Abstract
The main goal of the present work is to test the hypothesis that small-scale turbulence affected physiological activities and the morphology of cyanobacteria in high turbulence environments. Using quantified turbulence in a stirring device, we conducted one set of experiments on cultures of two strains of cyanobacteria with different phenotypes; i.e., unicellular Microcystis flos-aquae and colonial Anabaena flos-aquae. The effect of small-scale turbulence examined varied from 0 to 8.01×10−2 m2s-3, covering the range of turbulence intensities experienced by cyanobacteria in the field. The results of photosynthesis activity and the cellular chlorophyll a in both strains did not change significantly among the turbulence levels, indicating that the potential indirect effects of a light regime under the gradient of turbulent mixing could be ignored. However, the experiments demonstrated that small-scale turbulence significantly modulated algal nutrient uptake and growth in comparison to the stagnant control. Cellular N and C of the two stains showed approximately the same responses, resulting in a similar pattern of C/N ratios. Moreover, the change in the phosphate uptake rate was similar to that of growth in two strains, which implied that growth characteristic responses to turbulence may be dependent on the P strategy, which was correlated with accumulation of polyphosphate. Additionally, our results also showed the filament length of A. flos-aquae decreased in response to high turbulence, which could favor enhancement of the nutrient uptake. These findings suggested that both M. flos-aquae and A. flos-aquae adjust their growth rates in response to turbulence levels in the ways of asynchronous cellular stoichiometry of C, N, and P, especially the phosphorus strategy, to improve the nutrient application efficiency. The fact that adaptation strategies of cyanobacteria diversely to turbulence depending on their physiological conditions presents a good example to understand the direct cause—effect relationship between hydrodynamic forces and algae.
Introduction
Accelerated eutrophication of freshwater bodies often leads to the excessive proliferation of phytoplankton, of which cyanobacteria tend to predominate. Rapid growth of cyanobacteria often leads to blooms that can have serious adverse effects on aquatic communities in lakes throughout the world [1]. Cyanobacteria bloom occurrence is affected by a complex set of physical, chemical, biological, hydrological, and meteorological conditions, so it is difficult to isolate specific causative environmental factors [2]. Previous investigations suggested that interactions among nutrient inputs, turbulent mixing, underwater light availability, and grazing pressure can strongly influence the components of phytoplankton species in the water column [3, 4].
Turbulence mixing has been widely accepted as a source of external energy to the planktonic environment, such as regulating light accessibility in the water column [5], bringing eutrophic water into contact with algal cells [6, 7], and affecting encounter possibilities between phytoplankton and predators [8]. In particular, small-scale turbulence could play an important role in determining which phytoplanktonic taxon dominates over other competitors in the ever-changing environment [9]. Phytoplankton in the water column are constantly exposed to small-scale turbulence, which may have a differential effect on the physiology of algae [10]. The positive effect of small-scale turbulence on phytoplankton has been reported to occur through changing diffusive sublayers and regulating nutrient fluxes of cells [11–13], while the negative impacts include mechanical damage, behavioral alteration and physiological impairment [10, 14–16]. However, to date, there is only a small body of literature on the direct effects of small-scale turbulence on cyanobacteria in the freshwater rivers and lakes.
Cyanobacteria have constantly been subjected to a complex field environment imposed by these small-scale turbulent conditions. Survival of different cyanobacteria species in the mixed water column depends on the combined effects of varying environmental factors [17]. Nutrients and light are crucial, but the turbulence and its intensity also affect growth strategies of cyanobacteria species in their competition for these basic commodities [18, 19]. Because of a variety of adaptive strategies, such as buoyancy [20], N2-fixation [21], light tolerance [22], and efficient utilization of phosphorus and inorganic carbon [23, 24], cyanobacteria can dominate over other competitors throughout the life cycle. Under certain environmental stressors, cyanobacteria adapt habitat energy and nutrients through the adjustment of their metabolism while keeping cellular carbon, nitrogen and phosphorus in an acceptable range [25, 26]. Nevertheless, this plasticity of adaptation regarding nutrients has not been well reported when cyanobacteria species encounter turbulence mixing.
Many studies have reported that heterogeneity in response to turbulence is often observed among phytoplankton taxa [27], and certain species may exhibit specific adaptive strategies to turbulence. For instance, genus-specific shear effects have been illustrated for cyanobacteria such that the non-heterocystous filamentous Oscillatoria appears to be more shear tolerant than the heterocystous Anabaena [28, 29]. Furthermore, the seasonal field studies of mixed phytoplankton communities and enclosure experiments also have shown that shear has either increased or decreased/arrested cyanobacterial abundance or activity [28–30]. Some researchers suggested these differences in sensitivity induced by turbulence depend on cell size. It has been reported that the increase in the nutrient diffusion rate into cells due to small-scale turbulence varies from negligible to significant as cell size increases [13].
Hard evidence of a direct cause—effect relationships between turbulence and algae is not easy to find [8, 31]. In the present study, we investigated the effect of small-scale turbulence on the physiology and morphology of two bloom-forming cyanobacteria with different phenotypes, i.e., unicellular Microcystis flos-aquae and colonial Anabaena flos-aquae. To our knowledge, few experiments have been performed employing quantified turbulence levels to evaluate the effect of small-scale turbulence on the physiology of cyanobacteria. Thus we developed a model to quantify turbulence exposure in the experiment and systematically investigate the change in photosynthesis and total cellular C, N and P of the two stains, and filament length of A. flos-aquae under different turbulent dissipation rates. The data from the study were analyzed to illustrate the mechanisms of cyanobacteria with different morphology adaptation strategies under small-scale turbulence mixing.
Materials and Methods
Strains and culture conditions
Unicellular M. flos-aquae FACHB1028 and colonial A. flos-aquae FACHB245 were obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB-Collection, Wuhan, China). Routine cultures of the two strains were maintained in BG11 medium with a phosphate concentration of 2 mg/L [32] under a cool-white fluorescent light intensity of 25 μmol m-2 s-1 with a 12:12 light:dark cycle at a temperature of 25±1°C, and they were manually shaken 3 times every day during incubation.
In the experiments, cells at their exponential growth phase were served with P free BG11 medium for a period of 10 days before the growing experiment. After that, they were inoculated into 1 L glass beakers with 2 mg/L phosphate concentration BG11 medium and exposed to different agitation intensities. A 1-L beaker was used as the reactor in the experiment. A self-designed impeller was located at the center of the beaker. Geometric dimensions are shown in Fig 1. The liquid height was 113 mm. Continuous stirring was performed at the start of the growing experiment at rotation speeds of 100, 200, 300, 400 and 500 rpm. The samples cultured in completely stagnant conditions were used as controls. The test was run in triplicate for two weeks under the conditions described above, and they were sampled every 2 days for the determination of varying parameters, as described below.
Fig 1. Geometric dimensions of the reactor (Unit: mm).
Modeling and calculation of turbulent dissipation rates
A two-phase flow Computational Fluid Dynamics (CFD) model was used to simulate hydrodynamics in the reactor under different rotation speed [33]. The whole reactor was considered as the computational domain and was discretized using unstructured grids, where finer grids were performed in impeller and shaft regions (maximum size = 1 mm). About 1,000,000 total computational grids (maximum size = 2 mm) were created using the tetra mesh option of ANSYS ICEM CFD (ANSYS Inc.) in order to get the grid independent solution for the flow. The simulation of the process was considered as a steady-state, and numerically solved by the commercial software package CFX 12.0 (ANSYS Inc.). The convergence criteria used in all the simulations was 1×10−4, which was a factor by which the initial mass flow residual reduced as the simulation progressed. The simulations were carried out on the 6 nodes, 96 processor SUGON cluster. A commercial 2D particle image velocimetry (PIV) system (ILA, German), including a laser (Leamtech, Nd:YAG, 200Mj, 15Hz), a PIV camera (PCO2000, 2048×2048 pixels), a synchronizer and Vidpiv 4.7 software, was employed to validate the model. The setup and data processing were the same as described earlier [34]. Detail information of its validation results were reported in S1 File. The integrated results of turbulence mixing rate and Kolmogorov microscale in different levels of rotation speed were shown in Table 1.
Table 1. Turbulent dissipation rate and Kolmogorov microscale in different levels of rotation speed estimated by the CFD hydrodynamic model.
| Turbulence | |||||
|---|---|---|---|---|---|
| Rotation speed (rpm) | 100 | 200 | 300 | 400 | 500 |
| Turbulent dissipation rate (m2s-3) | 1.51×10−3 | 6.63×10−3 | 2.26×10−2 | 5.06×10−2 | 8.01×10−2 |
| Kolmogorov microscale (mm) | 0.186 | 0.126 | 0.092 | 0.074 | 0.065 |
Determination of growth rates and chlorophyll a (Chla) of cyanobacteria
Two strains were batch cultured under different agitation intensities (0 rpm to 500 rpm) for two weeks and were sampled every 2 days for cell counting under a microscope (Olympus CX 43, Japan) using the appropriate magnification and a hemocytometer. Prior to cell counting, A. flos-aquae colonies were treated with ultrasonication for 30 s or 60 s at 20 kHz and 100 W (JY92-2D, SCIENTZ, China) to obtain single cells. The growth rate was measured during the exponential growth phase according to the exponential growth equation: μ = (lnBt2-lnBt1)/Δt [35], where μ is the specific growth rate, Bt2 and Bt1 represent the cell concentration at the end (t2) and beginning (t1) of the log phase, respectively, and Δt = t2-t1. For Chla measurements, the samples were centrifuged at 6,000 rpm for 15 min, and cell pellets were extracted with 80% acetone/20%water.
Determination of photosynthetic activity of cyanobacteria
In vivo chlorophyll fluorescence was measured with a phytoplankton analyzer (PHYTO-PAM, Walz GmbH, Germany). The samples were dark-adapted for at least 15 min before measuring the fluorescence parameters (photosystem II activity, PSII). The electron transport rate of PSII (ETR) was calculated according to [36]. Relative ETR = (((Fm- Ft) /Fm)×0.84×0.5×PAR (m-2s-1)). The maximum effective quantum yield of PSII was calculated as Fv/Fm = (Fm-F0)/Fm [37]. Fv is the difference between Fm and F0, which are the maximum and minimum fluorescence values of the dark adapted stage of PSII.
Analysis of the carbon, nitrogen and phosphorus contents of cyanobacteria
To measure the carbon, nitrogen and phosphorus contents of the two strains, culture suspensions were taken from each sample, filtered through GF/F filters (Whatman, UK) and dried at 65°C for 24 h. Each filter was split for analysis of C, N, and total cellular phosphorus (TCP). C and N concentrations were measured using a Vario El cube elemental analyzer (Elementar, Germany). The filtrates were used for measuring soluble reactive phosphorus (SRP). The filtered extracts were diluted with distilled water, and TCP was determined by digestion [38, 39] followed by colorimetric analysis [40]. The polyphosphate (polyP) was extracted using the hot water method according to [41].
Uptake rate of phosphate
Phosphate uptake rates were calculated by the change in phosphate concentration in the culture media during 72 h batch cultures at the same culture conditions as mentioned above. Phosphate levels were determined using ascorbic acid-molybdenum blue spectrophotometric methods [42]. The uptake rate was calculated according to [43]: phosphate uptake rate = (W0-Wt) · V · N0-1· t-1, where W0 is the initial concentration of phosphate, Wt is the concentration of the phosphate after t hours, N0 is the initial level of cell density and V is the volume of culture medium.
Measurement of the filament size of Anabaena
Anabaena filaments were examined using an Olympus BX 53 light microscope with a digital camera (Olympus DP 71, Japan). The sizes of the filaments were measured in microphotographs using image analysis Olympus DP-Soft. One hundred filaments were chosen at random from each sample to capture the images, and then the surface area (S), width (d), and length (l) of individual filaments were obtained using Olympus DP-Soft, which was calibrated separately for each magnification.
Statistical analysis
The data in this study are presented as the mean ± standard deviation (SD). Significant differences between controls and treated samples were determined with an ANOVA. All statistical analyses were carried out with Origin 8.0 (OriginLab, USA). Differences were considered to be significant at P < 0.05.
Results
Effect of turbulence on the growth rate of cyanobacteria
Specific growth rates of unicellular M. flos-aquae and colonial A. flos-aquae at different turbulent dissipation rates are shown in Table 2. The growth curve was plotted to roughly ascertain the period of the exponential phase of each strain, and thus the growth rates of individual strains were calculated in the exponential phase accordingly. For M. flos-aquae, the growth rate increased with the elevation of the turbulent dissipation rate. At a turbulent dissipation rate of 1.51×10−3 m2s-3, the growth rate of M. flos-aquae was approximately 3.4 times than that in the stagnation condition. When the turbulent dissipation rate was above 5.06×10−2 m2s-3, the growth rate of M. flos-aquae remained relatively unchanged (ANOVA, P > 0.05). On the other hand, the growth rate of A. flos-aquae increased dramatically (ANOVA, P < 0.05) at lower turbulent dissipation rates and reached the max at 2.26×10−2 m2s-3, while the growth rate decreased with increases in the turbulence intensity at higher levels. Overall, the growth rates of A. flos-aquae at all levels of turbulence intensity were lower than that of M. flos-aquae.
Table 2. The specific growth rates of M. flos-aquae and A. flos-aquae under different levels of turbulence.
| Turbulent dissipation rate (m2s-3) | ||||||
|---|---|---|---|---|---|---|
| Strain | 0 | 1.51×10−3 | 6.63×10−3 | 2.26×10−2 | 5.06×10−2 | 8.01×10−2 |
| M. flos-aquae | 0.039±0.003 | 0.133±0.002 | 0.144±0.001 | 0.223±0.005 | 0.261±0.005 | 0.286±0.004 |
| A. flos-aquae | 0.009±0.001 | 0.014±0.002 | 0.027±0.001 | 0.043±0.002 | 0.040±0.003 | 0.029±0.003 |
The data are the mean ± SD (n = 3)
Photosynthetic parameters and cellular Chla concentrations of cyanobacteria
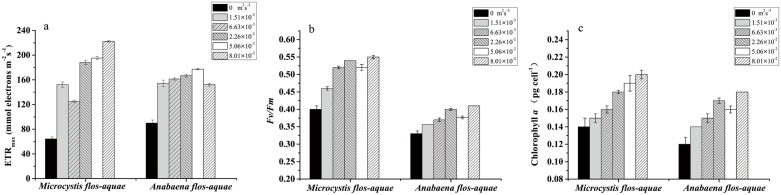
After incubation with varied levels of turbulent dissipation rates, the maximum electron transport rate (ETRmax) and the maximum quantum yield (Fv/Fm) of M. flos-aquae and A. flos-aquae showed considerable differences (Fig 2a and 2b). The parameters for the photosynthetic responses to changes in turbulence intensity of M. flos-aquae were significantly higher than in the control (non-turbulent) treatment (ANOVA, P < 0.05). Among all the turbulent mixing treatments, markedly low values of Fv/Fm of M. flos-aquae were found in cells grown at a turbulence intensity of 1.51×10−3 m2s-3. The values of ETRmax and Fv/Fm were not found to be significantly different among levels of turbulence intensities from 2.26×10−2 to 8.01×10−2 m2s-3 (ANOVA, P > 0.05). Coincident with the elevation in the ETRmax of M. flos-aquae, the changes in the ETRmax of A. flos-aquae showed similar trends to that of M. flos-aquae (ANOVA, P < 0.05). However, it appears that there was no significant difference in the Fv/Fm of A. flos-aquae between all the turbulent treatments and the controls (ANOVA, P > 0.05). The cellular Chla showed a similar pattern to the Fv/Fm ratios, although the cellular Chla was relatively higher at higher turbulent dissipation rates, probably due to the reduction in cell density at the highest turbulent dissipation rates (Fig 2c).
Fig 2. Changes in the maximum electron transfer rate (ETRmax) (a), Fv/Fm (b) and cellular chlorophyll a (Chla) (c) of M. flos-aquae and A. flos-aquae at different turbulent dissipation rate levels in the logarithmic growth phase. The data are the mean ± SD (n = 3).
Phosphate uptake of cyanobacteria
The time course of SRP in the culture medium and phosphate uptake rate of M. flos-aquae and A. flos-aquae cultured at different turbulent dissipation rate levels are shown in Fig 3, respectively. It was observed that the SRP concentrations of the two strains decreased significantly with culturing time at all turbulence intensity levels. The residual P concentrations in the M. flos-aquae cultures were affected by the turbulent dissipation rate. The concentration of SRP in the cultures treated with higher turbulent dissipation rates (8.01×10−2 m2s-3) were significantly lower than that in the turbulent dissipation rates of 1.51×10−3 and 6.63×10−3 m2s-3 at the end of the test (ANOVA, P < 0.05). However, the SRP concentration in the A. flos-aquae culture with a turbulence intensity of 2.26×10−2 m2s-3 was the greatest at the end of the test, whereas no significant differences were observed among the other treatment cultures (ANOVA, P > 0.05). The phosphate uptake rate was analyzed as a function of the external phosphate concentration. It showed similar trends to that of SRP (Fig 4). Under the turbulent dissipation rate of 2.26×10−2 m2s-3, the uptake rates of phosphate in the two strains increased with the elevation of turbulence intensity. When the turbulent dissipation rate was above 2.26×10−2 m2s-3, the phosphate uptake rate of M. flos-aquae remained relatively unchanged, while the phosphate uptake rate of A. flos-aquae decreased dramatically (ANOVA, P < 0.05).
Fig 3. The time course of soluble reactive phosphorus (SRP) in the culture medium of M. flos-aquae (a) and A. flos-aquae(b) at different turbulent dissipation rate levels.
The data are the mean ± SD (n = 3).
Fig 4. Uptake rate for phosphate and polyphosphate content of M. flos-aquae (a) and A. flos-aquae (b) cultured at different turbulent dissipation rate levels in the logarithmic growth phase.
The data are the mean ± SD (n = 3).
Effect of turbulence on the elemental composition of cyanobacteria
To further explore the cellular stoichiometry changes, cellular C, N and P in response to different turbulent dissipation rates were analyzed (Table 3). There were significant differences in cellular C, N, and P among different levels of turbulent mixing (ANOVA, P < 0.05). For M. flos-aquae, cellular C was the highest under the turbulent dissipation rate of 5.06×10−2 m2s-3, with an increase of 11% compared with the control treatment. However, the cellular N concentration was significantly decreased with increasing turbulence intensity, except under the turbulent dissipation rate of 1.51×10−3 m2s-3. Furthermore, as dissipation rates increased, there was also an increase in cellular P, and the maximum value was observed under the turbulent dissipation rate of 2.26×10−2 m2s-3, which was 2.0-fold compared with that at the stagnation condition. Additionally, the changes in cellular PolyP showed similar trends to that of cellular P (Fig 4). In A. flos-aquae, the highest concentrations of cellular C, N and P were found at the turbulent dissipation of 2.26×10−2 m2s-3. The cellular PolyP kept increasing along with increases in turbulence intensity at the rate of 0–2.26×10−2 m2s-3. Then, a decline of cellular PolyP was observed under the dissipation rate of 5.06×10−2 m2s-3 and above (ANOVA, P < 0.05).
Table 3. Cellular stoichiometry of M. flos-aquae and A. flos-aquae under different levels of turbulence in the logarithmic growth phase.
| Turbulent dissipation rate (m2s-3) | M. flos-aquae | A. flos-aquae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pg/cell | mole ratio | pg/cell | mole ratio | |||||||
| C | N | P | C/N | N/P | C | N | P | C/N | N/P | |
| 0 | 6.14 | 1.58 | 0.29 | 4.53 | 12.06 | 6.13 | 1.38 | 0.25 | 5.18 | 12.22 |
| 1.51×10−3 | 6.70 | 1.89 | 0.31 | 4.13 | 13.50 | 7.27 | 1.48 | 0.19 | 5.73 | 17.24 |
| 6.63×10−3 | 6.73 | 1.38 | 0.26 | 5.68 | 11.75 | 6.38 | 1.31 | 0.28 | 5.68 | 10.35 |
| 2.26×10−2 | 6.36 | 1.49 | 0.59 | 4.97 | 5.59 | 8.17 | 1.55 | 0.33 | 6.14 | 10.40 |
| 5.06×10−2 | 6.89 | 1.16 | 0.43 | 6.92 | 5.97 | 6.81 | 1.38 | 0.21 | 5.75 | 14.55 |
| 8.01×10−2 | 5.52 | 0.91 | 0.33 | 7.07 | 6.11 | 7.22 | 1.49 | 0.2 | 5.65 | 16.49 |
Changes in molar ratios of C/N and N/P of M. flos-aquae and A. flos-aquae are also shown in Table 3. The results from our experiment showed plasticity of cellular ratios in M. flos-aquae and A. flos-aquae in response to small-scale turbulence. There was an increase in the C/N ratio and a decrease in the N/P ratio in M. flos-aquae at the dissipation rate of 6.63×10−3 m2s-3 and above, compared with the controls. Variations in the C/N ratio in A. flos-aquae were similar to that in M. flos-aquae. The maximum C/N ratio was recorded at the turbulent dissipation rate of 2.26×10−2 m2/s3. However, the N/P ratio in A. flos-aquae decreased only at the turbulent dissipation rates of 6.63×10−3 and 2.26×10−2 m2s-3, compared with the control treatment, which was due to the increase in cellular P.
The filament length of A. flos-aquae
The filament length of A. flos-aquae varied with different turbulent dissipation rates (Fig 5a). It was observed that the geometric mean of the filament lengths of A. flos-aquae decreased with the elevation of turbulence intensity. Compared with the stagnation condition, the filament length of A. flos-aquae decreased by 69% and 47% at the turbulent dissipation rates of 2.26×10−2 and 5.06×10−2 m2s-3, respectively. The frequency distribution of each filament length range under the different turbulence intensities is shown in Fig 5b. The result also demonstrated that the frequency of 0–50 μm lengths was increased with increasing dissipation rates and reached the maximum at the 2.26×10−2 m2s-3, while the large filament (>200 μm) was the lowest. The frequencies of 100–200 μm lengths decreased at the turbulent dissipation rates of 1.51×10−3 and 6.63×10−3 m2s-3, while for the high turbulence intensities of 8.01×10−2 m2s-3, the frequencies of large filaments (>200 μm) also decreased significantly.
Fig 5. Box and whisker plots (a) and frequency distribution (b) of A. flos-aquae filament length as a response to various turbulence intensities at the end of the test.
a, filament lengths are presented in box plots showing the 5, 10, 25, 50, 75, 90 and 95 percentiles; whiskers represent minimum and maximum values, and dots inside the boxes are the mean values.
Discussion
Turbulence is ubiquitous in aquatic systems and thus can potentially influence a wide scope of phytoplankton and eco-physiological processes. It is still often referred to as one of the unsolved problems in field studies due to the interactions with other variables, such as light penetration and supply of nutrients, and their consequences for organism physiology and behaviors [44]. Some of the current knowledge has been derived from laboratories with devices to generate controlled turbulence; however, these should correctly evaluate the response of phytoplankton to a certain level of turbulence in natural conditions [45]. A gradient of increasing levels of dissipation rates was used in our study to investigate the shape and character of potential relationships between small-scale turbulence, nutrient conditions and cyanobacteria. Turbulent dissipation in the device varied from 10−4 to 10−2 m2s-3. On the Kolmogorov microscale, it was in the higher range of turbulences in natural aquatic ecosystems. However, it covered the range of turbulence intensities experienced by cyanobacteria in the field [2].
In most studies described in the literature to date, the effect of turbulence on phytoplankton was determined by changes in growth and the viability of the organism [46, 47]. In this study, responses of Microcystis and Anabaena to turbulence intensities were quite different as inferred by growth. The turbulent regimes of 1.51×10−3 to 2.26×10−2 m2s-3 favored the growth of the two strains. However, in regard to the higher dissipation rate of 8.01×10−2 m2s-3, turbulence had a negative impact on the growth rate of A. flos-aquae but did not affect the growth of M. flos-aquae, suggesting that unicellular M. flos-aquae was better adapted to growing under high turbulent dissipation rates.
The photosynthetic system is a good indicator system because it is very sensitive to any changes in living conditions [48]. Fv/Fm and ETRmax are considered as the sensitive photosynthetic parameters to environmental stress [49]. At the used range of turbulent mixing, the changes in Fv/Fm and ETRmax in the exponential phase support the hypothesis that the photosynthetic activity of A. flos-aquae significantly increases when compared with stagnant conditions but might not change in contrast to the unimodal response of a growth rate to a gradient of turbulent mixing. Similar results were also discussed by [50], indicating that at saturating irradiances, photosynthesis was less sensitive than growth to changes in turbulence mixing. Additionally, the Chla content in both M. flos-aquae and A. flos-aquae increased with turbulence even under a higher level, indicating that the photosynthetic apparatus of algae showed little or no disruption in response to turbulence. Therefore, the potential indirect effects of a light regime under the gradient of turbulent mixing, e.g., the shading effect due to an increase in the population in the reactors, could be ignored.
A great majority of studies reported that turbulence may have beneficial effects on phytoplankton due to an increase in the diffusive transport of nutrients toward the cell surface and, hence, an increase the nutrient uptake rate [14, 51]. Under turbulent mixing in our study, cellular N and C of the two stains showed approximately the same responses, resulting in a similar pattern of C/N ratios in response to turbulent mixing. Unlike cellular C and N, P uptake and assimilation in cells did not show a unimodal response. Turbulence is reported to affect the algal growth by changing their nutrient acquisition traits [52]. The fact that the change in phosphate uptake rates was similar to that of growth in both of the strains implied that different growth characteristic responses to turbulence may be dependent on the P strategy. This finding was consistent with previous research [47] in that the phosphate uptake rate of algae was approximately similar to the growth rate.
Formation and storage of PolyP is commonly regarded as a nutritional strategy of microalgae when encountering environmental stress [53]. According to Fig 4, it was shown that luxury uptake of phosphorus by cyanobacteria was mostly applied to accumulate PolyP, implying that accumulation of PolyP at high dissipation rates was the strategy for cyanobacteria to maintain phosphorus concentrations in unfavorable conditions. However, compared with M. flos-aquae, the concentration of PolyP in A. flos-aquae at dissipation rates of 2.26×10−2 to 5.06×10−2 m2s-3 were much higher than those with a dissipation rate of 1.51×10−3 m2s-3, indicating that the reallocation of cellular P in A. flos-aquae could be a response to the changed turbulent conditions, and the filament integrity in A. flos-aquae was more sensitive to turbulence than in unicellular M. flos-aquae. Furthermore, our results also showed that the filament length of A. flos-aquae decreased in response to turbulence. It seemed that turbulence negatively affects cyanobacterial morphology prior to physiological activity. We stress that this may be related to the nutrient acquisition strategies, which was depended on algal size. Theoretical predictions regarding the thickness of external diffusion boundary layer is equal to the equivalent spherical diameters of the organism [54]. Thus, larger organism increased the restriction on nutrient uptake from low external concentrations, probably because the greater thickness of the diffusion boundary layers around itself. As small filaments have higher rates of nutrient uptake and lower metabolic requirement, which allow them to grow at much lower resource concentrations than larger ones [55], we inferred that with the exhaustion of SRP in the culture, the decreased filaments of A. flos-aquae at higher turbulent dissipation rates could favor the resource gain. Our findings support what had been suggested by [47], such that, in the lotic diatoms where the cells were smaller, there was higher P affinity. Overall, our study also highlights the need for further investigation of the relationship between the boundary layer around filaments and turbulence mixing.
A gradient of increasing levels of dissipation rates is useful when attempting to investigate the shape and character of potential relationships between small-scale turbulence, nutrient conditions and planktonic organisms [56]. Small-scale shearing affects physiological activities and the morphology of phytoplankton in high turbulence environments. The variety of physiological and morphological properties of phytoplankton may be largely considered as adaptations to different scales and characteristics of turbulent motions in the aquatic environment [57]. In concert with earlier investigations, the results from the two types of cyanobacteria in our study illustrated that both M. flos-aquae and A. flos-aquae adjust their growth rates in response to turbulence levels in the ways of asynchronous cellular stoichiometry of C, N, and P, especially the phosphorus strategy, to improve the nutrient application efficiency. Additionally, the colonial A. flos-aquae could change the effective filament length to enhance the nutrient uptake under high turbulent conditions. Again, these evidences supported the hypothesis that hydrodynamic regimes may impact eco-physiology of cyanobacteria to form certain adaptive strategies, which should be carefully considered in elucidating mechanisms of cyanobacteria blooms. Clearly, future studies are warranted to assess the effects of turbulence on nutrient flux of cells including different morphological cyanobacteria species.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Natural Science Foundation (No. 51309220, 51679226), the Chongqing Natural Science Program (cstc2015jcyjBX0006), and the Western Action Program founded by the Chinese Academy of Sciences (No. KZCX2-XB3-14).
References
- 1.Sivonen K. Cyanobacterial toxins and toxin production. Phycologia. 1996; 35: 12–24. [Google Scholar]
- 2.Reynolds CS. The ecology of phytoplankton. UK: Cambridge University Press; 2006. [Google Scholar]
- 3.Becker V, Huszar VLM, Crossetti LO. Responses of phytoplankton functional groups to the mixing regime in a deep subtropical reservoir. Hydrobiologia. 2009; 628: 137–151. [Google Scholar]
- 4.Litchman E, De Tazanos Pinto P, Klausmeier CA, Thomas MK, Yoshiyama K. Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia. 2010; 653: 15–28. [Google Scholar]
- 5.Yu Q, Chen YC, Liu ZW, van de Giesen N, Zhu DJ. The influence of a eutrophic lake to the river downstream: spatiotemporal algal composition changes and the driving factors. Water. 2015; 7: 2184–2201. [Google Scholar]
- 6.Ebert U, Arrayas M, Temme N, Sommeijer B, Huisman J. Critical conditions for phytoplankton blooms. B. Math. Biol. 2001; 63: 1095–1124. [DOI] [PubMed] [Google Scholar]
- 7.Huisman J, Arrayas M, Ebert U, Sommeijer B. How do sinking phytoplankton species manage to persist? Am. Nat. 2002; 159: 245–254. 10.1086/338511 [DOI] [PubMed] [Google Scholar]
- 8.Peters F, Marrasé C. Effects of turbulence on plankton: an overview of experimental evidence and some theoretical considerations. Mar. Ecol. Prog. Ser. 2000; 205: 291–306. [Google Scholar]
- 9.Smayda TJ, Reynolds CS. Community assembly in marine phytoplankton: application of recent models to harmful dinoflagellate blooms. J. Plankton Res. 2001; 23: 447–461. [Google Scholar]
- 10.Sullivan JM, Swift E, Donaghay PL, Rines JEB. Small-scale turbulence affects the division rate and morphology of two red-tide dinoflagellates. Harmful Algae. 2003; 2: 183–199. [Google Scholar]
- 11.Savidge G. Studies of the effect of small-scale turbulence on phytoplankton. J. Mar. Biol. Assoc. U.K. 1981; 61: 477–488. [Google Scholar]
- 12.Lazier JRN, Mann KH. Turbulence and the diffusive layers around small organisms. Deep-Sea Res. 1989; 36: 1721–1733. [Google Scholar]
- 13.Karp-Boss L, Boss E, Jumars PA. Nutrients fluxes to planktonic osmotrophs in the presence of fluid motion. Oceanogr. Mar. Biol. 1996; 34: 71–107. [Google Scholar]
- 14.Estrada M, Berdalet E. Phytoplankton in a turbulent world. Sci. March 1997; 61: 125–140. [Google Scholar]
- 15.Karp-Boss L, Boss E, Jumars PA. Motion of dinoflagellates in a simple shear flow. Limnol. Oceanogr. 2000; 45: 1594–1602. [Google Scholar]
- 16.Juhl AR, Latz MI. Mechanisms of fluid shear-inducted inhibition of population growth in a red-tide dinoflagellate. J. Phycol. 2002; 38: 683–694. [Google Scholar]
- 17.Oliver RL, Ganf GG. Freshwater blooms In: Whitton BA, Potts M, editors. The ecology of cyanobacteria: their diversity in time and space. Dordrecht: Kluwer Academic; 2000. pp. 105–194. [Google Scholar]
- 18.Guven B, Howard A. Modelling the growth and movement of cyanobacteria in river systems. Sci. Total Environ. 2006; 368: 898–908. 10.1016/j.scitotenv.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 19.Li FP, Zhang HP, Zhu YP, Xiao YH, Chen L. Effect of flow velocity on phytoplankton biomass and composition in a freshwater lake. Sci. Total Environ. 2013; 447: 64–71. 10.1016/j.scitotenv.2012.12.066 [DOI] [PubMed] [Google Scholar]
- 20.Visser PM, Passarge J, Mur LR. Modelling vertical migration of the cyanobacterium Microcystis. Hydrobiologia. 1997; 349: 99–109. [Google Scholar]
- 21.Burford MA, Mcneale KL, Mckenzie-Smith FJ. The role of nitrogen in promoting the toxic cyanophyte Cylindrospermopsis raciborskii in a subtropical water reservoir. Freshwater Biol. 2006; 51: 2143–2153. [Google Scholar]
- 22.Sommaruga R, Chen YW, Liu ZW. Multiple strategies of bloom-forming Microcystis to minimize damage by solar ultraviolet radiation in surface waters. Microb. Ecol. 2009; 57: 667–674. 10.1007/s00248-008-9425-4 [DOI] [PubMed] [Google Scholar]
- 23.Paerl HW. Partitioning of CO2 fixation in the colonial cyanobacterium Microcystis aeruginosa: mechanism promoting formation of surface scums. Appl. Environ. Microb. 1983; 46: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Song LR. Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia. 2007; 592: 475–486. [Google Scholar]
- 25.Sterner RW, Elser JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press; 2002. [Google Scholar]
- 26.Klausmeier CA, Litchman E, Daufresne T, Levin SA. Phytoplankton stoichiometry. Ecol. Res. 2008; 23: 479–485 [Google Scholar]
- 27.Sullivan JM, Swift E. Effects of small-scale turbulence on net growth rate and size of ten species of marine dinoflagellates. J. Phycol. 2003; 39: 83–94. [Google Scholar]
- 28.Reynolds CS, Wiseman SW, Godfrey BM, Butterwick C. Some effects of artificial mixing on the dynamics of phytoplankton populations in large limnetic enclosures. J. Plankton Res. 1983; 5: 203–234. [Google Scholar]
- 29.Steinberg CW, Tille-Backhaus R. Re-occurrence of filamentous planktonic cyanobacteria during permanent artificial destratification. J. Plankton Res. 1990; 12: 661–664. [Google Scholar]
- 30.Petersen JE, Sanford LP, Kemp WM. Coastal plankton responses to turbulent mixing in experimental ecosystems. Mar. Ecol. Prog. Ser. 1998; 171: 23–41. [Google Scholar]
- 31.Zhu YP, Zhang HP, Li FP, Chen L. Enclosure experiments about the hydrodynamics effects on the plankton. Environ. Sci. 2010; 31: 69–75. [PubMed] [Google Scholar]
- 32.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strains histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979; 111: 1–61. [Google Scholar]
- 33.Li C, Xia JY, Chu J, Wang YH, Zhuang YP, Zhang SiL. CFD analysis of the turbulent flow in baffled shake flasks. Biochem. Eng. J. 2013; 70: 140–150. [Google Scholar]
- 34.Xie MH, Xia JY, Zhou Z, Chu J, Zhuang YP, Zhang SL. Flow pattern, mixing, gas hold-up and mass transfer coefficient of triple-impeller configurations in stirred tank bioreactors. Ind. Eng. Chem. Res. 2014; 53: 5941–5953. [Google Scholar]
- 35.Guillard RRL. Division rates In: Stein JR, editor. Handbook of phycological methods—culture methods and growth measurements. New York: Cambridge University Press; 1973. pp. 289–311. [Google Scholar]
- 36.Wu ZX, Song LR, Li RH. Different tolerances and responses to low temperature and darkness between water bloom forming cyanobacterium Microcystis and a green alga Scenedesmus. Hydrobiologia. 2008; 596: 47–55. [Google Scholar]
- 37.Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000; 51: 659–668. [DOI] [PubMed] [Google Scholar]
- 38.Parkinson J, Allen S. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plan. 1975; 6: 1–11. [Google Scholar]
- 39.O'halloran IP, Cade-Menun BJ. Total and organic phosphorus In: Carter MR, Gregorich EG, editors. Soil Sampling and Methods of Analysis, 2nd ed Canadian Society of Soil Science and CRC Press; 2008. Chapter 24. [Google Scholar]
- 40.Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962; 27: 31–36. [Google Scholar]
- 41.Eixler S, Selig U, Karsten U. Extraction and detection methods for polyphosphate storage in autotrophic planktonic organisms. Hydrobiologia. 2005; 533: 135–143. [Google Scholar]
- 42.APHA. Standard methods for the examination of water and wastewater, 19th ed Washington, D.C: American Public Health Association; 1995. [Google Scholar]
- 43.Harrison PJ. Determining phosphate uptake rates of phytoplankton In: Lobban CS, Chapman DJ, Kremer BP, editors. Experimental phycology: a laboratory manual New York: Cambridge University Press; 1988. pp. 186–195. [Google Scholar]
- 44.Prairie JC, Sutherland KR, Nickols KJ, Kaltenberg AM. Biophysical interactions in the plankton: A cross-scale review. Limnol. Oceanogr.–Fluids Environ. 2012; 2: 121–145. [Google Scholar]
- 45.Guadayol Ò, Peters F, Stiansen JE, Marrasé C, Lohrmann A. Evaluation of oscillating grids and orbital shakers as means to generate isotropic and homogeneous small-scale turbulence in laboratory enclosures commonly used in plankton studies. Limnol. Oceanogr.–Meth. 2009; 7: 287–303. [Google Scholar]
- 46.Michels MHA, Goot AJ, Norsker NH, Wijffels RH. Effects of shear stress on the microalgae Chaetoceros muelleri. Bioproc. Biosyst. Eng. 2010; 33:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang PL, Shen H, Xie P. Can hydrodynamics change phosphorus strategies of diatoms?—nutrient levels and diatom blooms in lotic and lentic ecosystems. Microb. Ecol. 2012; 63: 369–382. 10.1007/s00248-011-9917-5 [DOI] [PubMed] [Google Scholar]
- 48.Leupold M, Hindersin S, Gust G, Kerner M, Hanelt D. Influence of mixing and shear stress on Chlorella vulgaris, Scenedesmus obliquus, and Chlamydomonas reinhardtii. J. Appl. Phycol. 2013; 25: 485–495. [Google Scholar]
- 49.Wu ZX, Gan NQ, Huang Q, Song LR. Response of Microcystis to copper stress—do phenotypes of Microcystis make a difference in stress tolerance? Environ. Pollut. 2007; 147: 324–330. 10.1016/j.envpol.2006.05.022 [DOI] [PubMed] [Google Scholar]
- 50.Thomas WH, Vernet M, Gibson CH. Effects of small-scale turbulence on photosynthesis, pigmentation, cell division, and cell size in the marine dinoflagellate Gomaulax polyedra (Dinophyceae). J. Phycol. 1995; 31: 50–59. [Google Scholar]
- 51.Barton AD, Ward BA, Williams RG, Follows MJ. The impact of fine-scale turbulence on phytoplankton community structure. Limnol. Oceanogr.–Fluids Environ. 2014; 4: 34–49. [Google Scholar]
- 52.Moisander PH, Ochiai M, Lincoff A. Nutrient limitation of Microcystis aeruginosa in northern California Klamath River reservoirs. Harmful Algae. 2009; 8: 889–897. [Google Scholar]
- 53.Eixler S, Karsten U, Selig U. Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia. 2006; 45: 53–60. [Google Scholar]
- 54.Beardall J, Allen D, Bragg J, Finkel ZV, Flynn KJ, Quigg A, et al. Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytol. 2009; 181: 295–309. 10.1111/j.1469-8137.2008.02660.x [DOI] [PubMed] [Google Scholar]
- 55.Yoshiyama K, Klausmeier CA. Optimal cell size for resource uptake in fluid: a new facet of resource competition. Am. Nat. 2008; 171: 59–70. 10.1086/523950 [DOI] [PubMed] [Google Scholar]
- 56.Iversen KR, Primicerio R, Larsen A, Egge JK, Peters F, Guadayol Ó, et al. Effects of small-scale turbulence on lower trophic levels under different nutrient conditions. J. Plankton Res. 2010; 32: 197–208. [Google Scholar]
- 57.Moisander PH, Hench JL, Kononen K, Paerl HW. Small-scale shear effects on heterocystous cyanobacteria. Limnol. Oceanogr. 2002; 47: 108–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.