Scale-up of a low-cost human papillomavirus testing implementation project in El Salvador showed that the follow-up completion rate with screen-and-treat management was twice that with colposcopy management.
Key Words: cervical cancer, human papillomavirus, screening and care of human papillomavirus
Abstract
Objective
The Cervical Cancer Prevention in El Salvador is a demonstration project to introduce a lower-cost human papillomavirus (HPV)-DNA test into a public sector project. Started in October 2012, The Cervical Cancer Prevention in El Salvador consists of 3 phases and will ultimately screen 30,000 women. Results of phase 2 of the project are presented. The objective of this project was to compare colposcopy and noncolposcopy-based management for HPV-positive women.
Material and Methods
In phase 2, a total of 8,050 women, aged 30 to 49 years, were screened; 6,761 provided both self- and provider-collected specimens and 1,289 provided only provider-testing specimens. HPV results from self-collected specimens were not used in clinical management decisions. Women with provider-collected HPV-positive results were treated based on the strategy assigned to their community; the strategy was colposcopy management (CM) or screen-and-treat (ST) management if they were cryotherapy eligible or colposcopy if not eligible. Outcomes were assessed 6 months after screening.
Results
Overall, 489 (12.3%) of 3,963 women receiving CM and 465 (11.4%) of 4,087 women receiving ST tested HPV positive. In the CM cohort, 216 (44.2%) of 489 completed their intervention (203 treated, 11 diagnosed negative, 2 pregnant). In the ST cohort, 411 (88.4%) of 465 completed their intervention (407 treated, 2 diagnosed negative, 1 pregnant). Overall agreement between HPV test results from self-collected and provider-collected specimens was 93.7%, with a κ value of 0.70 (95% CI = 0.68–0.73).
Conclusions
Human papillomavirus testing with ST management resulted in an approximately twice completion rate compared with CM management. Agreement between self- and provider-based sampling was good and might be used to extend screening to women in areas that are more difficult to reach.
Ninety percent of new cases of cervical cancer occur in low-resource settings.1 Human papillomavirus (HPV) tests used for cervical cancer screening by high-resource settings are often not accessible to low-resource settings because of cost and lack of infrastructure. The careHPV test (Qiagen, Germantown, Md), a low-cost high-risk HPV screening test, was developed specifically for low-resource settings.
Because lower-cost HPV testing is a relatively new tool, the most effective strategy for public sector project implementation is unknown. The World Health Organization (WHO) has endorsed both colposcopy referral and immediate treatment as management strategies after a positive HPV test result.2 Strategies that employ HPV testing and immediate treatment benefit low-resource settings because they are less costly and more feasible than cytology-based methods and result in a higher proportion of women with cervical precancer receiving appropriate treatment.3–5 Several studies have been conducted with careHPV6,7; however, these primarily investigated clinical outcomes. The public-sector implementation program presented in this article was initiated by the government, with the intention of national scale-up.
The Cervical Cancer Prevention in El Salvador (CAPE) project was launched in 2012 to identify best practices for incorporating HPV-based screening into the national cervical cancer prevention project. The CAPE is a 3-phase, 30,000-woman demonstration project that assesses the feasibility and cost-effectiveness of a screening intervention using the low-cost HPV test. The CAPE is conducted by the Salvadoran Ministry of Health (MOH), with technical support provided by the nonprofit organization Basic Health International (BHI). Phase 1 of the project screened 2,000 women. Women testing positive for HPV received 1 of 2 treatment strategies: colposcopy management (CM) consisting of colposcopy and management per local guidelines, or screen-and-treat (ST) management using visual inspection with acetic acid to determine cryotherapy eligibility, with eligible women undergoing immediate cryotherapy and ineligible women undergoing CM. In phase 1, more women in the ST cohort received treatment within 6 months compared with those in the CM cohort (117/119 [98.3%] vs 64/93 [68.8%], p < .001). Furthermore, ST was the most cost-effective strategy.8–10 During phase 2, a total of 8,000 women were included and the same screening strategies were used. The objective of phase 2 was to scale up the project and compare the CM and ST strategies using a larger sample size. The secondary aims were to assess the feasibility of self-sampling and to perform quality control of the local pathology system.
MATERIALS AND METHODS
Women in the Paracentral region were contacted between October 2013 and July 2014. The 4 health units that participated in phase 1 (San Pedro Perulapán, San Rafael Cedros, Apastepeque, and San Sebastián) were included in phase 2, and 4 health units (Candelaria, Tecoluca, Suchitoto, and Periferica de San Vicente) that provide primary preventive care in the Paracentral region of El Salvador were added. The health units were selected with the goal of contacting 10,000 women to meet the target of screening 8,000 women, assuming 80% follow-up. According to the 2007 national census, there were 21,968 women aged 30 to 49 years (the age range targeted in CAPE) living in these health units' catchment areas.
Women were excluded if they were known to be pregnant, had a hysterectomy, had any history of loop electrosurgical excision procedure (LEEP) or cryotherapy, or previously had cervical precancer or cancer. According to government census, the 8 health units served populations similar in age, poverty level, and education.
All women were to have both self- and provider sampling with the careHPV test. However, because of a power failure that interrupted refrigeration of the tests and invalid runs caused by human error, a limited number of testing kits were available for self-sampling. For these reasons, self-sampling was not available for all women.
Case management was based on HPV results from provider-collected samples only, because self-sampling is not yet an approved method of screening. Women who tested HPV positive were managed by either the CM or ST strategy. Human papillomavirus–negative women were instructed to repeat screening in 5 years.
Treatment strategies were not randomized because CAPE was designed as an implementation project rather than as a research study. Assignments were solely based on population size. Women in El Salvador attend specific health units based on the community in which they live, and treatment strategies were based on health units. We first calculated the size of the eligible population and then determined which communities would be assigned which treatment strategy to obtain 4,000 of women in each cohort.
One of the 8 health units was significantly larger than the others; of the 17 communities served by this health unit, 3 were assigned the CM strategy and the other 14 were assigned the ST strategy. In total, the CM cohort consisted of 4 health units and the 3 communities from the large health unit, and the ST cohort consisted of 3 health units and the 14 communities from the large health unit.
Human papillomavirus–positive women in the CM cohort were referred to colposcopy; treatment was based on colposcopy biopsy results. In accordance with recent WHO treatment guidelines for regions lacking sufficient infrastructure for management, all eligible HPV-positive women received immediate cryotherapy even if no lesion is visualized. Human papillomavirus–positive women in the ST cohort were referred to a follow-up visit with a physician gynecologist who performed visual assessment for treatment (VAT). The purpose of VAT is to assess for contraindications to cryotherapy, which include pregnancy, large cervical lesion or lesion that extends into the endocervical canal, or suspected cancer. Referrals for colposcopy and further management were provided for women with any contraindications.
In both cohorts, follow-up for HPV-positive women was considered completed if treatment occurred within 6 months of screening. For women referred for colposcopy (ie, either in the CM cohort or in the ST cohort and ineligible for cryotherapy), follow-up was completed if 1 of 3 outcomes occurred within 6 months of diagnosis: (1) normal colposcopic impression (no biopsy or endocervical curettage [ECC] performed), (2) normal biopsy and/or ECC, or (3) treatment based on biopsy or ECC results was completed. Following Ministry of Health guidelines, women with cervical intraepithelial neoplasia grade 1 (CIN 1) were treated with cryotherapy. Women with CIN grade 2 or 3 (CIN 2/3) had cryotherapy, LEEP, or a hysterectomy, as determined by the managing physician. For women in the ST cohort, follow-up was considered completed once they were treated with cryotherapy. The BHI research team actively managed the treatment strategies. Five nurses were hired specifically to monitor loss to follow-up. If a patient did not attend her colposcopy appointment after 6 months, a BHI staff member contacted a Ministry of Health supervisor.
χ2 tests, Fisher exact tests, and 2 sample t tests were used to assess associations across recruitment periods and management strategies between demographic variables (age, education attainment, residential area, and number of children); sexual history (age at sexual initiation and number of lifetime sexual partners); time since last screening; HPV positivity; and follow-up compliance. Logistic regression was used to explore relationships between HPV positivity and demographic and screening characteristics, recruitment period, and management strategy. Variables with p values of less than .10 in unadjusted models were entered into the multivariate model using backward elimination. The κ statistic and McNemar tests were used to compare agreement between provider- and self-collected sampling methods. The significance level was set at 0.05, and all statistical analyses were conducted using Stata Version 12 (StataCorp LP, 2011, College Station, Tex).
The national ethical review board of El Salvador and the Cleveland Clinic institutional review board approved the study.
RESULTS
A total of 8,205 women were contacted and asked to participate in the screening program at the local health center within 15 days, and 81.1% participated (6,656/8,205). Additional recruitment strategies were used to achieve the target goal of screening 8,000 women: (1) providers visited women at home and offered at-home testing with the sample collected by a provider (n = 332) and (2) opportunistic screening when eligible women presented to health centers for other reasons (n = 1,062). As a result of these 3 strategies, a total of 8,050 women were screened.
Table 1 presents demographic screening characteristics stratified by management cohort. Women in the ST and CM cohorts were statistically but not meaningfully different in age, highest education level, parity, age at first sexual intercourse, number of lifetime sexual partners, time since last screen, screening location, and residence. Women in the ST cohort were more likely to have no education than women in the CM cohort (515/3,342 [15.4%] vs 650/3,419 [19.0%], p < .001). Women in the CM cohort were more likely than women in the ST cohort to have been screened within the past 3 years (2,514/3,963 [64.1%] vs 2,293/4,087 [57.6%], p < .01) and to have been screened at a health unit (614/3,963 [15.5%] vs 416/4,087 [10.2%], p < .01).
TABLE 1.
Demographic and Screening Characteristics of Participants by Management Strategy Cohort and Recruitment Period
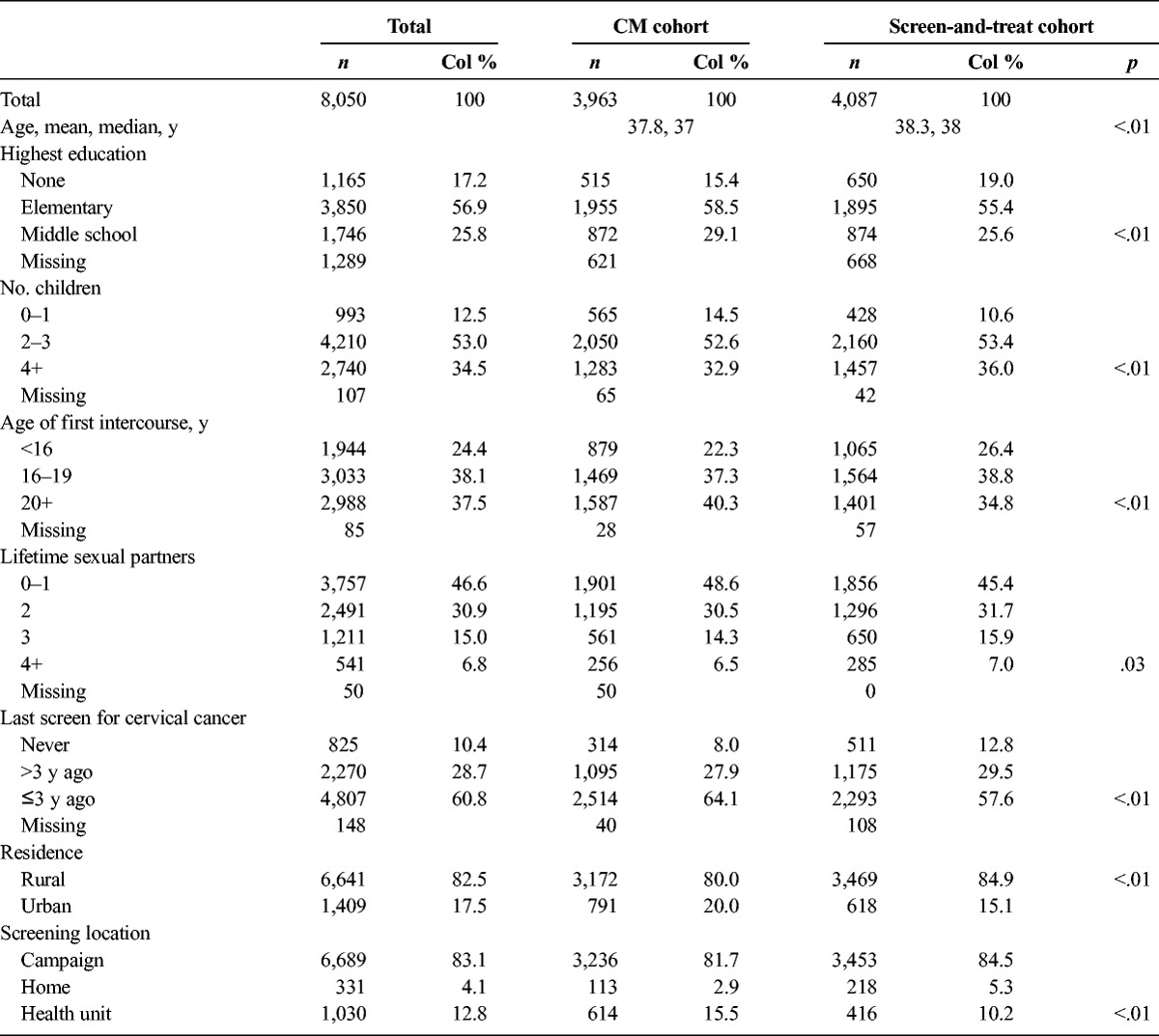
Table 2 presents summaries of outcomes within 6 months of screening by management cohort (a more detailed description is provided in Figures 1A, B). Women in the CM cohort were more likely to attend their screening appointments than women in the ST cohort (90.0% vs 74.3%, p < .001). Overall, 489 (12.3%) of 3,963 women in the CM cohort and 465 (11.4%) of 4,087 women in the ST cohort screened HPV positive (p = .18).
TABLE 2.
Compliance by Management Cohort
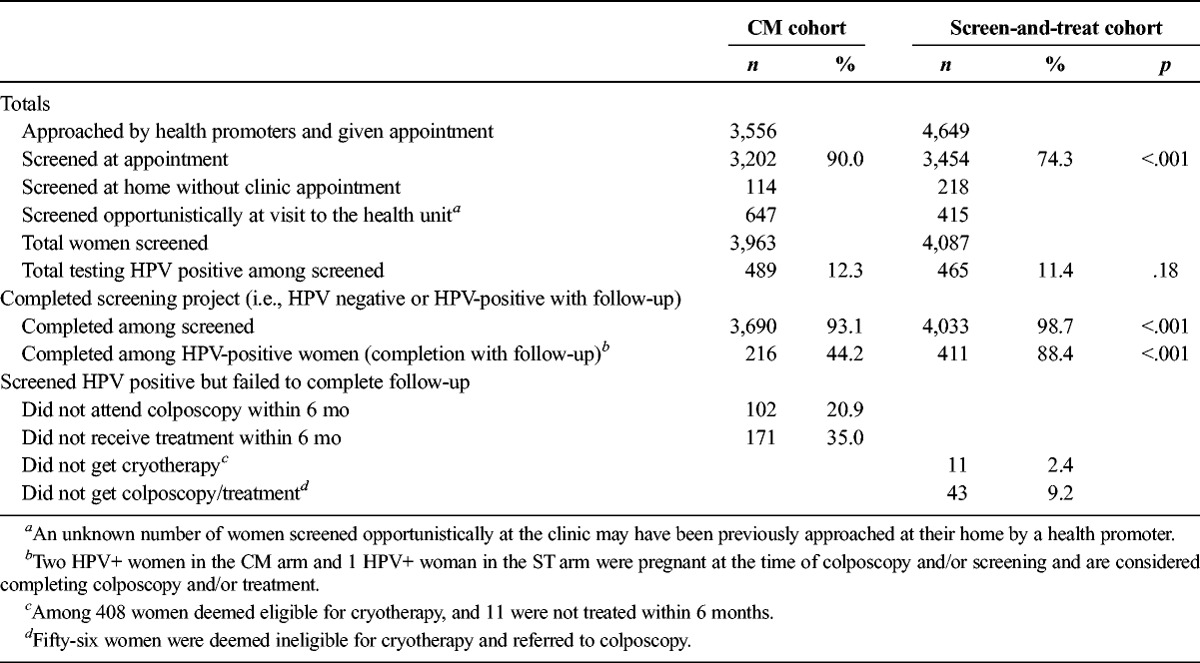
FIGURE 1.
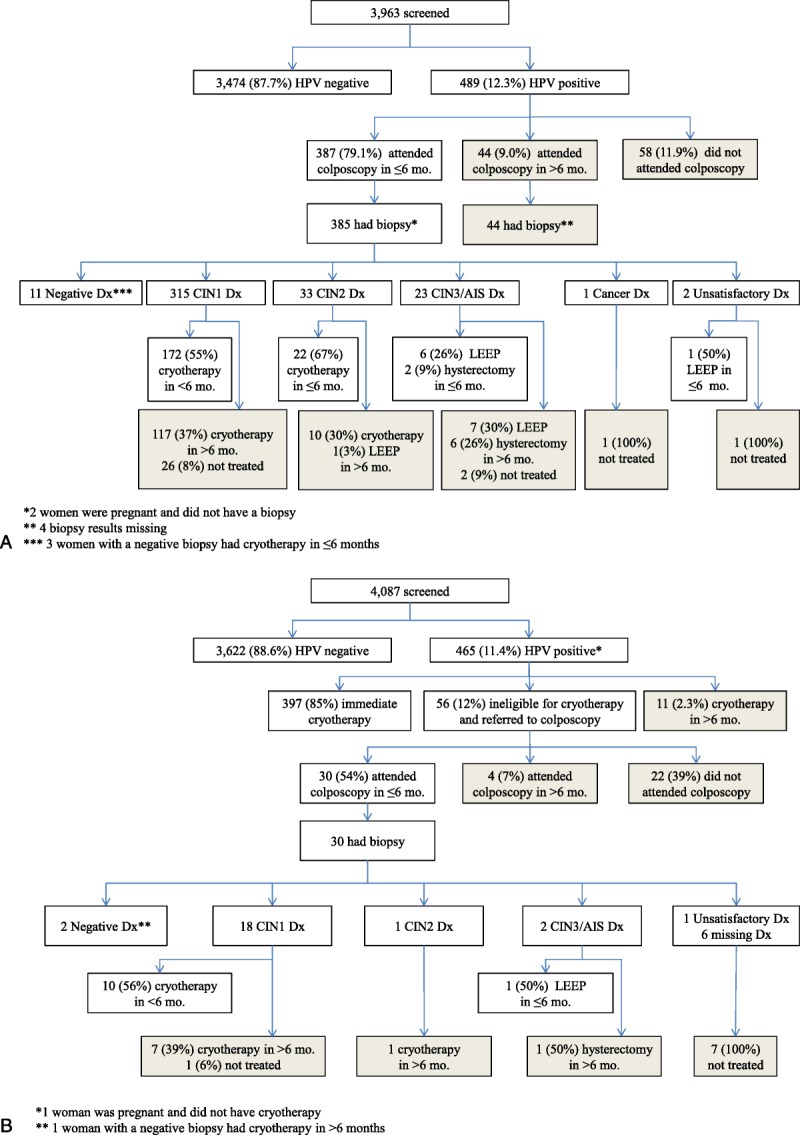
A, Colposcopy management cohort flow chart. B, Screen-and-treat management cohort flow chart.
Of the HPV-positive women, 216 (4.2%) of 489 in the CM cohort and 411 (88.4%) of 465 in the ST cohort completed their intervention (p < .001). In the CM cohort, 102 (20.9%) of 489 HPV-positive women did not attend colposcopy within 6 months and 171 (35.0%) of 489 were not treated in this same time span. In the ST cohort, 11 (2.4%) of 465 HPV-positive women did not receive cryotherapy and 43 (9.2%) of 465 either did not attend their colposcopy appointment or did not receive treatment within 6 months. Overall, 3,690 (93.1%) of 3,963 women screened in the CM cohort and 4,033 (98.7%) of 4,087 women screened in the ST cohort completed the recommended intervention and follow-up on the basis of HPV status (p < .001).
The flow charts for both management cohorts (see Figures 1A, B) illustrate the sequence of screening, diagnosis, and treatment. As shown in Figure 1A, 99.5% (385/387) of women in the CM cohort who attended colposcopy within 6 months had biopsy specimens taken. The local pathologist diagnosed 315 (81.8%) of 385 women with CIN 1; 172 (54.6%) of 315 had cryotherapy within 6 months because local guidelines recommend treatment for CIN 1. Of the 33 (8.6%) of 385 women with CIN 2, 22 (66.7%) of 33 were appropriately treated within 6 months. Of the 23 (6.0%) of 385 with CIN 3 or adenocarcinoma in situ, 8 (35.0%) of 23 were appropriately treated within 6 months. One woman had invasive cancer and was treated. Two women had unsatisfactory colposcopy; one was treated and one was lost to follow-up.
As shown in Figure 1B, 85% (397/465) of women screened in the ST cohort were treated without colposcopy after visual assessment triage (VAT). Among the remaining 68 women, 12 (2.6%) of 465 did not receive immediate treatment, because of either available treatment (11/12) or pregnancy (1/12); 56 (12.0%) of 465 were not eligible for cryotherapy and were referred to colposcopy. Of those referred to colposcopy, 30 (54%) of 56 attended and biopsies were taken in all examinations. Among the 3 women diagnosed with CIN 2 or CIN 3/adenocarcinoma in situ, one was treated within 6 months.
Table 3 presents the expert pathology readings for biopsies from colposcopy examinations in both cohorts. In the CM cohort, 423 (86.5%) of 489 HPV-positive women had biopsy results. In the ST cohort, because only women ineligible for cryotherapy were referred for colposcopy, 32 (6.9%) of 465 HPV-positive women had biopsy results. Expert pathologist diagnosis found that percentages of CIN 2+ and CIN 3+ diagnoses were higher among women who had biopsies in the ST cohort than among those in the CM cohort, although the difference was only statistically significant for CIN 3+ diagnoses (CIN 2+: 10/32 [31.2%] vs 82/423 [19.4%], p = .11; CIN 3+: 9/32 [28.1%] vs. 46/423 [10.9%], p = .004). Data for compliance with treatment based on expert pathologist diagnosis among women in the CM cohort are presented in Table 4.
TABLE 3.
Expert Pathologist Biopsy Readings Among Women Attending Colposcopy by Management Cohort
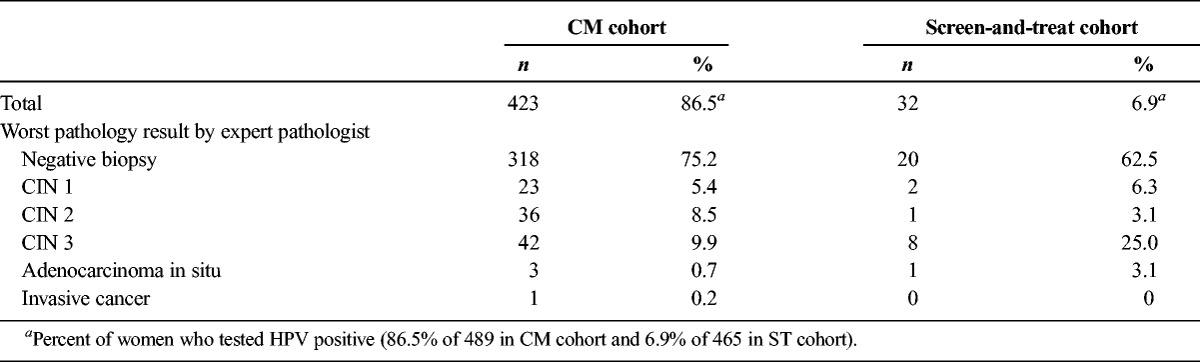
TABLE 4.
Treatment Given Expert Pathologist Biopsy Readings Among Women Attending Colposcopy in the CM Cohort
All slides were reviewed by an expert pathologist. This review showed that the local pathologist was significantly more likely to diagnose CIN 1 (303/365 [83.0%] vs 18/365 [4.9%]) and less likely to diagnose CIN 2/3 than the expert pathologist. The local pathologist diagnosed 27 (39.7%) of 68 CIN 2+ cases found by the expert pathologist (p < .01). κ agreement between the pathologists was 0.06 (95% CI = 0.04–0.07). After the review, the expert pathologist and local pathologist together examined all discrepant cases using a multiheaded microscope. During this process, the local pathologist observed the presence of CIN 2/3 in biopsies in which he had previously made a diagnosis of CIN 1. In most of these cases, the local pathologist's locator skills failed to identify small fragments of detached CIN 2/3 present in the slides.
More than 85% of all women had both self- and provider sampling; 1,289 women had only provider sampling because of the shortage of testing kits available for self-sampling. Overall agreement between HPV test results from self-collected and provider-collected samples was 93.7%, with a κ value of 0.70 (95% CI = 0.68–0.73). Twenty-seven percent (25/92) of women with CIN 2+ tested positive with the provider-collected specimen but not with the self-collected specimen. We do not know the true sensitivity of self-collection because we did not further investigate cases in which HPV was detected in self-collected samples and not detected in provider-collected specimens.
DISCUSSION
The results of phase 2 of CAPE were similar to the results of phase 1, only on a larger scale.11 Both cohorts had very high percentages of women who initiated appropriate follow-up after screening (93% for CM and 99% for ST). The substantial difference between the percentages of women who completed the intervention at 6 months in the 2 cohorts was conclusive. Only 44% of women completed appropriate follow-up in the CM cohort, compared with 88% of women in the ST group. Because the CM strategy required an additional visit, fewer women were able to complete the follow-up process.
Although the 2 treatment strategies were not randomized, the women in the 2 cohorts were similar according to national census data, indicating that the data are likely generalizable to the general population.12–14 The cohorts did not differ significantly in important variables such as age at first intercourse, number of lifetime partners, or number of live births.
The WHO recommendations for cervical cancer screening in low-resource settings include HPV screening followed by immediate triage and treatment. Although the ST strategy introduces the risk of overtreatment, the CM strategy poses a risk of undertreatment because of the increased likelihood of noncompliance with 1 or more of the multiple visits required to complete the management protocol. We believe that the benefits of the ST strategy far outweigh the risk of overtreatment because cryotherapy is a relatively benign procedure that potentially decreases women's risk of developing cancer and is associated with few adverse effects.15–18 Furthermore, the CM and ST strategies would likely result in equal numbers of cryotherapy treatments because the Salvadoran MOH guidelines advise cryotherapy even for CIN 1, a common diagnosis for women who screen HPV positive. Most women (81%) in the CM cohort referred for colposcopy were diagnosed with CIN 1. In fact, only 2.8% had a negative diagnosis. This fact is the rationale for the MOH decision to only use the ST strategy in the third and final phase of the project.
Most women in the CM cohort attended the colposcopy appointment; however, 15% of women with CIN 2 and 65% of women with CIN 3 did not receive appropriate treatment within 6 months. This finding underscores the importance of minimizing the number of visits necessary for women to receive treatment. In this study, most highest-risk women (those diagnosed with CIN 2 and CIN 3) did not return for appropriate management, leaving them at risk for invasive cancer.
This implementation project had higher follow-up rates than expected. Ministry of Health empowerment, involvement of community health providers, and the continuous technical support and active surveillance by nonprofit organization staff were key factors in encouraging appropriate follow-up.
Pathology diagnosis is challenging even in a highly regulated national project. The local pathologist diagnosed only 39.7% of the high-grade lesions identified by the expert pathologist upon secondary review. Even if all women underwent colposcopy, inaccurate pathology diagnosis may limit the effectiveness of a colposcopy-based strategy, because the success of that approach is dependent on pathology diagnosis.19,20 The importance of pathology diagnosis in cervical cancer prevention and treatment underscores the need for more resources to be directed toward pathology training.
Phase 2 of the implementation program demonstrated that the self-sampling process is feasible among a larger population than participated in phase 1. Overall agreement between provider sampling and self-sampling was good. Because provider sampling has better sensitivity and there is currently no label approval for self-sampling, the MOH decided to only use provider sampling in the third phase. Trials are underway investigating feasibility of self-sampling for women who do not attend screening appointments with provider sampling. We believe that the most effective screening algorithm will include self-sampling for women who are unable or unwilling to be tested by a provider.
The CAPE is the first public sector implementation of low-cost HPV screening in a low-resource setting. This implementation project demonstrates the feasibility and effectiveness of a paradigm that decreases the number of visits required and increases the percentage of women that complete treatment. The Salvadoran MOH successfully implemented a ST strategy consistent with recent WHO recommendations and will use only that strategy in phase 3. The decisive evidence of the success of ST management has encouraged stakeholders and decision makers to adopt this approach as the prevailing cervical cancer prevention strategy.

ACKNOWLEDGMENTS
The authors thank the Einhorn Family Charitable Trust and the Union for International Cancer Control for their support of this project.
Footnotes
The authors have declared they have no conflicts of interest.
M.C. is a paid consultant for Merck and has received honoraria as a speaker. J.K. is a paid consultant for Basic Health International. P.E.C. has received commercial HPV tests for research at a reduced or no cost from Roche, Qiagen, Norchip, Arbor Vita Corporation, BD, and mtm. P.E.C. has been compensated financially as a member of Merck Data and Safety Monitoring Board for HPV vaccines. P.E.C. has been a paid consultant for Gen-Probe/Hologic, Roche, Cepheid, ClearPath, Guided Therapeutics, Teva Pharmaceutics, Genticel, Inovio, and GE Healthcare. P.E.C. has received honoraria as a speaker for Roche and Cepheid. Dr. Gage has received HPV testing for research at no cost from Roche and BD.
The national ethical review boards of El Salvador and the Cleveland Clinic granted institutional review board approval.
REFERENCES
- 1. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; 2013.
- 2.WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 3.Goldie SJ, Kuhn L, Denny L, et al. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA 2001;285:3107–15. [DOI] [PubMed] [Google Scholar]
- 4.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 2005;353:2158–68. [DOI] [PubMed] [Google Scholar]
- 5.Campos NG, Maza M, Alfaro K, et al. The comparative and cost-effectiveness of HPV-based cervical cancer screening algorithms in El Salvador. Int J Cancer 2015;137:893–902. [DOI] [PubMed] [Google Scholar]
- 6.Bansil P, Wittet S, Lim JL, et al. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health 2014;14:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenzi AT, Fregnani JH, Possati-Resende JC, et al. Self-collection for high-risk HPV detection in Brazilian women using the careHPV™ test. Gynecol Oncol 2013;131:131–4. [DOI] [PubMed] [Google Scholar]
- 8.Campos NG, Castle PE, Wright TC, Jr, et al. Cervical cancer screening in low-resource settings: a cost-effectiveness framework for valuing tradeoffs between test performance and program coverage. Int J Cancer 2015;137:2208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny L, Kuhn L, De Souza M, et al. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA 2005;294:2173–81. [DOI] [PubMed] [Google Scholar]
- 10.Denny L, Kuhn L, Hu CC, et al. Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst 2010;102:1557–67. [DOI] [PubMed] [Google Scholar]
- 11.Cremer ML, Maza M, Alfaro KM, et al. Introducing a high-risk HPV DNA test into a public sector screening program in El Salvador. J Low Genit Tract Dis 2016;20:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum AJ, Gage JC, Alfaro KM, et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet 2014;126:156–60. [DOI] [PubMed] [Google Scholar]
- 13.Agurto I, Sandoval J, De La Rosa M, et al. Improving cervical cancer prevention in a developing country. Int J Qual Health Care 2006;18:81–6. [DOI] [PubMed] [Google Scholar]
- 14.Bruni L B-RL, Albero G, Aldea M, et al. Human papillomavirus and related diseases in El Salvador. Summary Report. ICO Information Centre on HPV and Cancer (HPV Information Centre); 2015.
- 15.Jacob M, Broekhuizen FF, Castro W, et al. Experience using cryotherapy for treatment of cervical precancerous lesions in low-resource settings. Int J Gynaecol Obstet 2005;89(suppl 2):S13–20. [DOI] [PubMed] [Google Scholar]
- 16.McClung EC, Blumenthal PD. Efficacy, safety, acceptability and affordability of cryotherapy: a review of current literature. Minerva Ginecol 2012;64:149–71. [PubMed] [Google Scholar]
- 17.Mitchell MF, Tortolero-Luna G, Cook E, et al. A randomized clinical trial of cryotherapy, laser vaporization, and loop electrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstet Gynecol 1998;92:737–44. [PubMed] [Google Scholar]
- 18.Chirenje ZM, Rusakaniko S, Akino V, et al. A randomised clinical trial of loop electrosurgical excision procedure (LEEP) versus cryotherapy in the treatment of cervical intraepithelial neoplasia. J Obstet Gynaecol 2001;21:617–21. [DOI] [PubMed] [Google Scholar]
- 19.Dalla Palma P, Giorgi Rossi P, Collina G, et al. The reproducibility of CIN diagnoses among different pathologists: data from histology reviews from a multicenter randomized study. Am J Clin Pathol 2009;132:125–32. [DOI] [PubMed] [Google Scholar]
- 20.Stoler MH, Schiffman M, Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001;285:1500–5. [DOI] [PubMed] [Google Scholar]


