Abstract
In the present work, the influence of intracellular injection of angiotensin-(1–12) [Ang-(1–12)] on the electrical properties of the intact left ventricle of Wistar Kyoto rats was investigated with electrophysiological methods. Particular attention was given to the role of chymostatin on the effect of the peptide. The results indicated that intra-cellular administration of the peptide elicited a depolarization of the surface cell membrane and an increase of duration of the action potential followed by the generation of early afterdepolarizations. The increment of action potential duration caused by Ang-(1–12) (100 nM) was due to a decrease of total potassium current recorded from single cardiomyocytes using the whole cell configuration of pCAMP. The decrease of potassium current was related to the activation of protein kinase C (PKC) because the specific inhibitor of kinase C, Bis-1 (10−9 M), abolished Ang-(1–12) effects on the potassium current. The question of whether the effect of Ang-(1–12) was related to the formation of Ang II by chymase was investigated. The results revealed that the intracellular administration of chymostatin, a chymase inhibitor (10−9 M) abolished the effect of intracellular Ang-(1–12) on the potassium current. Moreover, intracellular Ang II (100 nM), by itself, reduced the potassium current, an effect decreased by intracellular valsartan (100 nM). Valsartan (10–9 M) dialyzed into the cell abolished the effect of Ang-(1–12) (100 nM). These observations demonstrate that the effect of Ang-(1–12) on potassium current was related to the formation of Ang II and that the peptide has arrhythmogenic properties.
Keywords: Intracellular angiotensin-(1–12), Cardiac excitability, Potassium current, Myocardial contractility, Valsartan
Introduction
Angiotensin-(1–12) [Ang-(1–12)] found in the plasma and several tissues of Wistar rats [1] contains a two amino acid extension of angiotensin I (Ang I) (Leu11–Tyr12) at the C terminus of the molecule. Isolated and identified from the rat small intestine, Ang-(1–12) is present in high concentrations in the heart, aorta, and kidney [2, 3]. Previous studies demonstrated that Ang-(1–12) is a functional substrate for Ang II formation and that the tissue levels of the peptide were unchanged by renin inhibition or after bilateral nephrectomy [2, 4]. In the heart, chymase, instead of ACE, functions as the major Ang-(1–12)-metabolizing enzyme [5], while in the circulation, ACE cleaves the Leu10–Leu11 bond of Ang-(1–12) to generate Ang I [3]. These observations lead to the concept that local synthesis of Ang-(1–12) in the heart might be relevant especially during pathological conditions. The enzyme(s) involved in the formation of Ang-(1–12) from angiotensinogen (Agt) are not known; possible candidates are cathepsins, the kallikrein-kinin system, or even chymase.
Evidence is available that there are local renin angiotensin systems (RAS) in the heart and other tissues and that components of the system are expressed in heart and kidney supporting the view that there is an intracrine RAS [6–11]. Indeed, evidence is available that Ang II or renin synthesized locally or due to internalization are able to alter cellular functions [7, 12]. The presence of AT1 receptors, Agt and Ang II in different cells [11, 12] supports the concept of a local RAS. In human beings, the gradients of Ang II across the heart were increased in patients with congestive heart failure [13], and in the normal pig hearts, as much as 75 % of cardiac Ang II is synthesized at tissue sites [14].
Intracellular Ang II or renin, for instance, impaired cell-to-cell communication in the heart and facilitates the generation of cardiac arrhythmias [6]. Recently, the endogenous localization of Ang-(1–12) in cultured cardiac myocytes [15] raised the possibility that Ang-(1–12) has intracellular actions by which the peptide alters the electrical properties of cardiac cell through changing the ionic conductance of the surface cell membrane. In the present work, the hypothesis that Ang-(1–12) has intracrine action through the formation of Ang II was investigated in the intact left ventricle and in isolated ventricular cells from adult Wistar Kyoto (WKY) rats.
Materials and methods
Ethic statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH publication No. 85–23 m, revised 1996). The protocol was approved by the Institutional Animal Care and Use Committee at the University of Puerto Rico, Medical Sciences Campus.
Animals
Adult male WKY rats weighing 125–150 g were kept in an air-conditioned facility on a normal laboratory animal diet and given tap water ad libitum. The animals were anesthetized with sodium pentobarbital (50 mg/kg IP), and the heart was removed with the animals under deep anesthesia.
Measurements of membrane potentials
Intracellular potassium chloride (KCl) microelectrodes connected to a high impedance DC amplifier (8100 single electrode amplifier) (Dagan Co.) were used to measure the transmembrane potentials. The left ventricular muscle was dissected and transferred to a bath through which oxygenated Krebs solution (36 °C) flowed continuously. The composition of Krebs solution was as follows (mM): NaCl-150; KCl-5.4; CaCl2-1.8; MgCl2-1; HEPES-5; glucose-5; and pH adjusted to 7.3. The solution was saturated with 100 % O2. The ventricle was stimulated with rectangular pulses (3 ms duration, 0.6 Hz) generated by an electronic stimulator and applied gently to the tissue through a bipolar platinum electrode. The determination of the stimulus strength was achieved by amplifying the voltage drop across a 10 MΩ resistor placed between the muscle and ground. Conduction velocity was measured by the latency period.
Measurements of refractoriness
To investigate the influence of intracellular Ang-(1–12) on cardiac refractoriness, strength–interval curves were obtained from the same left ventricular cell before and after the injection of the peptide. The refractoriness was measured by applying a second current pulse of constant duration (3 ms) and variable intensity at different moments of the action potential and determining the minimal current strength required to elicit a clear propagated response. The intervals were previously selected and kept constant. The current strength was plotted against the interval, and strength–interval curves were obtained for control and experimental conditions.
Injection of Ang-(1–12) in the intact ventricle
Intracellular Ang-(1–12) injections were performed following a procedure similar to that described previously [16]. A Picospritzer (General Valve Corp, Fairfield, NJ) connected to a compressed nitrogen tank was employed for pressure regulation (up to 100 psi) and Ang-(1–12) injection. Microelectrode filled with Ang-(1–12) solution (100 nM) was connected to an 8100 single electrode amplifier (Dagan Co., Minneapolis, MN) which permitted to inject current pulses and record the action potential from the same cell simultaneously. The injection micropipette connected to the compressed nitrogen tank was beveled to a resistance of 10 MΩ or less and filled with a solution containing Ang-(1–12). The beveling of the micropipettes at an appropriate degree facilitates the ejection of the solution into the cell. Pressure pulses (40–75 psi) were applied to the injection electrode for 25 ms to inject the peptide. As previously described [16], the volume ejected and the intensity of pulse pressure were linearly related and positive results were obtained in about 85 % of the injected cells. Experiments in which an increase of electrode resistance due to clogging of the microelectrode occurred were rejected. The ejection volumes were quantified by measuring the diameter of the aqueous droplets delivered into mineral oil previously to cell impalement. The micropipette was filled with 3 M KCl solution containing Ang-(1–12) (10–8 M). A precise quantification of the injected volume was difficult due to expected differences between injection into the air and intracellularly (see also De Mello [16]). The use of micropipettes with a resistance of 8–9 MΩ reduced this problem.
The protocol used in these experiments consisted in impaling the cell and recording the action potential from the same endocardial left ventricular fiber before and after the intracellular injection of Ang-(1–12); during the injection of Ang-(1–12), the microelectrode was kept inside the cell. Three experimental conditions were essential for the results to be acceptable: (a) the value of the resting potential must be maintained during and immediately after the injection of Ang-(1–12) indicating no cell injury; (b) the ejection of a droplet of fluid from the tip of the micropipette into air (about 210 nL) was observed under the microscope during pressure pulses (40 psi for 20 ms) before impalement of the micropipette; and (c) injection of the KCl solution into cardiac cells (using pressure of 40–70 psi, for 25 ms) in the absence of Ang-(1–12) must be unable to change the electrical parameters.
Measurements of potassium current
Cell isolation procedure
The heart was removed and immediately perfused with normal Krebs’ solution containing (mM) NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, NaH2PO4 0.3, NaHCO3 11.9, glucose 5.5, and HEPES 5, with pH adjusted to 7.3. After 20 min, a calcium-free solution containing collagenase (0.4 %; Worthington Biochemical Corp, Lakewood, NJ) was recirculated through the heart for 1 h. The collagenase solution was washed out with 100 mL of recovery solution containing (mM) the following reagents: taurine 10, oxalic acid 10, glutamic acid 70, KCl 25, KH2PO4 10, glucose 11, and EGTA 0.5, with pH adjusted to 7.4. All solutions were oxygenated with 100 % O2. The ventricles were minced (1- to 2-mm-thick slices), and the resultant solution was agitated gently using a Pasteur pipette. The suspension was filtered through nylon gauze, and the filtrate was centrifuged for 4 min at 22 g. The cell pellets were then resuspended in normal Krebs’ solution. All experiments were conducted at 36 °C.
Experimental procedures
Suction pipettes were pulled from microhematocrit tubing (Clark Electromedical Instruments, Hamden, CT) by means of a controlled puller, and their tips were polished with a microforge (Narishige International Inc., East Meadow, NY). The pipettes, which were prepared immediately before the experiment, were filled with the following solution (mM): potassium aspartate 120, NaCl 10, MgCl2 3, EGTA 10, Na2ATP 5, and HEPES 5, with pH adjusted to 7.3. The resistance of the pipettes varied from 2 to 2.5 MΩ. To measure the influence of Ang-(1–12) on total potassium current, ventricular myocytes were depolarized using pulses steps of 10 mV from the holding potential of −80 mV. On these experiments, the cells were incubated in a modified Krebs’ solution containing no Ca or Na ions.
Immunochemistry studies
Immunohistochemistry was performed on sections from formalin-fixed paraffin-embedded LV. 5-μm sections were mounted on slides, deparaffinized in xylene, and rehydrated in a graded series of ethanol. After blocking with 1× PBS/1 % Casein and overnight incubation at 4 °C with the rat (1:1000; affinity purified custom-made antibody, generated for us by AnaSpec Co., Fremont, CA) antibody, slides were incubated for 1 h. with the desmin antibody (Abcam, Cambridge, MA, 1:200) at room temperature. Alexa Fluor 594- and Alexa Fluor 488-conjugated secondary antibodies (1:700; Invitrogen, Eugene, OR) were applied to visualize Ang-(1–12) (red) and desmin (green) in the tissue. Nuclei were stained (blue) with DAPI (1.5 μg/ml; Vector Laboratories, Burlingame, CA). Image acquisition (×63 objective, ×2500 video-screen magnification) was performed on a Leica DM6000 epifluorescence microscope using SimplePCI software (Compix, Inc., Cranberry Township, PA). Images were adjusted appropriately to remove background fluorescence.
Drugs
The rat sequence of Ang-(1–12) was custom made for us by GenScript Co. (Piscataway, NJ); the protein kinase C inhibitor bisindolylmaleimide I (Bis-1) and chymostatin were obtained from Sigma Chemical Co (St. Louis, MO). Valsartan was kindly provided by Novartis Pharmaceuticals Inc.
Data analysis
The output of the preamplifier was filtered at 10 kHz, and data acquisition and command potentials were controlled using PCLAMP software (Axon Instruments).
Statistical analysis
Numerical data were expressed as mean ± SEM. Student’s t test was used to estimate statistical significance. Measurements of variance were performed using ANOVA. Differences were considered significant when P < 0.05.
Results
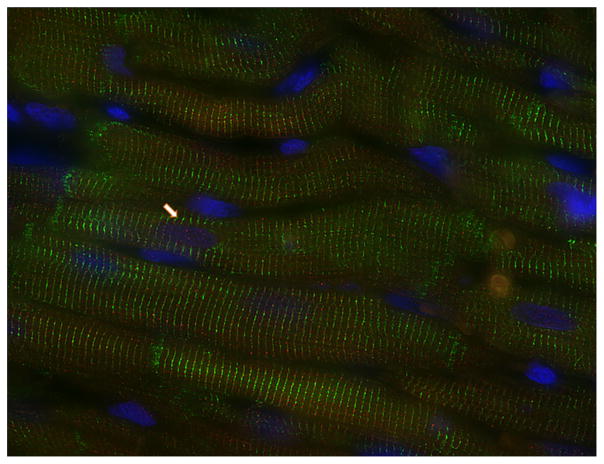
In agreement with previous findings [17], immunoreactive Ang-(1–12) fluorescence was visualized in cardiac myocytes obtained from the left ventricle of adult WKY rats. Discrete punctiform Ang-(1–12) immunofluorescence is found inside cardiac myocytes often at or near the Z line of the sarcomere. In addition, immunofluorescence for Ang-(1–12) is also sporadically found in the nuclei of cardiac myocytes (Fig. 1). Immunofluorescence is not present when preimmune rabbit serum was used and was blocked after preincubating antibodies with antigenic peptide.
Fig. 1.
Detection of Ang-(1–12) by immunohistochemistry in cardiac myocytes from the left ventricle of a Wistar Kyoto rat. The immunofluorescent is associated with sarcomeres and also sporadically observed in the nuclei (arrow)
Effect of intracellular injection of Ang-(1–12) in the intact ventricle
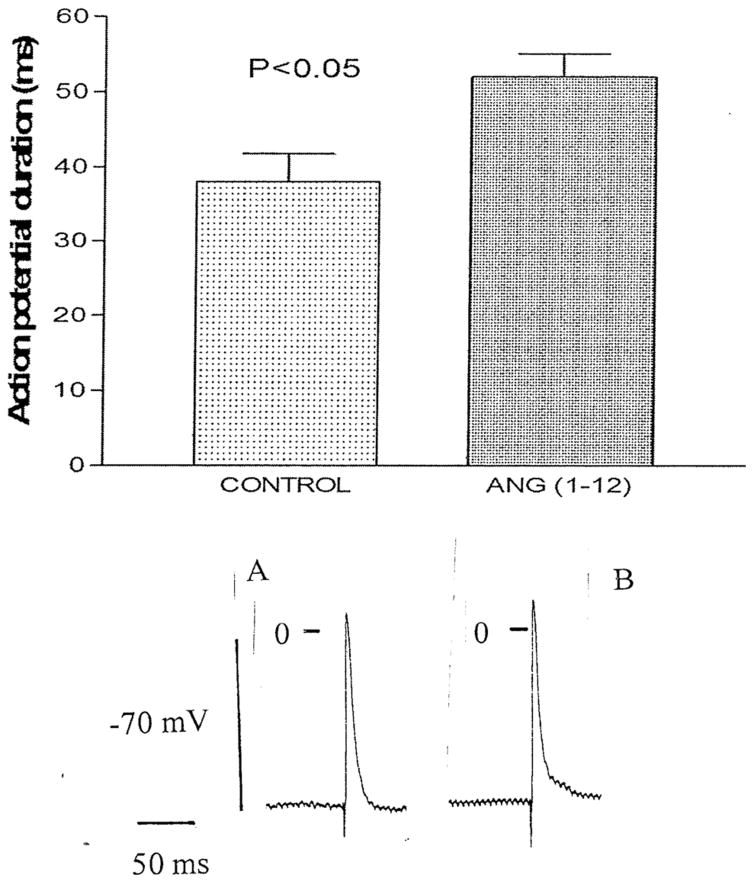
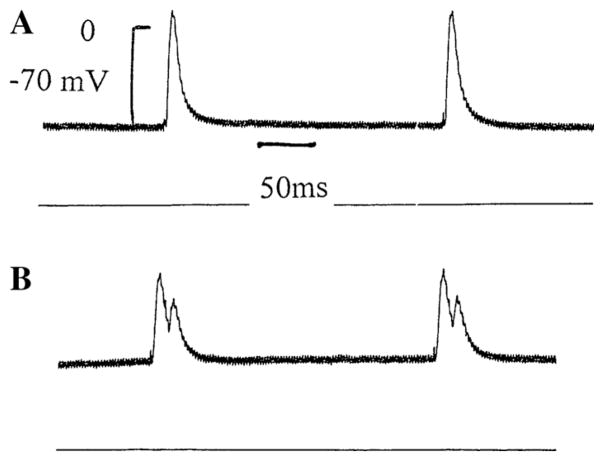
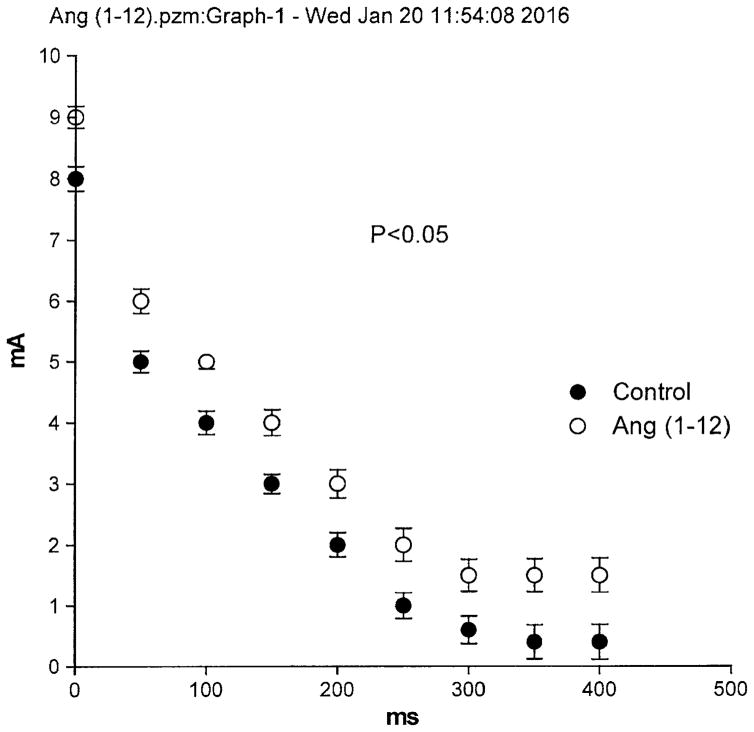
To investigate the influence of intracellular Ang-(1–12) on the electrical properties of cardiac ventricular cells, microelectrodes containing the peptide (100 nM) were introduced into a cell from the intact left ventricle and the resting as well as the action potential were measured before and after Ang-(1–12) intracellular injection for 15 ms. In some experiments, 4 successive injections were performed. The intracellular injection of Ang-(1–12) performed into the ventricle caused a decrease of the resting potential (7 ± 3.2 mV (n = 40) (6 animals) (P <0.05) and an increase of the action potential duration at 90 % repolarization (Fig. 2). These effect of intracellular Ang-(1–12) appeared within 3 min of injection reaching a stable value around 12 min. Concurrently with the increase in duration of the action potential, triggered activity was generated (Fig. 3). An important change induced by intracellular Ang-(1–12) was the displacement of the strength–duration curve to the right which means that the cardiac refractoriness was significantly increased (Fig. 4). No change in refractoriness or action potential duration was seen with repeated intracellular injections of KCl without Ang-(1–12) inside the pipette.
Fig. 2.
Top Influence of intracellular administration of Ang-(1–12) (100 nM) into a myocyte from the intact left ventricle of a WKY rat. Each bar is the average from 24 cells (5 animals). Vertical line at each bar SEM (P < 0.05). Bottom Action potential recoded from a single fiber of the intact left ventricle before and after intracellular injection of Ang-(1–12). Vertical line at each point SEM. Voltage calibration −70 mV; time calibration 50 ms
Fig. 3.
Early afterdepolarization (b) elicited by intracellular injection of Ang-(1–12) (100 nM) into a single ventricular fiber of the left ventricle. a Control. b 8 min after beginning of Ang-(1–12) injection. Voltage calibration −70 mV; time calibration 50 ms
Fig. 4.
Strength–duration curves showing displacement of the curve to the right caused by intracellular injection of the peptide indicating an increase in cardiac refractoriness. Each point is the average from 22 fibers. Vertical line at each point SEM (P < 0.05)
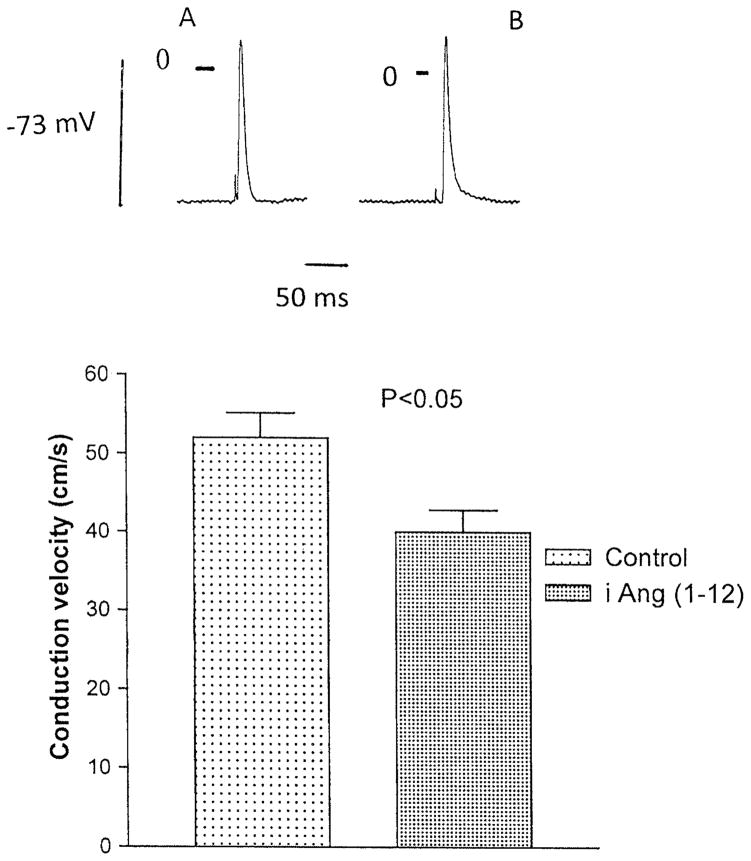
Measurements of conduction velocity calculated from latency time made before and after 15 min of intracellular injection of Ang-(1–12), indicated an average value of 52 ± 3.2 cm/s (n = 20 cells from 4 animals) for controls and 40 ± 2.8 cm/s (n = 24 cells from 4 animals) for Ang-(1–12) (P <0.05) (Fig. 5).
Fig. 5.
Bottom Influence of intracellular injection of Ang-(1–12) on the conduction velocity in fibers from the left ventricle. Each bar is the average from 24 fibers (5 animals). Vertical line at each point SEM (P < 0.05). Top Action potential recorded from single ventricular fiber before and after intracellular injection of the peptide. Voltage calibration −73 mV; time calibration 50 ms
Intracellular Ang-(1–12) reduces the total potassium current in isolated heart cells
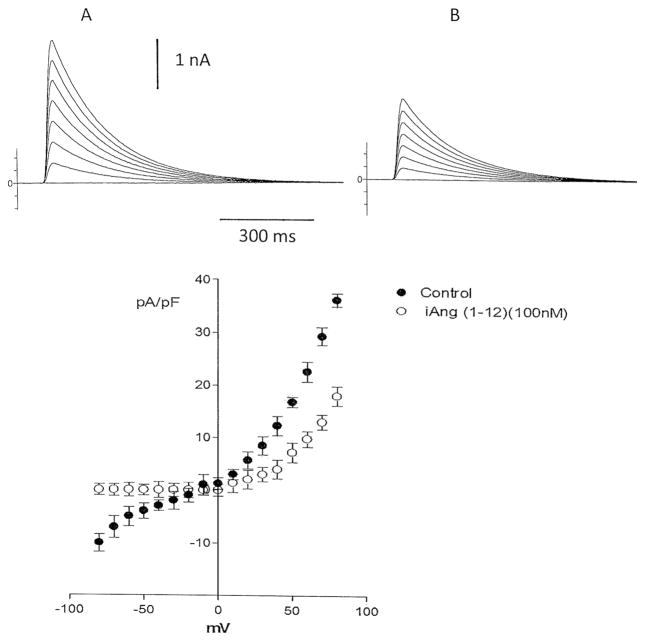
It is known that the potassium current is involved in the repolarization of the action potential in cardiac muscle. Since the peptide enhanced the action potential duration, it is important to investigate if Ang-(1–12) alters the outward potassium current in isolated cardiac myocytes. For this, myocytes from the left ventricle were isolated as described in “Materials and methods” section. The peptide (100 nM) was added to the pipette solution and then dialyzed into the cell using the whole cell configuration of path clamp. As shown in Fig. 6, Ang-(1–12) (100 nM), administered intracellularly, reduced the total potassium current.
Fig. 6.
Top Influence of intracellular dialysis of Ang-(1–12) (100 nM) on total potassium current recorded from a single isolated cell from the left ventricle before (a) and after 6 min of Ang (1–12) administration (b). Bottom Current–voltage curve showing a decrease of potassium current elicited by the intracellular dialysis of the peptide (100 nM). Each point is the average from 22 cells (4 animals). Vertical line at each point SEM (P < 0.05). Holding potential −80 mV
PKC activation is involved in the effect of the peptide
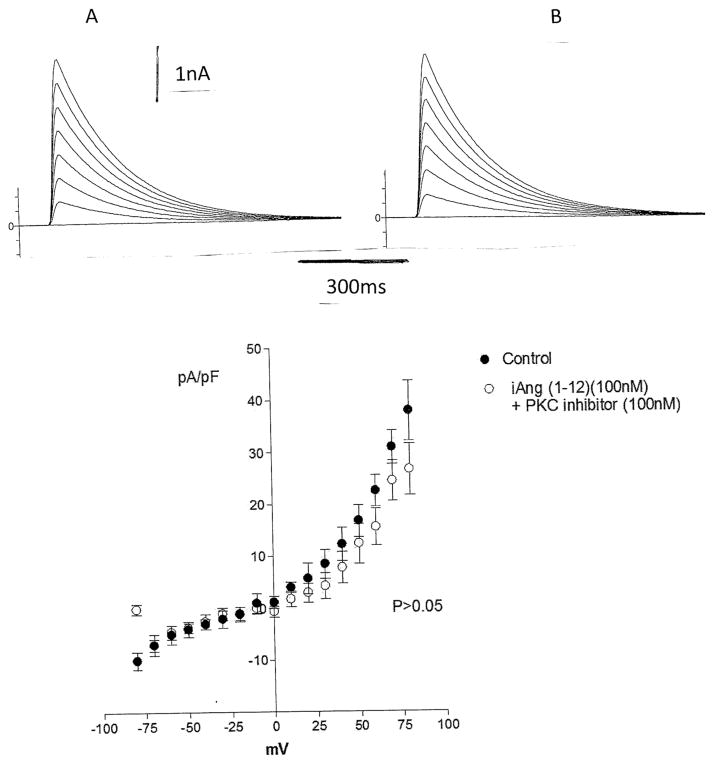
Because PKC activation is involved in the modulation of K current in the heart, we investigate if PKC has a role on the change of potassium current induced by Ang-(1–12). For this, Bis-1 (10−9 M), which is a selective PKC inhibitor, was added to the bath and after 12 min, Ang-(1–12) (100 nM) was dialyzed into the cytosol. As shown in Fig. 7, PKC inhibition blocked the effect of the peptide on total potassium current.
Fig. 7.
Inhibition of the effect of intracellular dialysis of Ang-(1–12) (100 nM) on total potassium current caused by PKC inhibition. Top Single cell before and after 6 min of intracellular dialysis of the peptide in presence of Bis-1 (10–9 M). Bottom Current–voltage curve showing the lack of action of the peptide on total potassium current caused by PKC inhibition. Each point is the average from 23 cells (4 animals). Vertical line at each point SEM (P > 0.05). Holding potential −80 mV
Is the generation of Ang II involved in the effect of Ang-(1–12)?
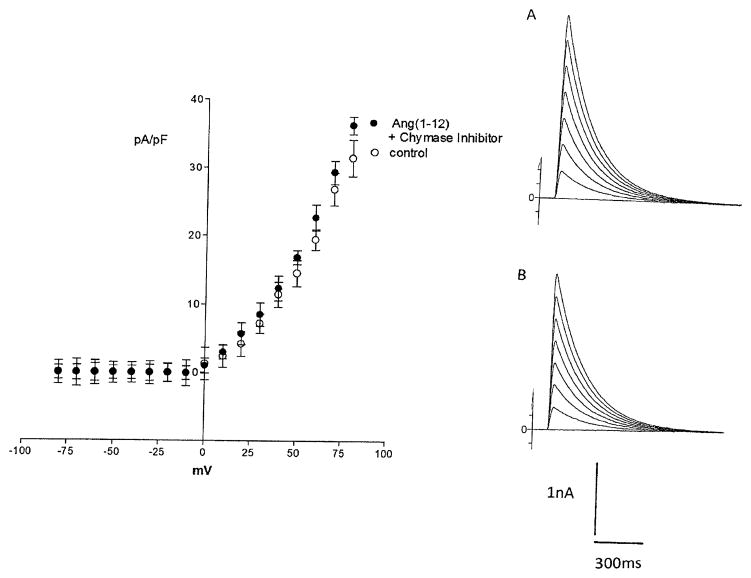
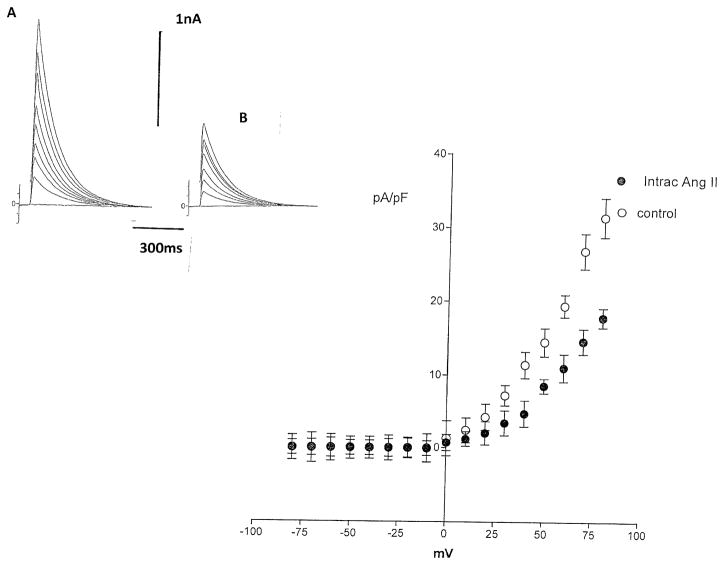
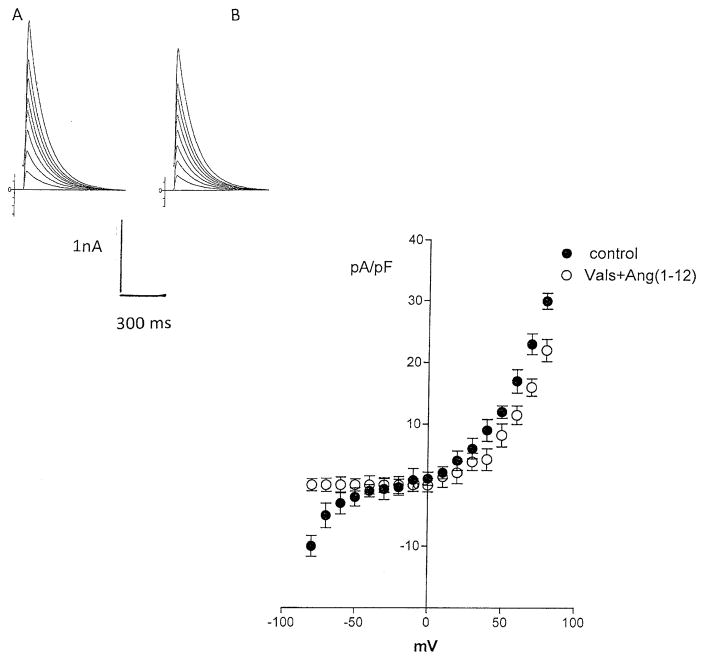
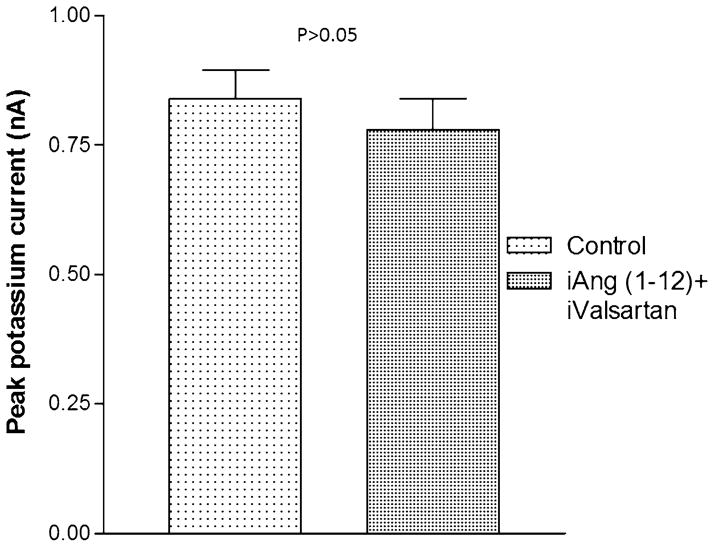
Previous studies indicated that chymase accounts for Ang II synthesis from Ang-(1–12) (13). To investigate if the effect of Ang-(1–12) on potassium current was related to formation of Ang II, experiments were performed in which a chymostatin (10−9 M) was added to the pipette solution and dialyzed into the cell together with Ang-(1–12) (100 nM). Figure 8 shows that the chymase inhibitor abolished the effect of Ang-(1–12) on total potassium current. Moreover, the intracellular administration of equimolar concentrations of Ang II (100 nM), by itself, reduced the potassium current as shown in Fig. 9, while that valsartan (100 nM) introduced into the cell decreased significantly the effect of intracellular Ang II on total potassium current (see Fig. 10). To investigate the role of AT1 receptor activation on the effect of Ang-(1–12), both the peptide and valsartan were dialyzed into the cell. As shown in Fig. 11, valsartan (10–9 M) abolished the effect of Ang-(1–12)(100 nM) on peak potassium current.
Fig. 8.
Top right Effect of intracellular administration of chymase inhibitor (chymostatin) (10–9 M) on the action of intracellular dialysis of Ang (1–12) (100 nM) on total potassium current recorded from single myocyte. a Control. b After intracellular administration of Ang II. Left Current–voltage curve showing the inhibition of the effect of intracellular dialysis of the peptide on potassium current in presence of the chymase inhibitor. Each point is the average from 19 cells (4 animals). Vertical line at each point SEM (P > 0.05)
Fig. 9.
Top left Effect of intracellular administration of Ang II (100 nM) on total potassium current recorded from single myocyte. a Control. b After intracellular administration of Ang II. Bottom right Current–voltage curve showing the effect of intracellular administration of Ang II (100 nM) on total potassium current. Each point is the average from 20 cells (4 animals). Vertical line at each point SEM (P < 0.05)
Fig. 10.
Top left Effect of intracellular administration of Ang II (100 nM) on total potassium current recorded from single myocyte dialyzed with valsartan (10–9 M). a Control. b After intracellular administration of Ang II. Bottom right Current–voltage curve showing the decline of the effect of intracellular Ang II (100 nM) on potassium current in presence of valsartan (10–9 M). Each point is the average from 21 cells (4 animals). Vertical line at each point SEM (P > 0.05)
Fig. 11.
Inhibition of the effect of intracellular Ang-(1–12) (100 nM) on peak potassium current elicited by intracellular administration of valsartan (10–9 M). Each bar is the average from 22 cells (3 animals). Vertical line at each bar SEM. P > 0.05
Discussion
The present results indicated that intracellular administration of Ang-(1–12) alters the electrical properties of the left ventricle of the normal heart in a manner similar to that obtained with Ang II [7]. The relevance of this observation is that the peptide changed the electrical properties in the intact ventricle in which the structure of cardiac muscle was preserved. The prolongation of the action potential induced by the peptide was found within minutes of intracellular injection, and concurrently with increment of action potential duration, triggered activity, like early afterdepolarizations (EADs), was generated. EAD is a pro-arrhythmic player [18–20] especially during pathological conditions or therapeutic procedures with drugs. The prolonged duration of the action potential can also be related to a change in the inactivation of the inward calcium current and the early afterdepolarization be due to reactivation of this current.
The intimate mechanism involved in the increase in duration of the action potential elicited by Ang-(1–12) involved a decrease of total potassium current as judged by the studies performed in isolated cardiomyocytes. The decrease of total potassium current was related to the activation of PKC because Bis-1 abolished the effect of Ang-(1–12) on potassium current. PKC belongs to a family of Ca2+-dependent serine kinases that based on sequence homology as well as Ca2+ and diacylglycerol stimulation, when activated reduces the potassium current [21].
The decline of conduction velocity caused by the intracellular injection of Ang-(1–12) in the intact ventricle, facilitates the generation of reentrant rhythms, and may be related to (a) a decline of gap junction conductance; (b) a fall of surface cell membrane resistance or (c) both [6]. Further studies will be needed to clarify which of these mechanisms are involved in the effect of Ang-(1–12).
The question whether the effect of Ang-(1–12) was related to the formation of Ang II merits special attention. Previous observations revealed that Ang-(1–12) is a substrate of Ang II [15] and that chymase was the enzyme involved in the formation of Ang II in the heart [2]. The present findings provide a more direct support for this conclusion because (a) a chymase inhibitor dialyzed intracellularly inhibited the effect of intracellular Ang-(1–12) on potassium current; (b) Ang II, by itself, administered to the cytosol caused a decline of potassium current similar to that seen with Ang-(1–12); and (c) the blunting of Ang II effects by intracellular administration of valsartan implicate AT1 receptors as mediators of the actions of Ang-(1–12);(d) valsartan administered intracellularly abolished the effect of intracellular Ang (1–12) on potassium current.
Experimental evidence supports the notion that local RAS are present in different organs including the heart and kidney [22] and that the synthesis of several components of the RAS or their uptake from plasma [8, 9] makes it possible to explain the synthesis of Ang II locally. Furthermore, the presence of AT1 receptors, Agt and Ang II in different cells [8, 23] supports the concept of local RAS. In the normal pig heart as much as 75 % of cardiac Ang II is synthesized at tissue sites [14], whereas in humans, the gradients of Ang II across the heart were increased in patients with congestive heart failure [13]. In addition, Ferrario et al. [24] reported that the antihypertensive effects of chronic administration of lisinopril, losartan, or both drugs combined did not alter cardiac content of Ang II. Rapid internalization of the Ang II–AT1 receptor complex, contributes significantly to the intracellular levels of the peptide [22]. All these observations support the notion of an intracrine RAS [12, 22]. The present findings substantiate the view that intracellular Ang II generated by Ang-(1–12) independently of renin, can exert a local intracrine effect [6–11, 22, 25] on cardiac excitability, and might be implicated in cardiac pathophysiology.
Clinical perspectives
The present findings showing that Ang-(1–12) reduces the total potassium current and increases the action potential duration has an important pathophysiological implication. Through these effects, the peptide can induce triggered activity and other types of cardiac arrhythmias in humans. This possibility is supported by previous studies showing that the peptide is expressed in human tissues [1].
Acknowledgments
The authors acknowledge the partial support that was provided for this study from Program Project Grant 2P01-HL051952 from the National Heart, Lung, and Blood Institute (NHLBI) to (F CM) and Institutional NIH GM61838 (WCM).
Footnotes
Author’s contribution Dr. WC De Mello performed the electro-physiological experiments and organized the text. Dr. CM Ferrario, Dr. J Varajic, and Dr. L D’Italia performed the immunochemistry studies and revised the text.
References
- 1.Nagata S, Varagic J, Kon ND, Wang H, Groban L, Simington SW, Ahmad S, Dell’Italia LJ, VonCannon JL, Deal D, Ferrario CM. Differential expression of the angiotensin-(1–12)/chymase axis in human atrial tissue. Ther Adv Cardiovasc Dis. 2015;9(4):168–180. doi: 10.1177/1753944715589717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Ahmad S, Varagic J, Groban L, Dell’Italia LJ, Nagata S, Kon ND, Ferrario CM. Angiotensin-(1–12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep. 2014;16(5):429. doi: 10.1007/s11906-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, et al. An evolving story of angiotensin II forming pathways in rodents and humans. Clin Sci. 2014;126:461–469. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng J, Wei CC, Hase N, Shi K, Killingsworth CR, Litovsky SH. Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog. PLoS One. 2014;9(4):e94732. doi: 10.1371/journal.pone.0094732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold AC, Isa K, Shaltout HA, et al. Angiotensin-(1–12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2010;299(3):H763–H771. doi: 10.1152/ajpheart.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Mello WC. Cardiac arrhythmias: the possible role of the renin-angiotensin system. J Mol Med. 2001;79(2–3):103–108. doi: 10.1007/s001090100195. [DOI] [PubMed] [Google Scholar]
- 7.De Mello WC. Is an intracellular renin angiotensin system involved in the control of cell communication in heart? J Cardiovasc Pharmacol. 1994;23:640–646. doi: 10.1097/00005344-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 8.De Mello WC, Danser AHJ. Angiotensin II and the heart on the intracrine renin angiotensin system. Hypertension. 2000;35:1183–1188. doi: 10.1161/01.HYP.35.6.1183. [DOI] [PubMed] [Google Scholar]
- 9.Kurdi M, De Mello WC, Booz GW. Working outside the system: an update on unconventional behavior of the renin angiotensin system components. Int J Biochem Cell Biol. 2005;37:1357–1367. doi: 10.1016/j.biocel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Re RN, Cook JL. The basis of an intracrine physiology. J Clin Pharmacol. 2008;48:344–350. doi: 10.1177/0091270007312155. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Singh VP, Baker KM. The intracellular renin angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 12.De Mello WC. On the pathophysiological implications of an intracellular renin receptor. Circ Res. 2006;99:1285–1286. doi: 10.161/01.RES.0000253141.65450.fc. [DOI] [PubMed] [Google Scholar]
- 13.Serneri GGN, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res. 2001;88:961–968. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 14.Danser AH, van Kats JP, Admiraal PJ, Derkx FH, Lamers JM, Verdouw PD, et al. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension. 1994;24(1):37–48. doi: 10.1161/01.HYP.24.1.37. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad S, Varagic J, Westwood BM, et al. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One. 2011;6(1):e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Mello WC. Intracellular renin alters the electrical properties of the intact heart ventricle of adult Sprague Dawley rats. Regul Pept. 2013;10(181):45–49. doi: 10.1016/j.regpep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294(6):H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy MN, Wiseman MN. Electrophysiologic mechanisms for ventricular arrhythmias in left ventricular dysfunction: electrolytes, catecholamines and drugs. J Clin Pharmacol. 1991;31:1053–1060. doi: 10.1002/j.1552-4604.1991.tb03672.x. [DOI] [PubMed] [Google Scholar]
- 19.Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 21.Seylera C, Zhangb W, Scherera D, Völkersa M, Bloehsa R, et al. Central role of PKCα in isoenzyme-selective regulation of cardiac transient outward current I to and Kv4.3 channels. J Mol Cell Cardiol. 2011;51:722–729. doi: 10.1016/j.yjmcc.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 22.De Mello WC, Frohlich ED. Clinical perspectives and fundamental aspects of local cardiovascular and renal renin-angiotensin systems. Front Endocrinol. 2014;19:5–16. doi: 10.3389/fendo.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuo JL, Li XC, Jeffrey L, Garvin L, et al. Intracellular Ang II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 25.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32:076–082. doi: 10.1161/01.HYP.32.6.976. [DOI] [PubMed] [Google Scholar]