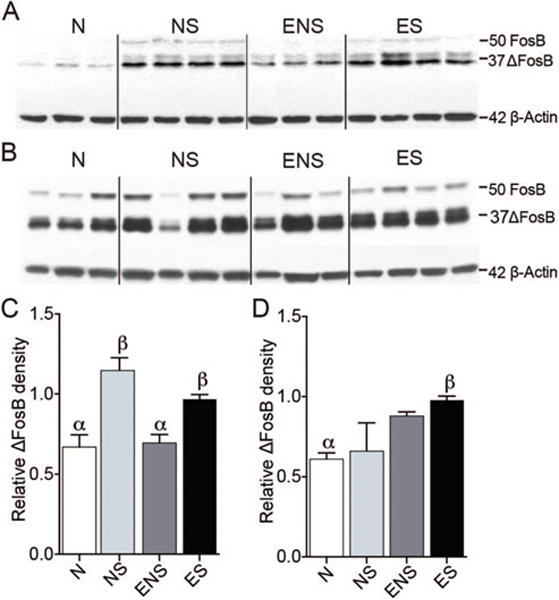
Sexual experience significantly affected the relative amount of 35–37 kDa ΔFosB in (A) MPOA and (B) NAc tissue punches, compared to 42 kDa β-actin control levels. (C) In the MPOA, NS and ES males had significantly more ΔFosB-ir at this band compared to naïve controls, and NS males had significantly more than ENS animals. (D) In the NAc, only ES males expressed more 35–37 kDa ΔFosB-ir than N controls. Bars designated with the same letter are not different from each other, but are different from those with the other letter. See the online article for the color version of this figure.
Effects of Cocaine Experience on ΔFosB Expression in the MPOA and NAc
In contrast to mating history, cocaine history did not significantly affect ΔFosB-ir in the MPOA or any of its subregions. Baseline ΔFosB expression was low in the MPOA (24.59 ± 3.72 cells/mm2) and did not vary with cocaine experience. However, this chronic cocaine regimen did increase ΔFosB-ir in the NAc, with a main effect of treatment observed in both the core (Figure 3B, F(3,12) = 5.520, p = .013) and shell (Figure 3C, F(3,12) = 3.868, p = .038), with this effect being the most pronounced in the core. In the NAc core, previous plus recent cocaine (CC) increased ΔFosB-ir, compared to control animals (SS, p = .045) and animals that received a single acute cocaine exposure (SC, p = .039). In the NAc shell, a significant increase was observed only among CS males (p = .033), compared to SS controls.
Figure 3.
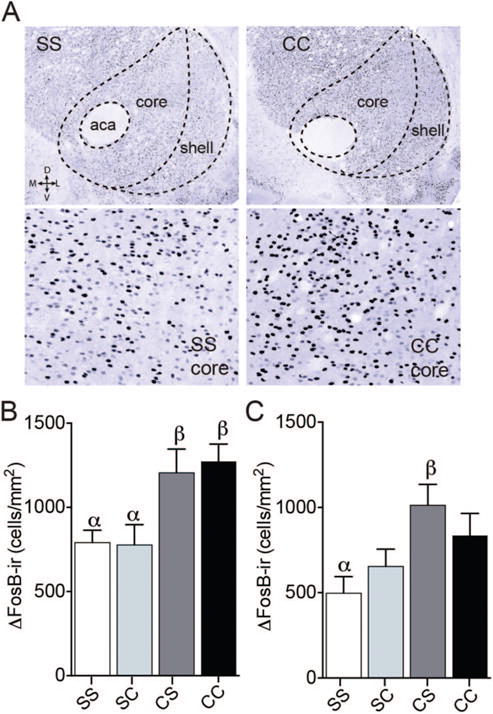
Effects of cocaine history on ΔFosB-ir in the NAc. (A) Representative coronal sections from the NAc of males that were cocaine naïve (Saline Saline, SS) or treated with previous and recent cocaine (Cocaine Cocaine, CC) on top. Bottom images are zoomed in representative images from the NAc core. There was a significant effect of the schedule of cocaine administration on ΔFosB-ir in the NAc shell (B) and core (C). In the NAc core, animals that received 15 thrice-weekly injections of cocaine with no period of abstinence (CC) had significantly more ΔFosB-ir than both control animals (SS) and animals that received a single acute cocaine exposure (Saline Cocaine, SC). In the NAc shell, animals that received 14 cocaine injections followed by an abstinence period (Cocaine Saline, CS) had significantly more ΔFosB-ir than controls (SS). See the online article for the color version of this figure.
Viral-Mediated Manipulation of ΔFosB in the MPOA
Viral-mediated gene transfer significantly affected copulatory frequencies, latencies, and efficiency most robustly on the first mating experience. Significant main effects of experience (F(2,153) = 4.42, p = 0.0136) and group (F(2,153) = 11.4, p < .0001) were observed for mount latency (see Table 1). Specifically, ΔFosB males had longer latencies to mount on the first mating test, compared to GFP controls (p = .0166) and ΔJunD males (p = .0015). Significant effects of experience (F(2,153) = 3.23, p = 0.0424) and group (F(2,153) = 6.76, p = .0015) were also observed for intromission latency, with ΔFosB males exhibiting longer intromission latencies on the first mating test than ΔJunD males (p = .0414) and controls (p = .0023) (Figure 4D). Since fewer than three ΔFosB males mated to ejaculation on the first test, no differences in ejaculation latency could be statistically detected for those animals. However, ΔJunD males had significantly shorter latencies to ejaculate than GFP-controls on the first mating test (t(34) = 5.05, p < .0001; Figure 4F). Differences in ejaculation latency were also observed on the second mating test (F(2,102) = 8.38, p = .0004), with ΔFosB males having longer latencies compared to controls (p = .0154) and ΔJunD males (p < .0001; Figure 4F).
Table 1.
Copulation Testing: Additional Parameters not Shown in Figure 4
| Copulation parameter | GFP | ΔFosB | ΔJunD | 2-Way ANOVA | GFP vs. ΔFosB | GFP vs. ΔjunD |
|---|---|---|---|---|---|---|
| Mount Latency Test 1 (s) | 316 ± 85 | 701 ± 173 | 210 ± 66 | F(2,153) = 11.40, p < .001 | p = .0061 | ns |
| Total Mounts Test 2 | 11 ± 2 | 3 ± 1.43 | 7 ± 1.87 | F(2,153) = 5.79, p = .0004 | p = .0109 | ns |
| Intromission Ratio Test 1 | .37 ± .07 | .08 ± .04 | .62 ± .09 | F(2,153) = 21.20, p < .001 | p = .0056 | p = .0193 |
| Intromission Ratio Test 2 | .43 ± .07 | .19 ± .08 | .54 ± .08 | F(2,153) = 21.20, p < .001 | p = .0272 | ns |
| (means ± SEM) | (F values, group effect) | (post-hoc values) | ||||
Note. Viral-mediated gene transfer significantly affected additional measures of copulatory latencies and efficiency. On Test 1, ΔFosB males had longer latencies to mount than GFP controls. On Test 1 and Test 2, ΔFosB males were also less efficient copulators (lower intromission ratios) than GFP controls. On Test 2, ΔFosB males had fewer mounts than GFP controls. In addition, ΔJunD males were more efficient copulators than GFP controls on Test 2. Copulation parameters are expressed as means ± SEM for each group. Two-way ANOVA F and p values are reported from observed group effects in addition to post-hoc comparisons between GFP controls and ΔFosB or ΔJunD males.
Figure 4.
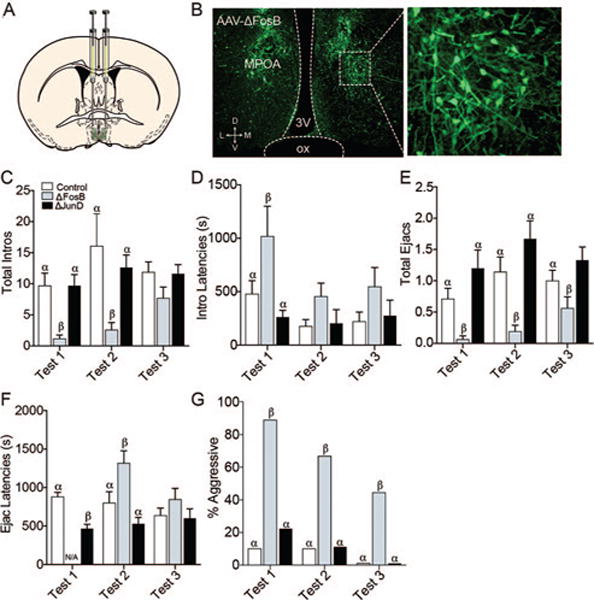
(A) Viral-mediated gene transfer in the MPOA. Diagram illustrates the site of injection at the level of the MPOA. (B) Photomicrograph exemplifies GFP-ir protein as a marker of AAV transfection in the MPOA, 20×. On test one, males receiving ΔFosB had fewer intromissions (C), longer intromission latencies (D), and fewer ejaculations (E) compared to ΔJunD males and GFP controls. Also, ΔJunD males had shorter ejaculation latencies than GFP controls (F). (There were fewer than 3 ΔFosB males ejaculating, so their latencies could not be compared statistically.) On Test 2, ΔFosB males had fewer intromissions (C), ejaculations (E), and longer ejaculation latencies (F) than ΔJunD males and GFP controls. There were no statistical differences in intromission or ejaculation latencies or numbers of intromissions on Test 3. (G) Across all three trials, animals that received ΔFosB were more likely to display female-directed aggression than animals receiving either GFP or ΔJunD. Bars designated with the same letter are not different from each other, but are different from those with the other letter within each test. See the online article for the color version of this figure.
There were also differences in the effects of mating experience (F(2,153) = 4.54, p = 0.0122) and of treatment (F(2,153) = 5.79, p = .0038) on total number of mounts, with ΔFosB males having fewer mounts (p = .0291) than GFP controls on the first mating test (see Table 1). There were also significant main effects of group for total intromissions (F(2,153) = 12.4, p < .0001; Figure 4C) and ejaculations (F(2,153) = 23.4, p < .0001; Figure 4E). Specifically, ΔFosB males had fewer intromissions than GFP controls and ΔJunD males on the first (p = .0276 for both) and second (p = .002; p = .0075, respectively) mating tests. In addition, ΔFosB males had fewer ejaculations on the first mating test compared to GFP controls (p = .0442) and ΔJunD males (p = .0003). On the second mating test ΔFosB males also had fewer ejaculations than GFP (p = .0034) and ΔJunD (p < .0001) males, and on the final mating test, fewer than ΔJunD males (p = .0224). Additional significant differences, including parameters of mating efficiency, are reported in Table 1. There were main effects of experience on copulatory efficiency (F(2,153) = 5.020, p = 0.0077) and main effects between groups (F(2,153) = 23.86, p < 0.0001; see Table 1).
An unexpected finding was that a greater percentage of ΔFosB animals showed aggression toward stimulus females on all three tests (chi-square, Test 1: , p = .0143; Test 2: , p = .0344; Test 3: , p = .0146; Figure 4G).
Discussion
The present experiments were conducted to test whether the transcription factor ΔFosB in the MPOA plays roles in the control of male rat sexual behavior and drug addiction similar to its roles in the NAc, where it has been shown to promote mating (Pitchers et al., 2010; Wallace et al., 2008) and drug addiction (reviewed in Nestler, 2015). Acute mating by both previously naïve (NS) and sexually experienced male rats (ES) increased ΔFosB expression in the MPOA. However, repeated mating, followed by sexual abstinence (ENS), did not. Neither acute nor repeated cocaine increased ΔFosB expression in the MPOA, but did in the NAc. Finally, viral-mediated overexpression of ΔFosB in the MPOA inhibited mating, but increased female-directed aggression.
The present immunohistochemical analyses reveal that mating-induced ΔFosB in the MPOA is expressed in a relatively transient manner, and this differs from that observed in the NAc. Western immunoblots corroborated this pattern and confirmed the presence of 35–37 kDa ΔFosB, distinguishing it from the full length ~50 kDa version of FosB. It is not clear what accounts for the relative transience of expression in the MPOA. This pattern differs from a report that five daily mating sessions increased ΔFosB in the NAc, medial prefrontal cortex, ventral tegmental area, and caudate putamen, but not in the MPOA (Pitchers et al., 2010). However, procedural differences may at least partially account for the disparity in results in the MPOA. Pitchers et al. used a lower antibody concentration, and their semiquantitative data showed only 2–10 cells in the MPOA of both naïve and experienced males, whereas the current data averaged ~175 cells/mm2 for NS males and ~150/mm2 for ES males, compared to ~5/mm2 for N males and ~70/mm2 for ENS males. The discrepancy may also be attributed to differences in the amount of sexual experience, time between mating and euthanasia, age, or strain. Collectively, this suggests that mating-induced ΔFosB is expressed in a time- and region-specific manner, and is sensitive to the amount of mating experience and duration of abstinence.
While others have reported similar effects of cocaine history on ΔFosB-ir in the NAc core and/or shell, none have examined expression in the MPOA (Marttila, Raattamaa, & Ahtee, 2006; Perrotti et al., 2008). Although mating increased ΔFosB in the MPOA, a cocaine regimen similar to that of our mating schedule did not. One potential reason for the MPOA’s relative unresponsiveness to cocaine is that it contains very few dopamine transporters (DAT) for cocaine to inhibit (Ciliax et al., 1995). While DAT is the primary dopamine transporter in the NAc and dorsal striatum, the unrelated and lower affinity plasma membrane monoamine transporter (PMAT) is the major dopamine transporter in the MPOA (Dahlin, Xia, Kong, Hevner, & Wang, 2007). However, in female rats, the MPOA may actually counteract the effects of drugs of abuse and block cocaine’s disruption of maternal behavior. In one such study, MPOA lesions increased both cocaine-induced place preference and cocaine-induced c-Fos expression in the NAc of female rats (Tobiansky et al., 2013). In addition, inactivation of the MPOA eliminated pup preference over cocaine in postpartum females (Pereira & Morrell, 2010). Thus, neurons in the MPOA may promote reproduction but inhibit motivation for drugs. While cocaine did not increase ΔFosB-ir in the MPOA, chronic, but not acute, cocaine exposure did increase it in the NAc. This is consistent with previous findings that repeated cocaine administration increases ΔFosB in the NAc (Lobo et al., 2013; Nye, Hope, Kelz, Iadarola, & Nestler, 1995; Ruffle, 2014), and that chronic but not acute cocaine self-administration increased ΔFosB in the NAc shell and core (Robison et al., 2013), where it appears to promote behavioral sensitization (Colby, Whisler, Steffen, Nestler, & Self, 2003; Kelz et al., 1999).
Overexpression of ΔFosB in the MPOA dramatically impaired mating, whereas overexpression of ΔJunD, the inhibitory partner of ΔFosB had relatively few effects on mating. These effects of ΔFosB overexpression were most pronounced on the first sexual experience, decreasing over subsequent tests. This is apparently different from the role of ΔFosB in the NAc, where overexpression of ΔFosB was reported to enhance male rat sexual behavior (Pitchers et al., 2010; Wallace et al., 2008) and female hamster sexual reward and copulation efficiency (Hedges et al., 2009). Conversely, ΔJunD overexpression in the NAc prevented female hamster sexual reward (Been et al., 2013) and attenuated sexual experience-induced facilitation of copulation in male rats (Pitchers et al., 2010, 2013). However, in the current experiment, ΔJunD overexpression did facilitate some aspects of copulation on the first test, increasing intromission ratios and decreasing ejaculation latencies, compared to controls. It is not clear why ΔJunD’s facilitative effects were not more dramatic; however, there may not have been enough natural ΔFosB produced for it to inhibit. In addition, mating induces ΔFosB in the MPOA under natural conditions and it is possible that viral-mediated gene transfer of ΔFosB in the MPOA differentially affects the timing and output of gene expression and/or targets different cell types.
Regional disparity in the induction and function of mating-related ΔFosB could result from differences between roles of the MPOA and NAc in mating and/or potential differences between cell types within these regions. Mating is a complex behavior that encompasses appetitive, consummatory, and refractory phases that are controlled by a number of neural circuits and cell types within the MPOA and NAc. Functional in vivo cell-specific and circuit-level approaches that target the MPOA or NAc during mating are still lacking, yet these two regions have been extensively examined and appear to have different and important roles in male mating behavior. The MPOA integrates hormonal and sensory input bidirectionally and orchestrates copulatory patterns (e.g., erection, mount, intromission, ejaculation) through a number of widespread projections to the paraventricular nucleus of the hypothalamus, the midbrain, and the hindbrain (reviewed in Hull & Dominguez, 2015). Inconsistencies remain as to whether the MPOA regulates only copulation or if it also promotes motivation for mating. However, a number of studies have shown that lesions, chemical inactivation, or dopamine antagonists in the MPOA inhibit measures of motivation in rats, ferrets, quail, and starlings (reviewed in Hull & Dominguez, 2015). Thus, while the MPOA is not essential for sexual motivation, it does contribute to such motivation. On the other hand, the NAc is part of the mesolimbic motivation circuit, receives dopaminergic input from the ventral tegmental area (VTA), and has a clear role in the motivational and reinforcing aspects of mating, although its role in copulation is less clear. These two regions likely communicate mating-related information, since the MPOA has projections to the VTA, and hormones can act in the MPOA to enhance drug-induced DA release in the NAc (Tobiansky et al., 2013). One possibility is that different cell types and circuits within the MPOA and NAc convey different aspects of mating. Thus, different phases of mating behavior and differences in the timing between or following mating bouts may lead to unique regional patterns of ΔFosB induction with different functional consequences depending on the region being targeted.
It remains unclear why expression of ΔFosB is transiently expressed in the MPOA after mating, yet overexpression here impairs mating. One possibility is that mating experience induces ΔFosB in a cell-specific and experience-dependent manner, and the current viral targeting approach in a rat model does not allow for genetically encoded and temporal specificity that might be more comparable to endogenous expression patterns. Electrophysiological recordings have detected putatively distinct MPOA neurons that respond during appetitive or consummatory phases of mating (Shimura, Yamamoto, & Shimokochi, 1994). Similarly, lesion and c-Fos studies have shown that more anterior portions of the MPOA of quail are more associated with appetitive behaviors, whereas more posterior areas are implicated in both (Balthazart & Ball, 2007). Mating-induced ΔFosB in the MPOA under natural conditions may be restricted to specific types of neurons within the MPOA, especially given the complex genetic heterogeneity of MPOA neurons (reviewed in McHenry, Rubinow, & Stuber, 2015) and the cellular specificity of ΔFosB induction observed in other brain regions. In the NAc, a variety of stimuli induce ΔFosB in dopamine D1-type or D2-type medium spiny neurons (MSNs), and cell-type selective expression of ΔFosB in D1-type or D2-type MSNs can result in different behavioral outcomes due to actions of ΔFosB AP-1 mediated transcription, either repressing or activating different subsets of genes (reviewed in Nestler, 2015). ΔFosB induced transcription has been explored in the NAc, where it enhances expression of the glutamate AMPA receptor subunit GluA2 and suppresses dynorphin. Further, Camk2a is a target gene for ΔFosB in D1-type medium spiny neurons (MSNs) in the NAc, demonstrating that upstream cell-specific signaling can lead to different cellular gene products in response to ΔFosB transcription. DNA expression arrays and microarrays have even been generated in a cell-specific manner within the NAc in response to manipulations of ΔFosB. Similar induction may occur in D1- or D2-like receptor containing neurons in the MPOA, but no studies have made such comparisons, and these types of arrays have not been generated for the MPOA. While it remains unknown whether ΔFosB expression in the MPOA is cell-type specific, the functional roles of different MPOA cell types in mating have been explored. For example, parasympathetically mediated erections are promoted by dopamine in the MPOA acting on D1-like receptors, whereas sympathetically mediated ejaculation is elicited by higher levels of MPOA dopamine acting via D2-like receptors (reviewed in Hull & Dominguez, 2015). Expressions of several genes in the MPOA are altered following repeated sexual experience. For example, sexually experienced males have more oxytocin receptors in the MPOA (Gil et al., 2013) and greater copulation-induced activation of DARPP-32, a marker of DA D1 receptor activation (McHenry et al., 2012). Sexual experience also increases the expression of neuronal nitric oxide synthase (nNOS), which produces nitric oxide (NO), which in turn promotes DA release in the MPOA and thereby enhances sexual ability (Dominguez, Gil, & Hull, 2006). These changes are often associated with cell-specific changes in transcription factor expression, as evidenced by the increased colocalization of Fos in D2-containing cells in sexually experienced animals after mating (Nutsch et al., 2016). However, it is unknown whether ΔFosB mediates any of these changes.
Overexpression of ΔFosB in the MPOA unexpectedly promoted female-directed aggression and may have stimulated an increase in sympathetic function, as evidenced by longer times to initiate copulation (which requires parasympathetic function). These differences diminished with repeated sexual experience. However, even on the third test, about 50% of ΔFosB-overexpressing males showed aggressive behaviors, compared to 0% in both control and ΔJunD-overexpressing males, and they also had longer mount latencies than the other two groups and fewer ejaculations than ΔJunD-overexpressing males. Prior studies have shown that MPOA lesions not only impair or abolish mating (Christensen, Nance, & Gorski, 1977; Hansen, Köhler, Goldstein, & Steinbusch, 1982; Larsson & Heimer, 1964; Lupo, Dessi-Fulgheri, Musi, & Larsson, 1983), but also result in increased activity of the sympathetic nervous system (Pfaff, 1980) and hypothalamic-pituitary-adrenal axis (Viau & Meaney, 1996; Williamson, Bingham, Gray, Innala, & Viau, 2010; Williamson & Viau, 2008). Because ejaculation is elicited by the sympathetic nervous system, and because D2-like receptors in the MPOA have been shown to elicit ejaculation, it is possible that ΔFosB is preferentially expressed in neurons containing D2-like receptors in the MPOA, which could explain its impairment of copulation and promotion of aggression.
Apart from autonomic and stress-hormone regulation, the MPOA is also implicated in the control of maternal and paternal aggression in female and male rodents (Bosch & Neumann, 2012; Gammie, 2005; Motta et al., 2013; Trainor, Finy, & Nelson, 2008) and aggressiveness by male mice during short photoperiod days (Laredo, Orr, McMackin, & Trainor, 2014). Furthermore, galanin-expressing neurons within the MPOA appear to be part of an inhibitory circuit capable of blocking intermale and pup-directed aggression in mice (Wu, Autry, Bergan, Watabe-Uchida, & Dulac, 2014). These same preoptic galanin neurons also promote parenting behavior in both male and female mice. Thus, disruption of genes in the MPOA that facilitate reproductive behaviors may not only impair mating or parental behavior but also stimulate aggressive behaviors. Optogenetic circuit studies in other mating-related regions have found that overlapping circuits can promote mating or aggression depending on the stimulation parameters of those circuits. For instance, ESR1 neurons in the ventromedial hypothalamus promote mounting behavior under weak stimulation, but increasing photostimulation switches this to attack behavior (Lee et al., 2014). Therefore, circuits within the MPOA may regulate the balance between aggressive and reproductive behaviors, as well as between sympathetic and parasympathetic influences. Thus, up-regulating ΔFosB in the MPOA may have offset its parasympathetic regulation or the functioning of circuitries that promote mating and suppress aggression. Future studies should also investigate possible roles of ΔFosB in the MPOA of females, which is outside the scope of the present study. While the MPOA governs reproductive behaviors in both sexes, there are many sex differences in these behaviors (Dulac, O’Connell, & Wu, 2014) and also potent sex differences resulting from gonadal steroid actions during development and in adulthood. For example, DA is released in the MPOA of both males and females to promote sexual behavior, but such release is influenced by the presence of gonadal steroids.
Castrated males actually have more DA in MPOA tissue punches, but much less in extracellular fluid, compared with intact males, indicating that testosterone is not required for DA synthesis, but is required for its release (Hull et al., 1999). Testosterone replacement restored MPOA DA release and copulation of male rats by upregulating nNOS in the MPOA (Sato, Braham, Putnam, & Hull, 2005). In ovariectomized female rats, DA is released in response to low-dose estradiol plus progesterone and rises during copulation (Matuszewich, Lorrain, & Hull, 2000). Furthermore, putatively different neurons in the MPOA of female rats regulate appetitive (sexual solicitation) versus consummatory (sexual receptivity, lordosis) behavior (reviewed in Veening, Coolen, & Gerrits, 2014). As previously mentioned, male MPOA neurons also seem to encode different aspects of mating, yet there are many sex differences in MPOA cell types that may lead to differences in up- and downstream mediators of mating related plasticity. Thus, it is possible that aspects of sexual behavior in males and females may be controlled in part by separate circuits in the MPOA that may be up- or down-regulated by Fos family transcription factors.
Collectively, the present data, along with the present literature, suggest that ΔFosB has region-specific effects, and can either promote or inhibit reproduction, aggression, stress, and drug addiction. The induction of ΔFosB in the MPOA appears to be stimulus-specific and sensitive to the schedule of reward and reward abstinence. Furthermore, viral overexpression of ΔFosB in the MPOA promotes female-directed aggression, rather than sexual behavior. These findings differ dramatically from those observed in the NAc, where both natural and drug rewards lead to a similar pattern of expression, and where ΔFosB expression may increase both copulation and motivation for cocaine. These data suggest that increased ΔFosB in the MPOA inhibits copulation, perhaps by activating the sympathetic nervous system via D2-containing neurons and/or inhibiting the parasympathetic nervous system via inhibition of D1-containing neurons. The fact that it is expressed during copulation, but that overexpression inhibits copulation, may be due to its normal expression during sympathetically mediated ejaculation, but overexpression may inhibit parasympathetically mediated erection and, possibly, sexual versus aggressive motivation.
Acknowledgments
This research was supported by the National Institutes of Health Grants R01 MH040826 to Elaine M. Hull, DA026854 to Carlos A. Bolaños-Guzmán, and R01 DA007359 and P50 MH096890 to Eric J. Nestler. Some of these data were presented at the 2010 Annual Meeting of the Society for Neuroscience. The authors thank Drs. Juan Dominguez and Xixi Jia for their guidance on immunohistochemical techniques, Dr. Frank Johnson for the use of his fluorescent microscope, Augi Colombo and Tim Arrant for their assistance with behavioral testing, Brandon L. Warren for his assistance with advanced biosafety laboratory procedures, and Katie Picotte-Grausam for her preliminary data.
Footnotes
Jenna A. McHenry is now at the Department of Psychiatry, University of North Carolina at Chapel Hill. Christopher L. Robison is now at the Department of Psychology, University of Texas at Austin. Genevieve Bell is now at the Department of Biological Sciences and Program in Neuroscience, Florida State University.
Contributor Information
Jenna A. McHenry, Department of Psychology and Program in Neuroscience, Florida State University
Christopher L. Robison, Department of Psychology and Program in Neuroscience, Florida State University
Genevieve A. Bell, Department of Psychology and Program in Neuroscience, Florida State University
Carlos A. Bolaños-Guzmán, Department of Psychology and Program in Neuroscience, Florida State University
Vincent V. Vialou, Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai and Neuroscience Paris Seine, UPMC CR18, INSERM U1130 CNRS UMR 8246, Pierre and Marie Curie University, Paris, France
Eric J. Nestler, Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai
Elaine M. Hull, Department of Psychology and Program in Neuroscience, Florida State University
References
- Balthazart J, Ball GF. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Frontiers in Neuroendocrinology. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. http://dx.doi.org/10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Hedges VL, Vialou V, Nestler EJ, Meisel RL. ΔJunD overexpression in the nucleus accumbens prevents sexual reward in female Syrian hamsters: ΔJunD prevents sexual reward. Genes, Brain & Behavior. 2013;12:666–672. doi: 10.1111/gbb.12060. http://dx.doi.org/10.1111/gbb.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. http://dx.doi.org/10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, Nestler EJ. Proteasome-dependent and -independent mechanisms for FosB destabilization: Identification of FosB degron domains and implications for ΔFosB stability: FosB degrons and ΔFosB stability. The European Journal of Neuroscience. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. http://dx.doi.org/10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- Christensen LW, Nance DM, Gorski RA. Effects of hypothalamic and preoptic lesions on reproductive behavior in male rats. Brain Research Bulletin. 1977;2:137–141. doi: 10.1016/0361-9230(77)90010-7. http://dx.doi.org/10.1016/0361-9230(77)90010-7. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Levey AI. The dopamine transporter: Immunochemical characterization and localization in brain. The Journal of Neuroscience. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. The Journal of Neuroscience. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. http://dx.doi.org/10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrazanski P, Noguchi T, Kovary K, Rizzo CA, Lazo PS, Bravo R. Both products of the fosB gene, FosB and its short form, ΔFosB/SF, are transcriptional activators in fibroblasts. Molecular and Cellular Biology. 1991;11:5470–5478. doi: 10.1128/mcb.11.11.5470. http://dx.doi.org/10.1128/MCB.11.11.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. The Journal of Neuroscience. 2006;26:1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. http://dx.doi.org/10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behavioral and Cognitive Neuroscience Reviews. 2005;4:119–135. doi: 10.1177/1534582305281086. http://dx.doi.org/10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gil M, Bhatt R, Picotte KB, Hull EM. Sexual experience increases oxytocin receptor gene expression and protein in the medial preoptic area of the male rat. Psychoneuroendocrinology. 2013;38:1688–1697. doi: 10.1016/j.psyneuen.2013.02.002. http://dx.doi.org/10.1016/j.psyneuen.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Köhler C, Goldstein M, Steinbusch HV. Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Research. 1982;239:213–232. doi: 10.1016/0006-8993(82)90843-5. http://dx.doi.org/10.1016/0006-8993(82)90843-5. [DOI] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. ΔFosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes, Brain & Behavior. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. http://dx.doi.org/10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Male sexual behavior. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s physiology of reproduction. Vol. 2. San Diego, CA: Academic Press; 2015. pp. 2211–2285. http://dx.doi.org/10.1016/B978-0-12-397175-3.00049-1. [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behavioural Brain Research. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. http://dx.doi.org/10.1016/S0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Jorissen HJMM, Ulery PG, Henry L, Gourneni S, Nestler EJ, Rudenko G. Dimerization and DNA-binding properties of the transcription factor ΔFosB. Biochemistry. 2007;46:8360–8372. doi: 10.1021/bi700494v. http://dx.doi.org/10.1021/bi700494v. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Nestler EJ. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. http://dx.doi.org/10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Laredo SA, Orr VN, McMackin MZ, Trainor BC. The effects of exogenous melatonin and melatonin receptor blockade on aggression and estrogen-dependent gene expression in male California mice (Peromyscus californicus) Physiology & Behavior. 2014;128:86–91. doi: 10.1016/j.physbeh.2014.01.039. http://dx.doi.org/10.1016/j.physbeh.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K, Heimer L. Mating behavior of male rats after lesions in the preoptic area. Nature. 1964;202:413–414. doi: 10.1038/202413a0. http://dx.doi.org/10.1038/202413a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Western blotting. In: Rosato E, editor. Methods in molecular biology. Vol. 362. Totowa, NJ: Humana Press; 2007. pp. 391–399. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Anderson DJ. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. http://dx.doi.org/10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nestler EJ. ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. The Journal of Neuroscience. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley LA, Hull EM. Effects of a D1 antagonist and of sexual experience on copulation-induced Fos-like immunoreactivity in the medial preoptic nucleus. Brain Research. 1999;829:55–68. doi: 10.1016/s0006-8993(99)01338-4. http://dx.doi.org/10.1016/S0006-8993(99)01338-4. [DOI] [PubMed] [Google Scholar]
- Lupo C, Dessi-Fulgheri F, Musi B, Larsson K. The effect of medial preoptic-anterior hypothalamic lesions on testosterone plasma levels and testosterone conversion in the hypothalamus of male rats. Neuroscience Letters. 1983;39:261–265. doi: 10.1016/0304-3940(83)90310-5. http://dx.doi.org/10.1016/0304-3940(83)90310-5. [DOI] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal FosB/ΔFosB and c-Fos expression in rats and mice. Neuropharmacology. 2006;51:44–51. doi: 10.1016/j.neuropharm.2006.02.014. http://dx.doi.org/10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Lorrain DS, Hull EM. Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behavioral Neuroscience. 2000;114:772–782. doi: 10.1037//0735-7044.114.4.772. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Bell GA, Parrish BP, Hull EM. Dopamine D1 receptors and phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in the medial preoptic area are involved in experience-induced enhancement of male sexual behavior in rats. Behavioral Neuroscience. 2012;126:523–529. doi: 10.1037/a0028707. http://dx.doi.org/10.1037/a0028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry JA, Rubinow DR, Stuber GD. Maternally responsive neurons in the bed nucleus of the stria terminalis and medial preoptic area: Putative circuits for regulating anxiety and reward. Frontiers in Neuroendocrinology. 2015;38:65–72. doi: 10.1016/j.yfrne.2015.04.001. http://dx.doi.org/10.1016/j.yfrne.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta SC, Guimarães CC, Furigo IC, Sukikara MH, Baldo MVC, Lonstein JS, Canteras NS. Ventral premammillary nucleus as a critical sensory relay to the maternal aggression network. Proceedings of the National Academy of Science of the United States of America. 2013;110:14438–14443. doi: 10.1073/pnas.1305581110. http://dx.doi.org/10.1073/pnas.1305581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. The Journal of Neuroscience. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: Role of ΔFosB. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. http://dx.doi.org/10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. ΔFosB: A transcriptional regulator of stress and antidepressant responses. European Journal of Pharmacology. 2015;753:66–72. doi: 10.1016/j.ejphar.2014.10.034. http://dx.doi.org/10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutsch VL, Will RG, Robison CL, Martz JR, Tobiansky DJ, Dominguez JM. Colocalization of mating-induced Fos and D2-like dopamine receptors in the medial preoptic area: Influence of sexual experience. Frontiers in Behavioral Neuroscience. 2016;10:75. doi: 10.3389/fnbeh.2016.00075. http://dx.doi.org/10.3389/fnbeh.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. The Journal of Pharmacology and Experimental Therapeutics. 1995;275:1671–1680. [PubMed] [Google Scholar]
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. http://dx.doi.org/10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. 2nd. New York, NY: Plenum Press; 1979. [Google Scholar]
- Pereira M, Morrell JI. The medial preoptic area is necessary for motivated choice of pup- over cocaine-associated environments by early postpartum rats. Neuroscience. 2010;167:216–231. doi: 10.1016/j.neuroscience.2010.02.015. http://dx.doi.org/10.1016/j.neuroscience.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of ΔFosB in reward-related brain structures after chronic stress. The Journal of Neuroscience. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. http://dx.doi.org/10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Nestler EJ. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. http://dx.doi.org/10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW. Hypothalamic mechanisms. In: Pfaff DW, editor. Estrogens and brain function. New York, NY: Springer; 1980. pp. 106–127. http://dx.doi.org/10.1007/978-1-4613-8084-9_7. [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. ΔFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes, Brain & Behavior. 2010;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. http://dx.doi.org/10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. The Journal of Neuroscience. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Nestler EJ. Behavioral and structural responses to chronic cocaine require a feedforward loop involving ΔFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. The Journal of Neuroscience. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffle JK. Molecular neurobiology of addiction: What’s all the (Δ)FosB about? The American Journal of Drug and Alcohol Abuse. 2014;40:428–437. doi: 10.3109/00952990.2014.933840. http://dx.doi.org/10.3109/00952990.2014.933840. [DOI] [PubMed] [Google Scholar]
- Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: Co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Research. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. http://dx.doi.org/10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Shimura T, Yamamoto T, Shimokochi M. The medial pre-optic area is involved in both sexual arousal and performance in male rats: Re-evaluation of neuron activity in freely moving animals. Brain Research. 1994;640:215–222. doi: 10.1016/0006-8993(94)91875-9. http://dx.doi.org/10.1016/0006-8993(94)91875-9. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Nestler EJ, Bale TL. ΔFosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biological Psychiatry. 2008;64:941–950. doi: 10.1016/j.biopsych.2008.06.007. http://dx.doi.org/10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky DJ, Roma PG, Hattori T, Will RG, Nutsch VL, Dominguez JM. The medial preoptic area modulates cocaine-induced activity in female rats. Behavioral Neuroscience. 2013;127:293–302. doi: 10.1037/a0031949. http://dx.doi.org/10.1037/a0031949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Finy MS, Nelson RJ. Paternal aggression in a biparental mouse: Parallels with maternal aggression. Hormones and Behavior. 2008;53:200–207. doi: 10.1016/j.yhbeh.2007.09.017. http://dx.doi.org/10.1016/j.yhbeh.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, Gerrits PO. Neural mechanisms of female sexual behavior in the rat; comparison with male ejaculatory control. Pharmacology, Biochemistry, and Behavior. 2014;121:16–30. doi: 10.1016/j.pbb.2013.11.025. http://dx.doi.org/10.1016/j.pbb.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. The Journal of Neuroscience. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Bolaños-Guzmán CA. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. The Journal of Neuroscience. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. Delta FosB regulates wheel running. The Journal of Neuroscience. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Gray M, Innala L, Viau V. The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. The Journal of Neuroscience. 2010;30:11762–11770. doi: 10.1523/JNEUROSCI.2852-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.2852-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Viau V. Selective contributions of the medial preoptic nucleus to testosterone-dependent regulation of the paraventricular nucleus of the hypothalamus and the HPA axis. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2008;295:R1020–R1030. doi: 10.1152/ajpregu.90389.2008. http://dx.doi.org/10.1152/ajpregu.90389.2008. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, Nestler EJ. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. The Journal of Neuroscience. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. http://dx.doi.org/10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. http://dx.doi.org/10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]


