Abstract
Aspirin (ASA) is known to alter the production of potent inflammatory lipid mediators, but whether it interacts with omega-3 fatty acids (FA) from fish oil to affect atherosclerosis has not been determined. The goal was to investigate the impact of a fish oil enriched diet alone and in combination with ASA on the production of lipid mediators and atherosclerosis. ApoE−/− female mice were fed for 13 weeks one of the four following diets: Omega-3 FA deficient (OD), Omega-3 FA Rich (OR) (1.8 g Omega-3 FAs/kg • diet per day), Omega-3 FA Rich plus ASA (ORA) (0.1 g ASA/kg • diet per day), or an Omega-3 FA deficient plus ASA (ODA) with supplement levels equivalent to human doses. Plasma lipids, atherosclerosis, markers of inflammation, hepatic gene expression and aortic lipid mediators were determined. Hepatic omega-3 FAs were markedly higher in OR (9.9-fold) and ORA (7-fold) groups. Mice in both OR and ORA groups had 40% less plasma cholesterol in VLDL and LDL fractions, but aortic plaque area formation was only significantly lower in the ORA group (5.5%) compared to the OD group (2.5%). Plasma PCSK9 protein levels were approximately 70% lower in the OR and ORA groups. Pro-inflammatory aortic lipid mediators were 50–70% lower in the ODA group than in the OD group and more than 50% lower in the ORA group. In summary, less aortic plaque lesions and aortic pro-inflammatory lipid mediators were observed in mice on the fish oil diet plus ASA versus just the fish oil diet.
Keywords: Atherosclerosis, Inflammation, Fish oil, Omega-3 Fatty Acids, Aspirin
1. Introduction
It is well known that elevated plasma levels of lipids and pro-inflammatory cytokines are strongly associated with cardiovascular disease (CVD) [1–3]. Based on numerous animal and human studies, omega-3 fatty acids (FAs), which are enriched in fish oils, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are considered to be atheroprotective, because of their potent lipid-lowering and anti-inflammatory effects [4, 5]. Acetylsalicylic acid (ASA), also commonly known as aspirin, is a cyclooxygenase 1, 2 (COX-1, 2) inhibitor and has also been shown to be useful in the prevention of CVD. This has largely been attributed to its anti-platelet effect [6], but possibly also because of its general anti-inflammatory and pro-resolving properties from the biosynthesis of aspirin-triggered lipid mediators derived from essential fatty acids [7, 8]. Recent clinical trials have found that treatment with fish oils in combination with ASA led to significant reduction in inflammatory cytokines and that these two agents can work synergistically in lowering CVD risk [9, 10].
Although it is generally agreed that treatment with fish oils plus ASA may result in beneficial lipid and anti-inflammatory changes, the mechanisms for this synergy is not fully understood. Some studies have suggested that the fish oil treatment effect on CVD is distinct from its effect on lowering plasma triglycerides and cholesterol and instead may be related to its ability to decrease the inflammatory response [11]. One possible interaction between ASA and fish oil is the ability of ASA to alter the production of specialized pro-resolution mediators (SPMs), such as resolvins, protectins and their aspirin-triggered forms [12], which are all derived from EPA and DHA. SPMs are key molecules involved in the resolution of the inflammatory process and may also have anti-atherosclerotic properties [13]. The acetylation of COX-2 by ASA inhibits prostaglandin formation but still allows the production of 15R-HETE from arachidonic acid and 17R-HDHA from DHA [7, 14]. Further conversion of these by leukocytes, for example, can lead to the production of aspirin-triggered lipoxins, which have potent anti-inflammatory actions [15] or from 17R-HDHA in the case of the D-series resolvins.
Thus, the endogenous biosynthesis and metabolism of prostaglandins and SPMs from EPA and DHA may differ in the presence and absence of ASA, and therefore ASA could significantly alter the anti-atherogenic effect of fish oil supplementation.
2. Materials and Methods
2.1. Animals and Diets
ApoE−/− mice on C57BL/6 background were obtained from Jackson Laboratory (strain 002052) at 12 weeks of age. The diets were obtained from the Teklad, Harlan Laboratories Inc, and the composition is shown in Supplement Table 1. The mass ratio of EPA/DHA of the Omega-3 FAs used in this study was approximately 1.5. After 2 weeks on an Omega-3 FA Deficient (OD) diet, mice were randomly assigned to one of 3 experimental diets (n=10): 1) OD diet, 2) Omega-3 FA Rich (OR) diet (1.8 g Omega-3 FAs/kg • diet per day), or 3) Omega-3 FA Rich + ASA (ORA) diet (0.1 g ASA/kg • diet per day) for another 13-week feeding period. Additional diet, 4) Omega-3 FA Deficient + ASA (ODA) (n=4) was included later under the same experiment conditions. Supplementation with EPA+DHA and ASA was calculated according to a metabolic body weight formula [16] to produce a human (70 kg) equivalent dose of 4 g/day of EPA+DHA and 100 mg/day of ASA. All feeds were stored in vacuum at 4°C and were changed twice a week. All procedures were approved by the Animal Care and Use Committee of the NHLBI (#H-0050R2). At the end of the 13-week feeding period, blood and organ samples were collected at sacrifice without fasting.
2.2. Analyses of the hepatic fatty acid composition
Liver lipids were extracted by the Folch method (17). In brief, a portion of liver sample in each mouse was homogenized with chloroform/methanol (2/1; v/v), followed by washing with 0.9% NaCl solution after recovered the liquid phase by centrifuge. Hepatic lipid aliquots were heated at 100°C for 1 h with methanol containing 14% BF3 to generate fatty acid methyl esters (FAME). An aliquot of FAME from each sample was injected onto a DB-FFAP fused silica capillary column (30 m×0.25 mm i.d.×0.25 µm, J&W Scientific, Folsom, CA) on HP-5890 (series II) gas chromatograph (Hewlett-Packard, Palo Alto, CA) coupled with a flame ionization detector. Injector and detector temperatures were set to 250°C, and the oven temperature program was as follows: 130 to 175°C at 4°C/min, 175 to 210°C at 1°C/min, and then to 245°C at 30°C/min, with a final hold for 15 min. The FAME of each fatty acid was identified by comparison with the retention times of a standard mixture containing 28 FAME (462; Nu-Chek-Prep, Elysian, MN, USA). The concentrations were calculated by comparing the integrated areas of each fatty acid peak in the gas chromatograms with that of the known amount of internal standard (C22:3n−3 free fatty acid) added in the sample prior to total lipid extraction. The data were expressed as the proportion of each fatty acid in the weight of the total identified fatty acids in each sample (wt%).
2.3. Analyses of the plasma lipids
Plasma collected in heparinized capillary tubes from the retro-orbital sinus were analyzed for total cholesterol (TC), triglycerides (TG), phospholipids (PL), free cholesterol (FC) and cholesteryl esters (CE) using enzymatic methods (18). Plasma lipoprotein profile from pooled plasma (n=10) were analyzed after separation by fast protein liquid chromatography (FPLC) chromatography [18].
2.4. Assessment of Atherosclerotic Lesions
After 13 weeks on the diets, the extent of atherosclerosis as the percentage of the aortic surface covered by lesions was assessed using an en-face analysis [19]. A second assessment of atherosclerosis in the aortic root and ascending aorta was histologically performed [20], using 5 slides/ten sections per animal (n=7). Slides were stained with hematoxylin and Movat and immuno-fluorescent staining was performed on frozen sections of the aortic sinus after 15 minutes of fixation in 4% paraformaldehyde. Non-specific binding was blocked with 10% goat serum in PBS, and sections were incubated with 1:50 rat anti-mouse monocyte+macrophage (MOMA-2) or 1:500 rat anti-mouse vascular cell adhesion molecule (VCAM-1) primary antibodies (Abcam Inc, USA) overnight at 4°C. Slides were then incubated with a FITC conjugated goat anti-rat secondary antibody (1:200 IgG, Jackson Immuno-Research Lab, USA) for 1 h at room temperature. Histopathological categorization of lesion severity by developmental stage was done in a blind manner by two investigators according to the Reddick classification [21, 22].
2.5. Quantification of endothelial progenitor cells (EPC) by Flow Cytometry
Bone marrow cells were harvested by flushing femurs and tibias harvested from mice. Red blood cells were lysed and four-color flow cytometry was performed for the markers CD45 (30-F11), CD34 (MEC14.7), CD117 (2B8), CD309 (Avas12) and Linage cocktail [23] with antibodies from BioLegend (USA), using a BD LSR II flow cytometer (BD Biosciences, USA).
2.6. Measurement of cytokines, PCSK9 and COX activity
Plasma cytokines (IL-1β, IL-12p70, IFN-γ, IL-6, mKC, IL-10, and TNF-α) were measured with a Multi-Spot® 96-Well-7 Spot ELISA (Meso Scale Discovery, USA) [24]. Sandwich-type ELISA from eBioscience (USA) was used to measure TGF-β and MCP-1. Plasma PCSK9 was assessed with a Mouse Proprotein Convertase 9/PCSK9 Quantikine ELISA kit (R&D Systems, Inc., USA). COX activity in tissue extracts was measured with COX Fluorescent Activity Assay Kit (Cayman Chemicals, USA).
2.7. Gene Expression Analysis
RNA analysis was done as previously described [25]. RNA had an A260:A230 ratio greater than 1.7; an A260:A280 ratio of approximately 2.1 ± 0.1; and a RIN number of 8.2 ± 0.2. Gene expression in liver of the mice was analyzed with Mouse Lipoprotein Signaling and Cholesterol Metabolism RT2 Profiler PCR Array (Qiagen, catalog No. PAMM-080E) for RT-PCR with an ABI 7900HT Real-Time PCR System (Life Technologies).
Expression of Ccl2 and Hmgcr was measured individually by RT-PCR with TaqMan assays, Mm00441242_m1 and Mm01282499_m1, respectively (Supplement Table 2) for OD, OR, ORA groups. Relative expression of the genes was calculated by the comparative CT (ΔΔCT) method [26], using software provided by manufacturer of the Array. Expression of several genes, as indicated in the text, was measured by RT-PCR with mouse β-actin and 28S rRNA genes used for normalization. Standard error of the mean was calculated with the REST 2009 software from Qiagen.
2.8. LC/MS/MS Analysis of Lipids
Identifications of SPMs by LC/MS/MS were done, using at least six diagnostic ions [27]. Spleen and lung tissue were removed from non-fasting mice within 5 min of sacrifice, whereas aortic tissue was isolated after approximately 10 min. Collected tissue was snapped frozen and stored at −80 °C before analysis. The LC/MS/MS system, QTrap 5500 (AB SCIEX, USA) used for the analysis was equipped with an Agilent HP1100 binary pump and diode-array detector. An Agilent Eclipse Plus C18 column was used with a gradient of methanol/water/acetic acid of 60:40:0.01 (vol:vol:vol) to 100:0:0.01 at 0.5 mL/min flow rate. To monitor and quantify lipid mediator (LM) levels, a multiple reaction monitoring method was developed with signature ion fragments for each molecule. Identification was conducted using published criteria [27] that included retention time and at least 6 diagnostic ions. Calibration curves were obtained using synthetic and authentic LM mixtures (d8-5S-HETE, d4-LTB4, d4-PGE2, resolvin (Rv) D1, RvD2, RvD5, maresin (MaR) 1, protectin D1 (PD1), as well as their synthetic aspirin triggered epimers (AT-RvD1, AT-LXA4) 4-hydroxydocosahexaenoic acid (HDHA), 7-HDHA, 14-HDHA, 17-HDHA, lipoxin (LX) A4, LXB4, LTB4, PGD2, PGE2, PGF2α, thromboxane (Tx) B2, 5-HETE, 12-HETE, 15-HETE, RvE1, RvE2, 5-hydroxyeicosapentaenoic acid (HEPE), 12-HEPE, 15-HEPE, 18-HEPE) at 2, 10, 40, 200 pg. Quantification was carried out based on area beneath the peak of the multiple reaction monitoring transition and the linear calibration curve for each compound.
2.9. Statistical Analysis
Unless otherwise indicated, all data are expressed as the means ± SEMs. Overall results were first analyzed by one-way ANOVA, or where indicated by two-factor ANOVA, using a fixed effects model. Differences between diet groups were assessed by paired two-tailed t-test for single comparisons, and P-values were adjusted for when there were unequal variances. Bonferroni’s post-hoc test correction was done for multiple comparisons. Data were considered statistically significant when P < 0.05.
3. Results
3.1. Hepatic fatty acid compositions
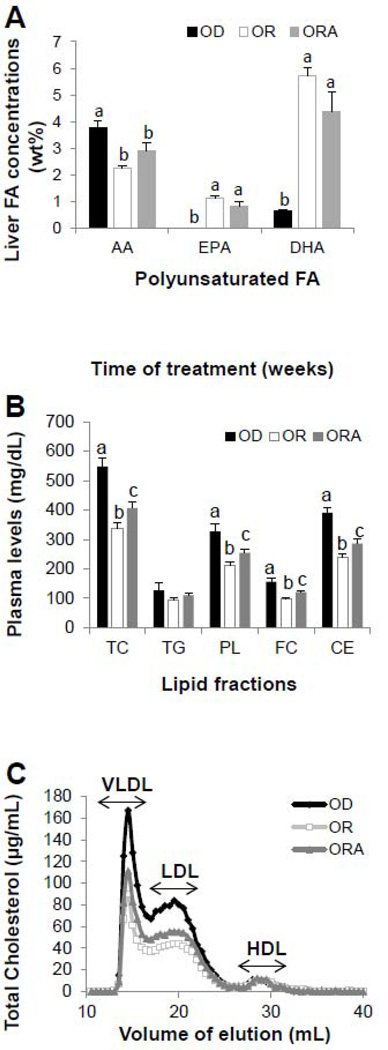
The complete hepatic fatty acid composition (wt%) for mice on the three different diets are shown in Supplemental Table 3. Although there were no significant differences in the concentrations of total saturated FA and monounsaturated FA, most omega-6 FA concentrations in OR and ORA diet groups were significantly (P < 0.05) less than that of the OD diet group. For example, compared to the OD diet group, arachidonic acid (AA) concentration in OR diet and ORA diet groups was significantly reduced (P < 0.001) by 40% and 22%, respectively. In contrast, omega-3 FAs concentration in OR and ORA diet groups were significantly greater than in the OD diet group (Fig. 1A). In particular, EPA concentration was higher (P < 0.0001) by 55- and 41-fold in OR and ORA diet groups, respectively, compared to the OD diet group. DHA concentration was also significantly (P < 0.0001) increased by 8- and 6-fold in OR and ORA diet groups, respectively.
FIGURE 1. Effect of diets on hepatic fish oil contents, plasma lipids and lipoproteins in apoE−/− mice.
A. Concentration of polyunsaturated FA contents in liver of apoE−/− mice after 13 weeks of OD, OR or ORA diets.
B. Level of plasma TC, triglycerides (TG), phospholipids (PL), free cholesterol (FC) and cholesteryl esters (CE) in apoE−/− mice after 13 weeks of OD, OR or ORA diets.
C. FPLC analysis of plasma cholesterol after 13 weeks on OD, OR or ORA diets. Values of pooled plasma from the each group were used for the data representation.
Values represent the means ± SEMs, n=10. Labeled means without a common letter differ, P < 0.05. OD, omega-3 FA deficient diet; OR, omega-3 FA rich diet; ORA, omega-3 FA rich plus aspirin diet.
3.2. Body Weight and Plasma Lipids
Mice in OR and ORA diet groups had significantly (P < 0.05) greater food intake compared to the OD diet group, and ORA diet group gained more weight at 13 weeks compared to the OR diet group (P < 0.05) (Supplemental Table 4). Plasma lipids and lipoproteins also showed a different response to the three different diets (Supplemental Table 5). By 13-weeks, TC was 40% (P < 0.0005) higher than baseline for mice on the OD diet. In contrast, TC was unchanged compared to baseline for the OR and ORA diet groups (Supplemental Table 5) and significantly lower than the OD group at 13-weeks (Fig. 1B). Plasma levels of PL, FC, and CE were also significantly less in mice fed the two diets enriched in omega-3 FA, with the OR diet group showing a greater decrease (Fig. 1B, Supplemental Table 5). No significant differences were observed in plasma TG levels among the three diet groups. Analysis of the lipoprotein profile by FPLC at 13 weeks revealed that plasma cholesterol decreased mainly in VLDL and LDL fractions in mice on OR and ORA diet groups (Fig. 1C). Mice on all three diet groups had relatively low levels of HDL-C, as have been previously described for apoE−/− mice [28].
3.3. Development of Atherosclerosis
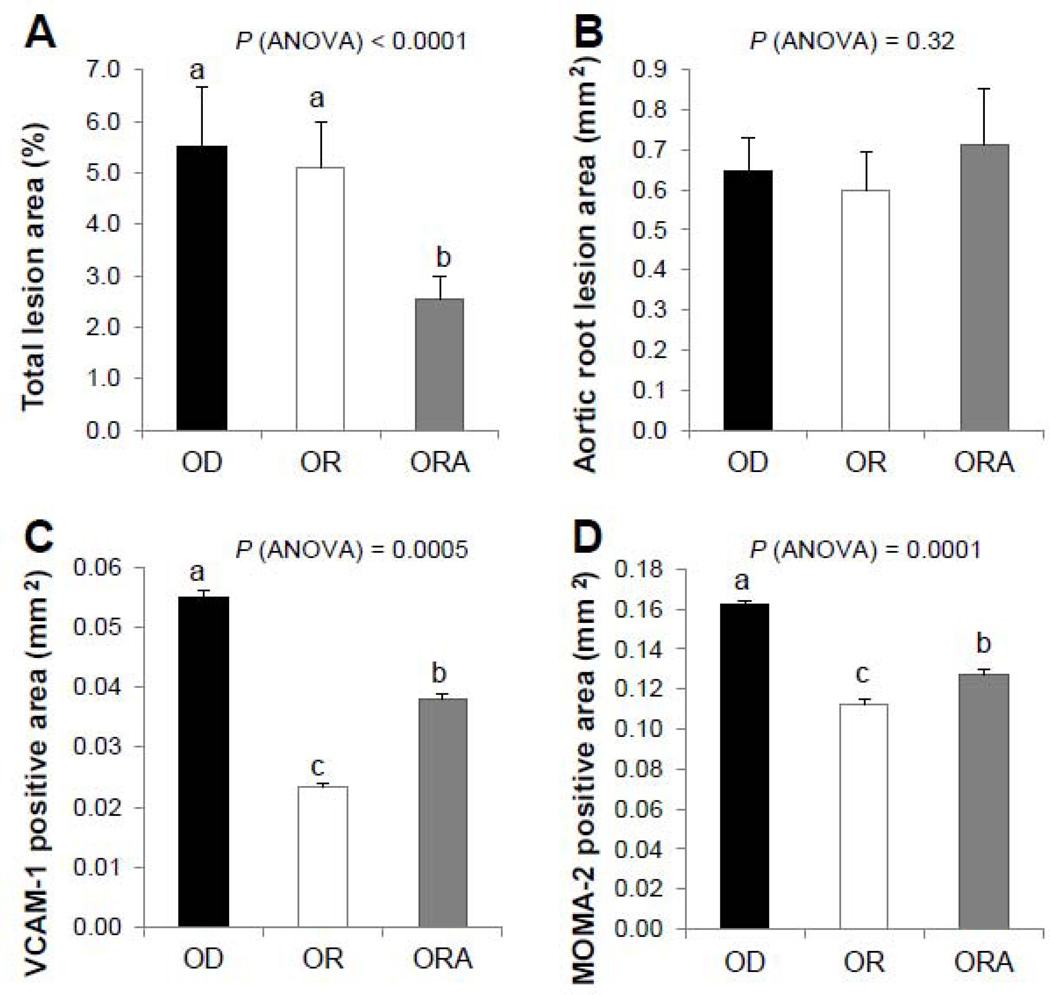
After 13 weeks, mice in the OD diet group compared to the other two diet groups had more complex lesions in their aortic root, with cholesterol clefts, and fibro-fatty nodules covered with fibrous plaques (Supplemental Fig. 1). Mice in the ORA diet group had significantly less total atherosclerotic lesion coverage in their aortas, as measured by en face analysis, than OD and OR diet groups (Fig. 2A). No significant differences were observed, however, among the three diet groups in Oil Red O-stained lesion area in the aortic root (Fig. 2B, Supplemental Fig. 2), the anatomic site that is most predisposed to atherosclerosis and is often resistant to various therapies [29]. Immunohistochemical differences, however, were also observed in the plaques of the aortic root (Fig. 2C and D). Aortic plaques for both OR and ORA diet groups showed less VCAM-1 positive staining and macrophage infiltration than the OD diet group.
FIGURE 2. Effect of diets on development of atherosclerosis in apoE−/− mice.
A. En face analysis of aortic lesions, n=7.
B. Analysis of aortic lesions in the aortic roots of the apoE−/− females after 13 weeks of the diet. The mean cross-sectional area of lesions in the aortic sinus assessed by the Oil Red O staining, n=7.
C. VCAM-1 expression in the aortic sinus lesions. Immunohistochemical analysis and quantitation were done in female ApoE−/− mice after 13 weeks on the experimental diets. Values represent the means ± SEMs, n=3.
D. MOMA-2 expression in the aortic sinus lesions. Immunohistochemical analysis and quantitation were done in female apoE−/− mice after 13 weeks on the OD, OR or ORA diet. Values represent the means ± SEMs, n=3.
Labeled means without a common letter differ, P < 0.05. OD, omega-3 FA deficient diet; OR, omega-3 FA rich diet; ORA, omega-3 FA rich plus aspirin diet.
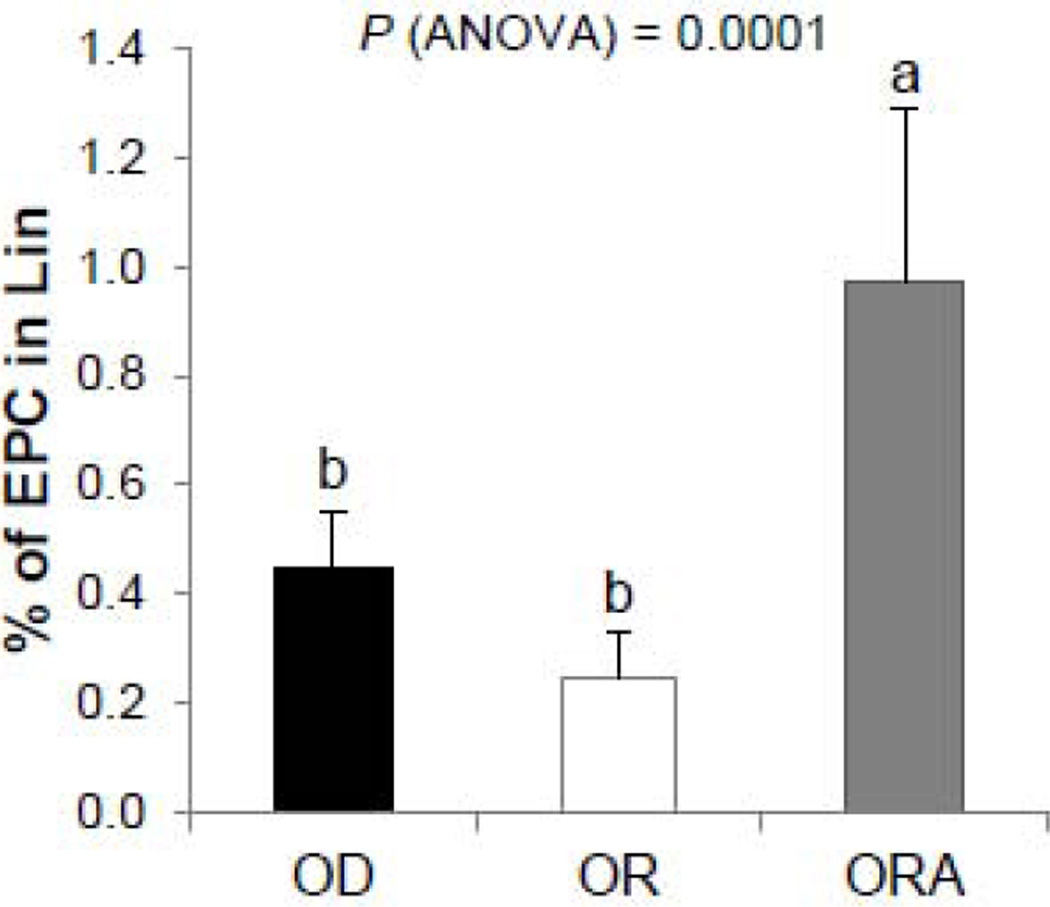
3.4. Endothelial Cell Proliferation
Because circulating endothelial progenitor cells have been described to effect the development of atherosclerosis [30], this was assessed for all three diet groups. After 13 weeks, mice in the ORA diet group had increased endothelial progenitor cells (EPC), as determined by the number of CD34+CD309+ bone marrow positive cells compared with OD and OR diet groups (Fig. 3).
FIGURE 3. Effect on diets on endothelial cells proliferation in apoE−/− mice.
EPC (defined as Lin-CD45−/CD34+/CD117+/CD309+ cells) mobilization from the bone marrow after 13 weeks of the diet study was determined by flow cytometry in apoE−/− mice. Values represent the means ± SEMs, n=10. Labeled means without a common letter differ, P < 0.05. OD, omega-3 FA deficient diet; OR, omega-3 FA rich diet; ORA, omega-3 FA rich plus aspirin diet.
3.5. Inflammation Markers
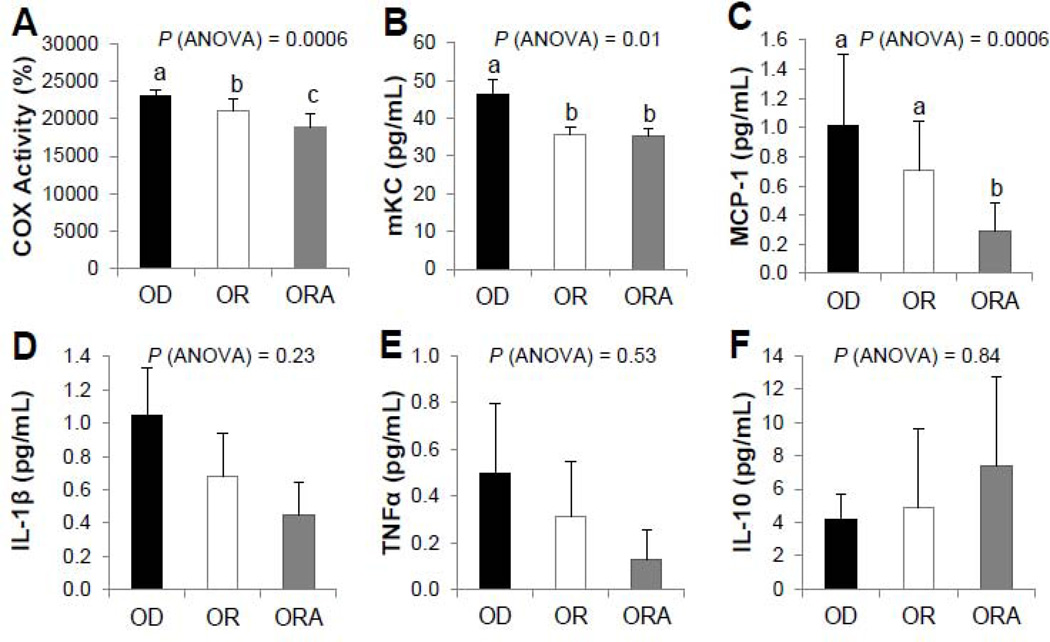
Differences in the inflammatory process between the three diet groups were first tested by measuring the level of COX enzyme activity in the aorta (Fig. 4A). COX activity in aortas from mice in both OR and ORA diet groups were significantly less than that of the OD diet group, and between the two fish oil enriched diet groups, the ORA group had the lowest level of COX enzyme activity.
FIGURE 4. Effect of diets on COX activity and cytokine levels in apoE−/− mice.
A. COX Activity Assay in aorta. Values represent the means ± SEMs, n=6.
B–F. Plasma cytokine levels. Values represent the means ± SEMs, n=10. Labeled means without a common letter differ, P < 0.05. OD, omega-3 FA deficient diet; OR, omega-3 FA rich diet; ORA, omega-3 FA rich plus aspirin diet.
Serum pro-inflammatory cytokines mKC and MCP-1 were significantly lower (P < 0.05) in OR and ORA diet groups compared to the OD diet group (Fig. 4B and 4C). No statistically significant changes were observed in other detectable cytokines (IL-1β, TNF-α and IL-10) (Fig. 4D, E, F). The other measured cytokines (IL-12p70, IFN-γ, and IL-6) were either not detectable or did not substantially differ between the different groups (data not shown).
3.6. Hepatic Gene Expression
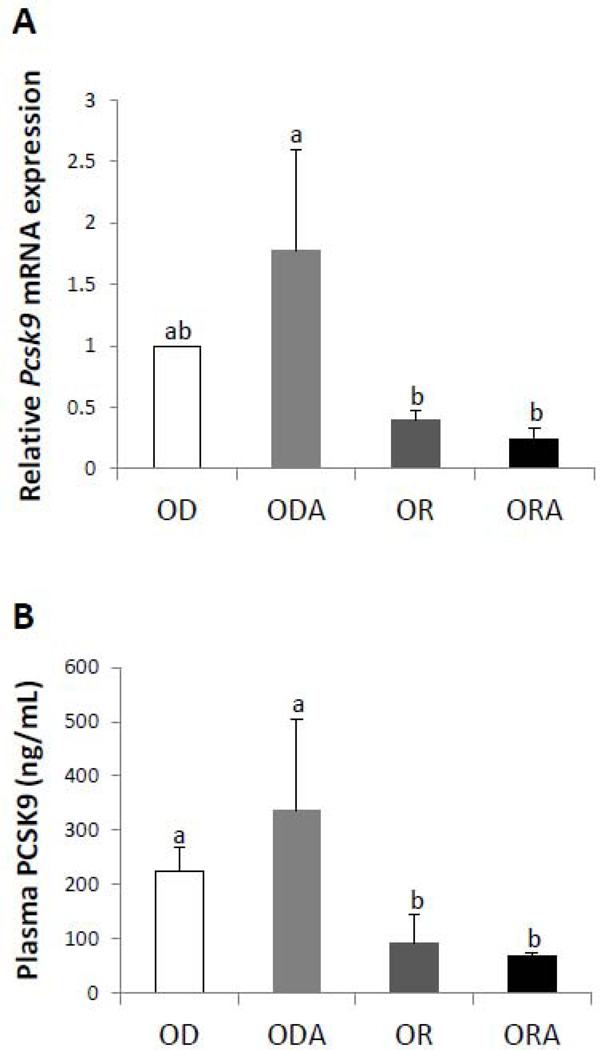
In order to gain further insight into the effect of the three diets on plasma lipoproteins, the expression of key hepatic genes involved in lipid synthesis and lipoprotein metabolism was measured (Supplemental Table 6). Multiple changes were observed for genes related to the function of the LDL receptor (combined in Group I), with the greatest change occurring with the down regulation of Pcsk9 (Fig. 5A). Pcsk9 encodes for a plasma secretory protein that down regulates the LDL-receptor (31), which could potentially explain the decrease in the plasma level of VLDL and LDL observed in mice in OR and ORA diet groups. In addition, mice in both OR and ORA diet groups also showed significantly lower PCSK9 protein in plasma than mice in the OD diet group, and this effect was further enhanced in the ORA diet group compared with the OR diet group (Fig. 5B). Adding ASA alone (ODA) did not lower PCSK9 plasma levels and in general had minimal effect on hepatic gene expression levels (Supplemental Table 6). To differentiate the observed findings the ODA group has been included in comparison showing distinguished effect of ASA supplementation with omega-3 FA on Pcsk9 expression changes (Fig. 5B). Genes (Apoa1, Apob and Lcat) involved in cholesterol transport and efflux (Group II) were induced in mice in OR and ORA diet groups. Several genes (Acaa2, Cyp7a1, Hmgcs2, Nsdhl, Stards3 and Tm7sf2) involved in cholesterol or fatty acid catabolism (Group III) were either significantly repressed or induced in mice in OR and ORA diet groups, and the most striking change was the 10.7-fold increase in Cyp7a1 expression. Cyp7a1 is the rate limiting step in the conversion of cholesterol to bile acids (32), and its induction would be predicted to increase the hepatic excretion of cholesterol. However, no additional effect in further inducing Cyp7a1 was observed in mice in the ORA diet group compared to the OR diet group. Genes related to cholesterol biosynthesis and metabolism (Group IV and V) were in general down regulated in mice in the both OR and ORA diet groups. For example, Dhcr7, Fdps, and Hmgcr were down regulated in mice in OR and ORA diet groups compared to the OD diet group. Srebf2, a key transcription factor that regulates many genes in the cholesterol biosynthetic pathway, and Scap, a protein involved in the release of the Srebf2 transcription factor, were also down regulated in the ORA but not the OR diet group. One gene related to inflammation (Group VI), namely Ccl2, which encodes for the MCP-1, a potent chemotactic factor, was down regulated in OR and ORA diet groups, which is consistent with the decrease observed in its plasma protein level (Fig. 4C). In summary, of the total of 25 hepatic gene changes observed in mice in the different diet groups, 11 of these gene changes occurred between the ORA versus the OR diet group with repeated control against the ODA diet group. Thus our findings showed the profound independent effect of ASA addition on the hepatic gene response to the EPA+DHA enriched diet.
FIGURE 5. Effect of diets on PCSK9 levels in apoE−/− mice.
A. Relative levels of Pcsk9 mRNA expression in liver of apoE−/− mice after 13 weeks of OD, OR or ORA diets.
B. Plasma PCSK9 protein levels of ApoE−/− mice after 13 weeks of OD, OR or ORA diets. Values represent the means ± SEMs, n=4. Labeled means without a common letter differ, P < 0.05. OD, omega-3 FA deficient diet; OR, omega-3 FA rich diet; ORA, omega-3 FA rich plus aspirin diet; ODA, omega-3 FA deficient diet plus aspirin.
3.7. Pro-Inflammatory and Pro-Resolving Lipid Mediators
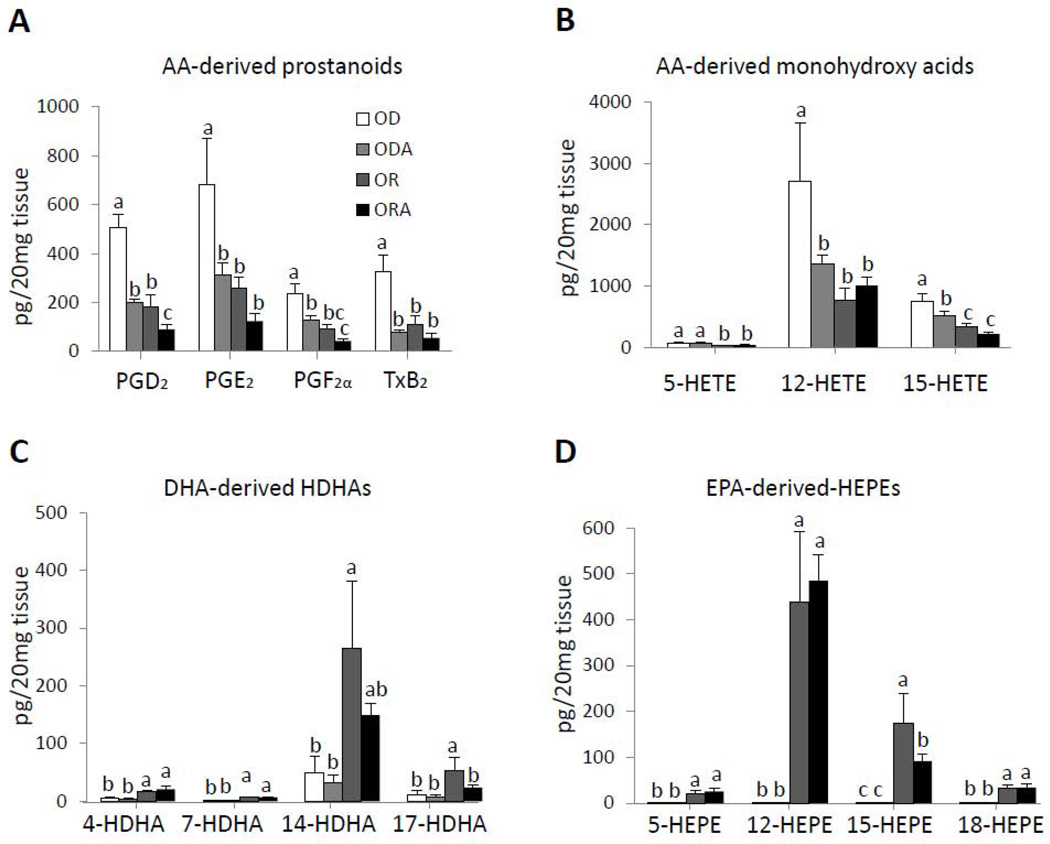
To determine if the changes observed in the mice treated with the three different diets could be a result of differential production of pro or anti-inflammatory lipid mediators, LC/MS/MS analysis was done on the aortas of the mice (Fig. 6, Supplemental Table 7). Arachidonic acid (AA)-derived pro-inflammatory prostanoids (PGD2, PGE2 PGF2α, and TxB2) and monohydroxy acids (5-HETE, 12-HETE and 15-HETE) were all significantly lower (P < 0.05) in ODA, OR and ORA diet groups compared to the OD diet group. For PGD2, PGE2 PGF2α and 15-HETE, the ORA group had lower values than either the ODA or OR groups (Fig. 6A and 6B). In contrast, DHA-derived HDHA series (4-, 7-, 14-, and 17-HDHA) and EPA-derived HEPE series (5-, 12-, 15-, and 18-HEPE), which are anti-inflammatory and pro-resolving [33], were largely only identified in OR and ORA diet groups enriched in EPA and DHA (Fig. 6C and 6D). We also measured several SPMs, such as RvD1, 2, 5 and MaR1 in the aorta, lung and spleen, which promote the resolution of inflammation [34]. All of these SPMs were identified in both the lung and spleen tissue (Supplemental Table 8 and 9), but not in the aorta. It is possible that the local metabolism of these lipid mediators leads to their rapid inactivation and or clearance from the aorta, during the time course of the sampling. The other lipid mediator changes in the lung and spleen in response to the diets, such as the prostanoids, were similar to the aorta and were diminished in mice in OR and ORA diet groups.
FIGURE 6. Effect of diets on lipid mediator (LM) levels in aortas of apoE−/− mice.
Tissues were harvested after 13 weeks on the diets and LM levels were assessed by LM metabololipidomics. Data were analyzed using one-way analysis of variation (ANOVA) and Bonferroni’s post hoc test.
A. AA-derived prostanoids; B. AA-derived monohydroxy acids; C. DHA-derived hydroxydocosahexaenoic acids (HDHA); D. EPA-derived hydroxyeicosapentaenoic acids (HEPE). Values represent the means ± SEMs, n=4. Labeled means without a common letter differ.
OD, omega-3 FA deficient diet; OR, omega-3 FA rich diet; ORA, omega-3 FA rich plus aspirin diet; ODA, omega-3 FA deficient diet plus aspirin.
4. Discussion
The main findings from this study are that less aortic atherosclerosis lesions and aortic pro-inflammatory lipid mediators were observed in apoE−/− mice on the EPA+DHA rich diet plus ASA versus just EPA+DHA rich diet. Although mice in both OR and ORA diet groups showed some histological improvement in atherosclerotic plaques in the aortic root, only mice in the ORA diet group showed a significant decrease in total atherosclerosis area by en face analysis of the aorta (Fig. 2A). As discussed below, multiple differences were observed in the response to fish oil supplementation with and without ASA, which could potentially account for the greater anti-atherogenic impact of the ORA diet.
As expected, the tissue concentration of omega-3 FAs markedly increased and most omega-6 FAs decreased in OR and ORA diet groups (Fig. 1A and Supplemental Table 3). In regard to the effect of OR and ORA diets on plasma lipids, it does not appear that ASA is mediating its anti-atherogenic effect by lowering pro-atherogenic lipoproteins (Fig. 1). Cholesterol in the pro-atherogenic VLDL and LDL lipoproteins was lower in both OR and ORA groups as compared to the OD group, but it was even lower in the OR group than in the ORA group. Pcsk9 mRNA and plasma PCSK9 protein, however, were lower in the ORA group than OR and OD groups (Fig. 5). This would be predicted to lead to a greater decrease in plasma LDL pool size in the ORA diet group, but this was not observed. One possible explanation is that the pool size of LDL is maintained despite the higher rate of hepatic catabolism because of a greater hepatic production rate perhaps due to a differential effect on hepatic gene expression (see below). Regardless of the mechanism, this is an important and novel observation and suggests at least in man in which PCSK9 has been shown to have a profound effect in lowering LDL-C that some of the beneficial effects of fish oil on lipoproteins could be mediated by changes in PCSK9. We did not observe a significant effect of fish oil (EPA+DHA) supplementation with or without ASA on triglycerides. In a study by Xu Z. et al, they showed that apoE−/− mice fed EPA and DHA have increased levels of plasma triglycerides and elevated or unchanged plasma total cholesterol concentration [35], but in other studies EPA was found to lower triglycerides [36, 37]. Of note, the diet used in these previous studies contained considerable amounts of cholesterol (~2000 ppm), whereas in our study we utilized a customized EPA+DHA deficient diet with a relatively low cholesterol content (<40 ppm).
Endothelial cells are known to play a key role in the development of atherosclerosis and in repair of vascular injury [38]. Interestingly, more circulating EPC were observed in mice in the ORA diet group compared to the OR diet group. It has been described that circulating EPC contributes to endothelial repair and EPC cell counts are inversely related to atherosclerosis in both animal models and in human studies [39]. How the addition of ASA to the EPA+DHA-rich diet leads to increased EPC is not known, but as discussed below, it could be related to changes in lipid mediators or in differential gene expression.
Mice in OR and ORA diet groups showed evidence of less inflammation, which could have contributed to their decrease in atherosclerosis. The major known biological property of ASA is related to its ability to inhibit the COX pathway, specifically COX-1 and COX-2. Previous findings showed that COX-2 inhibition is crucial for the angiogenesis. For example, incubation of endothelial cells with DHA reduces the tube-like network formation in a collagen matrix possibly through modulation of SPM levels [40, 41]. We observed a modest but statistically significant lower total COX activity from the aorta of mice in the OR diet group as compared with the OD diet group, and an even a greater decrease in the ORA group. Consistent with these findings, we observed significantly lower levels of mKC, and MCP-1. These two pro-inflammatory cytokines are known to play a key role in the development of atherosclerosis [2]. Ccl2 gene expression and its encoded protein, MCP-1, was lower in mice in OR and ORA diet groups compared to the OD diet group, and this effect was further enhanced in the ORA diet group compared to the OR diet group. Previous studies have shown that MCP-1 ligates CC chemokine receptor 2 to promote macrophage adhesion and infiltration, which is critical in atherosclerosis initiation and progression [42].
It has been previously established that fish oil supplementation is also able to affect the expression of numerous transcription factors that play a major roles in carbohydrate, fatty acid, triglyceride, cholesterol and bile acid metabolism, such as peroxisome proliferator activated receptor (α, β and γ), liver X receptors (α and β), retinoic X receptor α, hepatic nuclear factor-4α and sterol regulatory element binding proteins (SREBPs or SREBF) 1 and 2 [43]. As can be seen in Supplemental Table 6, the addition of ASA, in many cases, further potentiated the gene changes observed in the OR diet group. For example, compared to OD and ODA diet groups, we observed a greater decrease in both mRNA and protein for PCSK9 in the ORA diet group than the OR diet group. In case of Srebf, no significant change was found in the OR diet group compared to the OD diet group, but Srebf2 was significantly down regulated in the ORA diet group.
Our original hypothesis that ASA by modulating the production of pro and anti-inflammatory lipid mediators from the EPA+DHA supplementation could contribute to the anti-atherogenic effect of fish oil was supported by the lipidomics findings (Fig. 6). It is well established that inhibition of AA pathway products reduces the inflammatory process and arteriosclerosis [12]. Consistent with our hypothesis, mice in both OR and ORA diet groups showed a decline of AA-derived prostanoids (PGD2, PGE2, PGF2α, and TxB2) and monohydroxy acids (5-HETE, 12-HETE and 15-HETE), the AA biosynthetic pathway markers. Part of this effect is likely due to decrease substrate availability, namely the depletion of AA in tissues and its replacement with EPA and DHA (Supplemental Table 1). There also was an additional effect due to ASA, because compared with OR and ODA diet groups, most of these pro-inflammatory lipid mediators were lower in the ORA diet group. This is most likely due to the inhibition of cyclooxygenases by ASA. In contrast, anti-inflammatory lipid mediators derived from omega-3 FAs, such as the HDHA series and HEPE series of lipids were more equally increased in OR and ORA diet groups, which is likely due to increase substrate availability. This time the presence of ASA did not differentially alter their production, which is consistent with previous studies that have shown that 17S-HDHA can be produced in murine exudates and human cells independently of the actions of ASA [7,14]. It is important to note, however, that we did not measure the bioavailability of ASA in this study, but the dose we used was designed to be comparable to a standard human dose and is similar to the dose used in many other mouse studies of ASA [44–46]. Together, these findings are consistent with the modulation of lipid mediator production by ASA contributing to the anti-atherogenic benefit of the ORA diet, but which of the many lipid mediator observed changes described in this study are most directly linked to pathogenesis of atherosclerosis is presently not known.
In summary, less aortic atherosclerosis lesions and lower levels of pro-inflammatory cytokines were seen in apoE−/− mice on a fish oil diet enriched in EPA+DHA rich diet plus ASA versus just a fish oil diet, possibly by altering lipid mediator production, as well as by other mechanisms, such as favorably altering endothelial function, decreasing pro-inflammatory cytokine production, and inducing anti-atherogenic hepatic genes. Results from this study should help inform future human clinical trials investigating the combined effect of fish oils and ASA on inflammation and atherosclerosis.
Supplementary Material
Acknowledgments
Funding sources: This study is supported by intramural research funding of National Heart, Lung, and Blood Institute. LC-MS-MS profiling was supported by National Institutes of Health (R01GM038765 and P01GM095467) to Charles N. Serhan.
The authors wish to thank Denis Sviridov, M.D., Ph.D., and Lita Freeman, Ph.D. from the Dr. Remaley’s Laboratory at NHLBI for their technical assistance. We are also grateful for the assistance of Chris Ramsden, M.D. from NIAA for the fatty acid analysis. Also we want to express our gratitude to Drs. Jesmond Dalli and Jonathan Fitzgerald of the Prof. Serhan’s Laboratory, Center for Experimental Therapeutics and Reperfusion Injury Brigham and Women’s Hospital, Harvard Medical School, for carrying out LC/MS/MS and analysis supported by National Institutes of Health (P01GM095467 to C.N.S.).
Abbreviations used
- AA
arachidonic acid
- Apo
apolipoprotein
- ASA
acetylsalicylic acid/aspirin
- COX
cyclooxygenase
- CVD
cardiovascular diseases
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FAs
fatty acids
- HDHA
hydroxydocosahexaenoic acid
- HDL-C
high-density lipoprotein-cholesterol
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- LDL-C
low-density lipoprotein-cholesterol
- LM
lipid mediator
- LT
leukotriene
- LX
lipoxin
- MaR
maresin
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PD
protectin
- PG
prostaglandin
- Rv
resolving
- SPMs
specialized pro-resolution mediators
- Tx
thromboxane
- VLDL-C
very low-density lipoprotein-cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures: A. V. Sorokin, Z.-H. Yang, B. L. Vaisman, S. Thacker, Z.-X. Yu, M. Sampson, C. N. Serhan, and A. T. Remaley, no conflicts of interest.
References
- 1.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 4.Shearer GC, Savinova OV, Harris WS. Fish oil -- how does it reduce plasma triglycerides? Biochim Biophys Acta. 2012;1821(5):843–851. doi: 10.1016/j.bbalip.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massaro M, Scoditti E, Carluccio MA, Campana MC, De Caterina R. Omega-3 fatty acids, inflammation and angiogenesis: basic mechanisms behind the cardioprotective effects of fish and fish oils. Cell Mol Biol (Noisy-le-grand) 2010;56(1):59–82. [PubMed] [Google Scholar]
- 6.Antithrombotic Trialists C, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang N, Serhan CN. Aspirin triggers formation of anti-inflammatory mediators: New mechanism for an old drug. Discovery medicine. 2004;4(24):470–475. [PubMed] [Google Scholar]
- 8.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13(4):632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Block RC, Dier U, Calderonartero P, Shearer GC, Kakinami L, Larson MK, Harris WS, Georas S, Mousa SA. The Effects of EPA+DHA and Aspirin on Inflammatory Cytokines and Angiogenesis Factors. World J Cardiovasc Dis. 2012;2(1):14–19. doi: 10.4236/wjcd.2012.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lev EI, Solodky A, Harel N, Mager A, Brosh D, Assali A, Roller M, Battler A, Kleiman NS, Kornowski R. Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation. J Am Coll Cardiol. 2010;55(2):114–121. doi: 10.1016/j.jacc.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Deckelbaum RJ, Leaf A, Mozaffarian D, Jacobson TA, Harris WS, Akabas SR. Conclusions and recommendations from the symposium, Beyond Cholesterol: Prevention and Treatment of Coronary Heart Disease with n-3 Fatty Acids. Am J Clin Nutr. 2008;87(6):2010S–2012S. doi: 10.1093/ajcn/87.6.2010S. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 13.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. Journal of nutrigenetics and nutrigenomics. 2011;4(1):12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 16.Hawk CT, Leary SL, Morris TH American College of Laboratory Animal Medicine. Formulary for laboratory animals. 3rd. Ames, Iowa: Blackwell Pub.; 2005. European College of Laboratory Animal Medicine. [Google Scholar]
- 17.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1956;226:497–509. [PubMed] [Google Scholar]
- 18.Vaisman BL, Klein HG, Rouis M, Berard AM, Kindt MR, Talley GD, Meyn SM, Hoyt RF, Jr, Marcovina SM, Albers JJ, et al. Overexpression of human lecithin cholesterol acyltransferase leads to hyperalphalipoproteinemia in transgenic mice. J Biol Chem. 1995;270(20):12269–12275. doi: 10.1074/jbc.270.20.12269. [DOI] [PubMed] [Google Scholar]
- 19.Basso F, Amar MJ, Wagner EM, Vaisman B, Paigen B, Santamarina-Fojo S, Remaley AT. Enhanced ABCG1 expression increases atherosclerosis in LDLr-KO mice on a western diet. Biochem Biophys Res Commun. 2006;351(2):398–404. doi: 10.1016/j.bbrc.2006.10.044. doi: S0006-291X(06)02271-6 [pii] 10.1016/j.bbrc.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68(3):231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 21.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 22.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15(9):1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 23.Khan SS, Solomon MA, McCoy JP., Jr Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry Part B, Clinical cytometry. 2005;64(1):1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 24.Hall JC, Priestley JV, Perry VH, Michael-Titus AT. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J Neurochem. 2012;121(5):738–750. doi: 10.1111/j.1471-4159.2012.07726.x. [DOI] [PubMed] [Google Scholar]
- 25.Vaisman BL, Demosky SJ, Stonik JA, Ghias M, Knapper CL, Sampson ML, Dai CL, Levine SJ, Remaley AT. Endothelial expression of human ABCA1 in mice increases plasma HDL cholesterol and reduces diet-induced atherosclerosis. J Lipid Res. 2012;53(1):158–167. doi: 10.1194/jlr.M018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 27.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 29.VanderLaan PA1, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24(1):12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 30.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82(10):671–677. 29. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 32.Marais DA, Blom DJ, Petrides F, Goueffic Y, Lambert G. Proprotein convertase subtilisin/kexin type 9 inhibition. Curr Opin Lipidol. 2012;23(6):511–517. doi: 10.1097/MOL.0b013e3283587563. [DOI] [PubMed] [Google Scholar]
- 33.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97(3–4):73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;10(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Riediger N, Innis S, Moghadasian MH. Fish oil significantly alters fatty acid profiles in various lipid fractions but not atherogenesis in apo E-KO mice. Eur J Nutr. 2007;46(2):103–110. doi: 10.1007/s00394-006-0638-3. [DOI] [PubMed] [Google Scholar]
- 36.Brown WV, Bays H, Harris W, Miller M. Using omega-3 fatty acids in the practice of clinical lipidology. J Clin Lipidol. 2011;5(6):424–433. doi: 10.1016/j.jacl.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto M, Sata M, Fukuda D, Tanaka K, Soma M, Hirata Y, Nagai R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis. 2008;197(2):524–533. doi: 10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Du F, Zhou J, Gong R, Huang X, Pansuria M, Virtue A, Li X, Wang H, Yang XF. Endothelial progenitor cells in atherosclerosis. Front Biosci (Landmark Ed) 2012;17:2327–2349. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 40.Massaro M, Scoditti E, Carluccio MA, De Caterina R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):109–115. doi: 10.1016/j.plefa.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Jiang Y, Liang Y, Tian X, Peng C, Ma KY, Liu J, Huang Y, Chen ZY. DPA n-3, DPA n-6 and DHA improve lipoprotein profiles and aortic function in hamsters fed a high cholesterol diet. Atherosclerosis. 2012;221(2):397–404. doi: 10.1016/j.atherosclerosis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Lin J, Kakkar V, Lu X1. Impact of MCP-1 in atherosclerosis. Curr Pharm Des. 2014;20(28):4580–4588. doi: 10.2174/1381612820666140522115801. [DOI] [PubMed] [Google Scholar]
- 43.Jump DB. Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr Opin Lipidol. 2002;13(2):155–164. doi: 10.1097/00041433-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Khvostov MV, Tolstikova TG, Borisov SA, Zhukova NA, Dushkin AV, Chistyachenko YS, Polyakov NE. Improving the efficiency and safety of aspirin by complexation with the natural polysaccharide arabinogalactan. Curr Drug Deliv. 2015 doi: 10.2174/1567201812666150605104944. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Vad NM, Kudugunti SK, Wang H, Bhat GJ, Moridani MY. Efficacy of acetylsalicylic acid (aspirin) in skin B16-F0 melanoma tumor-bearing C57BL/6 mice. Tumour Biol. 2014;35(5):4967–4976. doi: 10.1007/s13277-014-1654-1. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Jiang D, Zhang S, Ou B. Aspirin inhibits fractalkine expression in atherosclerotic plaques and reduces atherosclerosis in ApoE gene knockout mice. Cardiovasc Drugs Ther. 2010;24(1):17–24. doi: 10.1007/s10557-009-6210-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.