Abstract
The continued success of efforts to reduce the global malaria burden will require sustained funding for interventions specifically targeting Plasmodium vivax. The optimal use of limited financial resources necessitates cost and cost-effectiveness analyses of strategies for diagnosing and treating P. vivax and vector control tools. Herein, we review the existing published evidence on the costs and cost-effectiveness of interventions for controlling P. vivax, identifying nine studies focused on diagnosis and treatment and seven studies focused on vector control. Although many of the results from the much more extensive P. falciparum literature can be applied to P. vivax, it is not always possible to extrapolate results from P. falciparum–specific cost-effectiveness analyses. Notably, there is a need for additional studies to evaluate the potential cost-effectiveness of radical cure with primaquine for the prevention of P. vivax relapses with glucose-6-phosphate dehydrogenase testing.
Introduction
Plasmodium vivax is the most widely distributed species of malaria across the world, with almost 3 billion people at risk and an estimated 13.8 (10.3–18.4) million clinical cases every year, mostly in Asia, the Horn of Africa, and South America.1–4 Almost half of all malaria cases outside Africa are attributable to P. vivax.5 The scale of the public health burden has been highlighted by the increasing evidence for the magnitude of severe and fatal disease caused by P. vivax.3 Over the past decade, the concerted scale-up of malaria control efforts, in particular long-lasting insecticidal nets (LLINs) and artemisinin combination therapies (ACT), has resulted in significant reductions in the burden of malaria, but with an increase in the ratio of P. vivax to Plasmodium falciparum cases in many areas where the two species coexist.6,7 In most countries in the malaria preelimination or elimination phases, P. vivax is the dominant species.5 It is likely that continued progress may require increased financing for interventions specifically directed toward the P. vivax hypnozoite reservoir and innovative vector control tools along with an improved understanding of the relationship between costs and public health benefit.
Much of the theoretical and empirical work on the costs and cost-effectiveness of malaria control interventions has focused on P. falciparum malaria,8,9 due to its high prevalence in sub-Saharan Africa where the majority of the burden of cases and deaths from malaria occur. The costs and cost-effectiveness of interventions for controlling P. falciparum are usually analyzed in isolation without the need to consider detailed interactions with other Plasmodium species, most notably P. vivax.8–10 In contrast, studies of P. vivax are often undertaken in areas that are coendemic with P. falciparum, thus requiring costs and benefits to be apportioned between the two species of malaria. Although some of the costs from studies focusing on P. falciparum can be used to estimate the costs related to P. vivax control, they cannot simply be extrapolated from an African setting to an Asian, western Pacific, or South American setting, nor can measures of effectiveness be extrapolated from P. falciparum to P. vivax. Furthermore, P. falciparum focused studies provide limited insight for P. vivax–specific interventions that target the hypnozoite reservoir.
The biology and epidemiology of P. vivax present a number of complicating factors that must be accounted for in studies for the evaluation of the costs and cost-effectiveness of certain interventions, most notably relapses due to hypnozoites, which are only killed by 8-aminoquinoline drugs11; and the risk of hemolysis in glucose-6-phosphate dehydrogenase (G6PD)–deficient patients after treatment with 8-aminoquinoline therapies.12 The impact of these phenomena on the effectiveness of P. vivax control interventions (vector control or treatment with antimalarials) has been documented in some cases,13 but the integration of the effects of relapses and hemolysis into studies of cost-effectiveness remains an ongoing challenge.
Herein, we review the evidence from published studies on the costs and cost-effectiveness of interventions for controlling P. vivax, and consider the economic consequences of controlling or failing to control P. vivax malaria.
Measuring Costs, Effects, and Cost-Effectiveness of Interventions
When evaluating the cost of interventions, the perspective taken can be that of the health-care provider, the patient, or both (the societal cost).14 Depending on the intervention, provider costs include the cost of consumables such as insecticide-treated bed nets (ITNs), drugs and rapid diagnostic tests (RDTs), and the costs associated with implementation. These can include start-up capital costs (such as buildings and vehicles), recurrent costs (such as personnel and overheads), and sometimes costs of starting up new interventions (such as training) or implementing supportive interventions (such as community sensitisation). Extending to a societal perspective requires inclusion of direct household costs of illness (such as travel, drugs, and consultation costs) which vary greatly and can be difficult to estimate.15 Costs can be expressed as the total costs of an intervention for a given area or population, or as cost per unit delivered or person protected. Furthermore, there is a distinction between financial and economic costs. Financial and economic costs reflect the unit cost of an intervention and the resources required for its delivery in terms of the actual expenditures incurred. The economic costs capture the opportunity cost of all resources used to provide an intervention, whether or not they incur a financial expenditure.
The cost-effectiveness of an intervention is the ratio of the cost to a relevant measure of its effect, and is often compared with a counterfactual of “doing nothing” or else the cost-effectiveness of an existing intervention (referred to as incremental cost-effectiveness when a comparison is made). The choice of outcome measure depends on the intervention and the perspective taken; for example, numbers correctly treated, numbers cured, number of cases and deaths averted, or the number of deaths or disability-adjusted life years (DALYs) averted. In particular, multiple effectiveness metrics can be defined for each intervention. Data on effects can come from routine health system data (e.g., number of clinical malaria cases treated and number of malaria-related deaths in the public health system) or research studies. However, studies measuring impact on transmission or mortality can be demanding to undertake, and therefore, economic and mathematical modeling is often used to predict the impact of interventions or packages of interventions on outcomes.16,17
A checklist for critical appraisal of health economic evaluation studies is provided by Drummond's criteria,14,18 which appraises whether costs and effects are adequately evaluated and compared with competing alternatives. An updated set of guiding principles, methodological specifications, and reporting standards to support cost-effectiveness evaluations of health interventions has recently been compiled by The Bill & Melinda Gates Foundation, the National Institute for Health and Care Excellence and partners, and presented in the Gates Reference Case.19 The reference case outlines a number of principles that should be considered during economic evaluations, including transparency, uncertainty, equity, time horizons, and appropriate measures of health outcomes. These principles provide a contemporary gold standard for the economic evaluation of health interventions.
Models for Estimating the Cost-Effectiveness of P. vivax Interventions
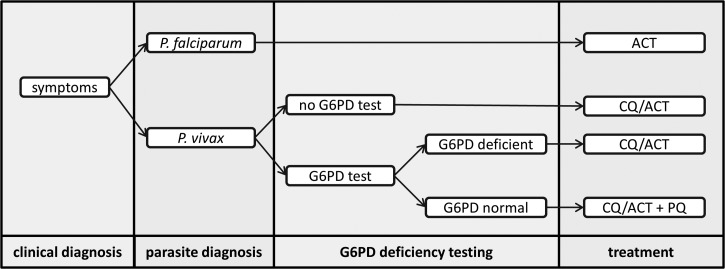
Cost-effectiveness analyses frequently utilize models to combine data with realistic assumptions such as diagnostic performance or treatment success rates. Decision tree models which track the progress of a patient through a treatment pathway are frequently used as they allow uncertainty to be handled robustly. They have been widely applied to the diagnosis and treatment of both P. falciparum and P. vivax.20–22 Figure 1 shows an example of a probabilistic decision tree model suitable for analyzing data from a study of treatment and diagnosis in a P. vivax and P. falciparum coendemic setting. At each node in the tree, the probability of progressing to the next node will be determined by the collected data or prior knowledge of the properties of the diagnostic tool (e.g., sensitivity and specificity). Running repeated simulations with the probabilities at each node varied within a range determined by a sensitivity analysis allows the uncertainty in estimates of costs and cost-effectiveness to be captured. Decision tree models can also be used to assess the feasibility of alternative strategies such as providing primaquine to all malaria cases in areas where P. falciparum infection is a good predictor of future P. vivax relapses.13,23
Figure 1.
Schematic of a decision tree model for cost-effectiveness analysis in a coendemic Plasmodium falciparum and Plasmodium vivax setting, with endpoints of correctly diagnosed and treated cases.
Markov models describe a number of states and likelihoods of moving from one state to another. Unlike decision tree models, Markov models allow loops back into previous states and are therefore often used in chronic diseases or relapsing diseases.24 Existing economic models of P. vivax have been based on decision trees20; however, the relapsing nature of the disease lends itself to a Markov model.
Overview of Existing Work on Cost-Effectiveness of P. vivax Interventions
Herein, we present an overview of the costs and cost-effectiveness of controlling P. vivax malaria based on the information available in the publicly available literature. A review of the published literature on the costs and cost-effectiveness of P. vivax control was conducted using the online database PubMed. The search term used was “vivax” and “cost” or “economic.” This was supplemented by reviews of the reference lists of relevant published papers. All studies reviewed focused either on diagnosis, treatment, or vector control. The results are presented in Table 1 (diagnosis and treatment) and Table 2 (vector control). All the identified studies were based on intervention trials, except for one study on the cost-effectiveness of diagnosis and treatment in the Brazilian Amazon.32 The number of studies on P. vivax was much less than the substantial literature on P. falciparum.10 For example, when van Vugt and others41 undertook a systematic review of the cost-effectiveness of malaria treatment and prophylaxis, they identified 17 studies, only two of which included estimates of the cost-effectiveness of testing or treating P. vivax. Studies scored highly when graded against Drummond's criteria18 (Table 1, Table 2, and Supplemental Table 1), indicating a good quality of evidence, particularly among studies published since 2000.
Table 1.
Studies of the costs and cost-effectiveness of diagnosing and treating Plasmodium vivax
| Study area (year) | Perspective and scope | P. vivax cases (%) | Drummond's criteria | Intervention | Cost 2013 USD | Cost-effectiveness 2013 USD |
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| Amazon, Brazil (2006)20 | Provider | 67 | 10/10 | Pf/Pv RDT (OptiMal) | 4.95 per test | 5.94 per case adequately diagnosed |
| Serv. incl. | Microscopy | Not provided | 7.85 per case adequately diagnosed | |||
| Microscopy vs. OptiMal | 635.45 per additional case adequately diagnosed | |||||
| Sri Lanka (2001–2002)25 | Provider | 70 | 8/10 | ICT Pf/Pv assay (70% sensitivity) | 4.93 per test | 14.36–143.62 per Pv infection detected |
| Serv. incl. | microscopy | 0.74–7.35 per Pv infection detected | ||||
| Manila, Palawan, Philippines (2009)26 | Provider | NA | NA | RDT (private sector: Panbio, Parascreen, Parabank, Paraview) | 4.13–13.03 per RDT | No effectiveness data |
| Diagnosis and treatment | ||||||
| Afghanistan (provider) (2009–2012)27 | Provider | ≥ 90 | 10/10 | Moderate transmission | ||
| Serv. incl. | Pf/Pv RDT (CareStart) | 2.08 per RDT | 2.82 per patient tested and treated | |||
| Microscopy | 2.05 per test by microscopy | 2.82 per patient tested and treated | ||||
| Low transmission | ||||||
| Pf/Pv RDT (CareStart) | 1.30 per RDT | 2.06 per patient tested and treated | ||||
| Microscopy | 8.32 per test by microscopy | 9.77 per patient tested and treated | ||||
| Afghanistan (societal) (2009–2012)27 | Societal | ≥ 90 | 10/10 | Moderate transmission | ||
| Serv. incl. | Pf/Pv RDT (CareStart) | 10.32 per patient tested and treated | ||||
| Microscopy | 10.64 per patient tested and treated | |||||
| Low transmission | ||||||
| Pf/Pv RDT (CareStart) | 14.66 per patient tested and treated | |||||
| Microscopy | 22.38 per patient tested and treated | |||||
| Tigray, Ethiopia (2006)28 | Provider | 31.5 | 9/10 | Presumptive treatment | 0.69–2.77 per course (AL) | 12.80 per malaria case treated |
| Pf RDT (Paracheck) + treatment | 0.68 per test | 5.38 per malaria case treated | ||||
| Pf/Pv RDT (Parascreen) + treatment | 1.21 per test | 7.88 per malaria case treated | ||||
| Thailand, near Myanmar border (2000–2001)29 | Societal | 50 | 9/10 | Microscopy + treatment | 13.23 per true-positive case | |
| Serv. incl. | 17.64 per Pv case | |||||
| ICT Pf/Pv assay + treatment | 4.46 per test | 10.17 per true-positive case | ||||
| 14.45 per Pv case | ||||||
| Pf/Pv RDT (OptiMal) + treatment | 4.24 per test | 8.36 per true-positive case | ||||
| 6.94 per Pv case | ||||||
| Madang, East Sepik, Papua New Guinea (2005–2007)30 | Societal | 30 | 10/10 | CQ + SP (base case) | 0.03 per treatment | 4.36 per Pv case treated |
| Serv. incl. | ARTS + SP | 0.43 per treatment | 4.98 per Pv case treated | |||
| DHA + PPQ | 0.27 per treatment | 4.25 per Pv case treated | ||||
| AL | 0.29 per treatment | 5.62 per Pv case treated | ||||
| ARTS+SP vs. CQ+SP | 0.62 per Pv case treated | |||||
| DHA+PPQ vs. to CQ+SP | −0.11 per Pv case treated | |||||
| AL vs. CQ + SP | 1.26 per Pv case treated | |||||
| Rakhine State, Myanmar (1998–1999)31 | Provider | 52 | 9/10 | EDAET (RDT + AL/[CQ+PQ]) | 0.30 per Pv treatment | 1.05 per child year |
| Serv. incl. | 0.62 per Pf treatment | 19 per DALY averted | ||||
| 0.80 per RDT | ||||||
| Amazon, Brazil (2009–2011)32,33 | Provider | 83 | 9/10 | Pv microscopy | 8.85–11.27 per test | |
| Serv. incl. | CQ (3 days) | 0.12 per treatment | ||||
| CQ (3 days) + PQ (7 days) | 0.23 per treatment | |||||
| CQ + prophylaxis (12 weeks) | 0.28 per treatment | |||||
| G6PD diagnosis: | ||||||
| CS-G6PD vs. routine | 4.14 per test | 4.30 per adequately diagnosed case | ||||
| BX-G6PD vs. routine | 9.81 per test | 9.96 per adequately diagnosed case | ||||
| CS-G6PD vs. BX-G6PD | 2.99 per adequately diagnosed case | |||||
AL = artemether–lumefantrine; ARTS = artesunate; BX = BinaxNow; CS = CareStart; CQ = chloroquine; DALY = disability-adjusted life year; DHA = dihydroartemisinin; EDAET = early diagnosis and effective treatment; G6PD = glucose-6-phosphate dehydrogenase; ICT = immunochromatographic test; Pf = Plasmodium falciparum; PPQ = piperaquine; PQ = primaquine; Pv = Plasmodium vivax; RDT = rapid diagnostic test; SP = sulfadoxine–pyrimethamine; USD = U.S. Dollar. All costs have been inflated to 2013 USD. All costs, unless otherwise indicated, are per person diagnosed or treated. Serv. incl. denotes that service delivery is included in the costing scope.
Cost saving (dominant).
Table 2.
Studies of the costs and cost-effectiveness of vector control interventions
| Study area (year) | Perspective and scope | Plasmodium vivax cases (%) | Drummond's criteria | Intervention | Cost 2013 USD | Cost-effectiveness 2013 USD |
|---|---|---|---|---|---|---|
| Rakhine State, Myanmar (1998–1999)31 | Provider serv. incl. | 52% | 9/10 | ITNs vs. control | 10.00 per net distributed 5.56 per person protected | 51 per DALY averted |
| ITNs + EDAET* vs. EDAET alone | 148 per DALY averted | |||||
| Thailand, villages near Myanmar border(1993–1994)34,35 | Provider | 20–33 | 9/10 | Control (surveillance only) | 3.43 per person at risk | |
| serv. incl. | ITNs (impregnation only) | 0.55 per person protected | 7.89 per case averted (compared with control) | |||
| IRS (DDT) | 1.32 per person protected | 16.54 per case averted (compared with control) | ||||
| ITNs (impregnation only) + control | 2.28 per person protected | −16.80 per case averted* | ||||
| IRS (DDT) + control | 2.80 per person protected | −7.88 per case averted* | ||||
| Gujarat, India (1997–1998)36 | Societal | NA | 10/10 | ITNs vs. control (EDPT†) | 3.52 per net distributed | 74.84 per case averted |
| Serv. incl. | 2.25 per person protected | |||||
| IRS (cyclthin) vs. control (EDPT) | 10.77 per house sprayed | 126.39 per case averted | ||||
| 2.05 per person protected | ||||||
| ITNs vs. IRS (cyclthin) | 32.36 per case averted | |||||
| Ninh Thuan, Vietnam forest (2012)37 | Societal serv. incl. | 47 | 10/10 | long-lasting insecticide treated hammock vs. control | 11.93 per hammock | 127.85 per case averted |
| Colombia (2000–2001)38 | Provider serv. incl. | NA | 6/10 | ITN impregnation (twice yearly) | 6.71–16.32 per net impregnated | |
| 4.87–11.19 per person protected | No effectiveness data | |||||
| IRS (lambdacyhalothrin, twice yearly) | 48.43–62.90 per house | |||||
| 9.74–13.55 per person protected | ||||||
| Solomon Islands (1989–1990)39 | Provider | NA | 6/10 | ITNs | 3.71 per person protected | No effectiveness data |
| Serv. incl. | IRS (DDT) | 8.22 per person protected | ||||
| Hoa Binh, Vietnam mountain (1996)40 | Provider | NA | 6/10 | ITNs (5-year duration) | 1.34 per person year | |
| Serv. incl. | ITN (impregnation only, twice yearly) | 0.48 per person year | No effectiveness data | |||
| IRS (lambdacyhalothrin, once yearly) | 0.70 per person year |
DALY = disability-adjusted life year; DDT = dichlorodiphenyltrichloroethane; ITN = insecticide-treated bed net; IRS = indoor residual spraying; NA = not applicable. All costs have been inflated to 2013 USD. Cases refer to both cases of Plasmodium vivax and Plasmodium falciparum. Unless stated otherwise, studies were costed from a provider perspective. Serv. incl. denotes that service delivery is included in the costing scope.
Cost saving (dominant). Early diagnosis (with RDTs) and early treatment (with ACTs).
Early diagnosis and prompt treatment.
Costs and Cost-Effectiveness of Diagnosis and Treatment
Episodes of P. vivax malaria can be treated using blood schizontocidal drugs such as chloroquine (CQ) (in areas free from resistance42) and ACTs.43 Blood schizontocidal drugs clear blood-stage parasites and reduce P. vivax associated morbidity, but do not affect the liver-stage hypnozoites responsible for relapses. There are few published studies on the costs and cost-effectiveness of case management specifically including P. vivax. Nine studies were identified (Table 1) with three focusing only on diagnostic testing. Only one study compared treatment with different blood schizontocidal regimens.30
In terms of diagnosis, the development and implementation of new diagnostic testing for P. vivax has lagged behind P. falciparum.44 Good-quality RDTs capable of detecting both species have become increasingly available in areas where the two species coexist.45 The cost of RDTs has been declining over time46 with pan-specific tests available in the range of 1–2 U.S. Dollars (USD).27 The cost-effectiveness studies that were surveyed used different costs and different measures of effectiveness such as “correctly treated” or “adequately diagnosed.” Two studies from different settings found RDTs to be a more cost-effectiveness option for diagnosis and treatment than microscopy (Table 1).20,27 In general, the cost-effectiveness of options for diagnosing P. vivax infections will depend on a number of factors including P. vivax prevalence, the operational accuracy of the different options (including presumptive and microscopy), cost of treatment, and provider adherence to the test result.20,27 A particularly important factor is the volume of patients: in a busy health center or hospital, microscopy may be very cost-effective per diagnosis, although there may be a trade-off in the time taken for diagnosis.
Plasmodium vivax infections can be treated with either CQ or ACTs.28,47 The decision to adopt treatment of P. vivax with ACTs (potentially as part of a unified treatment strategy) will depend on economic factors. The cost per full adult course of ACT has been estimated to be in the range 0.92–3.85 USD, compared with 0.07–0.10 USD for CQ.43 However, the cost of ACTs has been steadily declining.48 A study on the cost-effectiveness of treatment of uncomplicated malaria in children from a P. vivax and P. falciparum coendemic region of Papua New Guinea found that, despite the increased costs of ACTs, the cost per case of P. vivax treated was comparable for ACTs and CQ + sulfadoxine–pyrimethamine30 (Table 1), and dihydroartemisinin–piperaquine was found to be the most cost-effective option for P. vivax using 42-day efficacy as the outcome measure. Despite this finding, further evidence on the cost-effectiveness of unified treatment strategies is needed.
Radical Cure and G6PD Deficiency Testing
Compared with assessing the cost-effectiveness of blood schizontocidal drugs, there are a number of additional challenges associated with providing radical cure with hypnozoitocidal therapy against liver-stage parasites, in particular the occurrence of relapses and the potential for hemolysis in G6PD-deficient patients treated with 8-aminoquinolines.12 Each relapse may cause a debilitating febrile illness with deepening risk of severe anemia or other complications associated with fatal outcomes, along with opportunities for continued transmission in the community.
Currently, primaquine, an 8-aminoquinoline, is the only drug currently available that effectively eliminates hypnozoites, resulting in radical cure and therefore with the potential to cause large reductions in morbidity and mortality. Primaquine is inexpensive, costing 0.15–0.60 USD per course.49–51 However, there are two major disadvantages to its use as a hypnozoitocidal drug: a standard regimen of 14 days resulting in poor adherence; and the potential to cause life-threatening hemolysis in patients with G6PD deficiency, an inherited disorder. Tafenoquine, another 8-aminoquinoline, is currently undergoing Phase 3 clinical trials, and is likely to be at least as efficacious as primaquine,52 but has a much longer half-life and will therefore only require a single dose. Although it is likely to be more expensive than primaquine, the single dose may provide better levels of adherence to a full therapeutic course. However, this promising new therapy also puts patients with G6PD deficiency at risk of potentially fatal hemolysis without the option of abandoning the treatment course midway. The problem of screening out vulnerable patients from receiving it will be a crucial task in terms of real-world access to the drug and its huge clinical and public health benefits.
Screening for G6PD deficiency is possible, but has previously been limited to higher level health facilities.53 A number of different point of care diagnostics are under development, with some experience of using them in the field.54,55 Costs per assay are in the range of 1.50–20.00 USD23; however, once implementation costs are included, the minimum cost per subject tested is unlikely to be less than 4.00 USD. It is possible that the production of currently available tests could be optimized so that the price becomes comparable to parasitological RDTs costing approximately 0.50 USD per test.56,57 Notably, the cost of widespread testing for G6PD deficiency may be less than the alternative of providing primaquine without testing and treating subsequent episodes of hemolysis. For example, in the Brazilian Amazon providing primaquine without prior G6PD testing to infected males may be associated with excess deaths, as well as an estimated annual cost to the health system of 4–5 million USD.33
The cost-effectiveness of 8-aminoquinoline treatment with and without prior G6PD deficiency testing will depend on a number of epidemiological, biological, behavioral, and drug factors. The effectiveness in different transmission settings is likely to be highest where the likelihood and frequency of relapses is highest and the proportion of cases attributable to new/reinfections relatively low. The effectiveness of the drug will be critically dependent on adherence which will depend on the choice of drug and dosing regimen. The risk of hemolysis after treatment will depend on a number of factors including12,58 1) the overall prevalence of different variants of G6PD deficiency in the population and the susceptibility of the variant to hemolysis, 2) the gender of the patient, and 3) the dosing regimen of the 8-aminoquinoline. Finally, the incremental cost-effectiveness of G6PD deficiency testing will depend on the cost of the test, sensitivity, and specificity for correctly diagnosing individuals at significant risk of severe hemolysis, and uptake and quality of use under operational settings.
Costs and Cost-Effectiveness of Vector Control
Targeting mosquitoes with vector control interventions can jointly combat P. vivax and P. falciparum malaria,31 as well as other vector-borne diseases.59 The potential impact of interventions will depend on the behavioral characteristics of local mosquito populations, such as the proportion of blood meals taken on humans and the proportion of exposure occurring indoors.60 In P. falciparum–endemic Africa, LLINs and indoor residual spraying (IRS) are the mainstays of vector control and are highly effective against Anopheles gambiae, the predominant vector which feeds almost exclusively on humans indoors during the hours of sleep. The wide range of vector species in P. vivax–endemic regions61 will result in substantial variation in the potential effectiveness of different vector control tools, with feeding on domesticated animals and early outdoor biting limiting the effectiveness of LLINs and IRS. Some interventions such as insecticide-treated hammocks and hammock nets have been specifically developed for outdoor use.62 These have been used in parts of Asia by populations at risk of P. vivax (e.g., forest workers, other highly mobile groups, and people living in traditional homes not suited to LLINs).63 Environmental control methods such as water drainage and larviciding can provide long-lasting cost-effective protection in areas with vulnerable local vector species.64
As has been reviewed elsewhere, there are few studies on the effectiveness of vector control interventions for reducing P. vivax transmission and morbidity65 (Hii and others, Vector control thematic review, AJTMH supplement 2016). There are even fewer studies on the cost-effectiveness of vector control against P. vivax, with none looking at P. vivax specifically. Table 2 presents the costs per case averted or person protected against malaria in six countries with both P. vivax and P. falciparum. Four studies focused on ITNs and IRS. Three studies found ITNs to be more cost-effective than IRS.34–38 One study modeled the cost-effectiveness of LLINs compared with the provision of early RDT diagnosis and treatment using community volunteers in Myanmar.31 Compared with no interventions, ITNs were estimated to avert one DALY for every 51 USD spent. When ITNs were implemented alongside early diagnosis and effective treatment (EDAET), the incremental cost per DALY averted compared with EDAET alone was estimated as 148 USD. It should be noted that these studies provide estimates of cost-effectiveness of interventions against all episodes of malaria and are not stratified according to numbers of P. falciparum and P. vivax cases.
A study on long-lasting insecticide-treated hammocks found them to prevent cases of malaria in individuals sleeping and working in forested areas.37 The cost per case of malaria averted (P. vivax or P. falciparum) was estimated at 126 USD. In addition to ITNs and IRS, individuals frequently protect themselves using methods such as mosquito coils, aerosol sprays, vaporizing mats, and repellents. Although these tools have been proven to reduce the number of mosquito bites, evidence of protection against malaria in programmatic settings is scarce.66 Annual household expenditure on these tools has been reported as 4–25 USD in Thailand,67 2.04–19.20 USD in rural Indian areas, and 15.60–26.40 USD in urban Indian areas.68,69
Procurement Costs
The costs informing the studies in Tables 1 and 2 are based on commodity prices during the year of the study. However, the global scale-up of malaria control over the past decade has led to substantial reductions in the cost of procuring drugs, tests, and LLINs.70 For example, procurement costs for LLINs in P. vivax–endemic countries can be obtained from The Global Fund's Price and Quality Reporting tool. The average cost of procuring an LLIN in P. vivax–endemic regions has been reported as approximately three USD, ranging from 1.65 to 5.80 USD. In addition to procurement costs, the cost of distribution must be accounted for, usually found to be in the range 1–4 USD per net.10 The total cost of LLIN procurement and delivery is therefore likely to range between 4 and 7 USD and vary across net types and products.
The Cost of Not Controlling P. vivax
The socioeconomic burden of P. vivax depends on a number of factors, most notably the estimated annual number of clinical cases and P. vivax–associated deaths1; the contribution of P. vivax to chronic anemia and; the economic costs of treatment, borne by either the individual patient or the health system; and the economic cost to a society associated with absence from work or school. There are likely to be substantial indirect costs due to lost productivity—three studies found that symptomatic episodes of malaria in Asia resulted in the loss of 4–15 days of work or school by the affected patient.71–73 In addition to effects that can be quantified economically, P. vivax has many other negative consequences. Recurrent episodes of P. vivax are also likely to have an adverse impact on school performance in children, the economic cost of which is difficult to estimate.74,75 Plasmodium vivax episodes are expected to result in anemia, malnutrition, growth retardation, and stunting of development, all of which lead to societal direct costs including health-care costs from provider and patient perspectives and indirect costs such as impaired economic productivity in later life.3
As well as affecting households on a microeconomic level, malaria is also likely to have macroeconomic effects. However, the associations between malaria burden and macroeconomic measures such as gross domestic product (GDP) growth rate are challenging to estimate due to issues of causality—do people get malaria because they are poor, or are people poor because they get malaria?76 In an analysis of the association between malaria and GDP growth, Sachs and others found that in countries with intense malaria, GDP growth was reduced by 1.3% per year77,78; however, this study predominantly focused on P. falciparum malaria in sub-Saharan Africa, and while its conclusions may not be extrapolated to P. vivax, it is likely that P. vivax has a nonnegligible impact on economic development. Finally, there is evidence that socioeconomic development is an effective intervention against malaria.79 Increased economic development will lead to strengthening of health systems and better treatment, and economic empowerment of individuals to better cope with episodes of malaria. Globally significant social and economic trends such as urbanization80 and improved road and transport networks are all likely to reduce malaria burden.
Discussion
Although there is an emerging consensus of the need to further reduce the burden of P. vivax malaria, particularly given the renewed enthusiasm for malaria elimination,81 evidence for the costs and cost-effectiveness of P. vivax control and elimination is lacking. An increased evidence base on the cost-effectiveness of P. vivax control is crucial to make the case for increased and sustained funding for malaria control, and to make the most efficient use of existing, limited resources.
Valid comparison between the results of different studies is hampered by variation in malaria transmission intensity between locations, differences in methodologies, and in how the results for costs, effects, and cost-effectiveness are expressed. Only one study attempted to compare the cost-effectiveness of vector control with diagnosis and treatment, an approach which is potentially useful for making resource allocation decisions.31 Furthermore, most cost-effectiveness studies are based on intervention trials, so estimates of cost-effectiveness must be generalized to larger programmatic settings. Expanding P. vivax control measures will probably lead to economies of scale10 where the cost per unit intervention decreases, but also more importantly, to economies of scope as P. vivax control measures are integrated alongside surveillance and control measures for P. falciparum and possibly other diseases. Although in practice, control strategies are often integrated across all Plasmodium species at the programmatic level, in theory, cost-effectiveness analyses often focus on a single species. Evaluating the cost-effectiveness of P. vivax control interventions without accounting for the additional effects on P. falciparum may lead to the benefits of effective control measures being undersold. As integrated malaria control strategies are rolled out, appropriate methods for evaluating the cost-effectiveness of intervention packages are badly needed.
A limitation of many economic models used for evaluation of the cost-effectiveness of P. vivax interventions is that they are static and only capture the benefit to the individual being protected or treated, and not the additional benefits accruing due to reductions in transmission. Accounting for changes due to reduced transmission requires a model of the transmission dynamics of the Plasmodium parasite between humans and mosquitoes. Although there are several examples of P. falciparum transmission models being applied to cost-effectiveness problems,16,17 the capacity for modeling P. vivax transmission dynamics is much more limited with few published models.82–85 Incorporating a model of P. vivax transmission into cost-effectiveness analyses would allow for the benefits of reduced transmission after treatment to be accounted for, in particular the reduction in relapses after radical cure with primaquine. An additional benefit of using transmission models is that they can account for mixes of interventions where effects may not be additive. For example, if ITNs and treatment programs incorporating primaquine are deployed simultaneously,31 the benefits of reducing community-level transmission and preventing relapses are not likely to be additive.
The adoption of a unified treatment strategy for P. falciparum and P. vivax provides an opportunity for further integration of malaria controls, as there are substantial clinical and logistical benefits to treating P. vivax with ACTs instead of CQ.28,34 The clinical benefits are compelling in regions where CQ resistance has been reported.27 The short half-life of artemisinin may lead to reduced efficacy of ACT treatment against P. vivax relapses; however, this effect may be mitigated by selection of a partner drug with a long half-life.29 In areas where P. vivax and P. falciparum are coendemic, a unified treatment strategy incorporating ACTs and primaquine with testing for G6PD deficiency may allow simplified treatment protocols.86
In order for cost-effectiveness analyses to be used appropriately, there are several key knowledge gaps that need to be addressed.56,87 First, the contribution of P. vivax infection to severe anemia and the probability of progression to episodes of severe malaria and mortality need to be better estimated.3,5 Second, the dynamics of P. vivax transmission need to be understood to correctly estimate the impact of control measures on incidence, prevalence, and morbidity.83 Third, the operational effectiveness of different primaquine treatment schedules needs to be evaluated. In some cases, directly observed treatment of primaquine may be affordable and result in significant increases in effectiveness in preventing relapses. Finally, better data are needed on the likelihood and severity of hemolysis in G6PD-deficient individuals and the ability of diagnostic tests to categorize patients into those who are and are not at significant risk of hemolysis.
Research into the cost-effectiveness of malaria control has predominantly focused on the evaluation of interventions for treatment and prevention in endemic regions. However, in the future, as malaria control programs transition from control to elimination, a corresponding shift in the research agenda will be required, with more focus on the costs of surveillance, prevention of reintroduction, and responses to malaria epidemics.88,89 This is particularly true in regions that can sustain P. vivax transmission where elimination efforts are likely to consist of a protracted surveillance campaign to detect and respond to infections arising from P. vivax relapses long after other species of malaria have been eliminated.
Supplementary Material
ACKNOWLEDGMENTS
Katherine Battle and Rosalind Howes are thanked for helpful discussions. Two anonymous reviewers are thanked for helpful comments. Michael T. White was supported by a Population Health Scientist Fellowship from the MRC.
Footnotes
Authors' addresses: Michael T. White, Department of Infectious Disease Epidemiology, Imperial College London, London, United Kingdom, E-mail: m.white08@imperial.ac.uk. Shunmay Yeung, Department of Global Health and Development, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mail: shunmay.yeung@gmail.com. Edith Patouillard, Global Malaria Programme, World Health Organization, Geneva, Switzerland, Swiss Tropical and Public Health Institute, Basel, Switzerland, and Universitat Basel, Basel, Switzerland, E-mail: patouillarde@who.int. Richard Cibulskis, Global Malaria Programme, World Health Organization, Geneva, Switzerland, E-mail: cibulskisr@who.int.
The authors are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the World Health Organization.
References
- 1.Gething PW, Elyazar IRF, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HFL, Price RN, Mueeller I, Baird K, Hay SI. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battle KE, Gething PW, Elyazar IRF, Moyes CL, Sinka ME, Howes RE, Guerra CA, Price RN, Baird JK, Hay SI. The global public health significance of Plasmodium vivax. In: Hay SI, Price R, Baird JK, editors. Advances in Parasitology, Vol 80: Epidemiology of Plasmodium vivax: History, Hiatus and Hubris, Part A. Elsevier; 2012. pp. 1–111. [DOI] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Malaria Report 2015. Geneva, Switzerland: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf Available at. Accessed November 23, 2016. [Google Scholar]
- 5.World Health Organization (WHO) Control and Elimination of Plasmodium vivax: A Technical Brief. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 6.Sattabongkot J, Tsuboi T, Zollner GE, Sirichaisinthop J, Cui LW. Plasmodium vivax transmission: chances for control? Trends Parasitol. 2004;20:192–198. doi: 10.1016/j.pt.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Coura JR, Suarez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection: a review. Mem Inst Oswaldo Cruz. 2006;101:229–237. doi: 10.1590/s0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- 8.Goodman CA, Coleman PG, Mills AJ. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet. 1999;354:378–385. doi: 10.1016/s0140-6736(99)02141-8. [DOI] [PubMed] [Google Scholar]
- 9.Morel CM, Lauer JA, Evans DB. Achieving the millennium development goals for health: cost effectiveness analysis of strategies to combat malaria in developing countries. BMJ. 2005;331:1299. doi: 10.1136/bmj.38639.702384.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions: a systematic review. Malar J. 2011;10:337. doi: 10.1186/1475-2875-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, Price RN. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11:280. doi: 10.1186/1475-2875-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution, genetic variants and primaquine therapy. In: Hay SI, Price R, Baird JK, editors. Advances Parasitol, Vol 81: Epidemiology of Plasmodium vivax: History, Hiatus and Hubris, Part B. Elsevier; 2013. pp. 133–201. [DOI] [PubMed] [Google Scholar]
- 13.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Suen CSNLW, Hofmann NE, Kinboro B, Waltmann A, Brewster J, Lorry L, Tarongka N, Samol L, Silkey M, Bassat Q, Siba PM, Schofield L, Felger I, Mueller I. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12:e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford, United Kingdom: Oxford University Press; 1997. [Google Scholar]
- 15.Attanayake N, Fox-Rushby J, Mills A. Household costs of ‘malaria' morbidity: a study in Matale district, Sri Lanka. Trop Med Int Health. 2000;5:595–606. doi: 10.1046/j.1365-3156.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 16.Drake TL, Devine A, Yeung S, Day NPJ, White LJ, Lubell Y. Dynamic transmission economic evaluation of infectious disease interventions in low- and middle-income countries: a systematic literature review. Health Econ. 2016;25:124–139. doi: 10.1002/hec.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maire N, Shillcutt SD, Walker D, Tediosi F, Smith T. Cost-effectiveness of the introduction of a pre-erythrocytic malaria vaccine into the expanded program on immunization in sub-Saharan Africa: analysis of uncertainties using a stochastic individual-based simulation model of Plasmodium falciparum malaria. Value Health. 2011;14:1028–1038. doi: 10.1016/j.jval.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313:275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claxton K, Revill P, Sculpher M, Wilkinson T, Cairns J, Briggs A. The Gates Reference Case for Economic Evaluation. Seattle, WA: 2014. The Bill & Melinda Gates Foundation. [DOI] [PubMed] [Google Scholar]
- 20.de Oliveira MRF, Gomes AD, Toscano CM. Cost effectiveness of OptiMal (R) rapid diagnostic test for malaria in remote areas of the Amazon Region, Brazil. Malar J. 2010;9:277. doi: 10.1186/1475-2875-9-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman CA, Coleman PG, Mills A. Economic Analysis of Malaria Control in Sub-Saharan Africa; Geneva, Switzerland: World Health Organization Global Forum for Health Research; 2000. [Google Scholar]
- 22.Coleman PG, Morel C, Shillcutt S, Goodman C, Mills AJ. A threshold analysis of the cost-effectiveness of artemisinin-based combination therapies in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71:196–204. [PubMed] [Google Scholar]
- 23.Ley B, Luter N, Espino FE, Devine A, Kalnoky M, Lubell Y, Thriemer K, Baird JK, Poirot E, Conan N, Kheong CC, Dysoley L, Khan WA, Dion-Berboso AG, Bancone G, Hwang J, Kumar R, Price RN, von Seidlein L, Domingo GJ. The challenges of introducing routine G6PD testing into radical cure: a workshop report. Malar J. 2015;14:377. doi: 10.1186/s12936-015-0896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 25.Fernando SD, Karunaweera ND, Fernando WP, Attanayake N, Wickremasinghe AR. A cost analysis of the use of the rapid, whole-blood, immunochromatographic P.f/P.v. assay for the diagnosis of Plasmodium vivax malaria in a rural area of Sri Lanka. Ann Trop Med Parasitol. 2004;98:5–13. doi: 10.1179/000349804225003064. [DOI] [PubMed] [Google Scholar]
- 26.Albertini A, Djalle D, Faye B, Gamboa D, Luchavez J, Mationg ML, Mwangoka G, Oyibo W, Bennett J, Incardona S, Lee E. Preliminary enquiry into the availability, price and quality of malaria rapid diagnostic tests in the private health sector of six malaria-endemic countries. Trop Med Int Health. 2012;17:147–152. doi: 10.1111/j.1365-3156.2011.02904.x. [DOI] [PubMed] [Google Scholar]
- 27.Hansen KS, Grieve E, Mikhail A, Mayan I, Mohammed N, Anwar M, Baktash SH, Drake TL, Whitty CJM, Rowland MW, Leslie TJ. Cost-effectiveness of malaria diagnosis using rapid diagnostic tests compared to microscopy or clinical symptoms alone in Afghanistan. Malar J. 2015;14:217. doi: 10.1186/s12936-015-0696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10:405–416. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bualombai P, Prajakwong S, Aussawatheerakul N, Congpoung K, Sudathip S, Thimasarn K, Sirichaisinthop J, Indaratna K, Kidson C, Srisuphanand M. Determining cost-effectiveness and cost component of three malaria diagnostic models being used in remote non-microscope areas. SE Asian J Trop Med Pub Health. 2003;34:322–333. [PubMed] [Google Scholar]
- 30.Davis WA, Clarke PM, Siba PM, Karunajeewa HA, Davy C, Mueller I, Davis TME. Cost-effectiveness of artemisinin combination therapy for uncomplicated malaria in children: data from Papua New Guinea. Bull World Health Organ. 2011;89:211–220. doi: 10.2471/BLT.10.084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smithuis FM, Moe Kyaw K, Phe UO, van der Broek I, Katterman N, Rogers C, Almeida P, Kager PA, Stepniewska K, Lubell Y, Simpson JA, White NJ. The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J. 2013;12:363. doi: 10.1186/1475-2875-12-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peixoto HM, Brito MAM, Romero GAS, Monteiro WM, de Lacerda MVG, de Oliveira MRF. Cost-effectiveness analysis of rapid diagnostic tests for G6PD deficiency in patients with Plasmodium vivax malaria in the Brazilian Amazon. Malar J. 2016;15:82. doi: 10.1186/s12936-016-1140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peixoto HM, Brito MAM, Romero GAS, Monteiro WM, de Lacerda MVG, de Oliveira MRF. G6PD deficiency in male individuals infected by Plasmodium vivax malaria in the Brazilian Amazon: a cost study. Malar J. 2015;14:26. doi: 10.1186/s12936-015-0647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamolratanakul P, Butraporn P, Prasittisuk M, Prasittisuk C, Indaratna K. Cost-effectiveness and sustainability of lambdacyhalothrin-treated mosquito nets in comparison to DDT spraying for malaria control in western Thailand. Am J Trop Med Hyg. 2001;65:279–284. doi: 10.4269/ajtmh.2001.65.279. [DOI] [PubMed] [Google Scholar]
- 35.Butraporn P, Kamolratanakul P, Prasittisuk M, Prasittisuk C, Indaratna K. Cost-effectiveness analysis of lambdacyhalothrin-treated nets for malaria control: the patients' perspective. SE Asian J Trop Med Pub Health. 1999;30:427–431. [PubMed] [Google Scholar]
- 36.Bhatia MR, Fox-Rushby J, Mills A. Cost-effectiveness of malaria control interventions when malaria mortality is low: insecticide-treated nets versus in-house residual spraying in India. Soc Sci Med. 2004;59:525–539. doi: 10.1016/j.socscimed.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Morel CM, Ngo Duc T, Erhart A, Nguyen Xuan X, Grietens KP, Le Xuan H, Le Khan T, Pham Van K, Nguyen Manh H, Coosemans M, D'Alessandro U, Mills A. Cost-effectiveness of long-lasting insecticide-treated hammocks in preventing malaria in south-central Vietnam. PLoS One. 2013;8:e58205. doi: 10.1371/journal.pone.0058205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroeger A, Ayala C, Lara AM. Unit costs for house spraying and bednet impregnation with residual insecticides in Colombia: a management tool for the control of vector-borne disease. Ann Trop Med Parasitol. 2002;96:405–416. doi: 10.1179/000349802125001159. [DOI] [PubMed] [Google Scholar]
- 39.Kere JF, Kere NK. Bed-nets or spraying: cost analyses of malaria control in The Solomon Islands. Health Policy Plan. 1992;7:382–386. [Google Scholar]
- 40.Verle P, Lieu TTT, Kongs A, Van der Stuyft P, Coosemans M. Control of malaria vectors: cost analysis in a province of northern Vietnam. Trop Med Int Health. 1999;4:139–145. doi: 10.1046/j.1365-3156.1999.00365.x. [DOI] [PubMed] [Google Scholar]
- 41.van Vugt M, van Beest A, Sicuri E, van Tulder M, Grobusch MP. Malaria treatment and prophylaxis in endemic and nonendemic countries: evidence on strategies and their cost-effectiveness. Future Microbiol. 2011;6:1485–1500. doi: 10.2217/fmb.11.138. [DOI] [PubMed] [Google Scholar]
- 42.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10:405–416. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alonso PL, Barnwell JW, Bell D, Hanson K, Mendis K, Moonen B, Newman RD, de Savigny D, Schapira A, Slutsker L, Tanner M, Teuscher T, malERA Consultative Group on Diagnoses and Diagnostics A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. 2011;8:e1000396. doi: 10.1371/journal.pmed.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abba K, Kirkham AJ, Olliaro PL, Deeks JJ, Donegan S, Garner P, Takwoingi Y. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database Syst Rev. 2014;12:CD011431. doi: 10.1002/14651858.CD011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.UNICEF . Malaria Rapid Diagnostic Tests Market and Supply Update. Copenhagen, Denmark: 2016. UNICEF Supply Division. [Google Scholar]
- 47.Bassat Q. The use of artemether-lumefantrine for the treatment of uncomplicated Plasmodium vivax malaria. PLoS Negl Trop Dis. 2011;5:e1325. doi: 10.1371/journal.pntd.0001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav P, Cohen JL, Alphs S, Arkedis J, Larson PS, Massaga J, Sabot O. Trends in availability and prices of subsidized ACT over the first year of the AMFm: evidence from remote regions of Tanzania. Malar J. 2012;11:299. doi: 10.1186/1475-2875-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilairatana P, Tangpukdee N, Kano S, Krudsood S. Primaquine administration after falciparum malaria treatment in malaria hypoendemic areas with high incidence of falciparum and vivax mixed infection: pros and cons. Korean J Parasitol. 2010;48:175–177. doi: 10.3347/kjp.2010.48.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bray PG, Deed S, Fox E, Kalkanidis M, Mungthin M, Deady LW, Tilley L. Primaquine synergises the activity of chloroquine against chloroquine-resistant P. falciparum. Biochem Pharmacol. 2005;70:1158–1166. doi: 10.1016/j.bcp.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization (WHO) WHO-CHOICE. Choosing Interventions That are Cost-Effective. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 52.Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, Arthur P, Chuenchom N, Moehrle JJ, Duparc S, Ugwuegbulam C, Kleim J-P, Carter N, Green JA, Kellam L. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383:1049–1058. doi: 10.1016/S0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- 53.Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis. 2003;37:1659–1667. doi: 10.1086/379714. [DOI] [PubMed] [Google Scholar]
- 54.Tinley KE, Loughlin AM, Jepson A, Barnett ED. Evaluation of a rapid qualitative enzyme chromatographic test for glucose-6-phosphate dehydrogenase deficiency. Am J Trop Med Hyg. 2010;82:210–214. doi: 10.4269/ajtmh.2010.09-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S, Nguon C, Guillard B, Duong S, Chy S, Sum S, Nhem S, Bouchier C, Tichit M, Christophel E, Taylor WRJ, Baird JK, Menard D. Performance of the CareStart™ G6PD deficiency screening test, a point-of-care diagnostic for primaquine therapy screening. PLoS One. 2011;6:e28357. doi: 10.1371/journal.pone.0028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Seidlein L, Auburn S, Espino F, Shanks D, Cheng Q, McCarthy J, Baird K, Moyes C, Howes R, Menard D, Bancone G, Winasti-Satyahraha A, Vestergaard LS, Green J, Domingo G, Yeung S, Price R. Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J. 2013;12:112. doi: 10.1186/1475-2875-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, Bansil P, Carter N, Cheng Q, Culpepper J, Eziefula C, Fukuda M, Green J, Hwang J, Lacerda M, McGray S, Menard D, Nosten F, Nuchprayoon I, Oo NN, Bualombai P, Pumpradit W, Qian K, Recht J, Roca A, Satimai W, Sovannaroth S, Vestergaard LS, Von Seidlein L. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12:391. doi: 10.1186/1475-2875-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, Hogg MM, Battle KE, Padilla CD, Baird JK, Hay SI. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reimer LJ, Thomsen EK, Tisch DJ, Henry-Halldin CN, Zimmerman PA, Baea ME, Dagoro H, Susapu M, Hetzel MW, Bockarie MJ, Michael E, Siba PM, Kazura JW. Insecticidal bed nets and filariasis transmission in Papua New Guinea. N Engl J Med. 2013;369:745–753. doi: 10.1056/NEJMoa1207594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiware SS, Chitnis N, Devine GJ, Moore SJ, Majambere S, Killeen GF. Biologically meaningful coverage indicators for eliminating malaria transmission. Biol Lett. 2012;8:874–877. doi: 10.1098/rsbl.2012.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IRF, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thang ND, Erhart A, Speybroeck N, Xa NX, Thanh NN, Ky PV, Hung LX, Thuan LK, Coosemans M, D'Alessandro U. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of central Vietnam. PLoS One. 2009;4:e7369. doi: 10.1371/journal.pone.0007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grietens KP, Xa Nguyen X, Ribera JM, Thang Ngo D, van Bortel W, Nhat Truong B, Ky Pham V, Xuan HL, D'Alessandro U, Erhart A. Social determinants of long lasting insecticidal hammock-use among the Ra-Glai ethnic minority in Vietnam: implications for forest malaria control. PLoS One. 2012;7:e29991. doi: 10.1371/journal.pone.0029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Utzinger J, Tozan Y, Singer BH. Efficacy and cost-effectiveness of environmental management for malaria control. Trop Med Int Health. 2001;6:677–687. doi: 10.1046/j.1365-3156.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 65.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 66.Syafruddin D, Bangs MJ, Sidik D, Elyazar I, Asih PBS, Chan K, Nurleila S, Nixon C, Hendarto J, Wahid I, Ishak H, Bogh C, Grieco JP, Achee NL, Baird JK. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am J Trop Med Hyg. 2014;91:1079–1087. doi: 10.4269/ajtmh.13-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mulla MS, Thavara U, Tawatsin A, Kong-Ngamsuk W, Chompoosri J. Mosquito burden and impact on the poor: measures and costs for personal protection in some communities in Thailand. J Am Mosq Control Assoc. 2001;17:153–159. [PubMed] [Google Scholar]
- 68.Snehalatha KS, Ramaiah KD, Kumar KNV, Das PK. The mosquito problem and type and costs of personal protection measures used in rural and urban communities in Pondicherry region, south India. Acta Trop. 2003;88:3–9. doi: 10.1016/s0001-706x(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 69.Babu BV, Mishra S, Mishra S, Swain BK. Personal-protection measures against mosquitoes: a study of practices and costs in a district, in the Indian state of Orissa, where malaria and lymphatic filariasis are co-endemic. Ann Trop Med Parasitol. 2007;101:601–609. doi: 10.1179/136485907X193897. [DOI] [PubMed] [Google Scholar]
- 70.Wafula F, Agweyu A, Macintyre K. Regional and temporal trends in malaria commodity costs: an analysis of Global Fund data for 79 countries. Malar J. 2013;12:466. doi: 10.1186/1475-2875-12-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Picard J, Mills A. The effect of malaria on work time: analysis of data from 2 Nepali districts. J Trop Med Hyg. 1992;95:382–389. [PubMed] [Google Scholar]
- 72.Konradsen F, van derHoek W, Amerasinghe PH, Amerasinghe FP, Fonseka KT. Household responses to malaria and their costs: a study from rural Sri Lanka. Trans R Soc Trop Med Hyg. 1997;91:127–130. doi: 10.1016/s0035-9203(97)90194-2. [DOI] [PubMed] [Google Scholar]
- 73.Mills A. The household costs of malaria in Nepal. Trop Med Parasitol. 1993;44:9–13. [PubMed] [Google Scholar]
- 74.Fernando SD, Gunawardena DM, Bandara M, De Silva D, Carter R, Mendis KN, Wickremasinghe AR. The impact of repeated malaria attacks on the school performance of children. Am J Trop Med Hyg. 2003;69:582–588. [PubMed] [Google Scholar]
- 75.Fernando D, De Silva D, Wickremasinghe R. Short-term impact of an acute attack of malaria on the cognitive performance of schoolchildren living in a malaria-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg. 2003;97:633–639. doi: 10.1016/s0035-9203(03)80093-7. [DOI] [PubMed] [Google Scholar]
- 76.Teklehaimanot A, Mejia P. Malaria and poverty. Reducing the impact of poverty on health and human development. Scientific Approaches. 2008;1136:32–37. [Google Scholar]
- 77.Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64:85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 78.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 79.Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, Lindsay SW. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet. 2013;382:963–972. doi: 10.1016/S0140-6736(13)60851-X. [DOI] [PubMed] [Google Scholar]
- 80.Qi Q, Guerra CA, Moyes CL, Elyazar IRF, Gething PW, Hay SI, Tatem AJ. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar J. 2012;11:403. doi: 10.1186/1475-2875-11-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alonso PL, Brown G, Tanner M, malERA Consultative Group on Integration Strategies A research agenda for malaria eradication: cross-cutting issues for eradication. PLoS Med. 2011;8:e1000404. doi: 10.1371/journal.pmed.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy M, Bouma MJ, Ionides EL, Dhiman RC, Pascual M. The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Negl Trop Dis. 2013;7:1979. doi: 10.1371/journal.pntd.0001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. eLife. 2014;3:04692. doi: 10.7554/eLife.04692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aguas R, Ferreira MU, Gomes MGM. Modeling the effects of relapse in the transmission dynamics of malaria parasites. J Parasitol Res. 2012;2012:921715. doi: 10.1155/2012/921715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chamchod F, Beier JC. Modeling Plasmodium vivax: relapses, treatment, seasonality, and G6PD deficiency. J Theor Biol. 2013;316:25–34. doi: 10.1016/j.jtbi.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price RN, Douglas NM, Anstey NM, von Seidlein L. Plasmodium vivax treatments: what are we looking for? Curr Opin Infect Dis. 2011;24:578–585. doi: 10.1097/QCO.0b013e32834c61e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 88.Sabot O, Cohen JM, Hsiang MS, Kahn JG, Basu S, Tang L, Zheng B, Gao Q, Zou L, Tatarsky A, Aboobakar S, Usas J, Barrett S, Cohen JL, Jamison DT, Feachem RGA. Malaria elimination. 4. Costs and financial feasibility of malaria elimination. Lancet. 2010;376:1604–1615. doi: 10.1016/S0140-6736(10)61355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mills A, Lubell Y, Hanson K. Malaria eradication: the economic, financial and institutional challenge. Malar J. 2008;7((Suppl 1)):S11. doi: 10.1186/1475-2875-7-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.