Abstract
The onion (Allium cepa L.) is widely planted worldwide as a valuable vegetable crop. The scales of an onion bulb are a modified type of leaf. The one-layer-cell epidermis of onion scales is commonly used as a model experimental material in botany and molecular biology. The lower epidermis (LE) and upper epidermis (UE) of onion scales display obvious differences in microscopic structure, cell differentiation and pigment synthesis; however, associated proteomic differences are unclear. LE and UE can be easily sampled as single-layer-cell tissues for comparative proteomic analysis. In this study, a proteomic approach based on 2-DE and mass spectrometry (MS) was applied to compare LE and UE of fleshy scales from yellow and red onions. We identified 47 differential abundant protein spots (representing 31 unique proteins) between LE and UE in red and yellow onions. These proteins are mainly involved in pigment synthesis, stress response, and cell division. Particularly, the differentially accumulated chalcone-flavanone isomerase and flavone O-methyltransferase 1-like in LE may result in the differences in the onion scale color between red and yellow onions. Moreover, stress-related proteins abundantly accumulated in both LE and UE. In addition, the differential accumulation of UDP-arabinopyranose mutase 1-like protein and β-1,3-glucanase in the LE may be related to the different cell sizes between LE and UE of the two types of onion. The data derived from this study provides new insight into the differences in differentiation and developmental processes between onion epidermises. This study may also make a contribution to onion breeding, such as improving resistances and changing colors.
Introduction
The onion (Allium cepa L.) is a member of the Liliaceae family and is widely planted worldwide as a valuable vegetable crop, with fleshy scales that form bulbs as the main edible parts. The onion bulb has a unique flavor and a long storage period. It contains important dietary flavonoids and alk(en)yl cysteine sulfoxides and has many activities that are beneficial to human health, e.g., anticancer properties, and antiplatelet, antithrombotic and anti-asthmatic activities [1]. Global onion production increased from 52.65 million tons in 2003 to 85.79 million tons in 2013; In 2013, China’s onion production reached 22.34 million tons and ranked first place worldwide (FAO). Currently, onions are the second most-grown vegetable in the world after tomatoes.
The scales of an onion bulb are modified leaves. The edges of the scales bond together around the bud and finally form the bulb. Due to the polarity of the leaf primordia during differentiation and development, onion scales also show dorsoventrality. Each scale consists of an upper epidermis (UE), an intermediate parenchyma tissue (mesophyll), and a lower epidermis (LE). The epidermis of onion scales forms a boundary between the plant and the external environment. It serves different functions, such as protecting against water loss, regulating gas exchange, secreting metabolic compounds, and absorbing water and mineral nutrients.
Previous studies showed that cell differentiation capacity and maintenance of differentiated phenotypes (e.g., stomata and trichome cells) in plant epidermis were regulated through the coordination of multiple genes (e.g., defective kernel 1, homeodomain-leucine zipper, Arabidopsis thaliana meristem 1, protodermal factor 2) [2–5]. However, the differentiation mechanisim between LE and UE in onion has largely unclear.
Both LE and UE in onion are simple tissues, consisting of one layer of closely-associated cells that play a mechanical, protective role. UE is colorless, transparent and easily peels off, whereas LE is yellow, red or white in color, due to flavonoid compounds (e.g., quercetin, anthocyanin) [6, 7]. Onion bulbs are categorized as yellow, red (purple), and white based on the color of their scales. In yellow and red onions, quercetin is the most abundant flavonoid [7]. Anthocyanins represent approximately 10% of the total flavonoids, with cyanine derivatives as the major anthocyanins among at least 25 different compounds [6, 8].
LE and UE display obvious differences in cell differentiation and pigment synthesis. Unique microscopic structures and physiological functions make the epidermis of onion scales a commonly used experimental material in botany and molecular biology. The epidermis of onion scales has been used in biosensors [9], transient transformation (detecting protein subcellular localization) [10], cellular and subcellular metabolite analysis [11], and growth anisotropy [12]. However, the associated proteomic differences between LE and UE are unclear. The onion epidermis is a suitable material to carry out single-layer-cell proteomic analysis.
Although onion genome sequencing has not been reported and the number of identified proteins in the onion is limited in databases, the increasing numbers of available expressed sequence tag (EST) databases and the high homology of the onion to the lily genome allow the successful identification of proteins, as shown by a recent research study [13]. The present study compared the proteomic differences between LE and UE in yellow and red onion scales using 2-DE followed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) Mass Spectrometry (MS), revealing a number of the differential accumulated proteins involved in pigment synthesis, stress response and cell division in the onion epidermis. Our results provided new insight into the differences in differentiation and developmental processes between onion epidermises. This study may also make a contribution to onion breeding, such as improving resistances and changing colors.
Materials and Methods
Plant materials
Mature bulbs of yellow and red onions with diameter approximately 7–9 cm were obtained from a local market (Henan, Zhengzhou, China). The fleshy scales of onion bulbs were cut into pieces, and LE and UE were manually separated (Fig 1). For one independent biological experiment, the samples were collected from one onion, in which the outer dry leaves and inner light-colored leaves (about 3–4 layers) were discarded. Fresh samples were immediately used for microscopic observation or frozen in liquid nitrogen and stored at -80°C until the pigment assay and proteomic analysis.
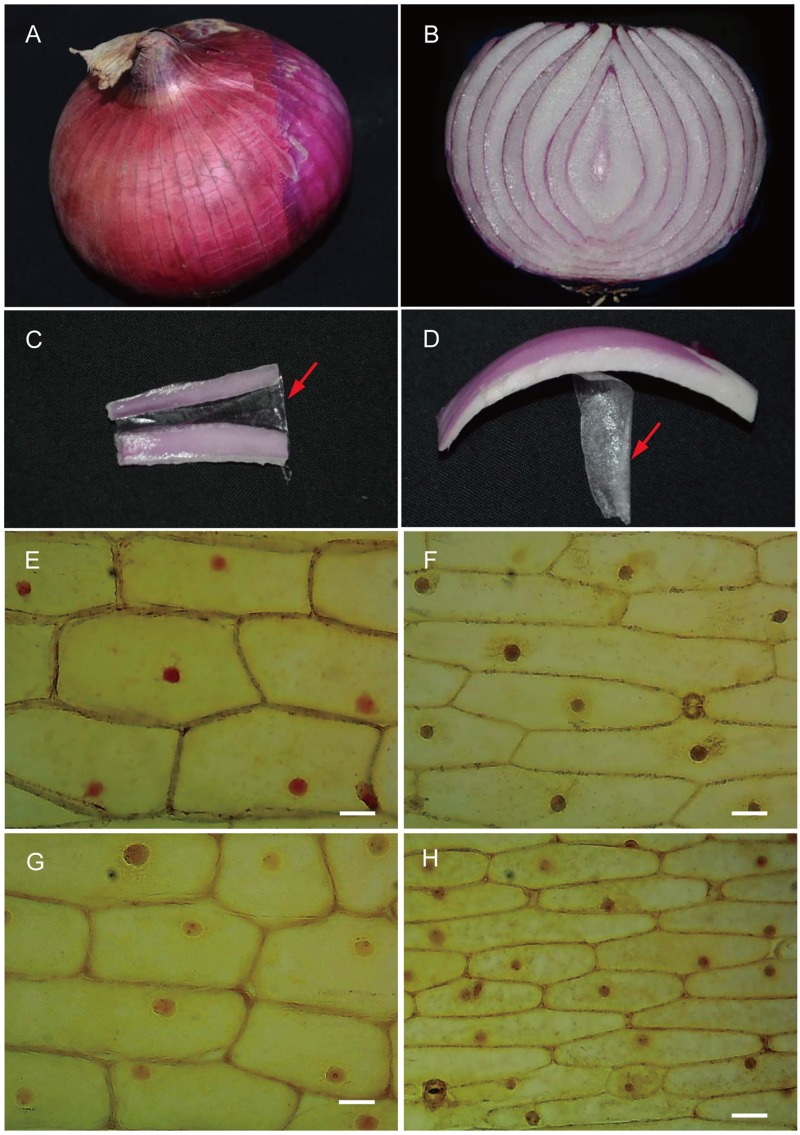
Fig 1. The epidermises of onion scales.
(A) Red onion bulb. B, Longitudinal section of a red onion scale. (C) The lower epidermis (LE) (arrow). (D) The upper epidermis (UE) (arrow). (E-H) Light microscopy of the LE (F and H) and UE (E and G) from a yellow onion scale (E and F) and a red onion scale (G and H). The bar = 2.0 μm.
Microscopic observation
Onion scale epidermises were stained with I2-KI and mounted onto temporary slides in Milli-Q water for light microscopy observation. The stained specimens were observed using a Phoenix PH50 microscope fitted with 4X lenses and were recorded and digitized using ToupView x86 software.
Pigment assay
Onion scale epidermises were ground to fine powder in liquid nitrogen, and homogenized in a mortar in distilled water (1 g/10 ml). The crude extract was shaken for 3–4 h [14] and then clarified through centrifugation. The absorbance spectra of pigment extracts were analyzed using a spectrophotometer UV-2550 (Shimadzu, Japan). The absorbance spectra were further recorded after titrating with 1 N HCl or 1 N NaOH.
Protein extraction and quantitation
Onion scale epidermises (ca. 1.0 g) were ground to fine powder in liquid nitrogen. Protein was extracted using the extraction solution (0.1 M Tris-HCl, pH 8.8, 5 mM DTT and 2 mM PMSF, 1.0 g/5 ml), and then subjected to phenol extraction as described previously [15]. For protein assay, protein precipitates were dissolved in a 2DE rehydration buffer without the IPG buffer to avoid its interference [16]. Protein concentrations were determined using Bradford method with bovine serum albumin as a standard.
2-DE and MS/MS
Approximately 800 μg of proteins were loaded into 11 cm linear pH 4–7 IPG strips in the Ettan III system (GE Healthcare, USA). 2-DE was performed as described before [17]. Digital images of the gels were analyzed using PDQuest 8.0 software (Bio-Rad, USA). The gels from three independent replicates were analyzed. Protein spots with at least 2-fold abundance change between the epidermises were excised from the gels, then reduced (10 mM DTT), alkylated (50 mM iodoacetic acid) and subsequently digested with 10 mg/ml of trypsin for 16 h at 37°C in 50 mM ammonium bicarbonate. The supernatants were vacuum-dried and dissolved in 5 μl 0.1% TFA followed by mixing in 1:1 ratio with a matrix consisting of a saturated solution of α-cyano-4-hydroxy-trans-cinnamic acid in 50% CAN and 1% TFA. The digested fragments were analyzed using a MALDI-TOF/TOF analyzer (Applied Biosystems, Foster City, USA). The MS/MS spectra were acquired in the positive ion mode and automatically submitted to Mascot 2.3 search engine (Matrix Science Ltd., London, U.K.) for peptide mass finger printing against the NCBInr 20150320 database (species, onion; protein database, 21872 sequences; EST database, 121224 sequences); type of search, MALDI-TOF ion search; enzyme, trypsin; variable modifications, acetyl (protein N-term), deamidated (NQ), dioxidation (W) and oxidation (M); fixed modifications, carbamidomethyl (C); mass values monoisotopic; protein mass, unrestricted; peptide mass tolerance, 100 ppm; fragment mass tolerance, 0.5 Da; max missed cleavages, 1; and instrument type, MALDI-TOF-TOF. Only significant scores greater than ‘identity’ defined through the probability analysis were considered for assigning protein identity. All of the positive protein identification scores were significant (p<0.05, score>32).
Bioinformatic analysis
By searching the key word “Allium cepa”, 1049 protein entries and 20204 ESTs entries were retrieved in the NCBInr database (version 20160428), and 364 onion protein entries retrieved in the UniProtKB database (version 20160428). This sequence information, together with homologous protein sequences from related monocotyledons (e.g., lily), allow for the successful identification of onion proteins by MS/MS [13]. Moreover, a BLASTX search can be applied to search for homologous proteins corresponding to onion ESTs from monocotyledons, such as liliaceous plants, maize, or rice.
Enzyme activity assay
The fresh onion epidermises were ground with 15 mM sodium acetate (pH 5.0, 0.2 g/1.0 ml). The homogenate was centrifuged at 12,000 g for 10 min. The supernatant was used for β-1,3-glucanase activity assay [18]. One unit of β-1,3-glucanase activity was defined as the concentration of the enzyme that produced 1 μmol glucose equivalents.
Semi-quantitative RT-PCR
The mRNA sequences of the onion chalcone-flavanone isomerase (CHI, AY541034.1) and actin (GU570135.2) were retrieved in the NCBI database. RNA was extracted from the epidermises of onion scales using TRIzol reagent (CW Biotech, China). cDNA was made with a HiFi-script first strand cDNA synthesis kit (CW Biotech, China). Primer sequences were actinF, AGAGCAGTATTCCCAAGC; actinR, TCTTCAGGAGCAACACGA; CHIF, TTTACGGCAATCGGCATCTA; CHIR, GCTTAGC AGCAGGTGATACA.
Statistical analysis
The results of the microscopic observation and enzyme assay were the means of three replicates. The mean values were compared by one-way analysis of variance with post hoc multiple comparisons using Fisher's least significant difference test (p < 0.05).
Results and Discussion
Comparative proteomic analysis of onion epidermises
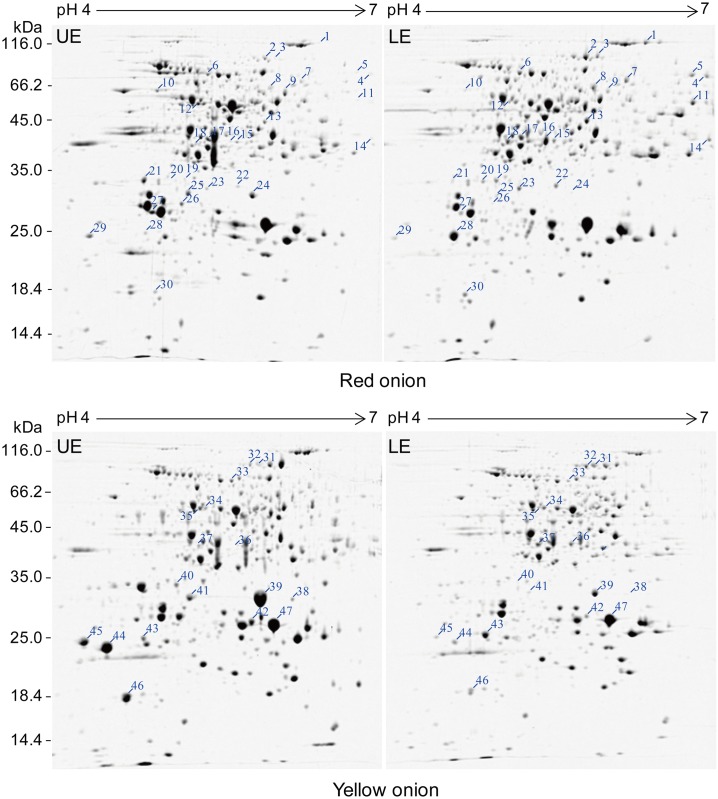
The protein yield in UE was higher than that in LE, and in red onion scale was higher than that in yellow one (Table 1). In 2-DE experiments, approximately 700–850 CBB-stained protein spots were detected in a range of pH 4–7 and 15–95 kDa, for both onions (Fig 2, Table 1, S1 Fig).
Table 1. Protein yield and 2-DE resolved spot number of epidermises in red and yellow onion scales.
| Onion types | Protein yield (mg/g dry weight) | Spot number (match rate)a | ||
|---|---|---|---|---|
| UE | LE | UE | LE | |
| Red | 5.91 ± 0.11 | 5.02 ± 0.19 | 764 ± 9 (66 ± 6%) | 845 ± 12 (98 ± 2%) |
| Yellow | 5.62 ± 0.06 | 4.78 ± 0.06 | 703 ± 18 (45 ± 4%) | 742 ± 10 (51 ± 5%) |
a Spot number and match rate were detected using automatic spot detection module of PDQuest software (Bio-Rad), using the gel image of LE of red onion scale as the master gel.
Fig 2. 2-DE detection of differentially abundant proteins between LE and UE in onion scales.
CBB-stained gels from two independent experiments. Differential spots with at least two-fold changes in volume are indicated.
A total of 62 CBB-stained protein spots in 2DE gels were detected with at least 2-fold abundance change between LE and UE in two types of onions from three biological replicates and selected for MS/MS identification. As a result, 47 protein spots were successfully identified (S1 File) and classified into 31 unique proteins, including 30 spots from red onion and 17 spots from yellow onion (Table 2). Among them, 4 spots (spots 9, 27, 28 and 43) were matched with distinct onion proteins, and 43 spots (27 in red onion and 16 in yellow onion) were matched with onion ESTs. The identified proteins were further characterized according to their homologs (in monocots, especially the lily family) in UniProtKB. All 47 protein spots were matched with their homologs at > 80% similarities. The identified proteins were classified into six categories according to the biological process involved (Table 3). Regarding subcellular location, 40% of the identified proteins existed in the cytoplasm, approximately 30% in the vacuole and extracellular space, 20% in plastids, and 10% in other compartments.
Table 2. The MS/MS identification of differentially abundance proteins between UE and LE in onion scales.
| Spot | Ratio a | NCBI accession | Protein name | Theorial Mr/ pI | Mascot Score | Coverage (%) | Matched peptides |
|---|---|---|---|---|---|---|---|
| Red scale | |||||||
| 1 | -8.1 | CF449626 | Translation elongation factor EF-2 subunit | 93.92/6.00 | 395 | 26 | DGNEYLINLIDSPGHVDFSSEVTAALR; ITDGALVVVDCIEGVCVQTETVLR; CFLELQVDGEEAYQTFSR |
| 2 | -8.6 | CF446696 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase | 84.67/5.93 | 296 | 21 | EVEDLEAAGVTVIQIDEAALR; SEHAFYLDWAVHSFR; YGAGIGPGVYDIHSPR |
| 3 | -6.4 | CF444585 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase | 84.67/5.93 | 493 | 27 | KLNLPILPTTTIGSFPQTVELR; EVEDLEAAGVTVIQIDEAALR; GMLTGPVTILNWSFVR; SEHAFYLDWAVHSFR |
| 4 | -6.9 | CF448000 | Malic enzyme | 62.93/5.52 | 183 | 15 | VHDELLLAASEALANEVTQEHYEK; GLIYPPFTNIR |
| 5 | -7.6 | CF448000 | Malic enzyme | 62.93/5.52 | 201 | 19 | VHDELLLAASEALANEVTQEHYEK; GLIYPPFTNIR; VYELGLATR |
| 6 | -1.0 | CF449636 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | 62.29/5.47 | 178 | 16 | AVGPIVDGDAVVTINFR; ALEYEDFNMFDR; YLVTPPEIER |
| 7 | -2.5 | CF451841 | D-3-phosphoglycerate dehydrogenase | 64.40/5.89 | 123 | 21 | GLGMHVIAHDPYAPADR; GGVIDEDALVR; IFNDETFGK |
| 8 | -4.0 | CF437616 | D-3-phosphoglycerate dehydrogenase | 64.80/6.73 | 253 | 26 | EAQEGVAIEIAEAVVGALK; GLIEPISDTHINLVNADFTAK; VLLDGSPENPLETIR; FASAMSETGDIR |
| 9 | 1.5 | ADA85889 | ATPase alpha subunit | 55.12/6.11 | 329 | 25 | VYGLNEIQAGEMVEFASGVK; GIALNLENENVGIVVFGSDTAIK; MTNFYTNFQVDEIGR; DNSMHALIIYDDLSK; EAFPGDVFYLHSR; AVDSLVPIGR; TAIAIDTILNQK; AAELTTLLESR; VVSVGDGIAR |
| 10 | 1.9 | CF437834 | Vacuolar ATP synthase subunit B | 54.06/5.07 | 245 | 26 | AVVGEEALSSEDLLYLEFLDKFER; KFVTQGAYDTR; TLDSFYSR |
| 11 | -10.2 | BQ580030 | Serine hydroxymethyltransferase | 51.63/6.87 | 145 | 21 | YYGGNEFIDQIENMCR;VSLETGYIDYDKMEEK; YSEGMPGNR |
| 12 | 1.8 | ES449575 | Enolase | 48.13/5.59 | 222 | 26 | GNPTVEADCHLSDGTLAR; AAVPSGASTGVYEALELR |
| 13 | -1.4 | CF438937 | GDP-mannose 3', 5'-epimerase 1 | 42.78/5.74 | 221 | 24 | VVGTQAPVQLGSLR; SFTFIDECVEGVLR; KLPIHHIPGPEGVR; EKAPAAFCR; ITYFWIK |
| 14 | -3.1 | CF443678 | Formate dehydrogenase 1 | 41.45/6.87 | 220 | 28 | AAAEAGLTVAEVTGSNTVSVAEDELMR; ANEYAAMNPNFVGCVEGSLGIR; NFLPGYHQVVNGEWNVAAIAHR |
| 15 | -1.3 | CF441553 | Glutamine synthetase root isozyme 5 | 39.26/5.52 | 100 | 20 | GGNNILVMCDCYTPAGEPIPTNHR; IIAEYIWIGGSGMDIR; AAEIFSHPDVVK |
| 16 | -7.1 | CF443460 | Trans-resveratrol di-O-methyltransferase-like | 40.27/5.38 | 336 | 32 | ELHNAQTHLWNILYNFMNSQTLK; SEQPSTAFEMYHGLYYWDATAK; WVLMPWSDEECVK; VILEDCADQFEGVK; CTLFELPHVIDAIEK |
| 17 | -1.0 | CF444706 | Flavone O-methyltransferase 1-like | 39.61/5.62 | 289 | 27 | AGPNAHLSPSQIADQLPTENPQAPVMIDR; AYGMSAFEYHGTDPR; AAIELDLFEIIKK; ILMESWYYLK |
| 18 | -3.4 | CF448455 | UDP-arabinopyranose mutase 1-like | 41.12/5.67 | 97 | 15 | NLLSPSTPYFFNTLYDPYTEGADFVR; VINVPEGFDYELYNR |
| 19 | -4.0 | CF437496 | Momilactone A synthase-like | 27.92/5.73 | 282 | 37 | FGHLDIMFNNAGITGPAIPDTTSYPLIDFKR; LFIQHGAQIVVADVQDK; VALITGGASGIGER; VIDINVTGPFLGIK |
| 20 | -5.5 | CF437496 | Momilactone A synthase-lik | 27.92/5.73 | 118 | 13 | VALITGGASGIGER; VIDINVTGPFLGIK |
| 21 | 5.7 | CF435027 | Chitinase, partial | 31.73/5.18 | 143 | 12 | QEQGNPPDYCQPSTQYPCAPGKK |
| 22 | -1.7 | CF437643 | Chitinase | 33.58/4.88 | 176 | 18 | GFYTYDAFIAAANSFIGFGTTGDTDTR; WSPSAADIGANR; NENSCPAR |
| 23 | -2.1 | CF437496 | Momilactone A synthase-like | 27.92/5.73 | 446 | 37 | FGHLDIMFNNAGITGPAIPDTTSYPLIDFKR; LFIQHGAQIVVADVQDK; VALITGGASGIGER; VIDINVTGPFLGIK |
| 24 | 10.2 | CF440559 | Beta-1,3-glucanase | 35.40/4.26 | 87 | 26 | VSTAVSLAVLGPSYPPSMGAFTSDAAQYLQPIVK; NNIQNYPSVSFK; SNSNFNGVR |
| 25 | 6.3 | CF445999 | Beta-1,3-glucanase | 35.40/4.26 | 118 | 24 | IVVSESGWPSAGGFAATVGNAQTYNTNLVNHVGK;GQGIESNFGLYYPNK |
| 26 | 3.5 | CF438948 | Salicylic acid-binding protein 2-like | 29.35/5.46 | 267 | 45 | RIEEVGTFSEYSQPLLNIMSSLPPNEK; TGLLYIEELSETPAFSK; VTVPDLAASGIDSR; CPEDLAITEDIQEK; LTAILFGPNFFASR; VAHVLESLGHR; EDIMLASTLVR; ENYGSIPR |
| 27 | 2.3 | BAC21275 | lachrymatory factor synthase | 19.33/5.02 | 33 | 11 | MELNPGAPAVVADSANGAR |
| 28 | -4.6 | AAS48418 | Chalcone isomerase | 24.27/5.10 | 541 | 58 | ALTQAVLESIIGEHGVSPAAK; LDVEGTAFDSVIIPPGSSK; FTNVTMILPLTGEQYSEK; AIGIYTDAEASAVDKFK; TGEELAGSLDFFR; VTENCVAYWK; THFLGGAGVR |
| 29 | 2.9 | CF450045 | Pathogenesis-related protein 5 | 23.53/4.98 | 318 | 27 | QLNSGDSWTVTANAGTTGGR; TDEYCCNSGSCGPTDFSR; CTADVNGQCPAQLR; AAGGCNNPCTVFK |
| 30 | -1.5 | CF438143 | Peroxiredoxin-5 | 17.32/4.85 | 349 | 45 | VILFGVPGAFTPTCSMQHVPGFISSAEVLK; VANIEQGGEFTISGAEEILK; AKGVDEILLVSVNDPFVMK; YTQELGLELDLTEK; FALLADDLKVK; RFALLADDLK |
| Yellow scale | |||||||
| 31 | -3.9 | CF444585 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase | 84.66/5.93 | 516 | 27 | LNLPILPTTTIGSFPQTVELR; EVEDLEAAGVTVIQIDEAALR; LQEELDIDVLVHGEPER; SEHAFYLDWAVHSFR |
| 32 | -2.3 | CF444585 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase | 84.66/5.93 | 532 | 27 | KLNLPILPTTTIGSFPQTVELR; EVEDLEAAGVTVIQIDEAALR; LQEELDIDVLVHGEPER; SEHAFYLDWAVHSFR |
| 33 | -1.6 | CF449636 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | 60.29/5.47 | 239 | 23 | NSDQYLPPFVIVDESGK; AVGPIVDGDAVVTINFR; ALEYEDFNMFDR; YLVTPPEIER |
| 34 | -1.9 | CF445718 | Enolase1 | 40.08/5.27 | 256 | 18 | YGQDATNVGDEGGFAPNIQENKEGLELLK; SFVADYPIVSIEDPFDQDDWTTYAK; LAMQEFMILPTGASSFK |
| 35 | 1.6 | ES449575 | Enolase | 48.13/5.59 | 179 | 26 | AAVPSGASTGVYEALELR; GNPTVEADCHLSDGTLAR |
| 36 | -8.2 | CF443460 | Trans-resveratrol di-O-methyltransferase-like | 40.27/5.38 | 122 | 19 | SEQPSTAFEMYHGLYYWDATAK; VILEDCADQFEGVK; CTLFELPHVIDAIEK |
| 37 | -2.5 | BQ580186 | Protein disulfide isomerase-like 2–2 | 39.82/5.67 | 116 | 33 | SEEDVVIANLDADNYKDLAEK; AGEDYDGGREVDDFVTFINEK; GQLTSQAGVVASLDTIVK; EVDDFVTFINEK; YGVSGYPTLK |
| 38 | 2.5 | CF444510 | Hydroxyacylglutathione hydrolasecytoplasmic -like isoform X1 | 28.59/5.55 | 64 | 12 | EGEDPAVFTGDTLFVAGCGK; FAATVEPENEK; VFCGHEYTEK; GHISYYVTSK |
| 39 | 11.5 | CF445999 | Beta-1,3-glucanase | 35.40/4.26 | 247 | 26 | IVVSESGWPSAGGFAATVGNAQTYNTNLVNHVGK; GQGIESNFGLYYPNKQPVYSI |
| 40 | 3.9 | CF435027 | Chitinase | 31.73/5.18 | 98 | 28 | GFYTYDAFIAAANSFSGFGTTGDTNTQKR; QEQGNPPDYCQPSTQYPCAPGKK |
| 41 | 7.2 | CF445999 | Beta-1,3-glucanase | 35.40/4.26 | 90 | 26 | IVVSESGWPSAGGFAATVGNAQTYNTNLVNHVGK; GQGIESNFGLYYPNKQPVYSI |
| 42 | 1.6 | CF444732 | Thaumatin-like protein 1b | 33.37/5.20 | 218 | 23 | FSCTSGDCGTGQVACNNAGGR; SACEAFNTDEYCCR; SWGTGGYLAGCK; AIGCYVDINAR |
| 43 | -2.5 | AAS48418 | Chalcone isomerase | 24.27/5.10 | 558 | 51 | LDVEGTAFDSVIIPPGSSKTHFLGGAGVR |
| 44 | 12.8 | CF450045 | Pathogenesis-related protein 5 | 23.53/4.98 | 409 | 27 | QLNSGDSWTVTANAGTTGGR; TDEYCCNSGSCGPTDFSR; CTADVNGQCPAQLR; AAGGCNNPCTVFK |
| 45 | 3.1 | CF450045 | Pathogenesis-related protein 5 | 23.53/4.98 | 362 | 21 | QLNSGDSWTVTANAGTTGGR; TDEYCCNSGSCGPTDFSR; CTADVNGQCPAQLR |
| 46 | 3.7 | CF443478 | Intracellular pathogenesis-related protein | 16.90/6.51 | 139 | 9 | AAPEVIASSTVLSGQGEVGSIR |
| 47 | -2.4 | CF446666 | Tau glutathione S-transferase | 25.69/6.24 | 127 | 7 | FWANYVDSKVWDAGANIWK |
a. Log2 ratio of relative abundance (UE/LE), Log2 ratio > 1 represents abundance change more than two folds.
Table 3. The differential proteins identified in the epidermises in red onion (spots 1–30) and yellow onion (spots 31–47).
| Spot | Homolog proteins(Plant species) | Accession a | Location b | Molecular function |
|---|---|---|---|---|
| Proteins in increased abundance in LE | ||||
| Pigment biosynthesis | ||||
| 17 | Flavone O-methyltransferase 1-like (Musa acuminata) | M0SIB3 | *Plastid | O-methyltransferase activity |
| 28 | Chalcone-flavanone isomerase (Allium cepa) | Q6QHK0 | Tonoplast, Nucleus, ER | Secondary metabolite (e.g., flavonoids) biosynthesis |
| 43 | Chalcone-flavanone isomerase (A. cepa) | Q6QHK0 | Tonoplast, Nucleus, ER | Secondary metabolite (e.g., flavonoids) biosynthesis |
| Stress response | ||||
| 13 | GDP-mannose 3',5'-epimerase 1 (Oryza sativa) | A3C4S4 | *Cytoplasm | GDP-mannose 3,5-epimerase activity, coenzyme binding |
| 14 | Formate dehydrogenase 1 (O. brachyantha) | J3ME94 | Plastid, Thylakoid | Oxidoreductase activity, NAD binding |
| 16 | Trans-resveratrol di-O-methyltransferase-like (Elaeis guineensis) | UPI00057A5A6F | *Cytoplasm, Plastid, Mitochondrion | Trans-resveratrol di-O-methyltransferase |
| 19, 20, 23 | Momilactone A synthase-like (Zea mays) | A0A096R062 | *Plastid | Oxidoreductase activity |
| 22 | Chitinase (Allium sativum) | Q38777 | *Extracellular space, Vacuole | Chitinase activity, chitin binding |
| 30 | Peroxiredoxin-5 (Z. mays) | B4FN24 | *Cytoplasm | Oxidoreductase activity |
| 36 | Trans-resveratrol di-O-methyltransferase-like (E. guineensis) | UPI00057A5A6F | *Cytoplasm, Plastid, Mitochondrion | Trans-resveratrol di-O-methyltransferase |
| 47 | Tau glutathione S-transferase (A. cepa) | F2ZC01 | *Cytoplasm | Transferase activity |
| Carbohydrate metabolism | ||||
| 4, 5 | Malic enzyme (O. sativa) | Q6T5D1 | *Cytoplasm, Plastid | Malate dehydrogenase (NAD+) (decarboxylating) activity, metal ion binding |
| 6 | 2,3-bisphosphoglycerat independent phosphoglycerate mutase (Z. mays) | C0HHU2 | Cytoplasm | Phosphoglycerate mutase activity, Mg2+ binding |
| 33 | 2,3-bisphosphoglycerat independent phosphoglycerate mutase (Z. mays) | C0HHU2 | Cytoplasm | Phosphoglycerate mutase activity, Mg2+ binding |
| 34 | Enolase1 (Z. mays) | K7V794 | Phosphopyruvate hydratase complex | Phosphopyruvate hydratase activity, Mg2+ binding |
| Cell division | ||||
| 18 | UDP-arabinopyranose mutase 1-like (O. brachyantha) | J3LQM6 | Golgi apparatus, Cytoplasm | Intramolecular transferase activity |
| Protein synthesis | ||||
| 1 | Translation elongation factor EF-2 subunit (Z. mays) | B6U0S1 | *Cytoplasm | GTPase activity, translation elongation factor activity, nucleotide binding |
| 2, 3 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase 2 (O. sativa) | Q2QLY4 | *Cytosol | 5-methyltetrahydropteroyltriglutamate-homocysteine S-methyltransferase activity, Zn2+ binding |
| 7 | D-3-phosphoglycerate dehydrogenase (O. sativa) | A3BE72 | *Plastid | Amino acid binding, NAD binding, phosphoglycerate dehydrogenase activity |
| 8 | D-3-phosphoglycerate dehydrogenase (Z. mays) | A0A096R8F6 | *Plastid | Amino acid binding, NAD binding, phosphoglycerate dehydrogenase activity |
| 11 | Serine hydroxymethyltransferase (O. brachyantha) | J3N865 | *Plasmodesma Cytoplasm, Membrane | Pyridoxal phosphate binding, glycine hydroxymethyltransferase activity |
| 15 | Glutamine synthetase root isozyme 5 (Z. mays) | P38563 | Cytoplasm | ATP binding, glutamate ammonia ligase activity |
| 31, 32 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase 2 (O. sativa) | Q2QLY4 | Cytosol | 5-methyltetrahydropteroyltriglutamate-homocysteine S-methyltransferase activity, Zn2+ binding |
| 37 | Protein disulfide isomerase like 2–2 (M. acuminata) | M0RS28 | ER | Isomerase activity |
| Proteins in increased abundance in UE | ||||
| Stress response | ||||
| 21 | Chitinase, partial (A. sativum) | Q38776 | *Vacuole, Extracellular space | Chitinase activity, chitin binding |
| 24, 25 | Beta-1,3-glucanase (Lilium) | Q1W6B9 | *Extracellular space | Hydrolase activity, hydrolyzing O-glycosyl compounds |
| 26 | Salicylic acid-binding protein 2-like (E. guineensis) | UPI00057B4D46 | *Plastid | Lipase activity, methyl salicylate esterase activity |
| 29 | Pathogenesis-related protein 5(A. sativum) | A0A0K1L9H1 | *Vacuole | Pathogenesis-related protein, plant defense |
| 38 | Hydroxyacylglutathione hydrolase cytoplasmic-like isoform X1 (O. brachyantha) | J3LNG8 | Cytosol | Hydroxyacylglutathione hydrolase activity |
| 39, 41 | Beta-1,3-glucanase (Lilium) | Q1W6B9 | *Extracellular space | Hydrolase activity, hydrolyzing O-glycosyl compounds |
| 40 | Chitinase (A. sativum) | Q38777 | *Vacuole, Extracellular space | Chitinase activity, chitin binding |
| 42 | Thaumatin-like protein 1b (Z. mays) | A0A096QLD7 | *Vacuole | Allergenic/antifungal thaumatin-like proteins |
| 44, 45 | Pathogenesis-related protein 5 (A. sativum) | A0A0K1L9H1 | Vacuole | Pathogenesis-related protein, plant defense |
| 46 | Intracellular pathogenesis related protein (Asparagus officinalis) | Q9SB87 | *Cytoplasm | Pathogenesis-related protein |
| Carbohydrate metabolism | ||||
| 9 | ATP synthase subunit alpha (A. cepa) | D2XT90 | Mitochondrion | Proton-transporting ATPase activity, ATP binding |
| 10 | Vacuolar ATP synthase subunit B (Z. mays) | B6T9C0 | Vacuole | ATP binding, hydrolase activity |
| 12 | Enolase (Z. mays) | B6T3P9 | Phosphopyruvate hydratase complex | Phosphopyruvate hydratase activity, Mg2+ binding |
| 35 | Enolase (Z. mays) | B6T3P9 | Phosphopyruvate hydratase complex | Phosphopyruvate hydratase activity, Mg2+ binding |
| Others | ||||
| 27 | Lachrymatory factor synthase (A. cepa) | P59082 | Vacuole | Lachrymatory factor synthase activity |
a UniProtKB/UniRef accession;
b Subcellular localization of proteins annotated in UniProtKB/Swiss-Prot (http://www.uniprot.org/) except those with asterisk mark which were predicte d in online Plant-mPLoc server (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/).
Differential protein expression related to pigment synthesis in onion epidermises
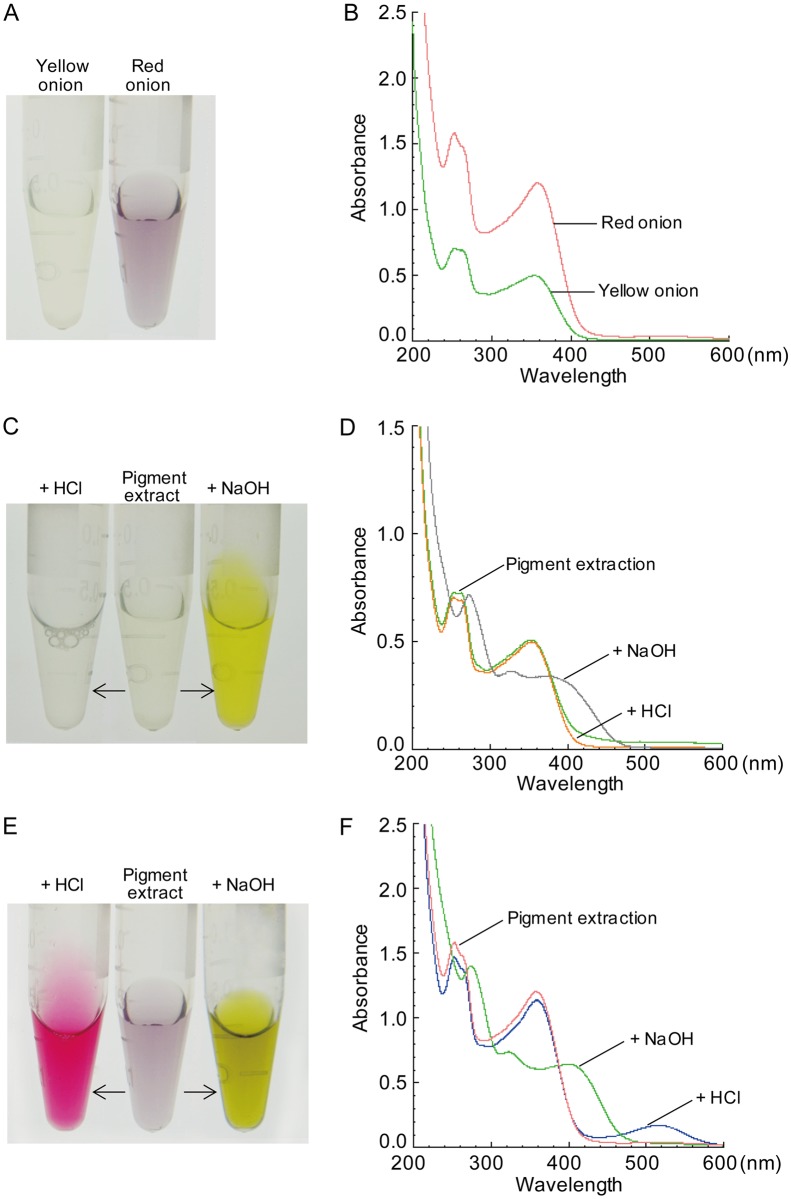
Color differences existed between LE and UE of onion scales, as well as between red and yellow onion scales (Fig 3A). Onion pigments (mainly flavonoids) can be dertemined using high performance liquid chromatography-mass spectrometry, thin-layer chromatography, capillary electrophoresis, spectrophotometry and so on [19, 20]. Here we selected a relatively quick and easy spectrophotometry to detect the main compositions of onion pigments.
Fig 3. Comparison of pigment abundance and absorbance spectra of LE extracts between red and yellow onions.
(A, B) The difference in color and spectra of pigment extracts of the LE between red and yellow onions. (C, D) Acid/base titration changed the color and spectra of LE pigment extracts from yellow onion scales. (E, F) Acid/base titration changed the color and spectra of LE pigment extracts from red onion scales.
The absorbance spectra of onion LE extracts were characteristic of two major peaks at 255 nm and 363 nm for red onion, and 253 nm and 360 nm for yellow onion (Fig 3B). The two peaks possibly corresponded to quercetin, which has a typical absorbance peak at 255 nm and 370 nm [21]. A previous study determined that quercetin is the main flavonoid in two onion scales [7], and trace amounts of another flavonoid, anthocyanin, were also found in red onions [6].
It has been reported that pH affected the absorption peaks of quercetin and anthocyanin [21]. Thus, the spectra of LE pigment extracts were scanned after acid/base titration. At pH < 7, the light yellow of yellow onion extract became colorless; at pH >7, the color of the extract became dark yellow (Fig 3C). At pH < 7, the light purple of red onion extract became red; at pH >7, the color of the red onion extract became yellow (Fig 3E). At pH < 7, red onion extract had an additional absorption peak at 520 nm (Fig 3F), which was identified as anthocyanin. However, there was no 520 nm peak in yellow onion extract at pH < 7 (Fig 3D). Moreover, there were no obvious absorption peaks in pigment extracts of the UE from both red and yellow onions between 200–900 nm (results not shown). Our results showed that quercetin was abundant in the LE of red and yellow onion scales while anthocyanin was found in LE of red onion scales.
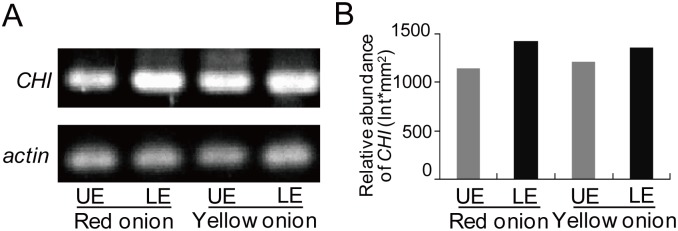
Comparative proteomic analysis revealed that two pigment synthesis-related proteins, chalcone-flavanone isomerase (CHI) (spots 28 and 43) and flavone O-methyltransferase 1-like (OMT1, spot 17), differentially accumulated between LE and UE (Table 3). CHI plays a part in the middle step of the flavonoid biosynthesis pathway by catalyzing the converse of chalcone into naringenin [22]. We checked the gene expression of CHI and found that it was up-regulated in LE compared with UE, especially in red onions (Fig 4), which was consistent with the abundance of this protein. The color difference between UE and LE and between red and yellow onions can be attributed to the differential accumulation of CHI, which increases flavonoid synthesis and the downstream production of quercetin and anthocyanin. OMT1, methylates 3’-OH residues in flavonoid compounds, such as quercetin, result in the formation of methoxy derivatives. Due to the effect of O-methylation on the solubility of flavonoids [23], highly abundant OMT1 in LE of red onions may contribute to the transformation of the tone of the onion by changing the solubility of flavonoids.
Fig 4. RT-PCR analysis of CHI expression in onion epidermises.
(A) Gels of RT-PCR. (B) Relative abundance of CHI PCR products.
Differential expression of stress-related proteins in onion epidermises
Both LE and UE of onion scales are the protective tissue, and play an important role in the process of various stress adaptation. In red onions, six stress-related proteins were more abundant in LE, whereas four were more abundant in UE (Table 3). Four stress-related proteins were the same between red and yellow onions, but showed abundance differences (Table 2).
The differentially accumulated proteins in LE of onions were previously proposed to resist various abiotic stresses (e.g., drought, cold, salt) [13, 24, 25] and biotic attacks (e.g., bacteria and fungi) [26, 27]. GDP-mannose 3',5'-epimerase 1 plays a protective role against reactive oxygen species, acid, drought and salt stresses in plants by regulating ascorbic acid biosynthesis [24]. In barley, formate dehydrogenase 1 responds to abiotic stresses (dark, chilling, drought, wounding) [28], and in pepper, it resists bacterial pathogens [26]. Momilactone A synthase-like and trans-resveratrol di-O-methyltransferase-like protect plants against pathogens by catalyzing the biosynthesis of plant phytoalexins, momilactone A [29] and pterostilbene [30]. Moreover, momilactone A synthase-like was induced to resist bacterial and fungal attacks in rice [31]. Chitinase, a member of pathogenesis related proteins (PRs), also enhanced disease resistance by degradation of chitin, which is the major component of fungal cell walls [27]. Peroxiredoxin-5 enhanced the resistance of abiotic and biotic stresses by catalyzing the decomposition of peroxides [32]. Tau glutathione S-transferase was found to participate in the freeze injury of onion scales [13], and the over-expressed tau glutathione S-transferase gene enhanced the resistance of transgenic tobacco to fluorodifen treatment, salt and drought [25]. Therefore, the above-mentioned seven highly abundant proteins in LE may contribute to the enhanced stress response of onion scales.
In the present study, such PRs as chitinase, β-1,3-glucanase and PR 5 were found to accumulate highly in UE of both onions but other PRs (e.g., intracellular PR and thaumatin-like protein 1b) were only in yellow onions (Table 3). Chitinase in red onions shown two species (spot 21 and 22) with a similar size but different pIs, which possibly results from post-translational modifications as found in tobacco [33]. Besides, PRs with diverse species were found to accumulate in UE of both onions. On one hand, PRs are known to protect plants against pathogens by attacking the molecules in the cell walls of bacteria or fungi or by spreading the ‘signal’ of the infection among plant cells [34]. PRs are known to promote the formation of local barricades in cell walls and slow the spread of pathogens in plants [35]. On the other hand, some PRs are also produced in response to abiotic stress. For instance, β-1,3-glucanase was up-regulated in response to aluminum toxicity [36], and thaumatin-like protein 1b enhanced plant tolerance to cold, drought, and salinity [37]. Salicylic acid-binding protein 2-like was reported to induce the production of PRs [38]. Hydroxyacylglutathione hydrolase cytoplasmic-like isoform X1 was up-regulated in response to abiotic stress in many plants [39]. For instance, an over-expression of the hydroxyacylglutathione hydrolase cytoplasmic-like isoform X1 gene in tobacco enhanced salt tolerance [40].
Epidermis differentiation and relevant differential proteins
Light microscopy showed that UE cells were larger (especially in width) than LE cells in both onions (Table 4). The ratio of length/width of LE cells in red onion was higher than that in yellow onion, and was also higher than that in UE cells of two onion types. Previously, the anisotropic expansion was found to be related to anisotropic cell wall structure and/or anisotropic stress distribution in the wall, suggesting cellulose microfibrils played an important role in anisotropic expansion [12]. Thus, the differences in cell size between UE and LE in both onion types may result from a combination of oriented cell divisions and anisotropic cell expansion.
Table 4. The average size of epidermis cells in red and yellow onion scales.
Values were means ± SD (n = 3).
| Cell Type | Length (μm) | Width (μm) | |
|---|---|---|---|
| Red onion: | LE | 29.28 ± 2.13 a | 6.20 ± 0.46C |
| UE | 31.69 ± 1.50 a | 13.19 ± 1.75A | |
| Yellow onion: | LE | 34.69 ± 3.77 a | 9.68 ± 1.90 B |
| UE | 35.38 ± 3.07 a | 13.96 ± 1.32A | |
Note: Values having different superscripts (lowercase or majuscule) are significantly different. Lowercase indicates p<0.05 and majuscule indicates p<0.01.
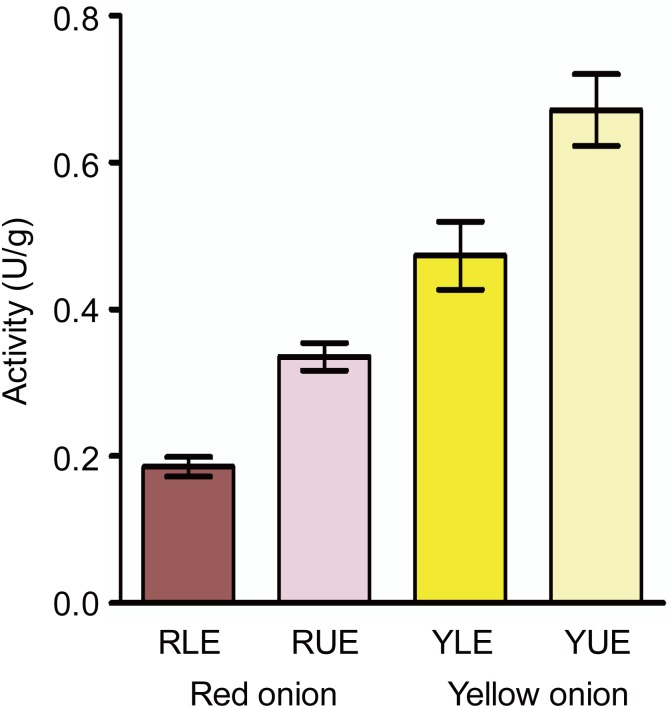
Comparative proteomic analysis revealed that two cell division related proteins, UDP-arabinopyranose mutase 1-like (UAM1, spot 18) and β-1,3-glucanase (spots 24, 25, 39 and 41), were differentially accumulated (Table 3). In the experiment of enzyme activity assay, the β-1,3-glucanase also showed a higher enzyme activity in UE than LE in both onions (Fig 5), which is consistent with the protein abundance. β-1,3-glucanase plays a vital role not only in defense mechanism of plants against pathogen infection, but in the growth and development of plants [41]. It catalyzes the decomposition of callose, which takes part in forming the cell-plate during cell division [42]. UAM1 participates in the essential reaction needed for the establishment of cell walls in plants [43]. The protein catalyzes the conversion of UDP-L-arabinopyranose to UDP-L-arabinofuranose. In Arabidopsis, non-expression of UAM1 causes several developmental retardation and dwarf phenotypes, and UAM knockout mutants produce approximately a 10% to 30% reduction of the monosaccharide content of the cell wall, which further restricts the formation of cell wall polysaccharide components [44]. Similarly, the differentially accumulated β-1,3-glucanase and UAM1 proteins in LE of red onion scales may result in smaller LE cells compared with LE cells of yellow onions and UE cells of both onions (Fig 1E–1H).
Fig 5. Comparison of β-1,3-glucanase activity in epidermises between red and yellow onions.
The error bar indicates the standard deviation.
In addition, 13 differential proteins were involved in protein synthesis and carbohydrate metabolic processes, of which only 2,3-bisphosphoglycerat independent phosphoglycerate mutase and enolase existed in both onions with a similar abundance change (Table 3).
Conclusions
In summary, comparative proteomic analysis in this study revealed that the proteome of UE and LE of onion scales were different. The differential proteins identified in the present study mainly participated in pigment synthesis (e.g., OMT1 and CHI), stress response (e.g., PRs), cell division (e.g., β-1,3-glucanase and UAM1), protein synthesis and carbohydrate metabolism. In addition, the differential proteins involved in cell division and stress response are worth to be further studied so as to reveal the mechanism of the differentiation and development of onion epidermis. The data derived from this study provides new insight into the differences in differentiation and developmental processes between onion epidermises, and may make a contribution to onion breeding, such as improving resistance and changing colors.
Supporting Information
CBB-stained gels from two independent experiments. Differential spots with at least two-fold changes in volume are indicated. A, B and C represent three independent replicates.
(PPT)
(RAR)
Acknowledgments
We acknowledge the financial support of the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (Grant No. 15IRTSTHN015).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We acknowledge the financial support of the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (grant no. 15IRTSTHN015) (http://www.haedu.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Suleria HA, Butt MS, Anjum FM, Saeed F, Khalid N. Onion: Nature protection against physiological threats. Crit Rev Food Sci. 2015; 55, 50–66. 10.1080/10408398.2011.646364 [DOI] [PubMed] [Google Scholar]
- 2.Galletti R, Johnson KL, Scofield S, San-Bento R, Watt AM, Murray JA, et al. DEFECTIVE KERNEL 1 promotes and maintains plant epidermal differentiation. Development. 2015; 142(11): 1978–83. 10.1242/dev.122325 [DOI] [PubMed] [Google Scholar]
- 3.San-Bento R, Farcot E, Galletti R, Creff A, Ingram G. Epidermal identity is maintained by cell-cell communication via a universally active feedback loop in Arabidopsis thaliana. Plant J. 2014; 77(1):46–58. 10.1111/tpj.12360 [DOI] [PubMed] [Google Scholar]
- 4.Glover BJ. Differentiation in plant epidermal cells. J Exp Bot. 2000; 51(344):497–505. [DOI] [PubMed] [Google Scholar]
- 5.Galletti R, Ingram GC. Communication is key: Reducing DEK1 activity reveals a link between cell-cell contacts and epidermal cell differentiation status. Commun Integr Biol. 2015; 29;8(5):e1059979 10.1080/19420889.2015.1059979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fossen T, Andersen ØM, Øvstedal DO, Pedersen AT, Raknes Å. Characteristic anthocyanin pattern from onions and other Allium spp. J Food Sci. 1996; 61, 703–706. 10.1111/j.1365-2621.1996.tb12185.x [DOI] [Google Scholar]
- 7.Rhodes MJC, Price KR. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 1996; 57, 113–117. 10.1016/0308-8146(96)00147-1 [DOI] [Google Scholar]
- 8.Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J. Agric. Food Chem. 2007; 55, 10067–10080. 10.1021/jf0712503 [DOI] [PubMed] [Google Scholar]
- 9.Kumar J, D'Souza SF. Immobilization of microbial cells on inner epidermis of onion bulb scale for biosensor application. Biosens Bioelectron. 2011; 26, 4399–4404. 10.1016/j.bios.2011.04.049 [DOI] [PubMed] [Google Scholar]
- 10.Xu KD, Huang XH, Wu MM, Wang Y, Chang YX, Liu K, et al. A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis. PLOS ONE. 2014; 9(1): e83556 10.1371/journal.pone.0083556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong XY, Zhao YY, Cai SQ, Fu SJ, Yang CD, Zhang SC, et al. Single cell analysis with probe ESI-mass spectrometry: detection of metabolites at cellular and subcellular levels. Anal Chem. 2014; 86, 3809–3816. 10.1021/ac500882e [DOI] [PubMed] [Google Scholar]
- 12.Suslov D, Verbelen JP. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. J Exp Bot. 2006; 57, 2183–2192. 10.1093/jxb/erj177 [DOI] [PubMed] [Google Scholar]
- 13.Chen K, Renaut J, Sergeant K, Wei H, Arora R. Proteomic changes associated with freeze-thaw injury and post-thaw recovery in onion (Allium cepa L.) scales. Plant Cell Environ. 2013; 36, 892–905. 10.1111/pce.12027 [DOI] [PubMed] [Google Scholar]
- 14.Bae SE. A study of onion skin pigments in the extracting solvents and residual pigments after dyeing the textiles. J Fashion Bus. 2009; 13, 109–117. [Google Scholar]
- 15.Wu X, Xiong E, Wang W, Scal M, Cresti M. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nat Protoc. 2014; 9: 362–374. 10.1038/nprot.2014.022 [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Gong F, Yang L, Hu X, Tai F, Wang W. Proteomic analysis reveals differential accumulation of small heat shock proteins and late embryogenesis abundant proteins between ABA-deficient mutant vp5 seeds and wild-type Vp5 seeds in maize. Front Plant Sci. 2015; 5:801 10.3389/fpls.2014.00801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959; 31(3), 426–428. 10.1021/ac60147a030 [DOI] [Google Scholar]
- 19.Glover BJ. Differentiation in plant epidermal cells. J Exp Bot. 2000; 51(344):497–505. [DOI] [PubMed] [Google Scholar]
- 20.Dmitrienko SG, Kudrinskaya VA, Apyari VV. Methods of extraction, preconcentration, and determination of quercetin. Journal of Analytical Chemistry. 2013; 67(4): 299–311. 10.1134/S106193481204003X [DOI] [Google Scholar]
- 21.Mabry TJ, Markham KR, Thomas MB. The systematic identification of flavonoids. Springer-Verlag; 1970. [Google Scholar]
- 22.Kim S, Jones R, Yoo KS, Pike LM. Gold color in onions (Allium cepa): a natural mutation of the chalcone-flavanone isomerasegene resulting in a premature stop codon. Mol Genet Genomics. 2004; 272, 411–419. 10.1007/s00438-004-1076-7 [DOI] [PubMed] [Google Scholar]
- 23.Kim BG, Lee Y, Hur HG, Lim Y, Ahn JH. Flavonoid 3'-O-methyltransferase from rice: cDNA cloning, characterization and functional expression. Phytochemistry. 2006: 67, 387–394. 10.1016/j.phytochem.2005.11.022 [DOI] [PubMed] [Google Scholar]
- 24.Ma LC,Wang YR,Liu WX, Liu ZP. Overexpression of an alfalfa GDP-mannose 3, 5-epimerase gene enhances acid, drought and salt tolerance in transgenic Arabidopsis by increasing ascorbate accumulation. Biotechnol Lett. 2014; 36, 2331–2341. 10.1007/s10529-014-1598-y [DOI] [PubMed] [Google Scholar]
- 25.Lo Cicero L, Madesis P, Tsaftaris A, Lo Piero AR. Tobacco plants over-expressing the sweet orange tau glutathione transferases (CsGSTUs) acquire tolerance to the diphenyl ether herbicide fluorodifen and to salt and drought stresses. Phytochem. 2015; 116, 69–77. 10.1016/j.phytochem.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Choi DS, Kim NH, Hwang BK. Pepper mitochondrial FORMATE DEHYDROGENASE1 regulates cell death and defense responses against bacterial pathogens. Plant Physiol. 2014; 166, 1298–1311. 10.1104/pp.114.246736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cletus J, Balasubramanian V, Vashisht D, Sakthivel N. Transgenic expression of plant chitinases to enhance disease resistance. Biotechnol Lett. 2013; 35, 1719–1732. doi: 10. 1007/s10529-013-1269-4 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Itai R, Suzuki K, Nakanishi H, Nishizawa NK, Yoshimura E, et al. Formate dehydrogenase, an enzyme of anaerobic metabolism, is induced by iron deficiency in barley roots. Plant Physiol. 1998; 116, 725–732. 10.1007/s10529-013-1269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozaki H, Hayashi K, Nishimura N, Kawaide H, Matsuo A, Takaoka D. Momilactone A and B as allelochemicals from moss Hypnum plumaeforme: first occurrence in bryophytes. Biosci Biotech Biochem. 2007; 71, 3127–3130. 10.1271/bbb.70625 [DOI] [PubMed] [Google Scholar]
- 30.Schmidlin L, Poutaraud A, Claudel P, Mestre P, Prado E, Santos-Rosa M, et al. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008; 148, 1630–1639. 10.1104/pp.108.126003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamogami S., and Kodama O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry. 2000; 54, 689–694. 10.1016/S0031-9422(00)00190-4 [DOI] [PubMed] [Google Scholar]
- 32.Bhatt I, Tripathi BN. Plant peroxiredoxins: catalytic mechanisms, functional significance and future perspectives. Biotechnol Adv. 2011; 29, 850–859. 10.1016/j.biotechadv.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 33.Kunze I, Nilsson C, Adler K, Manteuffel R, Horstmann C, Bröker M, et al. Correct targeting of a vacuolar tobacco chitinase in Saccharomy cescerevisiae post-translational modifications are dependent on the host strain. Biochim Biophys Acta. 1998; 1395, 329–344. 10.1016/S0167-4781(97)00163-2 [DOI] [PubMed] [Google Scholar]
- 34.van Loon LC. Pathogenesis-related proteins. Plant Mol Biol. 1985; 4: 111–116. 10.1007/BF02418757 [DOI] [PubMed] [Google Scholar]
- 35.Campbell NA, Reece JB. Biology. Benjamin Cummings; 2005. [Google Scholar]
- 36.Zhang H, Shi WL, You JF, Bian MD, Qin XM, Yu X, et al. Transgenic Arabidopsis thaliana plants expressing a β-1, 3-glucanase from sweet sorghum (Sorghum bicolor L.) show reduced callose deposition and increased tolerance to aluminium toxicity. Plant Cell Environ. 2015; 38, 1178–1188. 10.1111/pce.12472 [DOI] [PubMed] [Google Scholar]
- 37.Yu XM, Griffith M. Winter rye antifreeze activity increases in response to cold and drought, but not abscisic acid. Plant Physiol. 2001; 112:78–86. 10.1034/j.1399-3054.2001.1120111.x [DOI] [PubMed] [Google Scholar]
- 38.Van Huijsduijnen RAMH, Alblas SW, De Rijk RH, Bol JF. Induction by salicylic acid of pathogenesis-related proteins and resistance to alfalfa mosaic virus infection in various plant species. J. Gen. Virol. 1986; 67, 2135–2143. 10.1099/0022-1317-67-10-2135 [DOI] [Google Scholar]
- 39.Mustafiz A, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL. Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct. Integr. Genomics. 2011; 11, 293–305. 10.1007/s10142-010-0203-2 [DOI] [PubMed] [Google Scholar]
- 40.Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 2008; 17, 171–180. 10.1007/s11248-007-9082-2 [DOI] [PubMed] [Google Scholar]
- 41.Leubner-Metzger G, Frundt C, Vogeli-Lange R, Meins F Jr. Class I β-1,3-glucanases in the endosperm of tobacco during germination. Plant Physiol. 1995; 109, 751–759. 10.1104/pp.109.3.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuels AL, Giddings TH Jr, Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol. 1995; 130, 1345–1357. doi: 10.1.1.273.3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konishi T, Takeda T, Miyazaki Y, Ohnishi-Kameyama M, Hayashi T, O’Neill MA, Ishii T. A plant mutase that interconverts UDP-arabinofuranose and UDP-arabinopyranose. Glycobiology. 2007; 17:345–354. [DOI] [PubMed] [Google Scholar]
- 44.Rautengarten C, Ebert B, Herter T, Petzold CJ, Ishii T, Mukhopadhyay A, et al. The interconversion of UDP-arabinopyranose and UDP-arabinofuranose is indispensable for plant development in Arabidopsis. Plant Cell. 2011; 23, 1373–1390. 10.1105/tpc.111.083931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CBB-stained gels from two independent experiments. Differential spots with at least two-fold changes in volume are indicated. A, B and C represent three independent replicates.
(PPT)
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.