Abstract
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity worldwide. Neuroinflammation is prominent in the short and long-term consequences of neuronal injuries that occur after TBI. Neuroinflammation involves the activation of glia, including microglia and astrocytes, to release inflammatory mediators within the brain, and the subsequent recruitment of peripheral immune cells. Various animal models of TBI have been developed that have proved valuable to elucidate the pathophysiology of the disorder and to assess the safety and efficacy of novel therapies prior to clinical trials. These models provide an excellent platform to delineate key injury mechanisms that associate with types of injury (concussion, contusion, and penetration injuries) that occur clinically for the investigation of mild, moderate, and severe forms of TBI. Additionally, TBI modeling in genetically engineered mice, in particular, has aided the identification of key molecules and pathways for putative injury mechanisms, as targets for development of novel therapies for human TBI. This Review details the evidence showing that neuroinflammation, characterized by the activation of microglia and astrocytes and elevated production of inflammatory mediators, is a critical process occurring in various TBI animal models, provides a broad overview of commonly used animal models of TBI, and overviews representative techniques to quantify markers of the brain inflammatory process. A better understanding of neuroinflammation could open therapeutic avenues for abrogation of secondary cell death and behavioral symptoms that may mediate the progression of TBI.
BACKGROUND
Traumatic brain injury (TBI) is a leading cause of death and long-term disability in the developed world. Each year, approximately 10 million people suffer a TBI event worldwide (Hyder et al, 2007; Ruff et al, 2012). Predictive analyses indicate that TBI will constitute the third greatest portion of the total global disease burden by 2020 (Hyder et al, 2007). Within the US alone, some 1.7 million people sustain a TBI annually, and around 5.3 million people live with a TBI-associated disability (Langlois et al, 2006; Prins and Giza, 2012). Of those TBIs that occur, by far the majority are mild to moderate in nature and comprise 80–95 % of cases, with severe TBI accounting for the balance (Tagliaferri et al, 2006). Consequent to increases in survival rate after initial injury, TBI can give rise to substantial and lifelong cognitive, physical, and behavioral impairments that necessitate long-term access to health care and disability services (Tagliaferri et al, 2006; Shi et al, 2013). Exceptionally vulnerable are the elderly, in which the very same injury can cause greater disability and can induce a dramatic rise in the risk of neurodegenerative (Gardner et al, 2014; Barnes et al, 2014) and neuropsychiatric disorders (Chen et al., 2014). Although TBI symptoms can intermittently resolve within a year after injury, some 70–90% of patients endure prolonged and often permanent neurocognitive dysfunctions. It is now established that TBI represents a process, that once initiated can extend either silently or symptomatically to neurodegeneration. This process can lead to early onset of dementia (Gardner et al, 2014; Barnes et al, 2014) as well as Parkinson’s disease (PD) and other degenerative conditions (Gardner et al., 2015; Gardner & Yaffe 2015). Particularly notable, TBI is a strong environmental risk factor for development of Alzheimer’s disease (AD). Recent gene expression studies have delineated the up regulation of key pathways leading to AD and PD provoked by mild, let alone moderate or severe forms of TBI (Greig et al, 2014; Tweedie et al 2013 A&B; Goldstein et al, 2012). Consequent to a current lack of any available therapeutic options (Moppett, 2007), it is imperative to understand the mechanisms that underlie head injury and the ensuing neuronal dysfunction and cognitive impairments to successfully develop possible therapeutics.
TBI-TRIGGERED PATHOLOGICAL PROCESSES
TBI instigates complex pathological processes that involve a broad spectrum of cellular and molecular pathways. TBI-associated brain damage can be classified into two main phases. First, an initial primary damage phase occurs at the moment of insult. This can involve contusion and laceration, diffuse axonal injury, brain swelling and intracranial hemorrhage, and invariably results in immediate (necrotic) cell death (Greig et al, 2014; LaPlaca et al, 2007, Cheng et al., 2012). This is followed by an extended secondary phase that involves cascades of biological processes initiated at the time of injury that may endure over much longer times, from days to numerous weeks (Maas et al., 2008; Zhang et al., 2008). This delayed phase, caused by a variety of cellular and molecular responses instigated in an effort to potentially restore the cellular homeostasis of the damaged tissue, is not particularly well controlled and often will lead to exacerbation of the primary injury damage, progressive neurodegeneration and delayed cell death (Kabadi and Faden, 2014; Lozano et al., 2015). Hallmarks of the secondary insult response can include blood-brain barrier (BBB) breakdown, oxidative stress, glutamate excitotoxicity, and neuroinflammation, which all can occur time-dependently following the primary mechanical insult (Bains and Hall, 2012; Das et al., 2012; Maas et al., 2008; Zhang et al., 2008).
Neuroinflammation is present in both primary (acute) and secondary (chronic) stages of TBI (Lozano et al., 2015) and appears to be responsible for both detrimental and beneficial effects, contributing to primary insult and secondary injury but also facilitating tissue repair (Woodcock and Morganti-Kossmann, 2013). In this regard, following the primary insult, cellular endogenous inflammatory responses are triggered at the injury site with the aim to repair the damaged tissue; however, the often excessive production of pro-inflammatory cytokines appear to become an important driving force for the pathological progression in TBI.
Consequent to recent medical and media interest in sport and military concussions, the concept of chronic neuroinflammation and white matter (WM) injury has been highlighted within the context of TBI. Historically, chronic neuroinflammation was generally associated with chronic neurological diseases, such as occurs in multiple sclerosis, rather than with acute injury, as occurs with TBI (Kutzelnigg & Lassmann, 2014). However, recent reports linking single and repeated TBI events to chronic WM outcomes have fortified association between TBI and neuroinflammation. Neuropathological studies of pre-clinical and clinical TBI cases has provided evidence that glial cells are a central component of the chronic WM degenerative process (Glushakova et al., 2014); (Mouzon et al., 2014); (Sajja et al., 2014).
NEUROINFLAMMATION IN TBI
TBI neuroinflammation is characterized by reactive gliosis
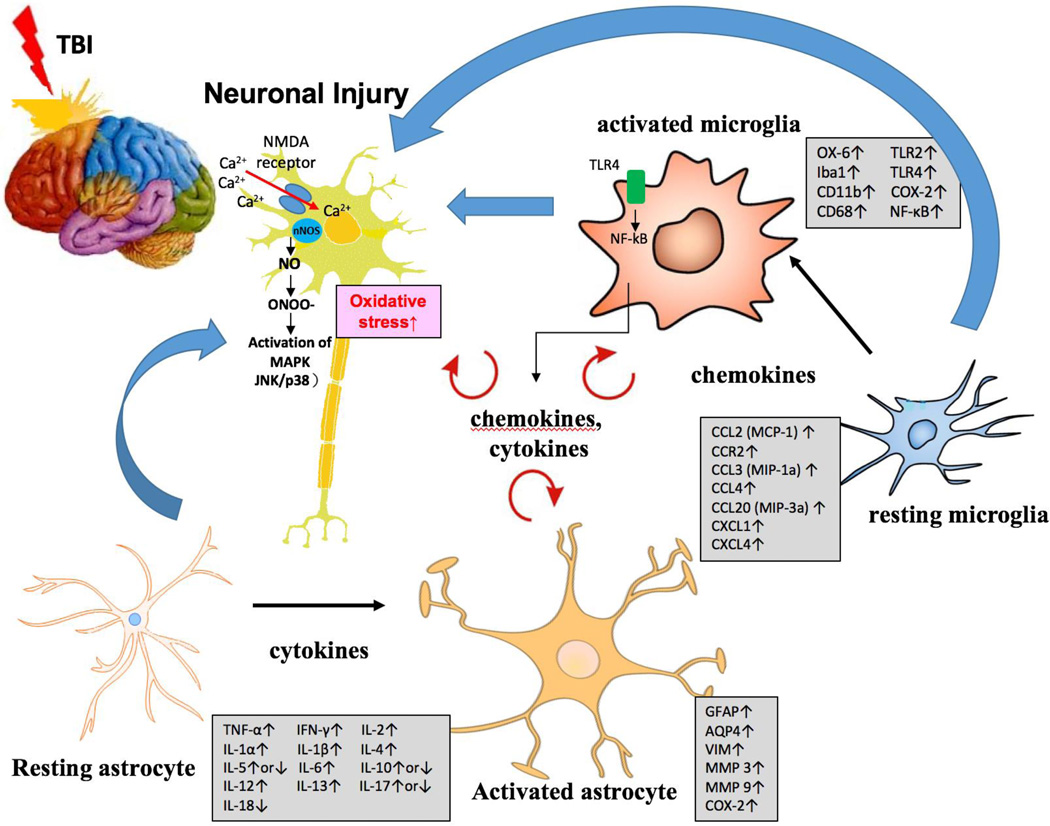
The development of neuroinflammation that follows TBI involves a complex process of cumulative changes that occur within the brain. Following a TBI insult, quiescent glial cells of multiple types become rapidly activated via a process termed “reactive gliosis”. This process involves activated microglia initiating and sustaining astrocytic activation via the generation and release of inflammatory mediators that, in turn, act on surrounding glia and neurons. Post-traumatic cerebral inflammation is characterized by glial activation, leukocyte recruitment, and upregulation and secretion of mediators such as cytokines and chemotactic cytokines (chemokines). (Morganti-Kossmann et al., 2001) (Figure 1).
Figure 1.
After a TBI event, resting glia of multiple types become rapidly activated in a process identified as “reactive gliosis.” This process involves activated microglia initiating and sustaining astrocytic activation via the generation and release of inflammatory markers/mediators (see Tables 1 and 2) that, in turn, act on surrounding glia and neurons via autocrine and paracrine functions (red arrows). Glial activation causes morphological and functional changes within the cells that impact critical neural–glial and glial–glial interactions. This can cause dysfunction of synaptic connections, imbalances of neurotransmitter homeostasis, potential axonal degeneration and neuronal death. Glial cells, most notably astrocytes and microglia, become activated. Their processes become hypertrophied. They upregulate cell surface immune modulatory proteins, and increase the synthesis and release of pro-inflammatory molecules including cytokines, chemokines (see Tables 3 and 4) and prostanoids.
These inflammatory mediators not only impact surrounding glia and neurons but additionally act to recruit peripheral immune cells, such as neutrophils, macrophages and lymphocytes, into brain. Thus acute neuroinflammation following an initial TBI insult not only functions to regulate both damaging and reparative events in the injured and recovering brain, but can also sensitize neurons to enable long-term degenerative processes (Mrak and Griffin, 2005; Streit et al., 2004; Tweedie et al., 2013, 2016). Glial activation induces morphological and functional alterations within the cells that impact neural–glial and glial–glial interactions. This change can cause dysfunction of synaptic connections, neurotransmitter homeostasis imbalance, and potential axonal degeneration and neuronal death ((Bal-Price and Brown, 2001). Astrocytes become activated (termed reactive astrogliosis) in response to CNS injury ((Buffo et al., 2010). Moreover, astrocytes are responsible for encapsulating damaged areas after injury, thereby partly separating injured from the healthy brain tissue ((Fitch and Silver, 2008); (Nimmerjahn, 2009). Astrocytic activation occurs consequent to a mechanism of hypertrophy and an upregulation of intermediate filaments, such as glial fibrillary acidic protein (GFAP) and vimentin ((Buffo et al., 2010); (Gervasi et al., 2008); (Seker et al., 2010). Classically, reactive astrocytes form locally at the primary site of injury, but quite possibly also migrate from distant sites ((Seker et al., 2010); (Turner et al., 1999). In this regard, astrocyte migration historically has been determined from animal models as well as human postmortem studies. However, more recent in vivo studies have questioned whether or not astrocyte migration substantially occurs or, alternatively, if selective astrocyte proliferation at the injury site takes place (Bardehle et al., 2013). Studies have shown the presence of a variety of cytokines involved with either the initiation or modulation of reactive astrogliosis (Farina et al., 2007); (John et al., 2003); Liberto, Albrecht, Herx, Yong, & Levison, 2004; (Rao et al., 2012). These include interleukin-1beta (IL-1β), tumor necrosis factor- alpha (TNF-α), and transforming growth factor beta- 1β (TGF-1β), for which astrocytes express receptors and their interaction triggers an astrocytic reactive response. Astrocyte-derived cytokines such as IL-1β and TNF-α are considered to promote neurotoxicity whereas TGF-β1 is thought to be neuroprotective (Farina et al., 2007); (John et al., 2003); (Liberto et al., 2004); (Rao et al., 2012). Key studies demonstrating astrocyte activation in animal models of TBI are highlighted in Table 1
TABLE 1.
Studies showing astrocyte activation in animal models of TBI
| Markers | model | Species | Findings | Reference |
|---|---|---|---|---|
| Increased Expression in Brain Tissue (Homogenate) | ||||
| GFAPa | CCI, WD, LFP |
Rat, Mice |
mRNA expression↑ (1 – 30 days) Protein expression↑ (3 – 14 days) |
(Fenn et al., 2014; Lagraoui et al., 2012; Liu et al., 2013; Mayeux et al., 2015; Sandhir et al., 2008; Singh et al., 2015; Su et al., 2014; Zhao et al., 2014) |
| AQP4b | CCI, WD, LFP |
Rat, Mice |
mRNA expression↑ (1 – 21 days) Protein expression↑ (2 days) |
(Lagraoui et al., 2012; Lopez-Rodriguez et al., 2015; Tomura et al., 2011) |
| VIMc | CCI, WD |
Mice | mRNA expression↑ (1 – 21 days) | (Lagraoui et al., 2012; Lopez-Rodriguez et al., 2015) |
| MMP 3d | CCI | Mice | mRNA expression↑ (1 day) | (Lagraoui et al., 2012) |
| MMP 9 | CCI | Mice | Protein expression↑ (1 day) | (Chen et al., 2012) |
| COX-2e | CCI | Mice | Protein expression↑ (1 day) | (Chen et al., 2012) |
| Increased Levels in Serum | ||||
| GFAP | WD | Mice | Protein expressionn Serumy) ez | (Yang et al., 2013b) |
GFAP: Glial Fibrillary Acidic Protein
AQP4: Aquaporin 4
VIM: Vimentin
MMP 3: Matrix Metalloproteinase-3
COX-2: Cyclooxygenase-2
TBI, micoglia and M1/M2 phenotypes
Microglia, comprising some 15% to 20% of total cells within the brain, actively survey the CNS environment to orchestrate changes to maintain homeostasis, meet changing physiological needs and respond to pathological events by serving as brain immune cells to coordinate innate immune responses (Harry, 2013). Their physical association with synapses implicates microglia with synaptic refinement and scaling, which is achieved by continuous sampling of specific signals derived from neuronal and astrocyte-derived factors that are sensed by the presence of numerous receptors and ion channels present on microglia. Signaling through these receptors can induce changes in membrane potential, intracellular calcium, cellular motility and cytokine release, accompanied by potential changes in phenotype from their relatively quiescent state to the activated one, evident in TBI. Microglia are adept at exhibiting a M1 pro-inflammatory phenotype, as ensues immediately after TBI, or a M2 anti-inflammatory phenotype which can be induced by IL-4 and is typified by the release of trophic factors such as insulin like growth factor-1, anti-inflammatory IL-10 (Harry, 2013; Suh et al., 2013), and the incretin GLP-1 (Kappe et al., 2012). The ability to time-dependently change phenotype as a consequence of appropriate microenvironmental queues accounts for the heterogeneity of microglial function (Harry, 2013).
As pathological sensors of TBI, microglia rapidly become activated (activated microglia), undergoing essential morphological changes from a branched phenotype to active amoeboid cells (Aihara et al., 1995). Accompanying this, there is an upregulation of a variety of membrane receptors that include those to support phagocytosis. In this regard, microglia are considered to protect neurons by migrating to the site of injury surrounding damaged or dead cells with the purpose of clearing debris, such as myelin debris that can act as a rate-limiting factor in the process of remyelination, and stimulating an M1 inflammatory response to initiate the healing process (Harry, 2013; Kotter et al., 2011). Critically, a rapid transition of this M1 response to one of resolution and repair is required, to provide trophic support by the release of various growth factors (M2 response) followed by quiescence. When appropriately queued, microglia can release nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3(NT-3) to augment neuronal growth and survival (Parekkadan et al., 2008).
Deficits in the ability of microglia to optimally perform these functions or to appropriately switch between M1 and M2 phenotypes detrimentally impacts brain function. Altered microglial morphology associated with a more reactive/activated (M1) phenotype has been reported in rodent and human brain as a function of age (Vaughan and Peters, 1974; Schuitemaker A, et al., 2010), with elevated activated microglia markers and basal pro-inflammation cytokine levels (Harry, 2013). Data on the M2 anti-inflammatory cytokine response is less clear, but is suggested to be reduced (Ye and Johnson 2001; Fenn et al., 2012). Hence age (where, as discussed, TBI events occur more often and induce greater damage) as well as other factors (e.g., the presence of aberrant misfolded proteins) can elevate induction of proinflammatory cytokines, particularly TNF-α and IL-1β, and impair the normal time-dependent transition to the reparative M2 phase, leading to detrimental effects, neuronal dysfunction and demise (Streit et al., 2004; Greig et al., 2014). Key studies demonstrating microglial activation in animal models of TBI are highlighted in Table 2.
TABLE 2.
Studies showing microglia activation levels in Animal Models of TBI
| Markers | model | Species | Findings | Reference |
|---|---|---|---|---|
| Increased Expression in Brain Tissue (Homogenate) | ||||
| OX-6a | CCI | Rat | Protein expression↑ (8 weeks) | (Acosta et al., 2013) |
| Iba1b | CCI | Rat, Mice |
mRNA expression↑ (1 – 28 days) Protein expression↑ (3 – 14 days) |
(Sandhir et al., 2008; Turtzo et al., 2014) |
| CD11bc | CCI | Mice | mRNA expression↑ (1 – 28 days) |
(Sandhir et al., 2008) |
| CD68 | CCI, LFP |
Rat | Protein expression↑ (3 h – 5 days) |
(Shojo et al., 2010; Zhang et al., 2012) |
| TLR2 | WD | Rat | mRNA expression↑ (5 days) Protein expression↑ (2 days) |
(Chen et al., 2008; Zhang et al., 2012) |
| TLR4 | CCI, WD |
Rat, Mice |
mRNA expression↑ (1 – 21 days) Protein expression↑ (1 – 21 days) |
(Chen et al., 2008; Dong et al., 2011; Wei et al., 2014; Ye et al., 2014; Zhang et al., 2012) |
| COX-2 | CCI | Mice | Protein expression↑ (1 day) | (Chen et al., 2012) |
| NF-κBd | CCI, WD |
Rat | mRNA expression↑ (1 day) Protein expression↑ (1 – 5 days) |
(Chen et al., 2012; Chen et al., 2008; Dong et al., 2011; Jin et al., 2010; Jin et al., 2008a; Jin et al., 2008b) |
OX-6: MHC Class II antibody
Iba1: Ionized Calcium Binding Adaptor Molecule 1
CD11b: Cluster of Differentiation Molecule 11b
NF-κB: Nuclear factor-κB
In contrast to microglial function, a central function of oligodendrocytes is to produce the myelin sheaths that surround CNS axons. Each oligodendrocyte may branch to form myelin on many axons within the surrounding tissue. Oligodendrocytes and their precursor cells (OPCs) are distributed throughout both gray and white matter of the CNS. They can provide trophic factors by the production of glial cell line-derived neurotrophic factor (GDNF), BDNF, and insulin-like growth factor-1 (IGF-1) (Dougherty et al., 2000; Du and Dreyfus, 2002). Oligodendrocytes, consequent to their multiple functions, have particularly high metabolic rates and are thus vulnerable to the molecular consequences of tissue insult (McTigue and Tripathi, 2008). Oligodendrocyte dysfunction and demise causes demyelination of WM tracts, resulting in impairment of axonal conduction, and eventually axon death that can underwrite memory impairment. Although phenotypically separate cells, oligodendrocytes, microglia and astrocytes collectively work together in a manner so that their combined dynamic responsibilities support the optimal functioning of the neuronal network. Subtle or blatant dysfunction of any or all of these glia, as occurs following TBI, is accompanied by morphological and functional changes that can impact both neural-glial and glial-glial interactions, and can thereby, result in a loss of homeostasis, imbalances of neurotransmitters and impaired synaptic function.
In synopsis, neuroinflammation is considered to have both beneficial and detrimental roles. Significant benefits can be achieved when the inflammation is controlled in a regulated manner and for a defined period of time. When sustained or excessive, however, inflammation can become a major cause of numerous neuropathologies and, thereby, drive neurodegenerative processes. Time-dependence is hence highly important when considering neuroinflammatory responses, as is their induction by specific experimental paradigms. This may explain the negative outcomes of anti-inflammatory agents in clinical trials of both head injury and other neurodegeneration diseases. Additionally, physiological and biochemical responses to TBI, including inflammation, may differ based on the species or strain, as well as the injury models used in the laboratory (Ziebell and Morganti-Kossmann, 2010). Finally, time-dependent changes ascertained in animal models may not necessarily directly translate in to humans (on a 1:1 ratio – quite possibly they may be slower in humans).
Neuroinflammation and acute production of inflammatory mediators in TBI
Within minutes of a traumatic insult, a robust neuroinflammatory response is generally provoked within the site of injury. The presence of a number of soluble markers of neuroinflammation is not only evident in the region of brain trauma but also in CSF, as typified by proinflammatory cytokines and chemokines (Karve et al., 2015; Kou and VandeVord, 2014; Lozano et al., 2015). As described, under pathological conditions in the CNS, chemokines are generated by activated resident local astrocytes and microglia and act to aid recruit peripheral immune cells into the brain parenchyma (Das et al., 2012; Semple et al., 2010b; Ziebell and Morganti-Kossmann, 2010). In addition to chemokines, various cytokines are also generated and secreted by activated glia following TBI (Das et al., 2012; Kumar and Loane, 2012; Ziebell and Morganti-Kossmann, 2010). These important chemokines and cytokines have been summarized in Tables 3 & 4.
TABLE 3.
Studies showing elevated cytokine levels in brain tissues, CSF and serum in acute stages of TBI in animal models
| Markers | model | Species | Findings | Reference |
|---|---|---|---|---|
| Increased Cytokines Expression in Brain Tissue (Homogenate) | ||||
| IL-1β | CCIa, WDb, LFPc |
Rat, Mice |
mRNA expression↑ (1 h – 7 days) Protein expression↑ (3 h – 24 h) |
(Kamm et al., 2006; Lagraoui et al., 2012; Dalgard et al., 2012; Chen et al., 2008) |
| IL-6 | CCI, WD, LFP |
Rat, Mice |
mRNA expression↑ (3 h – 7 days) Protein expression↑ (1 – 3 days) |
(Lagraoui et al., 2012) |
| TNF-α | CCI, WD, LFP |
Rat, Mice |
mRNA expression↑ (3 h – 5 days) Protein expression↑ (1 – 7 days) |
(Lagraoui et al., 2012; Dalgard et al., 2012; Chen et al., 2008; Zhao et al., 2014; Mukherjee et al., 2011) |
| IL-10 | CCI, WD, LFP |
Rat, Mice |
mRNA expression↑ (3 h – 7 days) Protein expression↑ (1 – 7 days) |
(Kamm et al., 2006; Lagraoui et al., 2012; Perez-Polo et al., 2013; Su et al., 2014) |
| CCI, WD, LFP |
Rat, Mice |
mRNA expression↓ (1 – 5 days) Protein expression↓ (1 – 3 days) |
(Lee et al., 2012; Mukherjee et al., 2011; Wang et al., 2011; Zhao et al., 2014) |
|
| IL-1α | WD, LFP |
Rat | mRNA expression↑ (3 h – 7 days) |
(Lu et al., 2005a; Lu et al., 2005b; Perez-Polo et al., 2013; Shojo et al., 2010; Su et al., 2014) |
| IL-4 | CCI, LFP |
Rat | mRNA expression↑ (7 days) Protein expression↑ (4 – 1 day) |
(Dalgard et al., 2012; Su et al., 2014) |
| IL-5 | CCI, LFP |
Rat | mRNA expression↑ (7 days) Protein expression↑ |
(Dalgard et al., 2012; Su et al., 2014) |
| LFP | Rat | mRNA expression↓ (1 day) | (Mukherjee et al., 2011) | |
| IL-2 | LFP | Rat | mRNA expression↑ (7 days) | (Su et al., 2014) |
| IL-12 | CCI | Mice | Protein expression↑ (3 days) | (Lagraoui et al., 2012) |
| IL-13 | CCI | Rat | Protein expression↑ (3 days) | (Dalgard et al., 2012) |
| IL-17 | LFP | Rat | mRNA expression↑ (7 days) | (Su et al., 2014) |
| mRNA expression↓ (1 day) | (Mukherjee et al., 2011) | |||
| IL-18 | LFP | Rat | mRNA expression↓ (1 day) | (Mukherjee et al., 2011) |
| IFN-γ | CCI, LFP |
Rat, Mice |
mRNA expression↑ (7 days) Protein expression↑ (4 – 12 h) |
(Dalgard et al., 2012; Lagraoui et al., 2012; Su et al., 2014) |
| Increased Cytokines levels in CSF | ||||
| Il-6 | CCI | Rat | Protein expression↑(8 h–2 days) | (Stover et al., 2000) |
| TNF-α | CCI | Rat | Protein expression00) h–2 | (Stover et al., 2000) |
| Increased Cytokines levels in Serum | ||||
| IL-6 | WD | Rat | Protein expressions levels | (Yang, Gangidine, Pritts, Goodman, & Lentsch, 2013; Yang, Gustafson, et al., 2013) |
| TNF-α | WD | Rat | mRNA expressionustafson, et | (Kamm et al., 2006; Singh et al., 2015) |
| Il-10 | WD | Rat | mRNA expression006; Sin | (Singh et al., 2015) |
CCI: Controlled Cortical Impact;
WD: Weight Drop;
LFP: Lateral Fluid Percussion
TABLE 4.
Studies showing elevated chemokine levels in brain tissues, CSF and serum in acute stages of TBI in animal models
| Markers | model | Species | Findings | Reference |
|---|---|---|---|---|
| Increased Chemokine Expression in Brain Tissue (Homogenate) | ||||
| CCL2a (MCP-1) |
CCI, WD, LFP |
Rat, Mice | mRNA expression↑ (4 h –10 days) Protein expression↑ (4 – 12 h) |
(Dalgard et al., 2012; Lagraoui et al., 2012; Liu et al., 2013; Mukherjee et al., 2011; Rau et al., 2014; Rhodes et al., 2009; Semple et al., 2010a; Teng and Molina, 2014) |
| CCR2 | WD | Rat | mRNA expression↑ (10 days) | (Liu et al., 2013) |
| CCL3 (MIP-1a) |
CCI, WD, LFP |
Rat, Mice | mRNA expression↑ (1 day) Protein expression↑ (1 day) |
(Lagraoui et al., 2012; Mukherjee et al., 2011; Su et al., 2014; Yang et al., 2013a) |
| CCL4 | CCI | Mice | mRNA expression↑ (1 – 20 days) | (Leonardo et al., 2012) |
| CCL20 (MIP-3a) |
CCI, LFP |
Rat | Protein expression↑ (2 days) | (Dalgard et al., 2012; Das et al., 2011) |
| CXCL1b | CCI | Rat, Mice | Protein expression↑ (4 h) | (Dalgard et al., 2012; Lagraoui et al., 2012) |
| CXCL4 | CCI | Mice | mRNA expression↑ (6 h) | (Lagraoui et al., 2012) |
| Increased Chemokine Levels in Serum | ||||
| CCL2 (MCP-1) |
CCI, WD, LFP |
Mice | mRNA expressionkine Level | (Probst et al., 2012) |
| CCL3 (MIP-1a) |
WD | Mice | Protein expression12) day) | (Yang, Gangidine, et al., 2013) |
CCL2: Chemokine (C-C Motif) Ligand 2
CXCL1: Chemokine (C-X-C motif) Ligand 1
Cytokines represent a diverse group of small proteins synthesized by a wide variety of cell types that act as autocrine and paracrine mediators of the inflammatory response to injury or infection. Notably, tumor necrosis factor-α (TNFα) is synthesized as a type II transmembrane protein (tmTNFα) of 26 kD. The metalloprotease, TNFα converting enzyme (TACE/ADAM17), can cleave tmTNFα from the plasma membrane of glia to release a soluble form (sTNF-α) of 17 kDa into the brain interstitial fluid (Frankola et al., 2011). Both tmTNFα and sTNFα can then assemble in biologically active homotrimers to bind and activate TNFα receptors (R), which exist in either of two distinct forms on the cell surface. TNF-R1 (p55 or p60) is ubiquitously and constitutively expressed, except on erythrocytes, whereas TNF-R2 (p75 or p80) expression is limited to myeloid and endothelial cells, myocytes, thymocytes, microglia, astrocytes, oligodendrocytes, and selected neurons (Speeckaert et al., 2012). Whereas the two receptors were originally attributed with opposing actions with TNF-R1 providing pro-apoptotic and TNF-R2 pro-survival roles, more recent research has demonstrated that both receptors are capable of driving cell survival and apoptosis depending on the microenvironment, cell type, intracellular signaling pathway and other factors present (Woodcock and Morganti-Kossmann, 2013; Ziebell and Morganti-Kossmann, 2010). Early experimental studies in models of brain injury have demonstrated that TNFα is upregulated in the brain within a few hours (Fan et al., 1996; Knoblach et al., 1999; Shohami et al., 1996; Trembovler et al., 1999), and our more recent studies have confirmed this (Baratz et al., 2015). Notably, the upregulation of TNFα mRNA is reported to occur prior to the time of peripheral immune cell infiltration into the injured site (Riva-Depaty et al., 1994; Ziebell and Morganti-Kossmann, 2010), indicating that resident brain immune cells (e.g., microglia, astrocytes and oligodendrocytes) are the source of cytokines, independent of peripheral immune cell activation. The dual role of TNFα as a pro-apoptotic and pro-survival cytokine in CNS inflammation remains controversial, and studies with TNFα as well as TNF-R1 and –R2 knockout mice suggest that a deficiency of TNFα is beneficial early after experimental brain injury but may be deleterious at a late stage (Scherbel et al., 1999) after TBI. Such time-dependence of lowering TNFα in brain can potentially be optimized pharmacologically by the early administration of a TNFα synthesis inhibitor following a TBI (Baratz et al., 2015).
ANIMAL MODELS OF TBI TO METHODOLOGICALLY EVALUATE NEUROINFLAMMATION
Three widely used TBI rodent models (fluid percussion injury, controlled cortical impact injury, and weight-drop impact acceleration injury) are also evaluated in this article, and these can reasonably mimic key parts of the diverse pathophysiology of human TBI. Each model has individual advantages and disadvantages, and although originally developed in rats, they have subsequently been effectively utilized in mice either with or without modifications. Clearly, mice are smaller, often less expensive, easier to handle, and provide a wide opportunity for genetic manipulations to understand mechanisms, using knock out and knock in technology. By contrast, rats have larger brains, and more readily allow time-dependent plasma as well as CSF sampling. Of note, rodent models of TBI require the use of anesthesthetic agents that clearly limit immediate postinjury behavioral evaluations in relation to orientation, memory, and level of consciousness. Although no single animal TBI model perfectly mimics the human condition (Marklund and Hillered 2011), cross-validation across animal models has demonstrated that neuroinflammation is a consistent feature of TBI and its early mitigation has benefits. In light of the lack of effective pharmacological treatments for TBI, developing drug-like compounds that safely and effectively achieve this and can translate their actions to the human condition is a critical current unmet medical need, and an increasing armamentarium of methodologies exist to support such work.
The weight drop (WD) injury model
The WD model is a clinically relevant animal model, which mimics cerebral contusion by dropping a fixed amount of weight from a pre-determined height onto the exposed dura (open skull WDI) or onto the intact calvarium (closed skull WD), which causes a shearing injury to the brain. The WD model was developed initially for use in the rat and has subsequently been applied to the mouse, either with or without modification(s) from the rat system. To prevent skull fractures caused by the dropped weight, a metallic disc is placed over the skull (generally in the rat) to dissipate the force over a larger area. The injury severity may be manipulated by adjusting the amount of weight as well as the height from which it is dropped. This model has been modified by some users by supporting the head with either a foam cushion, resin mold, or stereotactic frame that creates different pathological and behavioral outcomes. Whereas the weight of the mass, the height of its drop, and the mode of head support may be standardized, the magnitude of the brain injury may also be influenced by the skull thickness and degree of calcification. Slight differences in the drop angle and resultant skull fractures may influence the brain injury characteristics. In an effort to limit the injury variability caused by differences in the skull anatomy, the injury may be produced through a craniotomy using the direct fluid percussion (FP) or lateral FP (LFP) technique to the brain (Xiong et al., 2013). The application of the WD model to mice as a closed skull injury results in diffuse neuronal loss, neuroinflammation, elevation of markers of apoptosis, and both short- and long-term cognitive impairments across a range of behavioral paradigms (Deselms et al., 2016).
The lateral fluid percussion (LFP) model
First established for use in rabbit and cats, the midline FP model of brain injury was later adapted to the rat (Dixon et al., 1987; McIntosh et al., 1987) and subsequently adapted to produce injury within a single hemisphere in rats and mice (McIntosh et al., 1989a). The technique involves a fluid pulse against the intact dural surface of the brain, which is applied through a small trephine opening in the parietal bone to generate a diffuse brain injury. The FP or LFP injury model generates vascular and axonal damage within the brain that is not influenced by either skull thickness or the degree of bone calcification. FP can create complex patterns of brain deformity, contingent on the location of the craniotomy and the path of the percussion waves. In rats, LFP injury generates an amalgamation of focal cortical contusion and diffuse subcortical neuronal injury (that includes injury within the hippocampus and thalamus). This occurs within minutes of the impact and progresses to neuronal loss within 12 hours, but does not markedly expand into other brain regions - as evaluated 7 days post-injury. Reproducibility of results with this model requires extensive and precise animal preparation and operative experience in order to replicate pressure and resulting injury. The LFP model has become one of the most widely used and well characterized models of experimental TBI (Laurer et al., 2002). Changes in injury severity can be achieved by adjusting the pendulum height, which defines the force of the fluid pressure pulse transmitted through the saline reservoir that impacts the dura over the brain. Modification of the injury device has included substitution of a high pressure pump to replace the pendulum and saline reservoir in order to create more reproducible patterns of brain compression. The model requires creation of a craniotomy of a precise shape, size, and location. Small differences in the craniotomy site or the angle of the trajectory can result in different FPI forces and variable brain injury. When undertaken optimally the method is ideal for studies of diffuse axonal injury (DAI) following. Injury is generally accompanied by ipsilateral cortical contusions, with axonal injury to the internal and external capsule and corpus callosum that are similar to those seen in humans (Xiong et al., 2013; Galgano et al., 2015).
The controlled cortical impact (CCI) model
The CCI model induces a measurable brain displacement by effectively utilizing a mechanical solid percussion device that is applied to the dura through a cranial opening. Briefly, in an anesthetized animal a craniectomy is undertaken to gain access to the animal's brain for deformation brain injury induced using a pneumatic or electromechanical CCI device. This system manifests a strong correlation between the magnitude of cortical deformation that is produced and the resulting histological damage to the brain, as well as longer-term behavioral changes. The method is relatively simple to employ, and thus widely used in TBI research. Whereas the majority of CCI studies have been undertaken in rats, numerous also have been published in mice (Xiong et al., 2013; Galgano et al., 2015). Several parameters, including the impactor velocity and depth of the brain deformation produce a precise focal brain injury that may be accurately controlled. In this regard, the impactor can be relatively small tipped – and make a precise and well defined lesion, or it can be of a larger diameter (e.g., 5 mm) to induce DAI and its long-term functional deficits.
A midline CCI lesion using a large 5 mm diameter impactor can induce readily measurable neurological deficits, as assessed by cognitive dysfunction, foot drop, reduced Rotarod test scores, an increased latency in the water maze test, and clumsiness when removing small adhesive paper circles placed on the forelimb paw. Hence, changing the impactor size can create a diversity of clinically relevant models. A limitation of the CCI model is the occasional occurrence of a dural laceration, which is more frequent in the mouse consequent to the delicacy of the dura and its vulnerability to puncture at the plunger impact site. Relatively severe swelling can occur when a large contusion is created through a large craniotomy. When the bone flap is not replaced an open craniotomy provides a release mechanism for the contused and swollen brain, detrimentally affecting the reproducibility of the model. Nevertheless, the model is both widely and effectively utilized in brain injury research, and the relatively mild to moderate forces associated with the CCI model generally do not produce significant brainstem injury to cause high mortality. Subsequent to its development in rats (Dixon et al., 1991), CCI has been commonly applied across species (from large to small) as the quantitative control it permits over injury force and velocity, and thus graded tissue deformation, allows the model’s adaption to numerous research questions. Specifically, CCI has been successfully modified for use across mice, rats, swine, and primates in a manner that perhaps few other methodologies have.
Concussion models (mild TBI)
As sports injuries, particularly with American football, are common in humans, models of such injuries are critical to evaluate the effects of concussion related injuries. Experimental methodologies that cause a cerebral concussion in rodents are essentially similar to those that generate a cerebral contusion (e.g., WD, LFP and CCI models), but are used with decreased force. Cerebral concussion in rodents is characterized by behavioral changes in the forced swimming test, depression-like behavior, and cognitive abilities (Zohar et al., 2011; Deselms et al., 2016). Diffuse neuronal loss is generally evident, along with markers of apoptosis (Tweedie et al., 2007; Tashlykov et al., 2009; Deselms 2016). Concussion models can be used in either mouse or rat, and can involve either a single or repeated injury (Kondo et al., 2015). Neuroinflammation is invariably evident (Faden and Loane 2015), as assessed by astrocytic/microglial reactivity, and can be accompanied with or without alterations in BBB and elevations in phospho-tau levels, particularly the selective cis phosphor-tau form (Kondo et al., 2015) that is evident in chronic traumatic encephalopathy associated with American footballers and other contact sports following multiple concussive and subconcussive injuries (Turner et al., 2013; Gandy et al., 2014). Such animal models, detailed above, are clearly essential for the screening of new therapies for mild concussive and mild to moderate contusion injuries that represent the most frequent types of TBIs, particularly as recent studies suggest that such injuries may be treatable both immediately following and perhaps long after the insult in relation to the neuroinflammation that accompanies them (Faden and Loane 2015).
METHODOLOGICALLY EVALUATING NEUROINFLAMMATION: WHAT CAN BE MEASURED?
As discussed above, extensive research has been undertaken to define the wide-ranging homeostatic changes that ensue following a TBI as a response to attempt to mitigate damage and initiate reparative processes – numerous of which are centered on inflammatory and anti-inflammatory signaling pathways. The earliest quantitative alterations involve cerebral gene expression changes in response to TBI. Transcriptional changes in the injured brain of rodent TBI models have been widely reported (Kobori et al., 2002; Natale et al., 2003; Israelsson et al., 2008 and 2009) in order to increase our understanding of the molecular responses to TBI. Such studies are optimally performed when changes are evaluated time-dependently consequent to the transient changes that occur across cellular and molecular pathways. As an example, Israelsson et al., (2008) evaluated the transcriptional changes that occurred from 1 hr to 7 days following CCI in mice by evaluating injured neocortex and hippocampus using the quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) and performing an unbiased genome-wide search for transcripts up and down regulated in injured brain, as compared to uninjured brain samples. Spatial distribution of enhanced expression was then characterized by the use of in situ hybridization to evaluate in which cell types (microglia, astrocytes, oligodendrocytes, etc.) transcriptional changes were found. Along with this, injured brains were fixed by perfusion, cryosectioned and subjected to immunohistochemistry in order to detect activated microglial (IB4-positive) cells (Israelsson et al., 2008). Particularly, notable in this study is that over 100 gene transcripts were up regulated 3-fold or more, each in an orchestrated time-dependent manner, and were associated with defined cell types. Over 50% of the evaluated genes up regulated 3 days after injury encoded proteins involved in inflammation, immunity, defense responses, cell migration and adhesion, or the encompassed extracellular matrix. Particularly evident was that chemokines and cytokines were upregulated within hours after injury, some (Ccl3, Ccl12) by log-folds.
Mitochondrial dysfunction contributes to cell death in TBI (Hiebert et al, 2015; Yokobori et al, 2014). Recent research has characterized new roles for mitochondria in the regulation of inflammatory processes. The release of signals from mitochondria in response to stress and infection advances the formation of the inflammatory signaling platform identified as inflammasomes. The activation of inflammasomes by damaged mitochondria results in caspase-1-dependent secretion of inflammatory cytokines IL-1β and IL-18, and triggers an inflammatory form of cell death known as pyroptosis (Gurung et al., 2015). Furthermore, mitochondrial MAVS (mitochondrial antiviral-signaling) recruits NLPR3 (nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3) inflammasome components to the mitochondria and is required for optimal NLPR3 inflammasome activity (Haneklaus and O'Neill, 2015). Thus, evaluation of mitochondria-associated inflammasome activity and of NLPR3 inflammasome components (NLPR3, ASV and Caspase-1) and MAVS, likely will bring a greater understanding of the possible signaling cascades that are triggered by, and then underpin, deficits evident in TBI. Recently, increases in reactive oxygen species (ROS) generation were found to be a key factor to activate NLRP3 inflammasomes (Bauernfeind et al., 2011). ROS, that can readily be measured in brain by multiple methodologies (Mattiasson 2004; Wojtala et al., 2014; Bar-Or et al., 2015), appear to be at the crossroads of inflammasomes and inflammation, and elevated markers of oxidative stress have been reported in TBI (Table 5).
TABLE 5.
Studies Showing Elevated Oxidative Stress markers in Animal Models of TBI
| Markers | model | Species | Findings | Reference |
|---|---|---|---|---|
| Increased Oxidative Stress markers in Brain Homogenate | ||||
| SOD | WD | Rat | mRNA expression↓ (1 – 3 days) Protein expression↓ (1 day) |
(Ding et al., 2014a; He et al., 2012; Lu et al., 2015; Wang et al., 2011) |
| NO | WD, LFP |
Rat | Protein expression↑ (3h – 1day) | (Ferreira et al., 2013; Wang et al., 2011; Wei et al., 2014) |
| iNOS | CCI, WD,L FP |
Rat | Protein expression↑ (1 – 3 days) | (Gunther et al., 2012; Jafarian-Tehrani et al., 2005; Jung et al., 2014; Wang et al., 2011; Wei et al., 2014) |
| MDAa | WD | Rat | Protein expression↑ (1 – 3 days) | (Ding et al., 2014a; He et al., 2012; Lu et al., 2015; Wang et al., 2011) |
| MPOb | CCI, WD |
Rat | Protein expression↑ (1 day) | (Chen et al., 2012; Clark et al., 1994) |
| GPxc | Rat | Rat | Protein expression↓ (1 day) | (Ding et al., 2014b) |
MDA: Malondialdehyde
MPO: Myeloperoxidase
GPx: Glutathione Peroxidase
As described above, measuring microglia M1 and M2 phenotypes can be used to determine inflammatory status and mechanisms. M1 is pro inflammation and M2 is anti-inflammation. Isolated microglia or frozen tissues can be evaluated by RT-PCR or protein analyses methods (ELISA as well as multiplex technologies) to measure expression of markers of M1: Marco, IL1β, IL-6 (low IL10), S100A8, 9 and M2: Arg1, Ym-1, FIZZ, IL10, IGF1, together with quantification of other key inflammatory markers such as TNFα and nitric oxide. Likewise, from an immunohistochemistry perspective, antibodies to key proteins such as IBA1, CD-68 and MHC-II (OX6) can be utilized to probe the inflammatory status of the brain time-dependently following injury in both mechanism-focused and drug evaluation studies. A recent notable study is that of Kumar et al., (2015) that involved a detailed phenotypic analysis of M1-and M2-like polarized microglia during the acute phase after CCI in mice. Both phenotypes were activated early after TBI, but the classical ensuing M2-like phenotype was replaced by M1-like and mixed transitional (Mtran) phenotypes that expressed high levels of NOX2 at 7 days post-injury. This shift towards M1-like and Mtran phenotypes, creating an imbalance, is associated with increased neurodegeneration. And thus, these studies indicate that notwithstanding activation of both M1-like and M2-like microglia/macrophages responses after TBI, early M2-like responses may become dysfunctional over time, triggering development of pathological M1-like and Mtran phenotypes that time-dependently drive neuroinflammation from an aberrant perspective.
CONCLUSION
Neuroinflammation in response to TBI involves the activation of glia, release of inflammatory mediators within the brain, and recruitment of peripheral immune cells. Multiple markers of neuroinflammation have been reported to occur across animal models of TBI (Tables 1–5) as well as in the human condition (Woodcock and Morganti-Kossmann, 2013; Di Battista et al., 2015) and, importantly, appear to associate with both patient and animal model outcome measures (Di Battista et al., 2016). Understanding the cellular and molecular mechanisms underpinning neuroinflammation by combining methodologies overviewed herein may beneficially aid the development of new therapies aimed at modulating the inflammatory response acutely post-injury, to mitigate the short- and long-term consequence of TBI.
Acknowledgments
This study was supported in part by (i) a grant from the Ministry of Science and Technology, Taiwan (MOST104-2923-B-038-001-MY3), (ii) the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, MD, USA.
REFERENCES
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond DM, Sanberg PR, Bickford PC, Kaneko Y, Borlongan CV. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PloS one. 2013;8:e53376. doi: 10.1371/journal.pone.0053376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara N, Hall JJ, Pitts LH, Fukuda K, Noble LJ. Altered immunoexpression of microglia and macrophages after mild head injury. Journal of neurotrauma. 1995;12:53–63. doi: 10.1089/neu.1995.12.53. [DOI] [PubMed] [Google Scholar]
- Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochimica et biophysica acta. 2012;1822:675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Wang JY, Rubovitch V, Luo W, Hoffer BJ, Greig NH, Pick CG. Transiently lowering tumor necrosis factor-α synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice. J Neuroinflammation. 2015;12:45. doi: 10.1186/s12974-015-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nature neuroscience. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, et al. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or D, Bar-Or R, Rael LT, Brody EN. Oxidative stress in severe acute illness. Redox Biol. 2015;4:340–345. doi: 10.1016/j.redox.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochemical pharmacology. 2010;79:77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Chen CC, Hung TH, Wang YH, Lin CW, Wang PY, Lee CY, Chen SF. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PloS one. 2012;7:e30294. doi: 10.1371/journal.pone.0030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shi J, Jin W, Wang L, Xie W, Sun J, Hang C. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Annals of clinical and laboratory science. 2008;38:65–74. [PubMed] [Google Scholar]
- Chen Y, Garcia GE, Huang W, Constantini S. The involvement of secondary neuronal damage in the development of neuropsychiatric disorders following brain insults. Front Neurol. 2014;5:22. doi: 10.3389/fneur.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. British journal of pharmacology. 2012;167:699–719. doi: 10.1111/j.1476-5381.2012.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. Journal of neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- Dalgard CL, Cole JT, Kean WS, Lucky JJ, Sukumar G, McMullen DC, Pollard HB, Watson WD. The cytokine temporal profile in rat cortex after controlled cortical impact. Frontiers in molecular neuroscience. 2012;5:6. doi: 10.3389/fnmol.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Leonardo CC, Rangooni S, Pennypacker KR, Mohapatra S, Mohapatra SS. Lateral fluid percussion injury of the brain induces CCL20 inflammatory chemokine expression in rats. Journal of neuroinflammation. 2011;8:148. doi: 10.1186/1742-2094-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Mohapatra S, Mohapatra SS. New perspectives on central and peripheral immune responses to acute traumatic brain injury. Journal of neuroinflammation. 2012;9:236. doi: 10.1186/1742-2094-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deselms H, Maggio N, Rubovitch V, Chapman J, Schreiber S, Tweedie D, Kim DS, Greig NH, Pick CG. Novel pharmaceutical treatments for minimal traumatic brain injury and evaluation of animal models and methodologies supporting their development. J Neurosci Methods. 2016 doi: 10.1016/j.jneumeth.2016.02.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Battista AP, Buonora JE, Rhind SG, Hutchison MG, Baker AJ, Rizoli SB, Diaz-Arrastia R, Mueller GP. Blood Biomarkers in Moderate-To-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front Neurol. 2015;6:110. doi: 10.3389/fneur.2015.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Battista AP, Rhind SG, Hutchison MG, Hassan S, Shiu MY, Inaba K, Topolovec-Vranic J, Neto AC, Rizoli SB, Baker AJ. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J Neuroinflammation. 2016;13:40. doi: 10.1186/s12974-016-0500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y, Zhu L, He J, Zhou M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2-ARE signaling pathway as a potential mechanism. Free radical biology & medicine. 2014a;73:1–11. doi: 10.1016/j.freeradbiomed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Ding K, Wang H, Xu J, Lu X, Zhang L, Zhu L. Melatonin reduced microglial activation and alleviated neuroinflammation induced neuron degeneration in experimental traumatic brain injury: Possible involvement of mTOR pathway. Neurochemistry international. 2014b;76:23–31. doi: 10.1016/j.neuint.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Dong XQ, Yu WH, Hu YY, Zhang ZY, Huang M. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2011;60:533–539. doi: 10.1007/s00011-010-0300-7. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiology of disease. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. Journal of neuroscience research. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- Faden AI, Loane DJ. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics. 2015;12(1):143–150. doi: 10.1007/s13311-014-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Brain research. Molecular brain research. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biological psychiatry. 2014;76:575–584. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced IL-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AP, Rodrigues FS, Della-Pace ID, Mota BC, Oliveira SM, Velho Gewehr Cde C, Bobinski F, de Oliveira CV, Brum JS, Oliveira MS, Furian AF, de Barros CS, Ferreira J, Santos AR, Fighera MR, Royes LF. The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: role of inflammatory and oxidative brain damage. Neurochemistry international. 2013;63:583–593. doi: 10.1016/j.neuint.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Experimental neurology. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10(3):391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgano M, Russell T, Mc Gillis S, Toshkezi G, Chin L, Zhao LR. A review of traumatic brain injury animal models: are we lacking adequate models replicating chronic traumatic encephalopathy? J Neurol Neurobiol. 2(1) doi http://dx.doi.org/10.16966/2379-7150.117. [Google Scholar]
- Gandy S, Ikonomovic MD, Mitsis E, Elder G, Ahlers ST, Barth J, Stone JR, DeKosky ST. Chronic traumatic encephalopathy: clinical-biomarker correlations and current concepts in pathogenesis. Mol Neurodegener. 2014;9:37. doi: 10.1186/1750-1326-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987–995. doi: 10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66(Pt B):75–80. doi: 10.1016/j.mcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi NM, Kwok JC, Fawcett JW. Role of extracellular factors in axon regeneration in the CNS: implications for therapy. Regenerative medicine. 2008;3:907–923. doi: 10.2217/17460751.3.6.907. [DOI] [PubMed] [Google Scholar]
- Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. Journal of neurotrauma. 2014;31:1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig NH, Tweedie D, Rachmany L, Li Y, Rubovitch V, Schreiber S, Chiang YH, Hoffer BJ, Miller J, Lahiri DK, Sambamurti K, Becker RE, Pick CG. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014;10(1 Suppl):S62–S67. doi: 10.1016/j.jalz.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M, Al Nimer F, Gahm C, Piehl F, Mathiesen T. iNOS-mediated secondary inflammatory response differs between rat strains following experimental brain contusion. Acta neurochirurgica. 2012;154:689–697. doi: 10.1007/s00701-012-1297-1. [DOI] [PubMed] [Google Scholar]
- Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21(3):193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneklaus M, O'Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- Harry G. Microglia during development and aging. Pharmacol Ther. 2013;139:313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Qu S, Wang J, He X, Lin W, Zhen H, Zhang X. Neuroprotective effects of osthole pretreatment against traumatic brain injury in rats. Brain research. 2012;1433:127–136. doi: 10.1016/j.brainres.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Hiebert JB, Shen Q, Thimmesch AR, Pierce JD. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350(2):132–138. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neuro Rehabilitation. 2007;22(5):341–353. [PubMed] [Google Scholar]
- Israelsson C, Bengtsson H, Kylberg A, Kullander K, Lewén A, Hillered L, Ebendal T. Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J Neurotrauma. 2008;25(8):959–974. doi: 10.1089/neu.2008.0562. [DOI] [PubMed] [Google Scholar]
- Israelsson C, Wang Y, Kylberg A, Pick CG, Hoffer BJ, Ebendal T. Closed head injury in a mouse model results in molecular changes indicating inflammatory responses. J Neurotrauma. 2009;26(8):1307–1314. doi: 10.1089/neu.2008.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarian-Tehrani M, Louin G, Royo NC, Besson VC, Bohme GA, Plotkine M, Marchand-Verrecchia C. 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2005;12:61–69. doi: 10.1016/j.niox.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Jin W, Ni H, Dai Y, Wang H, Lu T, Wu J, Jiang J, Liang W. Effects of tert-butylhydroquinone on intestinal inflammatory response and apoptosis following traumatic brain injury in mice. Mediators of inflammation. 2010;2010:502564. doi: 10.1155/2010/502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Wang H, Ji Y, Hu Q, Yan W, Chen G, Yin H. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine. 2008a;44:135–140. doi: 10.1016/j.cyto.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Xu L, Wang X, Zhao X, Yang X, Chen G, Ji Y. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators of inflammation. 2008b;2008:725174. doi: 10.1155/2008/725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- Jung CS, Wispel C, Zweckberger K, Beynon C, Hertle D, Sakowitz OW, Unterberg AW. Endogenous nitric-oxide synthase inhibitor ADMA after acute brain injury. International journal of molecular sciences. 2014;15:4088–4103. doi: 10.3390/ijms15034088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabadi SV, Faden AI. Neuroprotective strategies for traumatic brain injury: improving clinical translation. International journal of molecular sciences. 2014;15:1216–1236. doi: 10.3390/ijms15011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K, Vanderkolk W, Lawrence C, Jonker M, Davis AT. The effect of traumatic brain injury upon the concentration and expression of interleukin-1beta and interleukin-10 in the rat. The Journal of trauma. 2006;60:152–157. doi: 10.1097/01.ta.0000196345.81169.a1. [DOI] [PubMed] [Google Scholar]
- Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjöholm Å. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation. 2012;9:276. doi: 10.1186/1742-2094-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. British journal of pharmacology. 2015 doi: 10.1111/bph.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunology. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res. Mol. Brain Res. 2002;104:148–158. doi: 10.1016/s0169-328x(02)00331-5. [DOI] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523(7561):431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease--can we wrap it up? Brain : a journal of neurology. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- Kou Z, Vande Vord PJ. Traumatic white matter injury and glial activation: from basic science to clinics. Glia. 2014;62:1831–1855. doi: 10.1002/glia.22690. [DOI] [PubMed] [Google Scholar]
- Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4268. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain, behavior, and immunity. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Lagraoui M, Latoche JR, Cartwright NG, Sukumar G, Dalgard CL, Schaefer BC. Controlled cortical impact and craniotomy induce strikingly similar profiles of inflammatory gene expression, but with distinct kinetics. Frontiers in neurology. 2012;3:155. doi: 10.3389/fneur.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Simon CM, Prado GR, Cullen DK. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- Lee HF, Lee TS, Kou YR. Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respiratory physiology & neurobiology. 2012;182:1–8. doi: 10.1016/j.resp.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Musso J, Das M, Rowe DD, Collier LA, Mohapatra S, Pennypacker KR. CCL20 Is Associated with Neurodegeneration Following Experimental Traumatic Brain Injury and Promotes Cellular Toxicity In Vitro. Translational stroke research. 2012;3:357–363. doi: 10.1007/s12975-012-0203-8. [DOI] [PubMed] [Google Scholar]
- Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. Journal of neurochemistry. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhang L, Wu Q, Wang T. Chemokine CCL2 induces apoptosis in cortex following traumatic brain injury. Journal of molecular neuroscience : MN. 2013;51:1021–1029. doi: 10.1007/s12031-013-0091-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, Garcia-Segura LM. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PloS one. 2015;10:e0128782. doi: 10.1371/journal.pone.0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatric disease and treatment. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Wang YW, Wo YY, Yang YL. Extracellular signal-regulated kinase-mediated IL-1-induced cortical neuron damage during traumatic brain injury. Neuroscience letters. 2005a;386:40–45. doi: 10.1016/j.neulet.2005.05.057. [DOI] [PubMed] [Google Scholar]
- Lu KT, Wang YW, Yang JT, Yang YL, Chen HI. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. Journal of neurotrauma. 2005b;22:885–895. doi: 10.1089/neu.2005.22.885. [DOI] [PubMed] [Google Scholar]
- Lu XY, Wang HD, Xu JG, Ding K, Li T. Deletion of Nrf2 Exacerbates Oxidative Stress After Traumatic Brain Injury in Mice. Cellular and molecular neurobiology. 2015;35:713–721. doi: 10.1007/s10571-015-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. The Lancet. Neurology. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Marklund N, Hillered L. Animal modeling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiasson G. Analysis of mitochondrial generation and release of reactive oxygen species. Cytometry A. 2004;62(2):89–96. doi: 10.1002/cyto.a.20089. [DOI] [PubMed] [Google Scholar]
- Mayeux JP, Teng SX, Katz PS, Gilpin NW, Molina PE. Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behavioural brain research. 2015;279:22–30. doi: 10.1016/j.bbr.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Annals of neurology. 2014;75:241–254. doi: 10.1002/ana.24064. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiology of aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Katki K, Arisi GM, Foresti ML, Shapiro LA. Early TBI-Induced Cytokine Alterations are Similarly Detected by Two Distinct Methods of Multiplex Assay. Frontiers in molecular neuroscience. 2011;4:21. doi: 10.3389/fnmol.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale JE, Ahmed F, Cernak I, Stoica B, Faden AI. Gene expression profile changes are commonly modulated across models and species after traumatic brain injury. J. Neurotrauma. 2003;20:907–927. doi: 10.1089/089771503770195777. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A. Astrocytes going live: advances and challenges. The Journal of Physiology. 2009;587:1639–1647. doi: 10.1113/jphysiol.2008.167171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, Berdichevsky Y, Irimia D, Leeder A, Yarmush G, Toner M, Levine JB, Yarmush ML. Cell-cell interaction modulates neuroectodermal specification of embryonic stem cells. Neuroscience letters. 2008;438:190–195. doi: 10.1016/j.neulet.2008.03.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Polo JR, Rea HC, Johnson KM, Parsley MA, Unabia GC, Xu G, Infante SK, Dewitt DS, Hulsebosch CE. Inflammatory consequences in a rodent model of mild traumatic brain injury. Journal of neurotrauma. 2013;30:727–740. doi: 10.1089/neu.2012.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Giza CC. Repeat traumatic brain injury in the developing brain. Int J Dev Neurosci. 2012;30:185–190. doi: 10.1016/j.ijdevneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA. Neuroinflammation and synaptic loss. Neurochemical research. 2012;37:903–910. doi: 10.1007/s11064-012-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau TF, Kothiwal A, Rova A, Rhoderick JF, Poulsen DJ. Phenoxybenzamine is neuroprotective in a rat model of severe traumatic brain injury. International journal of molecular sciences. 2014;15:1402–1417. doi: 10.3390/ijms15011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JK, Sharkey J, Andrews PJ. The temporal expression, cellular localization, and inhibition of the chemokines MIP-2 and MCP-1 after traumatic brain injury in the rat. Journal of neurotrauma. 2009;26:507–525. doi: 10.1089/neu.2008.0686. [DOI] [PubMed] [Google Scholar]
- Riva-Depaty I, Fardeau C, Mariani J, Bouchaud C, Delhaye-Bouchaud N. Contribution of peripheral macrophages and microglia to the cellular reaction after mechanical or neurotoxin-induced lesions of the rat brain. Experimental neurology. 1994;128:77–87. doi: 10.1006/exnr.1994.1114. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Riechers RG. Effective treatment of traumatic brain injury: learning from experience. JAMA. 2012;308:2032–2033. doi: 10.1001/jama.2012.14008. [DOI] [PubMed] [Google Scholar]
- Sajja VS, Perrine SA, Ghoddoussi F, Hall CS, Galloway MP, VandeVord PJ. Blast neurotrauma impairs working memory and disrupts prefrontal myo-inositol levels in rats. Molecular and cellular neurosciences. 2014;59:119–126. doi: 10.1016/j.mcn.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Experimental neurology. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW, McIntosh TK. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seker E, Berdichevsky Y, Begley MR, Reed ML, Staley KJ, Yarmush ML. The fabrication of low-impedance nanoporous gold multiple-electrode arrays for neural electrophysiology studies. Nanotechnology. 2010;21:125504. doi: 10.1088/0957-4484/21/12/125504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010a;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010b;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HY, Hwang SL, Lee KT, Lin CL. Temporal trends and volume-outcome associations after traumatic brain injury: a 12-year study in Taiwan. J Neurosurg. 2013;118(4):732–738. doi: 10.3171/2012.12.JNS12693. [DOI] [PubMed] [Google Scholar]
- Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Shojo H, Kaneko Y, Mabuchi T, Kibayashi K, Adachi N, Borlongan CV. Genetic and histologic evidence implicates role of inflammation in traumatic brain injury-induced apoptosis in the rat cerebral cortex following moderate fluid percussion injury. Neuroscience. 2010;171:1273–1282. doi: 10.1016/j.neuroscience.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Singh K, Trivedi R, Devi MM, Tripathi RP, Khushu S. Longitudinal changes in the DTI measures, anti-GFAP expression and levels of serum inflammatory cytokines following mild traumatic brain injury. Experimental neurology. 2015 doi: 10.1016/j.expneurol.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Stover JF, Schoning B, Beyer TF, Woiciechowsky C, Unterberg AW. Temporal profile of cerebrospinal fluid glutamate, interleukin-6, and tumor necrosis factor-alpha in relation to brain edema and contusion following controlled cortical impact injury in rats. Neuroscience letters. 2000;288:25–28. doi: 10.1016/s0304-3940(00)01187-3. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. Journal of neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, Huang H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience. 2014;266:56–65. doi: 10.1016/j.neuroscience.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Suh H, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation. 2013;10:37. doi: 10.1186/1742-2094-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006;148(3):255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, Zohar O, Pick CG. Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci. 2009;37(1):16–24. doi: 10.1007/s12031-008-9094-2. [DOI] [PubMed] [Google Scholar]
- Teng SX, Molina PE. Acute alcohol intoxication prolongs neuroinflammation without exacerbating neurobehavioral dysfunction following mild traumatic brain injury. Journal of neurotrauma. 2014;31:378–386. doi: 10.1089/neu.2013.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura S, Nawashiro H, Otani N, Uozumi Y, Toyooka T, Ohsumi A, Shima K. Effect of decompressive craniectomy on aquaporin-4 expression after lateral fluid percussion injury in rats. Journal of neurotrauma. 2011;28:237–243. doi: 10.1089/neu.2010.1443. [DOI] [PubMed] [Google Scholar]
- Trembovler V, Beit-Yannai E, Younis F, Gallily R, Horowitz M, Shohami E. Antioxidants attenuate acute toxicity of tumor necrosis factor-alpha induced by brain injury in rat. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1999;19:791–795. doi: 10.1089/107999099313640. [DOI] [PubMed] [Google Scholar]
- Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral astrocyte response to micromachined silicon implants. Experimental neurology. 1999;156:33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- Turner RC, Lucke-Wold BP, Robson MJ, Omalu BI, Petraglia AL, Bailes JE. Repetitive traumatic brain injury and development of chronic traumatic encephalopathy: a potential role for biomarkers in diagnosis, prognosis, and treatment? Front Neurol. 2013;3:186. doi: 10.3389/fneur.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. Journal of neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH. Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res. 2007;85(4):805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Kim DS, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Pick CG, Greig NH. Mild traumatic brain injury-induced hippocampal gene expressions: The identification of target cellular processes for drug development. J Neurosci Methods. 2016 doi: 10.1016/j.jneumeth.2016.02.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Li Y, Holloway HW, Lehrmann E, Zhang Y, Becker KG, Perez E, Hoffer BJ, Pick CG, Greig NH. Blast traumatic brain injury-induced cognitive deficits are attenuated by preinjury or postinjury treatment with the glucagon-like peptide-1 receptor agonist, exendin-4. Alzheimers Dement. 2016;12(1):34–48. doi: 10.1016/j.jalz.2015.07.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Zhang Y, Becker KG, Perez E, Hoffer BJ, Pick CG, Greig NH. Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol Dis. 54:1–11. doi: 10.1016/j.nbd.2013.02.006. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol. 1974;3:405–429. doi: 10.1007/BF01098730. [DOI] [PubMed] [Google Scholar]
- Wang GH, Jiang ZL, Li YC, Li X, Shi H, Gao YQ, Vosler PS, Chen J. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. Journal of neurotrauma. 2011;28:2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Zhang Y, Yang C, Wang Q, Zhuang Z, Sun Z. Neuroprotective effects of ebselen in traumatic brain injury model: involvement of nitric oxide and p38 mitogen-activated protein kinase signalling pathway. Clinical and experimental pharmacology & physiology. 2014;41:134–138. doi: 10.1111/1440-1681.12186. [DOI] [PubMed] [Google Scholar]
- Wojtala A, Bonora M, Malinska D, Pinton P, Duszynski J, Wieckowski MR. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014;542:243–262. doi: 10.1016/B978-0-12-416618-9.00013-3. [DOI] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Frontiers in neurology. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Gangidine M, Pritts TA, Goodman MD, Lentsch AB. Interleukin 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock. 2013a;40:471–475. doi: 10.1097/SHK.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Gustafson J, Gangidine M, Stepien D, Schuster R, Pritts TA, Goodman MD, Remick DG, Lentsch AB. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. The Journal of surgical research. 2013b;184:981–988. doi: 10.1016/j.jss.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Johnson R. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Ye Y, Xu H, Zhang X, Li Z, Jia Y, He X, Huang JH. Association between toll-like receptor 4 expression and neural stem cell proliferation in the hippocampus following traumatic brain injury in mice. International journal of molecular sciences. 2014;15:12651–12664. doi: 10.3390/ijms150712651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobori S, Mazzeo AT, Gajavelli S, Bullock MR. Mitochondrial neuroprotection in traumatic brain injury: rationale and therapeutic strategies. CNS Neurol Disord Drug Targets. 2014;13(4):606–619. doi: 10.2174/187152731304140702112805. [DOI] [PubMed] [Google Scholar]
- Zhang YB, Li SX, Chen XP, Yang L, Zhang YG, Liu R, Tao LY. Autophagy is activated and might protect neurons from degeneration after traumatic brain injury. Neuroscience bulletin. 2008;24:143–149. doi: 10.1007/s12264-008-1108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Immunolocalization of Toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. Neuroimmunomodulation. 2012;19:10–19. doi: 10.1159/000326771. [DOI] [PubMed] [Google Scholar]
- Zhao GW, Wang Y, Li YC, Jiang ZL, Sun L, Xi X, He P, Wang GH, Xu SH, Ma DM, Ke KF. The neuroprotective effect of modified "Shengyu" decoction is mediated through an anti-inflammatory mechanism in the rat after traumatic brain injury. Journal of ethnopharmacology. 2014;151:694–703. doi: 10.1016/j.jep.2013.11.041. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp (Wars) 2011;71(1):36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]