Abstract
Dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) are richly innervated by GABAergic neurons. The postsynaptic effects of GABA on SNc DA neurons are mediated by a mixture of GABAA and GABAB receptors. Although activation of GABAA receptors inhibits spike generation, the consequences of GABAB receptor activation are less well characterized. To help fill this gap, perforated patch recordings were made from young adult mouse SNc DA neurons. Sustained stimulation of GABAB receptors hyperpolarized SNc DA neurons, as previously described. However, transient stimulation of GABAB receptors by optical uncaging of GABA did not; rather, it reduced the opening of small-conductance, calcium-activated K+ (SK) channels and increased the irregularity of spiking. This modulation was attributable to inhibition of adenylyl cyclase and protein kinase A. Thus, because suppression of SK channel activity increases the probability of burst spiking, transient co-activation of GABAA and GABAB receptors could promote a pause-burst pattern of spiking.
Introduction
SNc DA neurons play an important role in goal directed movement [1–3] and reward-based learning [4–7]. As a consequence, their electrophysiological properties have been intensively studied [8–12]. Much of this effort has been focused on defining the role of synaptic connections in determining the types of activity patterns seen in vivo [13–18].
While a number of studies have explored the excitatory glutamatergic regulation of SNc dopaminergic spiking [14,19–21], far less attention has been paid to GABAergic synapses [18,22,23] despite roughly 70% of the synapses on SNc dopaminergic neurons being GABAergic [24,25]. Like most neurons in the brain, the effects of GABA on SNc dopaminergic neurons are mediated by ionotropic GABAA receptors and G-protein coupled GABAB receptors. In spite of the fact that the GABAA receptor reversal potential is relatively depolarized [26] in SNc DA neurons, their activation clearly slows pacemaking [22,27]. However, the effects of synaptically released GABA on postsynaptic GABAB receptors has been more difficult to ascertain [14,28,29]. What is known is that stimulation of GABAB receptors with exogenous application of agonists leads to activation of Kir3 K+ channels, hyperpolarizing SNc dopaminergic neurons [30–32].
Although they provide a framework for understanding the effects of GABA on SNc dopaminergic neurons in vivo, these experiments have two limitations. First, the time course of receptor activation is much more prolonged than that expected to occur during a phasic burst of activity at GABAergic synapses. Second, much of the work has been conducted with a recording configuration that disrupts the oscillations in cytosolic Ca2+ concentration that accompany pacemaking [33,34]. As intracellular Ca2+ is a potent modulator of both plasma membrane ion channels and signaling pathways coupled to G-protein coupled receptors, this recording configuration might distort normal cellular responses.
To overcome these limitations, two experimental steps were taken. First, SNc dopaminergic neurons were recorded using the perforated patch technique with gramicidin D. This method only allows monovalent cations to pass between the cell and the electrode [35]. Second, GABA was applied transiently by optically releasing it from a chemical cage [36]. These experiments revealed that transient GABAB receptor stimulation preferentially suppressed SK K+ channel currents by inhibiting constitutively active adenylyl cyclase and down-regulating protein kinase A activity. This suppression of SK K+ channel currents induced a seconds long period of irregularity in the spiking of SNc dopaminergic neurons. Moreover, given previous work linking SK channels and burst spiking in SNc dopaminergic neurons, GABAB receptor signaling could promote burst spiking after a GABAA receptor induced pause.
Materials and Methods
Mice and brain slice preparation
All experiments were performed in accordance with the Northwestern University Animal Care and Use Committee and NIH guidelines. For electrophysiology experiments, male and female P30-45 C57Bl/6 mice were anesthetized with a ketamine/xylazine mixture and then intracardially perfused with ~4°C, high-sucrose, high-Mg2+ slicing aCSF containing (in mM) 50 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 CaCl2, 10 MgCl2, 25 glucose, pH 7.3 (~310 mOsm/L) just prior to decapitation and brain extraction. Coronal brain slices containing the SNc were cut at 300 μm with a Leica VT1200 S vibratome in slicing aCSF, and placed in a RT recovery solution containing (in mM) 82.5 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1.5 CaCl2, 5.5 MgCl2, 25 glucose, pH 7.3 (~310 mOsm/L) until recording. All solutions were oxygenated with a mixture of 95% O2/5% CO2.
Electrophysiology
For patch-clamp recordings, slices were washed with aCSF containing (in mM) 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 25 glucose, pH 7.3 (~310 mOsm/L) and then placed in a recording chamber perfused continuously at ~2 mL/min with oxygenated aCSF kept at ~35–37°C and allowed to acclimate for a minimum of 15 minutes before recording. Cells were visualized on an Olympus BX51 upright microscope outfitted with IR DIC optics with a 40x water-immersion objective (NA 0.8). Patch pipettes were pulled from thick-walled borosilicate glass with a Sutter P-1000 puller. Pipettes had a tip resistance between 1.5–3 MΩ when filled with internal solution. To prevent dialysis with the patch electrode all experiments, with the exception of those measuring Ca2+ channel currents, were performed using the gramicidin-D perforated patch configuration. Pipettes used for perforated patch recordings were first front-filled with a solution containing (in mM) 126 KMeSO4, 14 KCl, 10 HEPES, 1 EGTA, 0.5 CaCl2, 3 MgCl2, pH 7.3 (~280 mOsm/L), and then back-filled with the same solution containing ~20 μg/mL gramicidin-D. Calcium current recordings were conducted in a whole-cell patch-clamp configuration with an internal solution containing (in mM) 120 CsMeSO3, 15 CsCl2, 10 HEPES, 0.2 EGTA, 3 ATP-Mg, 0.3 GTP-Na, 10 TEA-Cl, pH 7.3 (~280 mOsm/L) and an external solution containing (in mM) 145 TEA-Cl, 2.5 CsCl2, 10 HEPES, 2 CaCl2, 1 MgCl2, 25 glucose, pH 7.3 (~310 mOsm/L), in addition to NBQX, (R)-CPP, SR95531, TTX, isradipine, and omega-conotoxin-GIVA at concentrations denoted in the pharmacology methods. Total calcium current was antagonized with cadmium [500 μM] and subtracted from analyzed traces. All recordings were digitized at 10 kHz and low-pass filtered with a 1 kHz cutoff Bessel.
GABA photolysis
RuBi-GABA [5μM] for uncaging experiments was purchased from Abcam (Cambridge, UK) and stock solutions were made and diluted within one week of use. Wide-field, 50 ms, 500 ms, and 1 min pulses from a CooLED (Andover, UK) pe-100 LED centered at 470nm (100% power) were used to uncage RuBi-GABA around the soma of the recorded cell.
Pharmacology
Electrophysiology and imaging experiments used a variety of pharmacology. Stocks of drugs were made according to manufacturer instructions and diluted ~1000x just prior to experiments. Experimental drugs were all purchased from R&D Systems (Minneapolis, MN) or Santa-Cruz Biotechnology (Dallas, TX) and concentrations were as follows: NBQX [5 μM], (R)-CPP [5 μM], SR95531 [5–25 μM], CGP55845 [2 μM], baclofen [5 μM], apamin [300 pM-200 nM], tetrodotoxin [1 mM], isradipine [20 μM], omeaga-conotoxin-GIVA [10 nM], H89 [10 μM], Rp-8-CPT-cAMPS [100 μM], mibefradil [10 μM], 8-bromo-cAMP [1 μM], CPA [10 μM], dantrolene [10 μM]. All experimental drugs were made to their suggested stock concentrations and stored according to their instructions. Drugs were diluted to their final concentrations just prior to experiments.
Endoplasmic reticulum(ER) imaging
For ER imaging experiments, P17-P21 C57Bl/6 mice were stereotaxically injected with 350 nl AAV9 packaged with TH-G-CEPIA1er construct (1.5e13 vp/ml, Virovek) in their right midbrain region (stereotaxic coordinates: DV: 4.5; ML: 1.3; RC: 3.1, adjusted for the size of each mouse according to the distance between Bregma and Lambda) and sacrificed at least 10 days after the injection (P26-P36) and coronal midbrain slices (220 μm) were collected and used for experiments.
2-Photon Laser Scanning Microscopy (2PLSM)
Brain slices were placed on an upright microscope heated chamber (~33°C) and perfused at a constant rate of ~2 mL/min with aCSF and allowed to stabilize for at least 15 mins before starting the acquisitions. Optical imaging of G-CEPIA1er signals were acquired using a 920-nm excitation beam (80-MHz pulse repetition frequency and ~250-fs pulse duration), in a fixed plane of focus with a pixel size between 0.18 and 0.21 μm and a 12-μs pixel dwell time. The G-CEPIA1er fluorescence was detected by a GaSP PMT and a Dodt contrast-detector system that provided a bright-field transmission image (Prairie Technologies). Images were acquired with an Olympus LUMPFL 60×/1.0 NA water-dipping objective lens. One or two cell bodies were defined as a region of interest (ROI) for each experiment. Twenty frames of the G-CEPIA1er signal were collected in one optical plane at a rate of 3–4 frames per second. Acquisitions were taken every 10 mins for the baseline and the recovery phases, more frequently (1–5 mins) during stimulations.
Electrophysiology and imaging analysis
Data were analyzed using a custom written python analysis package (Neurphys; https://github.com/surmeierlab/neurphys) and MatLab scripts. Figures were created with Matplotlib [37] and Adobe Illustrator. All box plots presented as median, first and third quartiles, and whiskers at 10th and 90th percentiles. Data outside that range is represented as individual points.
TRAP tissue preparation
All experiments were approved by the Rockefeller University Institutional Animal Care and Use Committee and performed in accordance with the guidelines described in the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed in rooms on a 12 h dark/light cycle at 22°C and maintained with rodent diet (Picolab) and water available ad libitum. 30 four-month-old male hemizygous Dat bacTRAP mice [38] were randomly divided into six groups of five mice. Brains were removed and sectioned using an ice-cold Adult Mouse Brain Slicer with 1 mm coronal slice intervals (Zivic Instruments). From the tissue section containing the midbrain, the SNpc and VTA regions were dissected and separated under a Nikon SMZ645 light microscope using a 10x lens.
RNA sequencing and analysis
Translated mRNAs were purified as described previously (Heiman et al. Nature Protocols 2014). TRAP samples underwent DNase digestion using the RNase-Free DNase Set (Qiagen) and were subsequently purified with the RNeasy MinElute Cleanup Kit (Qiagen). Eluted RNA samples were analyzed on a 2100 Bioanalyzer (Agilent) using RNA Pico Chips (Agilent) to confirm RNA integrity, followed by the measurement of RNA concentrations with the Quant-iT RiboGreen RNA Assay Kit (Life Technologies). cDNAs were prepared with the Ovation RNA-Seq System V2 kit (NuGEN), using an input of 1 ng RNA. 500 ng cDNA from each sample were fragmented on a Covaris S2 Focused Ultrasonicator using the operating conditions recommended by the manufacturer for a target fragment size of 200 bp. Fragment size was confirmed on a 2100 Bioanalyzer using High Sensitivity DNA Chips (Agilent). Libraries for RNA sequencing were prepared with the TruSeq RNA Sample Preparation v2 kit (Illumina), starting the manufacturer’s low-throughput protocol with the end repair step. The concentration of the RNA-Seq libraries was determined on a 2100 Bioanalyzer using High Sensitivity DNA Chips. Subsequently, two libraries with different adapters were multiplexed for sequencing. After confirming the concentration of the multiplexed samples on a 2100 Bioanalyzer using High Sensitivity DNA Chips, samples were analyzed on an Illumina HiSeq 2000 sequencer using 100 bp single-end sequencing. RNA-Seq reads were mapped to the Mus musculus assembly 10 reference genome using TopHat version 2.0.11. FPKM values for all genes in each sample were calculated with Cufflinks version 2.2.1. To analyze differential gene expression between samples, DESeq version 1.14.0 was used under the standard comparison mode. P values were reported by DESeq, adjusted for multiple testing using the Benjamini-Hochberg procedure.
Results
Transient stimulation of GABAB receptors has distinct effects
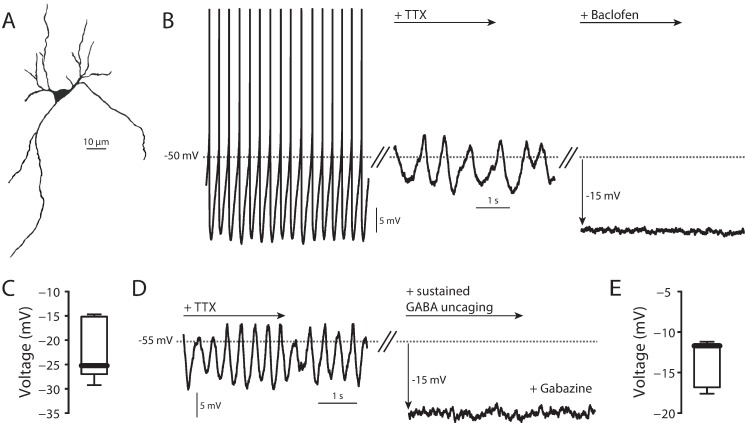
In ex vivo, coronal midbrain slices taken from P30-P45 mice, SNc DA neurons (Fig 1A) recorded using the perforated patch configuration exhibited regular, pacemaking activity (1–6 spikes/s) (Fig 1B) [31,39–41]. When the NaV1 channel antagonist tetrodotoxin (TTX, 1 μM) was added to the bath, slow oscillatory potentials (SOPs) appeared, which had a dominant frequency slower than that of pacemaking (Fig 1B) [33,34,42,43]. These SOPs were dependent upon opening of CaV1 Ca2+ channels, as bath application of dihydropyridines at low micromolar concentrations eliminated them [33,34]. Bath application of the GABAA receptor antagonist gabazine (25 μM) did not alter either basal spiking rate or SOPs, suggesting that there was no tonic GABAergic tone with superfused slices (data not shown). As shown previously, bath application of the GABAB receptor agonist baclofen (5 μM) induced membrane hyperpolarization (Fig 1B). Sustained uncaging of Rubi-GABA (5 μM) in the presence of the GABAA receptor antagonist SR-95531 (gabazine, 25 μM) [44,45] also induced a consistent membrane hyperpolarization (Fig 1D). These effects are in agreement with the previously described coupling of GABAB receptors through Gi/o proteins to Kir3 K+ channels [30,46].
Fig 1. SNc DA neuron physiology.
(A) 2P reconstruction of SNc DA neuron. (B) Left, normal pacemaking of a SNc DA neuron. Middle, TTX (1 μM) application uncovered slow oscillatory potential (SOP). Right, 5 μM baclofen application hyperpolarized the cell. (C) Summary of hyperpolarization due to application of 5 μM baclofen (n = 8, median = -25.24 mV). (D) Sustained uncaging of 5 μM RuBi-GABA in the presence of 25 μM gabazine (to block GABAA receptors) hyperpolarized cells in a manner similar to that seen following baclofen application. (E) Summary of hyperpolarization due to sustained 5 μM RuBi-GABA uncaging (n = 7, median = -11.71 mV).
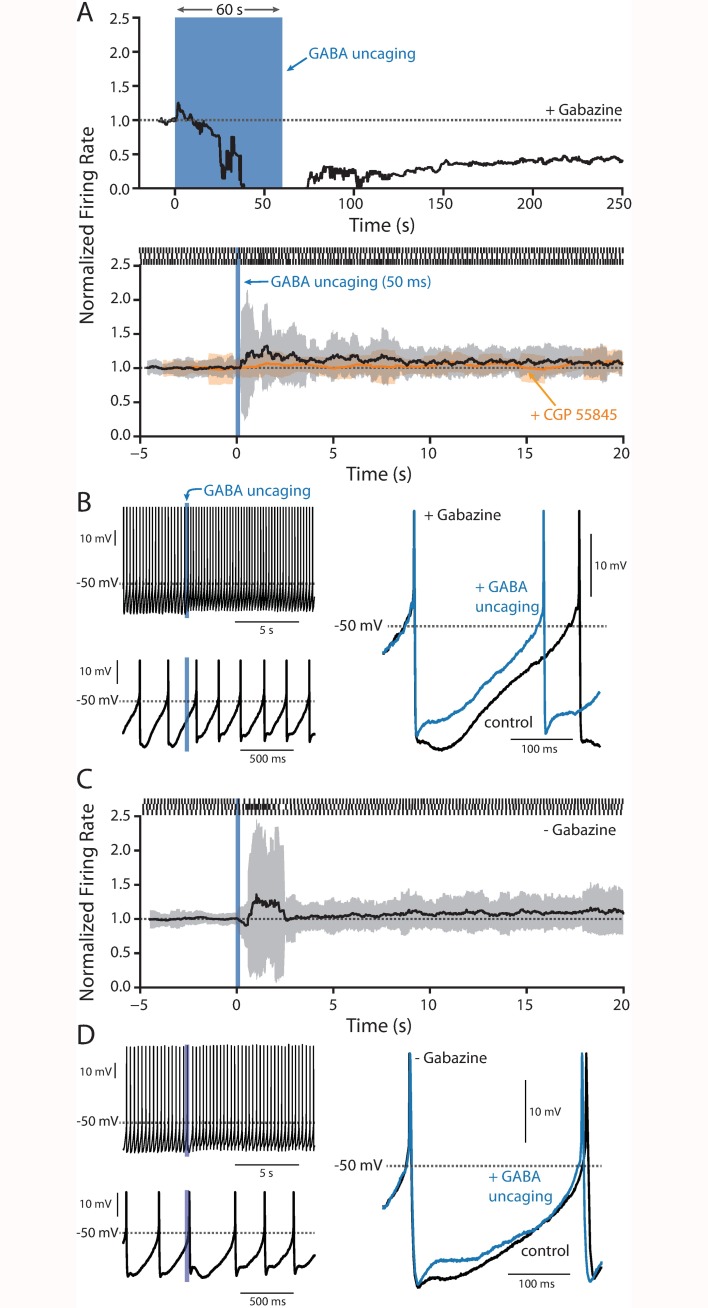
To determine if the duration of GABAB receptor activation had an impact on the physiology of SNc neurons, RuBi-GABA (5 μM) was uncaged for varying periods of time using full-field LED illumination in the presence of gabazine (25 μM) to block GABAA receptors, and the effect on pacemaking monitored. A one minute uncaging pulse led to the expected slow hyperpolarization of the membrane and the eventual cessation of firing seen with bath application of baclofen (Fig 2A). The onset of the hyperpolarization was slow, consistent with either a concentration dependence or a kinetically slow coupling mechanism. In contrast, a single 50 ms uncaging pulse caused a small, but consistent increase in discharge rate (Fig 2A) with a more pronounced increase in the irregularity of spiking as measured by interval standard deviation (Fig 2A). Within seconds, spiking returned to its normal rate and regularity. Bath application of the GABAB receptor antagonist CGP 55845 (2 μM) prior to GABA uncaging blocked changes in firing frequency and regularity (Fig 2A). Moreover, omission of the GABAA receptor antagonist gabazine during GABA uncaging did restore a brief delay in the next spike latency attributable to GABAA receptors; however, it did not qualitatively change the subsequent increase in discharge rate and irregularity (Fig 2C and 2D).
Fig 2. RuBi-GABA uncaging.
(A) Top, plot of the normalized firing rate before during and after a 60 s uncaging pulse (blue bar) of 5 μM RuBi-GABA in the presence of 25 μM gabazine (n = 4). Bottom, plot of normalized firing rate (black line) and running standard deviation (grey area) before, during and after a 50 ms uncaging pulse in the presence of 5 μM RuBi-GABA and 25 μM gabazine (n = 12); application of 2 μM CGP 55845 blunted the changes in spiking induced by RuBi-GABA uncaging (orange line, n = 4). Example raster plots are shown at the top of the panel. (B) Left, two different time scales showing action potentials just prior to and after GABA uncaging. Right, overlaid action potentials from just prior to and after GABA uncaging showing a clear reduction in the mAHP. (C-D) As in panel A (bottom) and B, but in the absence of gabazine (n = 9).
Closer inspection of the spikes revealed that GABA uncaging reduced the magnitude of the medium afterhyperpolarization (mAHP) (Fig 2B–2D). The omission of gabazine did not alter the ability of GABA uncaging to reduce the mAHP (Fig 2D). In SNc dopaminergic neurons, the mAHP is dominated by currents through SK K+ channels [33,47–49]. These channels modulate both the rate and regularity of spiking [48–52] and have a well-established role in the SOP [33,53].
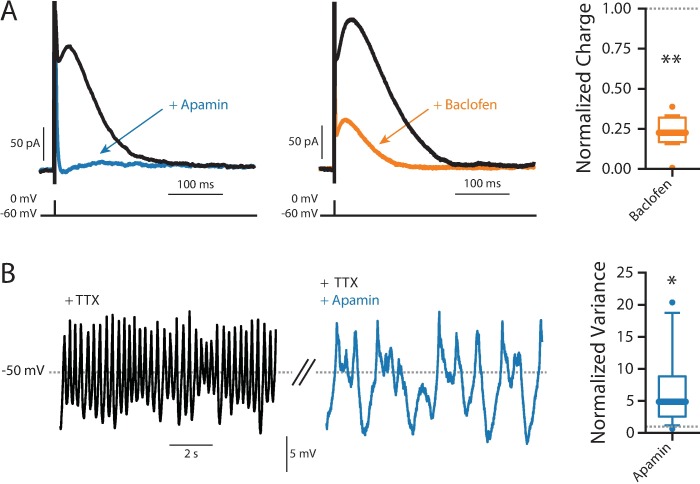
To directly assess the ability of GABAB receptor signaling to modulate SK channels, a hybrid clamp technique was used. In perforated patch recordings, a brief current pulse was used to evoke a spike and the cell was voltage clamped at -60 mV, allowed SK K+ currents to be reliably measured [48]. The SK channel antagonist apamin almost completely eliminated the post-spike current (Fig 3A). Baclofen (5 μM) also dramatically reduced the post-spike current, providing direct evidence of SK channel modulation by GABAB receptors (Fig 3A).
Fig 3. SK channels.
(A) Example SK voltage-clamp recordings showing baseline (black), and responses to 200 nM apamin (blue) and 5 μM baclofen (orange). Right, summary of normalized response to baclofen (n = 8, Wilcoxon signed rank test, p = 0.0078). (B) SOPs (black) are greatly slowed and increase in amplitude with exposure to 200 nM apamin (blue). Right, summary of the normalized variance (n = 9, Wilcoxon signed rank test, p = 0.0151).
As mentioned above, SK channels have also been implicated in the regulation of the SOP [33,53]. In agreement with previous studies using sharp electrodes [53], in perforated patch recordings apamin (200 nM) caused the SOP to slow and become more irregular (Fig 3B).
GABAB receptor signaling inhibited adenylyl cyclase
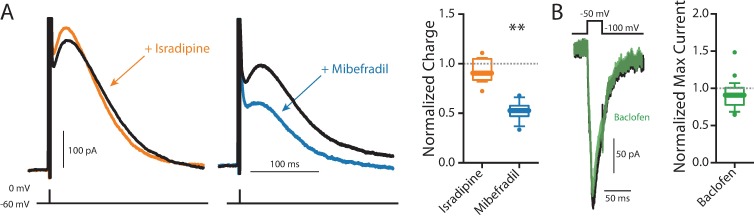
GABAB receptors couple to Gi/o signaling pathways. In addition to activating Kir3 K+ channels, Gi/o-protein coupled receptors can inhibit voltage-dependent Ca2+ channels [54,55]. This type of modulation could mediate the GABAB receptor suppression of Ca2+ activated SK channels. In agreement with previous work [48], an inhibitor of CaV3 (mibefradil, 10 μM) but not CaV1 (isradipine, 10 μM) Ca2+ channels decreased SK channel currents measured with the hybrid clamp (Fig 4A). However, there was no measureable effect of baclofen (5 μM) on CaV3 channel currents measured with a voltage step from -100 mV to -50 mV (Fig 4B). Although it is possible that incomplete voltage clamp obscured a modest modulation of these currents by GABAB receptor activation, the more plausible interpretation is that the SK channel modulation was mediated by some other mechanism.
Fig 4. VGCC contribution to SK.
(A) Application of 10 μM isradipine (orange) to inhibit CaV1 channels did not reduce total SK charge (n = 8, Wilcoxon signed rank test, p = 0.3828), while inhibiting CaV3 channels with 10 μM mibefradil (blue) inhibited roughly half the charge (n = 8, Wilcoxon signed rank test, p = 0.0078). (B) 5 μM baclofen (green) application did not inhibit T-type calcium current (n = 12, Wilcoxon signed rank test, p = 0.1099).
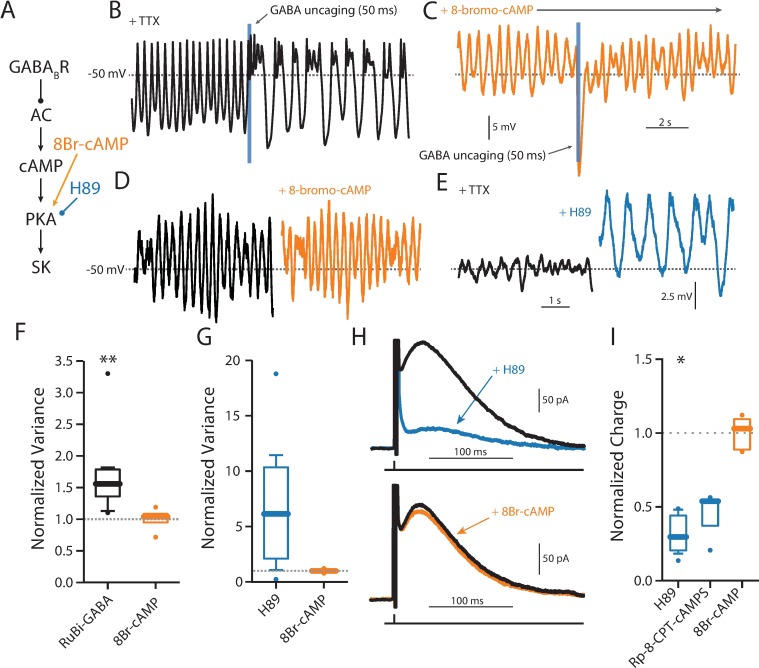
To narrow the range of potential targets, an attempt was made to determine the signaling elements downstream of GABAB receptors. One of the best described targets of Gi/o signaling is adenylyl cyclase (AC) [56,57]. SNc dopaminergic neurons express AC1 isoforms that are stimulated by Ca2+ (S1 Table), raising the possibility that this form of AC is constitutively active during pacemaking. To test this hypothesis, the ability of GABAB receptors to modulate the SOP was examined before and after perturbing AC signaling. First, as shown above, a single, brief uncaging of GABA induced a slowing of SOP frequency and an increased irregularity in the oscillation (Fig 5A). Bath application of a membrane permeable analog of cyclic adenosine monophosphate (cAMP) (8-bromo-cAMP, 1 μM) blunted this modulation (Fig 5B and 5C). However, bath application of 8-bromo-cAMP (1 μM) had no discernible effect on SOPs (Fig 5D), consistent with the proposition that AC was constitutively active and GABAB receptors were having their effect by transiently suppressing this activity.
Fig 5. PKA activation prevents GABAB modulation of SK.
(A) Schematic diagram showing the hypothesized signaling pathway from GABAB receptor activation to SK channels, and the site of action of 8-bromo-cAMP and H-89 in that pathway. (B) A 50 ms uncaging pulse elicited an immediate and significant change in SOP variance (black, n = 10, Wilcoxon signed rank test, p = 0.002). (C) The same was not seen when cells were incubated in 1 μM 8-bromo-cAMP (orange, n = 4, Wilcoxon signed rank test, p = 0.875). (D) Directly activating PKA with 1 μM 8-bromo-cAMP does not have an effect on SOP variance (n = 4, Wilcoxon signed rank test, p = 0.75). (E) Inhibiting PKA with 10 μM H89 increases SOP variance (n = 6, Wilcoxon signed rank test, p = 0.0938). (F) Summary data for panels A-B. (G) Summary data for panels C-D. (H) Top, inhibiting PKA with 10 μM H89 significantly decreases SK current (n = 6, Wilcoxon signed rank test, p = 0.0313). Bottom, directly activating PKA with 1 μM 8-bromo-cAMP does not have an effect on SK current (n = 5, Wilcoxon signed rank test, p = 1.00). (I) Summary data for PKA modulators from panel H and Rp-8-CPT-cAMPS (n = 3, Wilcoxon signed rank test, p = 0.25).
The cAMP generated by AC activates protein kinase A (PKA), leading to phosphorylation of a wide range of substrates. If GABAB receptor signaling was inhibiting AC, it should result in diminished PKA activity. Thus, PKA inhibition should mimic GABAB receptor signaling. To test this idea, the PKA inhibitor H-89 (10 μM) was bath applied. As predicted, H-89 slowed the SOP and increased its irregularity and amplitude (Fig 5E and 5G–5I). To directly test the role of AC signaling in regulating SK channels, the hybrid clamp was used. As expected, 8-bromo-cAMP had no effect on SK channel currents (Fig 5H). However, H-89 dramatically suppressed SK channel currents (Fig 5H), arguing that constitutive AC and PKA activity was critical to maintaining SK activity and that GABAB receptor inhibition of AC could mediate SK channel inhibition. Similar results were seen with another PKA antagonist, Rp-8-CPT-cAMPS (100 μM) (Fig 5I).
Depletion of intracellular Ca2+ stores did not block SK modulation
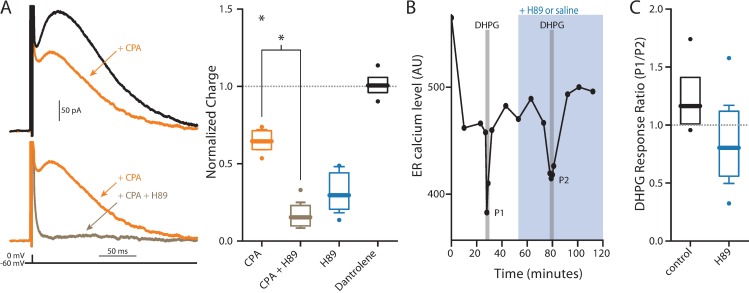
SK channels in SNc dopaminergic neurons also are known to be regulated by Ca2+ released from intracellular stores controlled by inositol 1,4,5-triphosphate (IP3) receptors [58,59]. PKA phosphorylation of IP3 receptors enhances their opening [60–62]. Thus, one potential way in which PKA might promote SK activation is by enhancing IP3 receptor mobilization of Ca2+. Depleting intracellular Ca2+ stores by inhibiting the smooth endoplasmic reticulum Ca2+ ATPase (SERCA) with cyclopiazonic acid (CPA, 10 μM) reduced SK channel currents measured with the hybrid clamp, in agreement with previous work [63]. However, in the presence of CPA, the PKA inhibitor H-89 (10 μM) continued to suppress SK channel currents (Fig 6A), suggesting that PKA was not constitutively effecting SK channels by enhancing IP3 receptor function.
Fig 6. ER Ca2+ contribution to SK current.
(A) Application of CPA to empty ER Ca2+ stores significantly reduced SK current (orange, n = 6, Wilcoxon signed rank test, p = 0.0313) and further application of 10 μM H89 to inhibit PKA activity further significantly reduced SK current (brown, n = 6, Wilcoxon signed rank test, p = 0.0313), but does not reduce it any further than H89 alone (Mann-Whitney U test, p = 0.1320). Inhibiting ryanodine receptors with 10 μM dantrolene did not change SK current (black, n = 4, Wilcoxon signed rank test, p = 1.00). (B) Left, an example experiment showing the effect of DHPG application on ER Ca2+ levels. Right, summary of normalized data showing a small, but not significant change in induced ER Ca2+ release after application of H89 (n = 4) compared to control (n = 7) recordings (Mann-Whitney U test, p = 0.2303).
To provide an additional test of this inference, the effects of PKA inhibition on the ability of group 1 metabotropic glutamate receptors (mGluRs) to deplete ER Ca2+ stores was assessed. If PKA was constitutively enhancing IP3 receptor function, inhibiting PKA should blunt the ability of mGluRs to activate IP3 receptors and deplete ER stores. To test this prediction, group 1 mGluRs were activated with (S)-3,5-dihydroxyphenylglycine (DHPG, 10 μM). DHPG produced a robust lowering of ER Ca2+ concentration measured in ex vivo brain slices with the genetically encoded ER Ca2+ probe CEPIA1er [64]. However, H-89 (10 μM) failed to significantly diminish the DHPG evoked drop in ER Ca2+ (Fig 6B), suggesting that PKA does not constitutively enhance IP3R-mediated Ca2+ release in SNc dopaminergic neurons. Application of the ryanodine receptor (RyR) antagonist dantrolene (10 μM) had no effect on SK currents, suggesting that RyRs do not contribute to SK channel activation. Taken together, these results argue that PKA is regulating SK channel gating through a mechanism that is independent of ER stores (Fig 7).
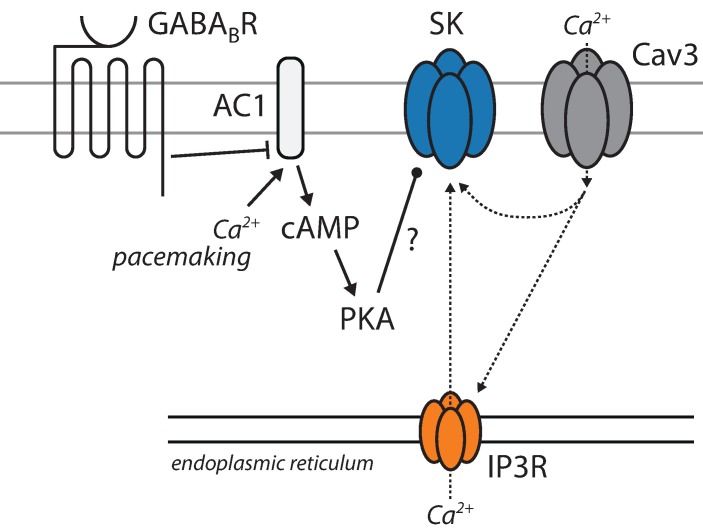
Fig 7. Schematic diagram depicting hypothesized signaling pathways involved in the GABAB receptor-mediated inhibition of SK channels.
GABAB receptor inhibition of AC by Gi signaling is hypothesized to be responsible for reduced cAMP levels and PKA signaling. The reduction in PKA activity is hypothesized to reduce SK channel opening through mechanism that are independent of either plasma membrane Ca2+ channels or release from intracellular stores.
Discussion
Our studies have identified a novel mechanism of GABAB receptor modulation of SNc dopaminergic neuron activity. While confirming that GABAB receptor signaling can activate Kir3 K+ channels and suppress spiking when stimulation is sustained, our results show that transient activation of GABAB receptors has a very different effect on ongoing pacemaking. Rather than inhibiting spiking, transient activation of GABAB receptors increased spiking rate and irregularity. This effect on spiking was mediated by suppression of SK channel currents. Given the well-established ability of SK channel inhibition to increase the propensity of SNc dopaminergic neurons to spike in bursts [19,51], a natural inference from our studies are that in vivo GABAB receptors signaling could create a seconds long window in which subsequent glutamatergic input could induce burst spiking more easily.
Transient elevation in GABA induced suppression of SK currents
Using bath application of ligands, previous studies of GABAB receptor effects on SNc dopaminergic neurons have repeatedly found that they are capable of activating Kir3 K+ channels, leading to hyperpolarization and the cessation of ongoing, autonomous pacemaking [30,31]. The ability of Gβγ proteins released by stimulation of Gi/o coupled receptors to increase the open probability of Kir3 K+ channels through a membrane delimited signaling pathway has been extensively characterized in both native and heterologous expression systems [65–67]. This is a robust modulation that is resistant to the alterations in intracellular environment brought about by whole cell or sharp electrode intracellular recording techniques. Using the perforated patch technique, which largely preserves the intracellular milieu, including the oscillation in intracellular Ca2+ concentration that accompanies pacemaking [34,68], this GABAB receptor modulation also was evident with bath application of GABAB receptor agonists. Thus, there is no apparent negative regulator of Kir3 K+ channel modulation in SNc neurons that are pacemaking and have normal fluctuations in intracellular Ca2+.
However, with brief stimulation of GABAB receptors enabled by optical uncaging of GABA, there was little evidence of Kir3 K+ channel activation in most cells (Fig 5C). Rather, uncaging GABA evoked a transient increase in discharge rate and a more prolonged period of irregularity in spiking. Both effects were attributable to a suppression in the mAHP generated by SK K+ channel currents. This was shown not only by inspection of the voltage-trajectory of the somatic membrane potential but also with use of hybrid clamp techniques that allowed spike generated outward currents to be isolated. In these experiments, the SK K+ channel blocker apamin mimicked the effects of GABAB receptor agonists. Apamin also is known to increase the irregularity of the SOP created by blocking NaV1 Na+ channels in SNc dopaminergic neurons. Again, this effect was mimicked by GABAB receptor agonists.
In contrast the modulation of Kir3 channels, the effect of GABAB receptors on SK channels appeared to be mediated by Gαi inhibition of adenylyl cyclase. There are several observations consistent with this conclusion. First, mRNA profiling of SNc DA neurons demonstrated they robustly express AC1, a isoform of adenylyl cyclase that is stimulated by Ca2+/calmodulin [69,70]. As pacemaking SNc DA neurons have high levels of intracellular Ca2+ [34], AC1 should be constitutively activated. Previous work with whole cell recording where this intracellular Ca2+ oscillation was disrupted could have missed the GABAB receptor modulation of SK channels because AC1 activity was reduced. Second, bath application of a membrane permeable cAMP analog (bypassing AC1) blunted the GABAB receptor modulation of SK channels. Moreover, as expected if there was constitutive activity of AC1, the cAMP analogue had no effect in the absence of GABAB receptor stimulation. Third, inhibiting PKA, a major target of cAMP signaling, mimicked the effects of GABAB receptor signaling and occluded the effects of GABAB receptor activation. All three of these observations point to a simple signaling model (Fig 7).
What is unresolved is how PKA signaling enhances SK K+ channel gating. Neither source of Ca2+ involved in SK K+ channel gating–CaV3 Ca2+ channels and IP3 receptor sensitive, ER Ca2+ stores [48,63]–appeared be affected by PKA inhibition. However, it is possible that our assays of PKA modulation of these targets was not sensitive enough. It is also possible that the SK channel itself is a target of PKA. SK2 and SK3 channels are expressed by SNc DA neurons [47,50] and both channels have serine/threonine phosphorylation sites [71,72]. Although these sites have been reported to control membrane trafficking, it is not clear whether they also affect channel gating.
Another unresolved question is why transient uncaging of GABA was effective in triggering modulation of SK channels but not Kir3 channels. As the duration of GABA uncaging will affect the concentration of GABA achieved in the extracellular space, one possibility is that there are high and low affinity GABAB receptors that differentially couple to SK and Kir3 channels, but there is no evidence for this kind of GABAB receptor heterogeneity. Another possibility is that differential scaffolding of targets in the neighborhood of GABAB receptors is responsible. It is possible that in SNc dopaminergic neurons the density of Kir3 channels in the neighborhood of GABAB receptors is low and that Gβγ subunits released by receptor binding have to diffuse a substantial distance to interact with them, slowing the modulation. AC1, on the other hand, could be held near GABAB receptors by scaffolding proteins [55], allowing Gi subunits to quickly inhibit enzymatic activity.
Could GABAB receptors contribute to burst spiking?
Based upon previous work identifying Kir3 channels as targets of GABAB receptor signaling, it is widely assumed that GABAB receptors inhibit SNc DA neuron spiking much like GABAA receptors. Our results suggest an alternative scenario. In vivo, SNc DA neurons often follow a pause in spiking with a period of increased spiking or bursting [16,27]. This pattern of activity, particularly bursting, is thought to have profound effects on target structures like the striatum by transiently elevating extracellular dopamine concentration. This transient elevation has been linked to reward prediction errors as well as the initiation of movement [4,73].
It is widely held that burst spiking in SNc DA neurons is driven by excitatory glutamatergic synaptic activity [14]. Much like apamin [19], the transient suppression of SK K+ channel currents by GABAB receptors should make it easier for glutamatergic synapse to drive bursting [19,20,23,74]. Thus, glutamatergic input to SNc DA neurons that temporally lagged a GABAergic volley from striatal spiny projection neurons that engaged GABAB receptors, would be very effective in evoking a burst of spikes. However, if the GABAergic input was maintained for a longer period of time, it could lead to engagement of Kir3 K+ channels and suppression of responsiveness to glutamatergic inputs.
Supporting Information
AC1-10 (n = 6).
(XLSX)
All data from figure boxplots.
(XLSX)
Acknowledgments
This work was supported by grants from the JPB Foundation (DJS, PG) and the National Institutes of Health (P50NS047085) (DJS). The Fisher Center for Alzheimer’s Research (PG) and the Leon Black Family Foundation (PG).
Data Availability
Data are contained in two Supporting Information excel files.
Funding Statement
This work was funded by JPB Foundation [www.jpbfoundation.org] (DJS PG), National Institutes of Health (P50NS047085) (DJS), The Fisher Center for Alzheimer’s Research [https://www.alzinfo.org/] (PG), and Leon Black Family Foundation (PG).
References
- 1.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1983;80: 4546–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graybiel A, Aosaki T, Flaherty A, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265: 1826–1831. [DOI] [PubMed] [Google Scholar]
- 3.Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Current opinion in neurobiology. 2000;10: 732–739. [DOI] [PubMed] [Google Scholar]
- 4.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997. [DOI] [PubMed] [Google Scholar]
- 5.Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. Journal of Neuroscience. 2008;28: 11673–11684. 10.1523/JNEUROSCI.3839-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. TRENDS in Neurosciences. 2009;32: 517–524. 10.1016/j.tins.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6: 24 10.1186/1744-9081-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kita T, Kita H, Kitai ST. Electrical membrane properties of rat substantia nigra compacta neurons in an in vitro slice preparation. Brain Research. 1986;372: 21–30. [DOI] [PubMed] [Google Scholar]
- 9.Cardozo DL, Bean BP. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. Journal of Neurophysiology. 1995;74: 1137–1148. [DOI] [PubMed] [Google Scholar]
- 10.Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. Journal of Neuroscience. 2002;22: 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. Journal of Neuroscience. 2007;27: 645–656. 10.1523/JNEUROSCI.4341-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amendola J, Woodhouse A, Martin-Eauclaire M-F, Goaillard J-M. Ca²⁺/cAMP-sensitive covariation of I(A) and I(H) voltage dependences tunes rebound firing in dopaminergic neurons. Journal of Neuroscience. 2012;32: 2166–2181. 10.1523/JNEUROSCI.5297-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CR, Tepper JM. Basal Ganglia Control of Substantia Nigra Dopaminergic Neurons Birth, Life and Death of Dopaminergic Neurons in the Substantia Nigra. Springer; Vienna; 2009. pp. 71–90. [DOI] [PubMed] [Google Scholar]
- 14.Paladini CA, Roeper J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience. 2014;282C: 109–121. [DOI] [PubMed] [Google Scholar]
- 15.Johnson SW, Wu Y-N. Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Research. 2004;1019: 293–296. 10.1016/j.brainres.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 16.Hyland BI, Reynolds JNJ, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114: 475–492. [DOI] [PubMed] [Google Scholar]
- 17.Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience. 1999;89: 799–812. [DOI] [PubMed] [Google Scholar]
- 18.Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15: 3092–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deister CA, Teagarden MA, Wilson CJ, Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. Journal of Neuroscience. 2009;29: 15888–15897. 10.1523/JNEUROSCI.4053-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blythe SN, Wokosin D, Atherton JF, Bevan MD. Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J Neurosci. 2009;29: 15531–15541. 10.1523/JNEUROSCI.2961-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearlstein E, Gouty-Colomer L-A, Michel FJ, Cloarec R, HAMMOND C. Glutamatergic synaptic currents of nigral dopaminergic neurons follow a postnatal developmental sequence. Front Cell Neurosci. 2015;9: 210 10.3389/fncel.2015.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paladini CA, Iribe Y, Tepper JM. GABAA receptor stimulation blocks NMDA-induced bursting of dopaminergic neurons in vitro by decreasing input resistance. Brain Research. 1999;832: 145–151. [DOI] [PubMed] [Google Scholar]
- 23.Lobb CJ, Wilson CJ, Paladini CA. A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons. Journal of Neurophysiology. 2010;104: 403–413. 10.1152/jn.00204.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henny P, Brown MTC, Northrop A, Faunes M, Ungless MA, Magill PJ, et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15: 613–619. 10.1038/nn.3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Research. 1990;529: 57–78. [DOI] [PubMed] [Google Scholar]
- 26.Gulácsi A, Lee CR, Sík A, Viitanen T, Kaila K, Tepper JM, et al. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. Journal of Neuroscience. 2003;23: 8237–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paladini CA, Tepper JM. GABAA and GABAB antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999. [DOI] [PubMed] [Google Scholar]
- 28.Charara A, Heilman TC, Levey AI, Smith Y. Pre- and postsynaptic localization of GABA(B) receptors in the basal ganglia in monkeys. Neuroscience. 2000;95: 127–140. [DOI] [PubMed] [Google Scholar]
- 29.Boyes J, Bolam JP. The subcellular localization of GABA(B) receptor subunits in the rat substantia nigra. Eur J Neurosci. 2003;18: 3279–3293. [DOI] [PubMed] [Google Scholar]
- 30.Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. The Journal of Physiology. 1988;401: 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts AE, Williams JT, Henderson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. Journal of Neurophysiology. 1996;76: 2262–2270. [DOI] [PubMed] [Google Scholar]
- 33.Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. The Journal of Physiology. 1993;466: 727–747. [PMC free article] [PubMed] [Google Scholar]
- 34.Guzman JN, Sanchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. Journal of Neuroscience. 2009;29: 11011–11019. 10.1523/JNEUROSCI.2519-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. Journal of Neuroscience Methods. 1995;57: 27–35. [DOI] [PubMed] [Google Scholar]
- 36.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Frontiers in Neural Circuits. 2008;2: 2 10.3389/neuro.04.002.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter JD. Matplotlib: A 2D graphics environment. Computing in science and engineering. 2007. [Google Scholar]
- 38.Brichta L, Shin W, Jackson-Lewis V, Blesa J, Yap E-L, Walker Z, et al. Identification of neurodegenerative factors using translatome-regulatory network analysis. Nat Neurosci. 2015;18: 1325–1333. 10.1038/nn.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience. 1983;10: 301–315. [DOI] [PubMed] [Google Scholar]
- 40.Llinás R, Greenfield SA, Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra—a possible mechanism for dendritic release. Brain Research. 1984;294: 127–132. [DOI] [PubMed] [Google Scholar]
- 41.Shepard PD, Bunney BS. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca2+-activated K+ conductance. Exp Brain Res. 1991;86. [DOI] [PubMed] [Google Scholar]
- 42.Harris NC, Webb C, Greenfield SA. A possible pacemaker mechanism in pars compacta neurons of the guinea-pig substantia nigra revealed by various ion channel blocking agents. Neuroscience. 1989;31: 355–362. [DOI] [PubMed] [Google Scholar]
- 43.Yung WH, Häusser MA, Jack JJ. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. The Journal of Physiology. 1991;436: 643–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, et al. Changes in firing rate and pattern of GABAergic neurons in subregions of the substantia nigra pars reticulata in rat models of Parkinson's disease. Brain Research. 2010;1324: 54–63. 10.1016/j.brainres.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 46.Labouèbe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10: 1559–1568. 10.1038/nn2006 [DOI] [PubMed] [Google Scholar]
- 47.Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. Journal of Neuroscience. 2001;21: 3443–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. Journal of Neuroscience. 2002;22: 3404–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji H, Hougaard C, Herrik KF, Strøbaek D, Christophersen P, Shepard PD. Tuning the excitability of midbrain dopamine neurons by modulating the Ca2+ sensitivity of SK channels. Eur J Neurosci. 2009;29: 1883–1895. 10.1111/j.1460-9568.2009.06735.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deignan J, Luján R, Bond C, Riegel A, Watanabe M, Williams JT, et al. SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience. 2012;217: 67–76. 10.1016/j.neuroscience.2012.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji H, Shepard PD. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience. 2006;140: 623–633. 10.1016/j.neuroscience.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 52.Tateno T. A small-conductance Ca2+-dependent K+ current regulates dopamine neuron activity: a combined approach of dynamic current clamping and intracellular imaging of calcium signals. NeuroReport. 2010;21: 667–674. 10.1097/WNR.0b013e32833add56 [DOI] [PubMed] [Google Scholar]
- 53.Ping HX, Shepard PD. Apamin-sensitive Ca(2+)-activated K+ channels regulate pacemaker activity in nigral dopamine neurons. NeuroReport. 1996;7: 809–814. [DOI] [PubMed] [Google Scholar]
- 54.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiological reviews. 2004;84: 835–867. 10.1152/physrev.00036.2003 [DOI] [PubMed] [Google Scholar]
- 55.Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 2010;58: 123–147. 10.1016/S1054-3589(10)58006-2 [DOI] [PubMed] [Google Scholar]
- 56.Federman AD, Conklin BR, Schrader KA, Reed RR, Bourne HR. Hormonal stimulation of adenylyl cyclase through Gi-protein beta gamma subunits. Nature. 1992;356: 159–161. 10.1038/356159a0 [DOI] [PubMed] [Google Scholar]
- 57.Nishikawa M, Hirouchi M, Kuriyama K. Functional coupling of Gi subtype with GABAB receptor/adenylyl cyclase system: analysis using a reconstituted system with purified GTP-binding protein from bovine cerebral cortex. Neurochem Int. 1997;31: 21–25. [DOI] [PubMed] [Google Scholar]
- 58.Morikawa H, Imani F, Khodakhah K, Williams JT. Inositol 1,4,5-triphosphate-evoked responses in midbrain dopamine neurons. Journal of Neuroscience. 2000;20: RC103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. Journal of Neuroscience. 2003;23: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2009;1793: 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeSouza N, Reiken S, Ondrias K, Yang Y-M, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277: 39397–39400. 10.1074/jbc.M207059200 [DOI] [PubMed] [Google Scholar]
- 62.Chaloux B, Caron AZ, Guillemette G. Protein kinase A increases the binding affinity and the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor type 3 in RINm5F cells. Biology of the Cell. 2012;99: 379–388. [DOI] [PubMed] [Google Scholar]
- 63.Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J Neurosci. 2007;27: 4776–4785. 10.1523/JNEUROSCI.0139-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun. 2014;5: 4153 10.1038/ncomms5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Q, Kawano T, Nakata H, Nakajima Y, Nakajima S, Kozasa T. Interaction of G protein beta subunit with inward rectifier K(+) channel Kir3. Molecular Pharmacology. 2003;64: 1085–1091. 10.1124/mol.64.5.1085 [DOI] [PubMed] [Google Scholar]
- 66.Berlin S, Keren-Raifman T, Castel R, Rubinstein M, Dessauer CW, Ivanina T, et al. G alpha(i) and G betagamma jointly regulate the conformations of a G betagamma effector, the neuronal G protein-activated K+ channel (GIRK). Journal of Biological Chemistry. 2010;285: 6179–6185. 10.1074/jbc.M109.085944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernández-Fernández JM, Abogadie FC, Milligan G, Delmas P, Brown DA. Multiple pertussis toxin-sensitive G-proteins can couple receptors to GIRK channels in rat sympathetic neurons when expressed heterologously, but only native G(i)-proteins do so in situ. Eur J Neurosci. 2001;14: 283–292. [DOI] [PubMed] [Google Scholar]
- 68.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. 'Rejuvenation‘ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447: 1081–1086. 10.1038/nature05865 [DOI] [PubMed] [Google Scholar]
- 69.Westcott KR, La Porte DC, Storm DR. Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proc Natl Acad Sci USA. 1979;76: 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villacres EC, Wu Z, Hua W, Nielsen MD, Watters JJ, Yan C, et al. Developmentally expressed Ca(2+)-sensitive adenylyl cyclase activity is disrupted in the brains of type I adenylyl cyclase mutant mice. J Biol Chem. 1995;270: 14352–14357. [DOI] [PubMed] [Google Scholar]
- 71.Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11: 170–177. 10.1038/nn2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clarysse L, Guéguinou M, Potier-Cartereau M, Vandecasteele G, Bougnoux P, Chevalier S, et al. cAMP-PKA inhibition of SK3 channel reduced both Ca2+ entry and cancer cell migration by regulation of SK3-Orai1 complex. Pflügers Arch—Eur J Physiol. 2014;466: 1921–1932. [DOI] [PubMed] [Google Scholar]
- 73.Howe MW, Dombeck DA. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016;535: 505–510. 10.1038/nature18942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lobb CJ, Wilson CJ, Paladini CA. High-frequency, short-latency disinhibition bursting of midbrain dopaminergic neurons. Journal of Neurophysiology. 2011;105: 2501–2511. 10.1152/jn.01076.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AC1-10 (n = 6).
(XLSX)
All data from figure boxplots.
(XLSX)
Data Availability Statement
Data are contained in two Supporting Information excel files.