This and Related “Classic” Articles Appear on Prsjournal.Com for Journal Club Discussions.
Abstract
Background:
Natrelle 410 silicone breast implants are approved in the United States for breast augmentation, reconstruction, and revision.
Methods:
In two ongoing, prospective, multicenter 10-year studies, 17,656 subjects received Natrelle 410 implants for augmentation (n = 5059), revision-augmentation (n = 2632), reconstruction (n = 7502), or revision-reconstruction (n = 2463). Capsular contracture, implant malposition, and late seroma were documented. Cox proportional hazards regression analyses evaluated potential associations between subject-, implant-, and surgery-related factors and these complications.
Results:
Median follow-up was 4.1, 2.6, 2.1, and 2.3 years in the augmentation, revision-augmentation, reconstruction, and revision-reconstruction cohorts, respectively. Incidence of capsular contracture across cohorts ranged from 2.3 to 4.1 percent; malposition, 1.5 to 2.7 percent; and late seroma, 0.1 to 0.2 percent. Significant risk factors for capsular contracture were subglandular implant placement, periareolar incision site, and older device age in the augmentation cohort (p < 0.0001), older subject age in the revision-augmentation cohort (p < 0.0001), and higher body mass index (p = 0.0026) and no povidone-iodine pocket irrigation (p = 0.0006) in the reconstruction cohort. Significant risk factors for malposition were longer incision size in the augmentation cohort (p = 0.0003), capsulectomy at the time of implantation in the reconstruction cohort (p = 0.0028), and implantations performed in physicians’ offices versus hospitals or standalone surgical facilities in both revision cohorts (p < 0.0001). The incidence of late seroma was too low to perform risk factor analysis.
Conclusions:
These data reaffirm the safety of Natrelle 410 implants. Knowledge of risk factors for capsular contracture and implant malposition offers guidance for reducing complications and optimizing outcomes.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Risk, II.
Breast implants have been available for cosmetic augmentation and postmastectomy breast reconstruction for more than 50 years. During this period, advances in shape, surface features, and composition have led to improved safety and high levels of patient satisfaction. The Natrelle 410 breast implant (Allergan plc, Dublin, Ireland) is a teardrop-shaped, textured, highly cohesive silicone gel implant designed to mimic the natural slope of the breast.1,2 It has a Biocell (Allergan) textured shell surface consisting of irregularly arranged depressions with a mean pore diameter of 300 mm (range, 100 to 600 mm), which is designed to reduce implant mobility and, therefore, minimize the risk of rotation.2–4 The Natrelle 410 breast implant was introduced initially in Europe in 1993 and was approved by the U.S. Food and Drug Administration in 2013. It is manufactured in 12 styles, combining different ratios of height (low, moderate, or full) and projection (low, moderate, full, or extra full).4 Each style is designated by two letters corresponding to the height and projection, respectively (e.g., FM indicates full height, moderate projection). The long-term safety and effectiveness of Natrelle 410 implants are supported by results from a 10-year, prospective multicenter study.5 At 10 years, satisfaction rates of subjects who received Natrelle 410 implants were 96 percent in primary augmentation, 93 percent in primary reconstruction, and more than 87 percent in revision procedures.5
Capsular contracture and implant malposition are common causes for revision surgery after breast implantation.6,7 In the long-term study, these complications occurred at lower rates with Natrelle 410 compared with prior reports of standard round gel implants.4,5,8,9 Late seroma is a rare complication that manifests as fluid collection in the periprosthetic space occurring more than 1 year after implantation.10 Interest in late seroma has increased because of its similar clinical presentation compared with breast implant–associated anaplastic large cell lymphoma.11,12 Most cases of late seroma have been observed in subjects receiving textured implants, but whether any relationship exists between a particular textured implant and late seroma is not clear.13 The present report analyzes potential risk factors for development of capsular contracture, implant malposition, and late seroma in subjects receiving Natrelle 410 breast implants in two ongoing, prospective, multicenter studies.
PATIENTS AND METHODS
These analyses were based on data collected through July 31, 2014, in the Continued Access and Continued Access Reconstruction/Revision Expansion clinical trials. Data from these ongoing 10-year trials were pooled, as they had similar study designs. Natrelle 410 devices were implanted by surgeons certified by the American Board of Plastic Surgery with experience placing silicone-filled implants. Investigators for the Continued Access trial were required to have participated in the 410 pivotal study, and investigators for the Continued Access Reconstruction/Revision Expansion trial were required to have participated in either the 410 pivotal study or another Allergan-sponsored study of shaped gel implants. All investigational sites had sufficient support staff to meet the study’s documentation objectives. Both studies were conducted in compliance with U.S. Food and Drug Administration requirements. Each site obtained approval from the relevant institutional review board before enrolling any subjects. All subjects provided written informed consent before surgery.
Subject Eligibility
The Continued Access study enrolled subjects presenting for primary breast augmentation, primary breast reconstruction, or breast implant revision surgery, whereas the Continued Access Reconstruction/Revision Expansion trial enrolled only subjects presenting for breast reconstruction or implant revision. Eligible subjects were aged 18 years or older, had adequate tissue available to cover the implants, and were willing to follow all study requirements. Subjects were excluded if they had advanced fibrocystic disease considered to be premalignant without accompanying subcutaneous mastectomy, breast cancer without mastectomy, an abscess or infection at the time of enrollment, any disease known to impact wound healing, tissue characteristics incompatible with mammaplasty, any condition that contributes unwarranted surgical risk, psychological characteristics incompatible with the surgical procedure or implant, or an unwillingness to undergo further surgery for revision if medically required. Subjects who were pregnant or nursing were also ineligible.
Data Collection
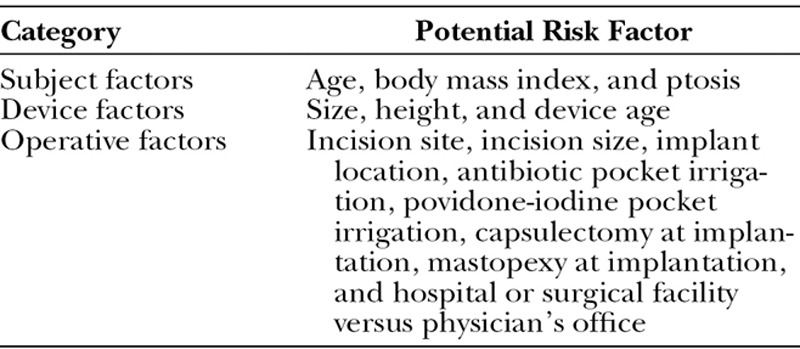
All subjects were scheduled for regular monitoring for 10 years after implantation. The development of capsular contracture, malposition, and late seroma (i.e., occurring >1 year after implantation) was documented on standardized case report forms at the time of occurrence and reported by investigators to the study sponsor. Potential risk factors related to the subject, implant, operation, and facility were collected on case report forms before implantation (Table 1).
Table 1.
Risk Factors Analyzed
Statistical Analysis
A Cox proportional hazards regression analysis was performed for possible associations with the development of capsular contracture, malposition, and late seroma in each of the four study cohorts: primary breast augmentation, revision-augmentation, primary breast reconstruction, and revision-reconstruction. Marginal and multivariable models were constructed as appropriate for each of the potential risk factors; marginal models resulting in a significance of p < 0.25 were entered into a multivariable model, with a stay criterion of p < 0.01, using the backward elimination technique. Categorical covariate factors (e.g., inframammary versus periareolar site) and continuous covariates (e.g., body mass index, incision size) were analyzed using analysis of variance, or Kruskal-Wallis tests when a categorical and a continuous covariate were compared, chi-square or Fisher’s exact tests when comparing two categorical covariates, or Pearson or Spearman correlation coefficients when comparing two continuous covariates (as appropriate). Statistically significant risk factors were presented as summary statistics. Unadjusted risk ratios were determined directly from the reported incidence of each complication for all marginal models, whereas the adjusted risk ratio and its 95 percent confidence interval were calculated from the multivariable model, which adjusts for the other significant predictors in the model.
RESULTS
Subjects
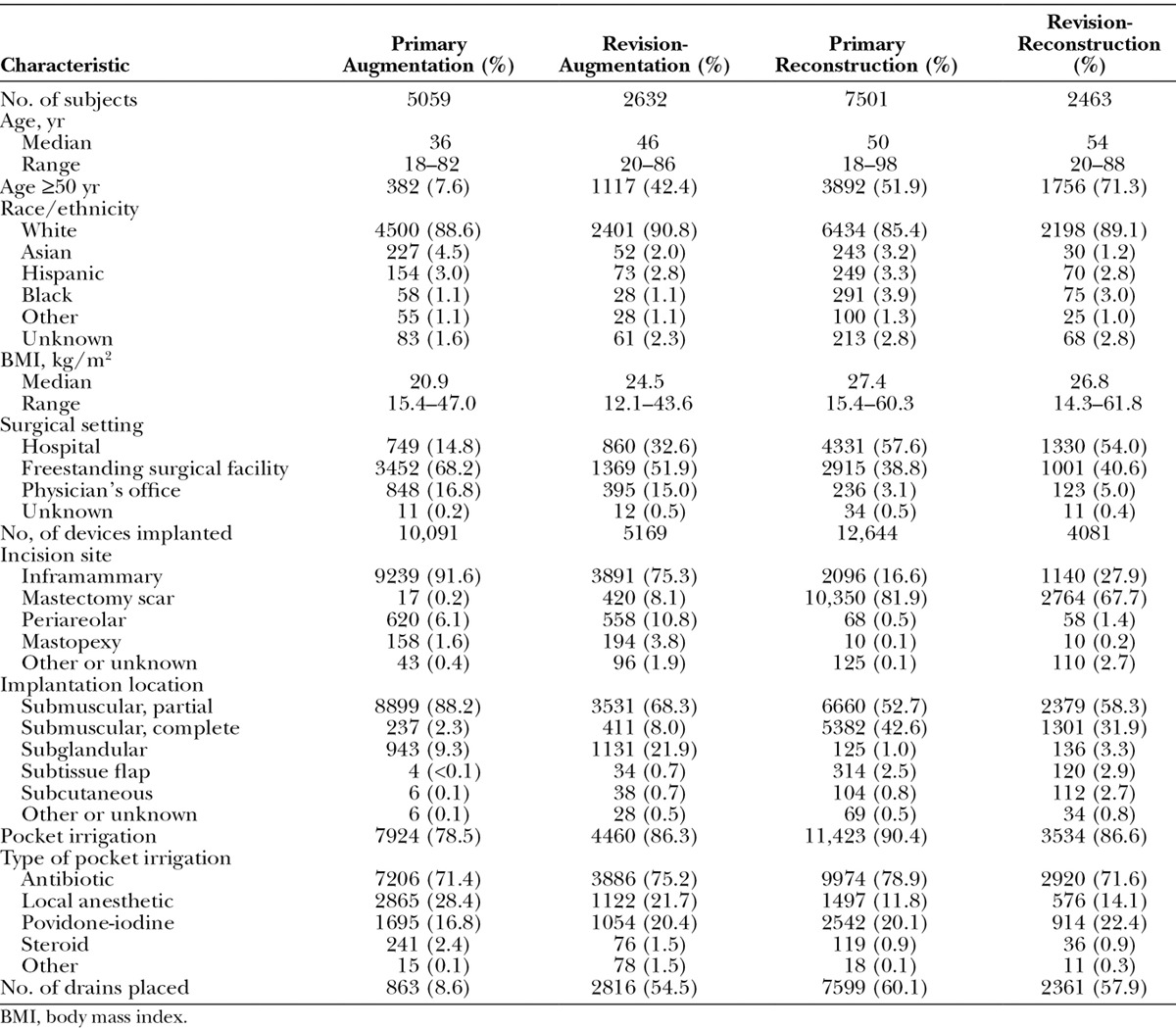
A total of 17,656 subjects received Natrelle 410 breast implants for augmentation (n = 5059), revision-augmentation (n = 2632), reconstruction (n = 7502), or revision-reconstruction (n = 2463) (Table 2). At the time of implantation, the median age in these cohorts ranged from 36 to 54 years, and the median body mass index ranged from 20.9 to 27.4 kg/m2. Subjects in each cohort were predominantly white. Because of the ongoing study enrollment, some subjects have been studied through 10 years of follow-up, whereas others were more recently enrolled. All subjects had surpassed the 1-year time point after implantation, and 93.9 percent had surpassed the 2-year time point. Mean follow-up after implantation was 4.1 years for the augmentation group, 3.7 years for the revision-augmentation group, 2.9 years for the reconstruction group, and 3.5 years for the revision-reconstruction group.
Table 2.
Subject and Surgical Characteristics
Devices
A total of 31,985 devices were implanted, including 10,091 in the augmentation cohort, 5169 in the revision-augmentation cohort, 12,644 in the reconstruction cohort, and 4081 in the revision-reconstruction cohort (Table 3). The styles of the Natrelle 410 device that were implanted varied by cohort, with most augmentations using full- or moderate-projection devices and most reconstructions performed with extra-full– or full-projection implants. The three most frequently used implants in the primary augmentation cohort were the full height, moderate projection, moderate height, full projection, and full height, full projection styles. In the revision-augmentation cohort, they were the full height, extra-full projection, moderate height, full projection, and full height, full projection styles. The full height, extra-full projection, moderate height, extra-full projection, and moderate height, full projection were the most commonly used in the primary reconstruction cohort, and the full height, extra-full projection, full height, full projection, and moderate height, full projection styles were used most frequently in the revision-reconstruction cohort.
Table 3.
Three Most Frequently Used Implant Sizes for Each Natrelle 410 Breast Implant Style
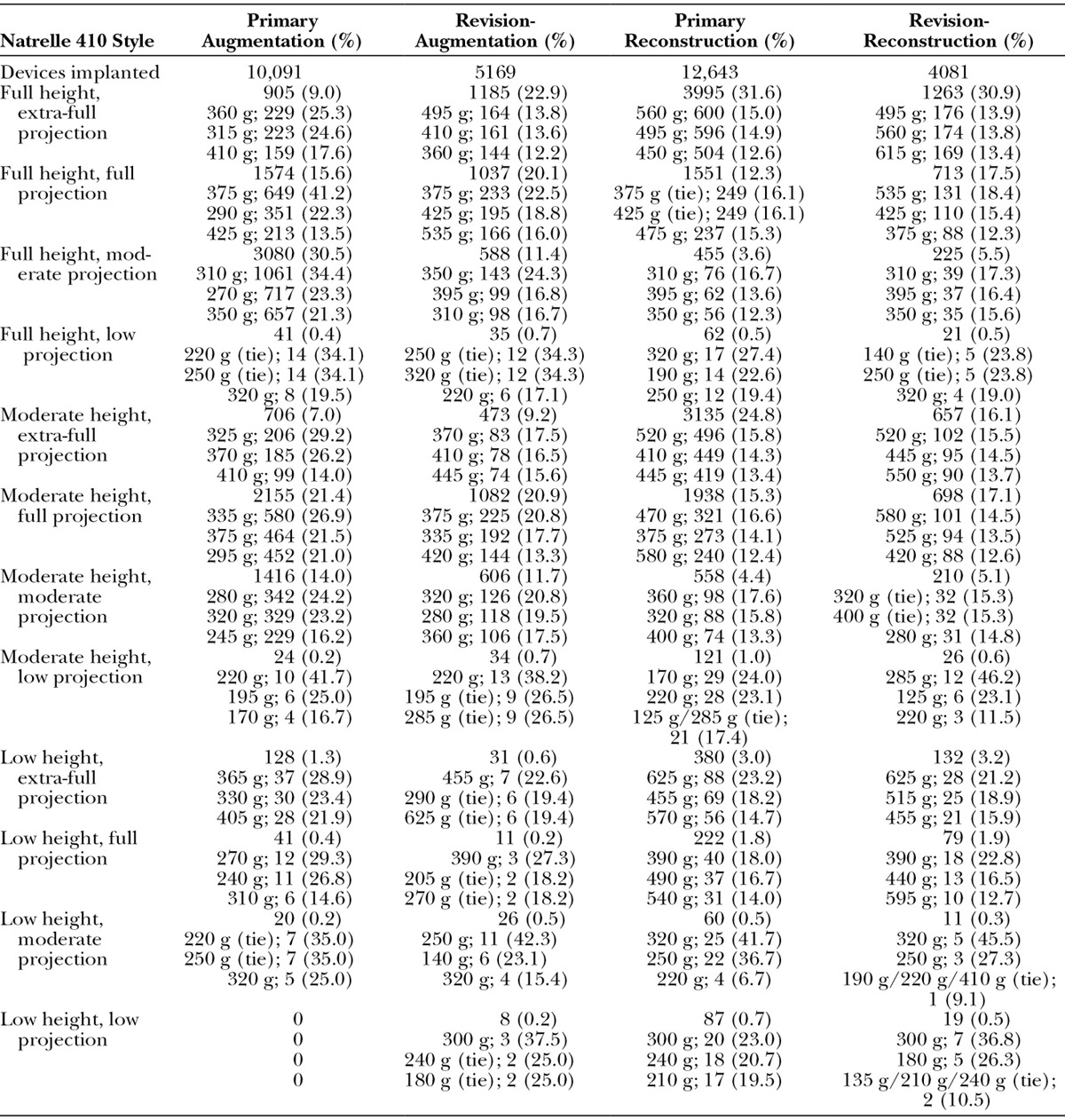
The most frequently used implant sizes for each implant style were similar across respective primary and revision procedures (Table 3). For example, for the full height, extra-full projection implants, the 360-cc and 495-cc sizes were favored for primary augmentation and revision-augmentation, respectively, whereas the 560-cc and 495-cc sizes were favored for primary reconstruction and revision-reconstruction, respectively. For the full-height, full-projection implants, the 375-cc size was favored for both primary augmentation and revision-augmentation, whereas the 375-cc and 425-cc sizes were equally favored for primary reconstruction and the 535-cc size was favored for revision-reconstruction.
Surgical Procedures
Augmentation procedures were mostly performed by means of an inframammary incision, and reconstruction procedures were mostly performed through an incision in the mastectomy scar (Table 2). In each cohort, the majority of devices were implanted using a partial submuscular placement ranging from 52.7 percent in the primary reconstruction cohort to 88.2 percent in the primary augmentation cohort. Complete submuscular placement was the next most common for both the primary reconstruction and revision-reconstruction cohorts, and subglandular was the next most common placement in both the primary augmentation and revision-augmentation cohorts. Pocket irrigation was used in the majority of implant surgeries, ranging from 78.5 percent for primary augmentation to 90.4 percent for primary reconstruction. Antibiotics were included in pocket irrigation in at least 70 percent of the implant surgeries in each cohort, with povidone-iodine included in 16.8 to 22.4 percent and local anesthetics included in 11.8 to 28.4 percent of cases. The majority of augmentation procedures were performed in freestanding surgical facilities, whereas most reconstruction procedures were performed in hospitals. A small percentage of procedures were performed in a physician’s office.
Capsular Contracture
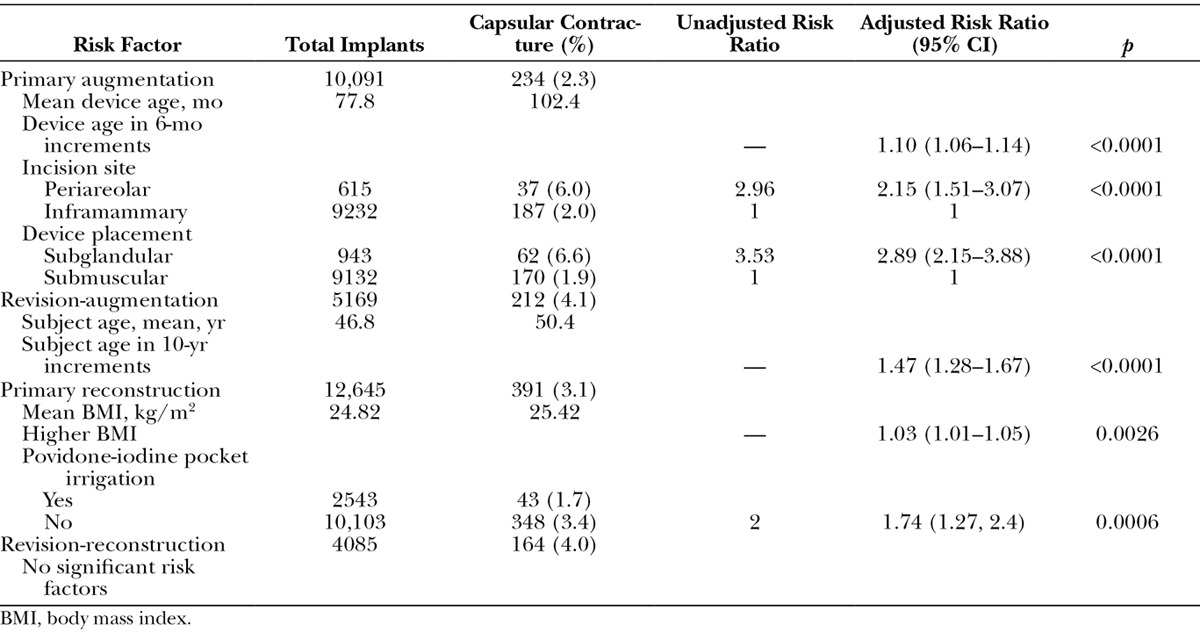
Capsular contracture events occurred for 234 implants (2.3 percent) in the augmentation cohort, 212 implants (4.1 percent) in the revision-augmentation cohort, 391 implants (3.1 percent) in the reconstruction cohort, and 164 implants (4.0 percent) in the revision-reconstruction cohort. Risk factors for development of capsular contracture varied across cohorts (Table 4). Notably, device size and height did not significantly affect risk in any cohort. In the primary augmentation cohort, subglandular placement of the device was the strongest univariate risk factor for development of capsular contracture (adjusted risk ratio, 2.89; p < 0.0001). Older device age and periareolar incision site were also significantly associated with capsular contracture risk (both p < 0.0001). No subject-related risk factors were identified in the primary augmentation cohort. Older subject age was the only factor associated with capsular contracture risk in the revision-augmentation group (p < 0.0001). Higher body mass index levels (p = 0.0026) and absence of povidone-iodine pocket irrigation (p = 0.0006) were risk factors associated with capsular contracture in the primary reconstruction cohort. No significant risk factor was identified in the revision-reconstruction group. Double capsule formation was not prospectively followed in these studies; however, one anecdotal report of this complication was recorded.
Table 4.
Risk Factors for Capsular Contracture by Indication
Malposition
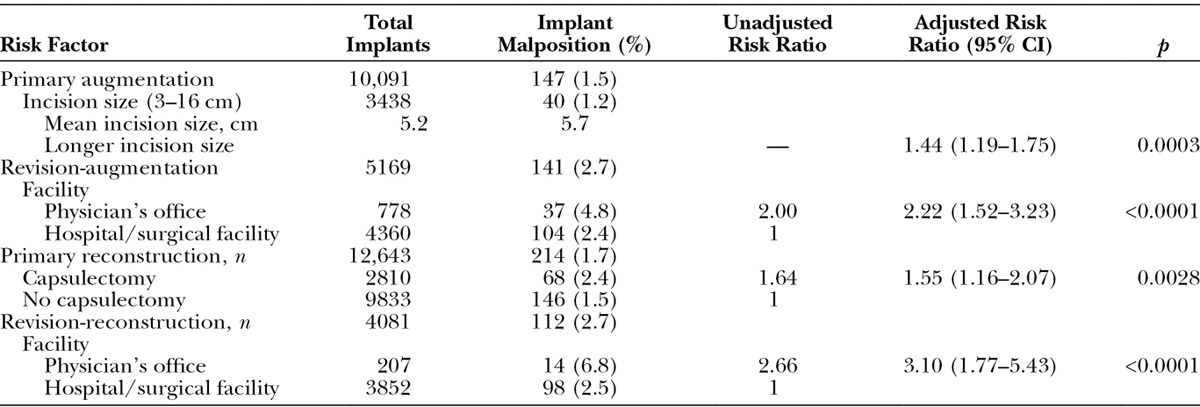
Malposition was reported for 147 implants (1.5 percent) in the primary augmentation cohort, 141 implants (2.7 percent) in the revision-augmentation cohort, 214 implants (1.7 percent) in the primary reconstruction cohort, and 112 implants (2.7 percent) in the revision-reconstruction cohort. Risk of malposition was not associated with any subject- or device-related factors, but was significantly associated with longer incision size in the primary augmentation cohort (p = 0.0003) and with capsulectomy performed at the time of implantation in the primary reconstruction cohort (p = 0.0028) (Table 5). In the two revision cohorts, risk of malposition was two to three times higher for implantations performed in a physician’s office compared with a hospital or standalone surgical facility (both p < 0.0001).
Table 5.
Risk Factors for Malposition by Indication
Late Seroma
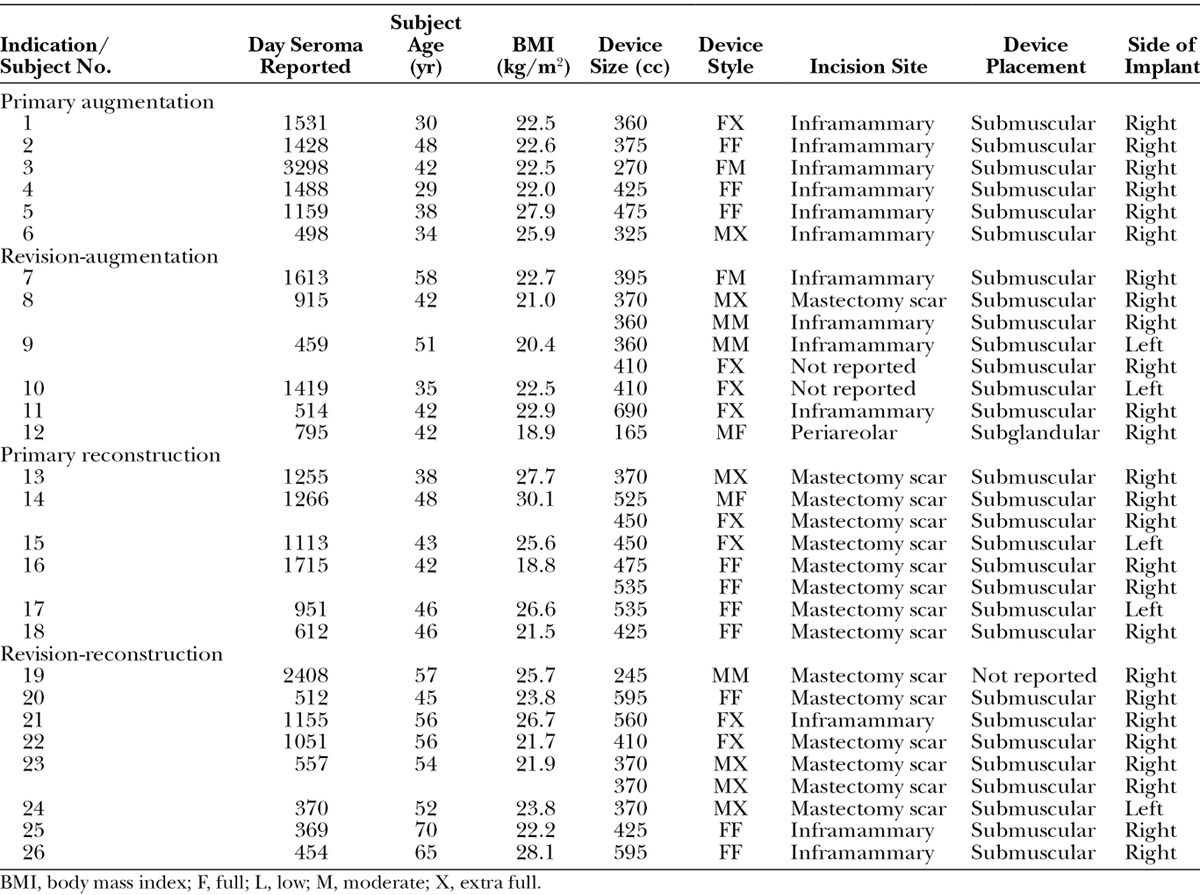
Overall, seroma was identified more than 1 year after implantation in 31 of the 31,985 implants (0.1 percent). Rates were 0.06 percent in the primary augmentation cohort, 0.15 percent in the revision-augmentation cohort, 0.06 percent in the primary reconstruction cohort, and 0.22 percent in the revision-reconstruction cohort (Table 6). Development of late seroma was bilateral in five subjects and unilateral in 21 subjects. All unilateral cases occurred in the right breast. The cases of late seroma were insufficient to perform a risk factor analysis. All cases occurred with submuscular device placement. No obvious trends toward the development of late seroma were observed in relation to subject age or body mass index, device size, style, or incision site. Four cases of breast implant–associated anaplastic large cell lymphoma were reported. One case each was reported in the augmentation, revision-augmentation, reconstruction, and revision-reconstruction cohorts. In these four subjects, breast implant–associated anaplastic large cell lymphoma was diagnosed from approximately 3.5 to 11.6 years after implantation.
Table 6.
Subjects with Unilateral or Bilateral Seroma Occurring More than 1 Year after Device Implantation
DISCUSSION
The present analysis reaffirms the safety of the Natrelle 410 breast implant in women undergoing primary breast augmentation, postmastectomy breast reconstruction, or revision after augmentation or reconstruction. Across the four cohorts, the incidence of capsular contracture ranged from 2.3 to 4.1 percent, implant malposition ranged from 1.5 to 2.7 percent, and late seroma ranged from 0.1 to 0.2 percent. The lower end of incidence rates was found in subjects who underwent primary breast augmentation, and the higher incidence rates were reported for subjects who underwent revision surgery. These rates are similar to those reported in earlier studies of Natrelle 410 breast implants at comparable follow-up time points.1,4
The risk of capsular contracture was associated with a periareolar incision site and subglandular device placement in the primary augmentation cohort, consistent with findings from other studies with large cohorts.8,14,15 In the studies by Namnoum et al.14 and Stevens et al.,15 the risk of capsular contracture was also significantly higher (p < 0.03) with smooth surface devices versus those having a textured surface, consistent with the low rate of capsular contracture associated with Natrelle 410 breast implants. Device age was also identified as a significant risk factor for capsular contracture in the primary augmentation cohort in the current study (p < 0.0001), reflecting the small increasing rate of this complication over time. Modest increases in capsular contracture rates over time have been reported with Natrelle 410 and other breast implants during long-term follow-up.5,8 Higher body mass index was identified as the only significant risk factor for capsular contracture in the primary reconstruction cohort. Other studies have shown capsular contracture risk to be associated with postmastectomy radiation therapy.16,17 However, information about postimplantation radiation therapy was not collected in the current study.
Triple-antibiotic pocket irrigation has been reported to reduce the incidence of capsular contracture after breast augmentation and reconstruction.18 Although antibiotic irrigation was delivered in the majority of cases in this study, the nature of the regimen was not collected. Because multiple bacteria have been implicated in producing capsular contracture, the irrigation antibiotics delivered may not necessarily have provided adequate coverage against these organisms.19
The risk of implant malposition was associated with one risk factor in each cohort. These factors included longer incision size in the primary augmentation cohort, capsulectomy in the reconstruction cohort, and performing the implantation in a surgeon’s office instead of a hospital or standalone surgical facility in the revision cohorts. In a previous analysis that included smooth and textured implants, the rate of moderate to severe malposition was lower with inframammary incision sites versus periareolar or axillary sites; with subpectoral versus subglandular placement; and with Natrelle 410 textured, shaped, highly cohesive silicone-filled breast implants versus smooth surface, round, silicone-filled breast implants.14
The rate of late seroma observed with the Natrelle 410 breast implant is consistent with the overall rate reported for breast implants in general,10 although higher rates of late seroma have been reported in smaller studies of subjects with breast implants.20,21 No significant risk factors for late seroma were identified with the Natrelle 410 breast implant, reflecting the small number of late seroma cases [26 of 17,656 subjects (0.15 percent) and 31 of 31,992 implanted devices (0.10 percent)]. It was recently suggested that late seroma may be a phenomenon related to textured implants.13 However, as the rate observed in this study is similar to that reported for breast implants in general, it is unlikely that the Natrelle 410 breast implant itself confers an incremental risk of late seroma relative to other implants.
Higher complication rates have been associated with extra-full projection implants compared with lower profile implants.22,23 However, the risk of capsular contracture, malposition, or late seroma was not associated with the size or the projection of the Natrelle 410 breast implants.
The findings of this study should be considered in light of the fact that results depended on the consistent reporting of complications by the investigators, thereby introducing a potential for bias. The investigators who participated in the Continued Access and the Continued Access Reconstruction/Revision Expansion studies were experienced surgeons who had participated in a previous pivotal trial of the Natrelle 410 breast implant or in another Allergan-sponsored study of shaped gel implants. Although individual investigators are likely to be consistent in their reporting of complications, differences in reporting may be expected between the many investigators who participated in these studies. Despite this limitation, the results of prospective multicenter studies are likely to be less biased than results obtained from retrospective analyses. In addition, although the incidence of capsular contracture was low, data on the course of radiation therapy in these subjects are limited. Therefore, conclusions about the impact of radiation therapy on capsular contracture cannot be drawn. The incidence of late seroma was also low in this analysis; however, definitive information may require follow-up at later time points. Although long-term follow-up in large cohorts of subjects presents substantial challenges, additional rigorous, prospective studies will continue to strengthen our understanding of the potential relationships between breast implant devices and procedures and late complications, such as seroma and capsular contracture. Recognition of the incidence of double capsule formation and its potential association with various breast implant procedures and devices was not well recognized when these lengthy studies were initiated, but evolved over the course of the studies.24 Double capsule formation was not prospectively followed; thus, no conclusions on incidence or associated risk factors can be drawn.
CONCLUSIONS
These findings reaffirm the low rates of complications in subjects receiving Natrelle 410 breast implants in primary and secondary surgical settings. Knowledge about the risk factors associated with capsular contracture and implant malposition may offer additional guidance to surgeons for reducing complication rates and optimizing outcomes.
ACKNOWLEDGMENTS
This study was sponsored by Allergan plc, Dublin, Ireland. Medical writing and editorial assistance was provided to the authors by Barry Weichman, Ph.D., and Michael L. Pucci, Ph.D., with Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan plc. Statistical analysis of the data was performed by Ramkumar Krish, M.S., of Allergan plc. All authors meet the International Committee of Medical Journal Editors authorship criteria. Neither honoraria nor payments were made for authorship.
Footnotes
Disclosure: P. McGuire is a consultant and speaker for Allergan plc. N.R. Reisman has no conflicts of interest to report. D.K. Murphy is an employee and stockholder of Allergan plc, Irvine, Calif.
REFERENCES
- 1.Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP Style 410 U.S. Core Clinical Study Group. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(Suppl 1):40S–48S. doi: 10.1097/01.prs.0000286666.29101.11. [DOI] [PubMed] [Google Scholar]
- 2.Hedén P. Breast augmentation with anatomic, high-cohesiveness silicone gel implants (European experience). In: Spear SL, Willey SC, Robb GL, Hammond DC, Nahabedian MY, editors. In: Surgery of the Breast: Principles and Art. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 1322–1345. [Google Scholar]
- 3.Barone FE, Perry L, Keller T, Maxwell GP. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;90:77–86. doi: 10.1097/00006534-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle style 410 form-stable silicone breast implants: Core study results at 6 years. Aesthet Surg J. 2012;32:709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell GP, Van Natta BW, Bengtson BP, Murphy DK. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35:145–155. doi: 10.1093/asj/sju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grewal NS, Fisher J. Why do patients seek revisionary breast surgery? Aesthet Surg J. 2013;33:237–244. doi: 10.1177/1090820X12472693. [DOI] [PubMed] [Google Scholar]
- 7.Forster NA, Künzi W, Giovanoli P. The reoperation cascade after breast augmentation with implants: What the patient needs to know. J Plast Reconstr Aesthet Surg. 2013;66:313–322. doi: 10.1016/j.bjps.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Spear SL, Murphy DK Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg. 2014;133:1354–1361. doi: 10.1097/PRS.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham B, McCue J. Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;33:440–444. doi: 10.1007/s00266-009-9364-6. [DOI] [PubMed] [Google Scholar]
- 10.Bengtson B, Brody GS, Brown MH, et al. Late Periprosthetic Fluid Collection after Breast Implant Working Group. Managing late periprosthetic fluid collections (seroma) in patients with breast implants: A consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011;128:1–7. doi: 10.1097/PRS.0b013e318217fdb0. [DOI] [PubMed] [Google Scholar]
- 11.Murphy S, Carroll S. Importance of histological analysis of seroma fluid. Aesthetic Plast Surg. 2013;37:187–188. doi: 10.1007/s00266-012-0007-y. [DOI] [PubMed] [Google Scholar]
- 12.Thompson PA, Prince HM. Breast implant-associated anaplastic large cell lymphoma: A systematic review of the literature and mini-meta analysis. Curr Hematol Malig Rep. 2013;8:196–210. doi: 10.1007/s11899-013-0164-3. [DOI] [PubMed] [Google Scholar]
- 13.Park BY, Lee DH, Lim SY, et al. Is late seroma a phenomenon related to textured implants? A report of rare complications and a literature review. Aesthetic Plast Surg. 2014;38:139–145. doi: 10.1007/s00266-013-0232-z. [DOI] [PubMed] [Google Scholar]
- 14.Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66:1165–1172. doi: 10.1016/j.bjps.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: A 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132:1115–1123. doi: 10.1097/01.prs.0000435317.76381.68. [DOI] [PubMed] [Google Scholar]
- 16.Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 17.Hvilsom GB, Hölmich LR, Steding-Jessen M, et al. Delayed breast implant reconstruction: Is radiation therapy associated with capsular contracture or reoperations? Ann Plast Surg. 2012;68:246–252. doi: 10.1097/SAP.0b013e318214e69c. [DOI] [PubMed] [Google Scholar]
- 18.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: Six-year prospective clinical study. Plast Reconstr Surg. 2006;117:30–36. [PubMed] [Google Scholar]
- 19.Adams WP., Jr Capsular contracture: What is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;36:119–126, vii. doi: 10.1016/j.cps.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Mazzocchi M, Dessy LA, Corrias F, Scuderi N. A clinical study of late seroma in breast implantation surgery. Aesthetic Plast Surg. 2012;36:97–104. doi: 10.1007/s00266-011-9755-3. [DOI] [PubMed] [Google Scholar]
- 21.Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg. 2011;35:303–314. doi: 10.1007/s00266-010-9607-6. [DOI] [PubMed] [Google Scholar]
- 22.Tebbetts JB, Teitelbaum S. High- and extra-high-projection breast implants: Potential consequences for patients. Plast Reconstr Surg. 2010;126:2150–2159. doi: 10.1097/PRS.0b013e3181f44564. [DOI] [PubMed] [Google Scholar]
- 23.Handel N. Secondary mastopexy in the augmented patient: A recipe for disaster. Plast Reconstr Surg. 2006;118(Suppl):152S–163S; discussion 164S. doi: 10.1097/01.prs.0000246106.85435.74. [DOI] [PubMed] [Google Scholar]
- 24.Hall-Findlay EJ. Breast implant complication review: Double capsules and late seromas. Plast Reconstr Surg. 2011;127:56–66. doi: 10.1097/PRS.0b013e3181fad34d. [DOI] [PubMed] [Google Scholar]


