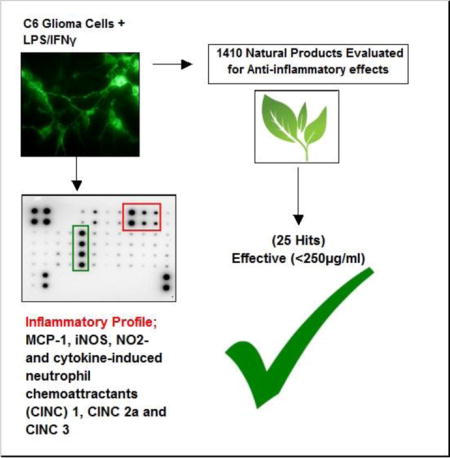
Abstract
Chronic or acute central nervous system (CNS) inflammation is carried out by glial cells, which can contribute to neurological injuries associated with head trauma, stroke, and infection, Parkinson’s or Alzheimer ’s disease. The process of aging combined with inflammation are also risk factors for developing glioblastoma multiforme (GBM) as well as perpetuating its malignant aggression. With growing public curiosity in complementary and alternative medicines (CAMs), in this work we conduct a high throughput (HTP) screening of >1400 natural herbs, plants and over the counter (OTC) products for anti-inflammatory effects on lipopolysaccharide (LPS)/interferon gamma (IFNγ) activated C6 glioma cells. Validation studies suggest a pro-inflammatory profile mediated by LPS [3μg/ml/IFNγ 3ng/ml] is consistent with elevation [> 8.5 fold] of MCP-1 and cytokine-induced neutrophil chemo-attractants (CINC) 1, CINC 2a and CINC3. The data show no evidence of changes to the following, IL-13, TNF-a, fracktaline, leptin, LIX, GM-CSF, ICAM1, L-Selectin, activin A, agrin, IL-1α, MIP-3a, B72/CD86, NGF, IL-1b, MMP-8, IL-1 R6, PDGF-AA, IL-2, IL-4, prolactin R, RAGE, IL-6, Thymus Chemokine-1, CNTF,IL-10 or TIMP-1. The data also show a LPS/IFNγ mediated rise in iNOS and NO2− as often reported. A HTP screening was conducted, where we employ an in vitro efficacy index (iEI) defined as the ratio of toxicity (LC50)/anti-inflammatory potency (IC50) to ensure biological effects were occurring in fully viable cells (ratio > 3.8). Using NO2− as a guideline molecule, the data show that 1.77 % (25 of 1410 tested) (at <250μg/ml) had anti-inflammatory effects with an iEI ratio >3.8. These include reference drugs (hydrocortisone, dexamethasone N6-(1-iminoethyl)-L-lysine and NSAIDS : diclofenac, tolfenamic acid), a histone deacetylase inhibitor (apicidin) and the following natural products; Ashwaganda (Withania somnifera), Elecampagne Root (Inula helenium), Feverfew (Tanacetum parthenium), Green Tea (Camellia sinensis), Turmeric Root (Curcuma Longa) Ganthoda (Valeriana wallichii), Tansy (Tanacetum vulgare), Maddar Root (Rubia tinctoria), Red Sandle wood (Pterocarpus santalinus), Bay Leaf (Laurus nobilis, Lauraceae), quercetin, cardamonin, fisetin, EGCG, biochanin A, galangin, apigenin and curcumin. The herb with the largest iEI was Ashwaganda where the IC50/LC50 was 11.1/>1750.0 μg/mL, and the compound with the greatest iEI was quercetin where the IC50/LC50 was 10.0/>363.6 ug/mL. These substances also downregulate the production of iNOS expression and attenuate CINC-3 release. In summary, this HTP screening provides guideline information as to efficacy of natural products that in the long term, could prevent inflammatory processes associated with neurodegenerative disease and aggressive glioma tumor growth.
Graphical abstract
Introduction
CNS Inflammation, Glial cells and Neurodegeneration
Age related central nervous system (CNS) degenerative diseases such as Parkinson’s (PD) and Alzheimer’s disease (AD) are becoming significant global health concerns. Inflammatory processes occurring within CNS (by glial cells) contribute vulnerability by initiating vascular damage (Doyle et al., 2015, Weaver et al., 2010) neurological insult (Bodnar et al., 2015, Garcia et al., 2016) and destruction to the blood brain barrier (BBB). (Adelson et al., 1998) While CNS glial cells exert an influential role in neurodevelopment, neuronal homeostasis and CNS detoxification, on the flip side, detrimental inflammatory processes can arise from head trauma (Lopez-Rodriguez et al., 2015), ischemia (Li et al., 2015c) infection (Ben Haim et al., 2015a) lysosomal storage diseases (Rama Rao and Kielian, 2015) and protein aggregates of amyloid β (Aβ) (Ben Haim, Carrillo-de Sauvage, 2015a) and α-synuclein A53T (Ben Haim, Carrillo-de Sauvage, 2015a, Yang et al., 2015) common to AD and PD, respectively. Once neurodegeneration ensues, reactive gliosis (Mohn and Koob, 2015) circumscribing degenerative neurons can occur due to debris generated as danger-associated molecular patterns (DAMPs), (Frank et al., 2016, Kigerl et al., 2014) leading to a cyclic perpetuated release of pro-inflammatory neurotoxic cytokines (Hammond et al., 2015, Mohn and Koob, 2015). Chronic CNS inflammation can manifest itself systemically, where high elevated cytokines are often reported in the cerebral spinal, synovial or serum in patients with PD (Bessler et al., 1999) or AD (Blum-Degen et al., 1995, Brodacki et al., 2008).
CNS Inflammation, Glial Cells and Malignancy
While aging and chronic inflammation are associated with major neurodegenerative disorders, both events are also risk factors for malignant glioblastoma multiforme (GBM), and its radiotherapeutic resistance.(Li and Liu, 2015) Glial tumors originate from sites of chronic irritation/inflammation and once formed, perpetuate release of pro-tumor cytokines as means to drive tumor cell proliferation, resistance, and immune escape (Salazar-Ramiro et al., 2016). Glioma cells have enormous capacity to release chemokines such as MCP-1, which traffic recruitment of tumor associated monocytes (TAMS) (Leung et al., 1997) to infiltrate the glioma tumor bed (Lindemann et al., 2015, Polyzoidis et al., 2015). These processes then drive aggressive malignancies common to both glioblastomas and astrocytomas (Liang et al., 2008, Lin et al., 2013).
There is growing public awareness about the potential use of complementary and alternative medicine (CAMS) to reduce risk of chronic disease. In this study, we use a high through put (HTP) screening procedure to test 1410 natural products (herbs, seeds, roots, leaves, stems) which are available to consumers worldwide as OTC nutraceuticals. The study design in employs the use of a C6 glioma cell line to conduct the HTP screenings. C6 cells are of malignant origin and widely used to investigate neuro inflammation relevant to brain disorders, depression, AD, PD, schizophrenia (de Souza et al., 2013, Kawashima et al., 2008, Lykhmus et al., 2015) defects in iron metabolism (di Patti et al., 2004) glutamate uptake (dos Santos et al., 2006) multiple sclerosis (MS)(Harzheim et al., 2003) as well as therapies and processes associate with aggressive malignant tumor growth and invasion (Adach-Kilon et al., 2011, Li et al., 2016, Liau et al., 1998, Pineda et al., 2005). Glioma tumor growth is advanced by an inflammatory microenvironment involving inducible nitric oxide synthase (iNOS) leading to enhanced vasodilation, proliferation (Munoz-Fernandez and Fresno, 1993) migration (Yeh et al., 2012) and the rapid growth of GBMs (Cobbs et al., 2003).
In this study, we explore a large number of nutraceutical products for evidence of capacity to inhibit inflammation within activated glioma. Using stringent controls to account for therapeutic effects in fully viable cells, the data show that only 25/1410 tested have confirmed anti-inflammatory effects with a relatively high level of confidence.
Methods and Materials
Hanks Balanced Salt Solution, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), ethanol, sufanilamide, 96 well plates, general reagents and supplies, were all purchased from Sigma-Aldrich (St Louis, MO) or VWR (Radnor, PA). Imaging probes were purchased from Life Technologies (Grand Island, NY). Natural products were purchased from Frontier Natural Products Co-op (Norway, IA), Monterey Bay Spice Company (Watsonville, CA), Mountain Rose Herbs (Eugene, OR), Mayway Traditional Chinese Herbs (Oakland, California), Kalyx Natural Marketplace (Camden, NY), Futureceuticals (Momence, IL), organic fruit and vegetable market (New Leaf, Tallahassee, FL), Florida Food Products Inc. (Eustis, FL), Patel Brothers Indian Grocery (Tampa, FL) and OpilGold from AgingSciences LLC (Wayland, MA). Elisa kits and cytokine antibody arrays were purchased from Assay Biotech (Sunnyvale, CA) and Raybiotech (Norcross, GA).
Cell Culture
C6 cells were purchased from American Type Culture Collection (Manassas, VA). Cells were brought up according to the manufacturers instruction, then sub cultured in DMEM high glucose media [glucose 4500mg/L] containing 5% FBS, 4 mM L-glutamine, and penicillin/streptomycin (100 U/0.1 mg/ml). Culture conditions : maintained at 37°C in 5% CO2/atmosphere. For experiments, plating media consisted of DMEM (minus phenol red) [glucose 4500mg/L], 2.5% FBS and penicillin/streptomycin (100 U/0.1 mg/ml). LPS O111:B4 was prepared in HBSS at 1 mg/ml and stored at −20°C. Rat Interferon gamma (IFNγ) was prepared according the manufacturer’s instructions and aliquoted in siliconized micro centrifuge tubes, then stored at −20°C. For experiments, LPS/IFNγ was added to the culture media at a working concentration of 3μg/3ng per ml.
Herbal, Compound and Drug Preparations
All natural chemicals and reference drugs were dissolved in DMSO [5–20mg/mL] and crude herbs in absolute ethanol [50mg/ml] after being diced, macerated and powdered then stored at −20°C. All dilutions were prepared in sterile HBSS + 5 mM HEPES, adjusted to a pH of 7.4 and solvent concentration of DMSO or Etoh was less than 0.5%.
Cell Viability
Cell Viability was quantified using resazurin [7-Hydroxy-3H-phenoxazin-3-one 10-oxide] (Alamar Blue) indicator dye. (Evans et al., 2001) A working solution of resazurin was prepared in sterile HBSS – phenol red (0.5 mg/ml) and added (15% v/v) to each sample. Samples were returned to the incubator for 2–4 hr, and reduction of the dye by viable cells (to resorufin, a fluorescent compound) was quantitatively analyzed using a Synergy HTX multi-mode reader (Bio-Tek, Winooski, VT) with settings at [550/580], [excitation/emission].
Nitrite (NO2−,)/iNOS Expression
Quantification of nitrite (NO2−) was determined by using the Greiss reagent. (Cendan et al., 1996) The Greiss reagent was prepared by mixing an equal volume of 1.0% sulfanilamide in 10% phosphoric acid and 0.1% N-(1-naphthyl)-ethylenediamine in deionized water, then added directly to the cell supernatant (experimental media consisting of DMEM – phenol red) and incubated at room temperature for 10 min. Controls and blanks were run simultaneously, and subtracted from the final value to eliminate interference. Samples were analyzed at 540 nm on a Synergy HTX multi-mode reader (Bio-Tek, Winooski, VT)
For iNOS protein expression, cells were fixed in 4% paraformaldehyde/permeabilized in 0.2% triton X-100 in phosphate buffered saline (PBS) and incubated with anti-iNOS, N-Terminal antibody produced in rabbit for 24 hours at 4°C in a casein blocking buffer. Samples were washed in PBS, then incubated with anti-rabbit Alexa Fluor® 488 conjugate for two hours at RT. Samples were photographically imaged using a fluorescent/inverted microscope, CCD camera and data acquisition using ToupTek View (TouTek Photonics Co, Zhejiang, P.R. China).
High-Through Put Methods
In vitro HTP screenings of natural plants can be informative, but require a number of critical controls to eliminate interfering variables such as inherent variation in pH, solvents and most important, cytotoxicity. In the case of natural products, cell death becomes a pivotal issue given the dual property of many natural products to incur both anti-cancer and anti-inflammatory effects through similar pathways for example the phosphorylation of extracellular signal-regulated kinase (ERK), c-jun NH2-terminal kinase (JNK) phosphorylation and p38 MAPK NF-κB (Amirghofran, 2012, Huang et al., 2007, Pan et al., 2015, Zhang et al., 2015). This aspect becomes important, in particular when using astrocyte cell lines of malignant and immune origin, such as rat C6 glioma cells. For this reason, we construct and use an in vitro efficacy index (iEI) defined as LC50 (toxic concentrations)/IC50 (anti-inflammatory concentrations), to where a higher ratio establishes a greater confidence for the anti-inflammatory proponent with little interfering effects of cytotoxicity. The data from this study, reveal that a very small percentage of natural compounds, at low concentration (<250μg/ml) have anti-inflammatory effects against LPS/IFNγ activation in C6 glioma cells at therapeutic concentrations comparable to NSAIDS.
Rat Cytokine Antibody Array
Rat Cytokine Antibody Arrays – (34 Targets/ab133992) (Abcam, Cambridge, MA) were used to profile the effects of LPS/IFNγ on C6 glioma cells. Each experiment was carried out in accordance with manufacturer’s instructions. Detection of spots using chemiluminescence was acquired using a VersaDoc Imager/Quantity One software (Bio-Rad).
Cytokine-Induced Neutrophil Chemoattractant 3-ELISA
Sample cell supernatants were evaluated for release of CINC-3 using a CINC-3 (CXCL2) Rat ELISA Kit (ab155463) (Abcam, Cambridge, MA). Quantification of CINC-3 release was performed according to the manufacturer’s guidelines. Data was quantified at 450 nm using a Synergy HTX multi-mode reader (Bio-Tek, Winooski, VT).
Data analysis
Statistical analysis was performed using Graph Pad Prism (version 3.0; Graph Pad Software Inc. San Diego, CA, USA) with significance of difference between the groups assessed using a one-way ANOVA, followed by Tukey post hoc means comparison test, or Student’s t test. IC50s and LC50s were determined by regression analysis using Origin Software (OriginLab, Northampton, MA).
Results
Validation studies were conducted to establish dominant inflammatory molecules in LPS 3μg/ml/IFNγ 3ng/ml activated C6 glioma cells (Figure 1A,B), where significant changes in CINC (1,2a and 3) release were observed. Release of NO2− was also significantly elevated in LPS/IFNγ treated cells vs. resting cells, then reduced by hydrocortisone and L-NIL (20μg/ml) (Figure 2A) the latter, without variation in cell viability (Figure 2B). This pattern coincided with iNOS protein expression, (Figure 2C), and 24 hour release of CINC-3 (Figure 2D) to the exception of L-NIL. These findings demonstrate that L-NIL appears to be target specific (enzyme inactivation of iNOS), offering little influence on the process of inflammation as a whole, unlike hydrocortisone which reduces both NO-2, iNOS and CINC3 release.
Figure 1A.
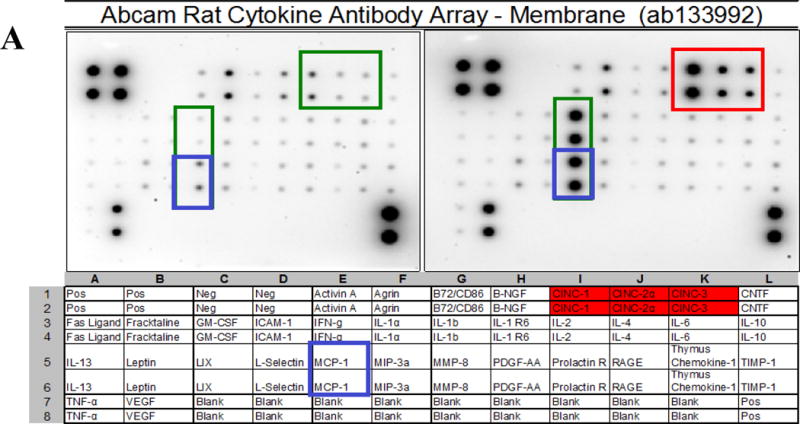
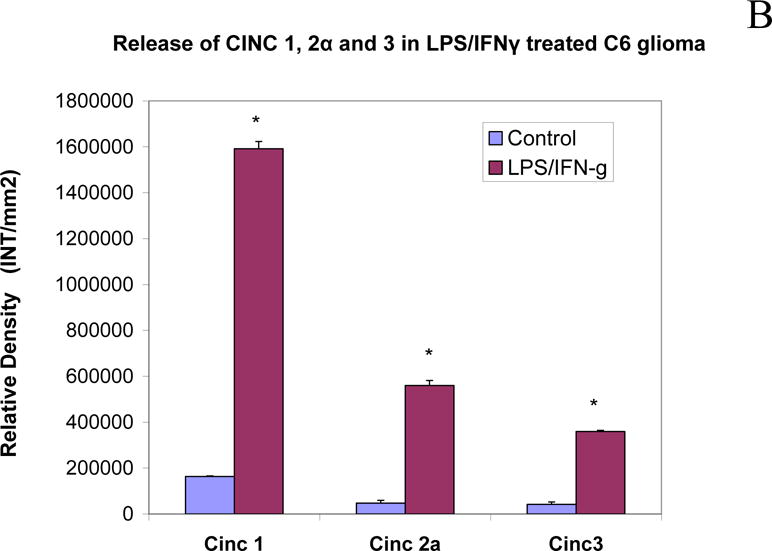
Cytokine release in LPS/IFNγ treated C6 glioma cells at 24h. The blot image (Top) and corresponding array grid layout (Bottom) are presented. (B) CINC1,2a and 3 release were significantly upregulated in LPS/IFNγ treated C6 glioma. The data represents relative density and are expressed as the Mean ± S. E. M., n=4. Differences between resting and activated cells were determined using a Student’s t-test, (*) P<.001.
Figure 2A.
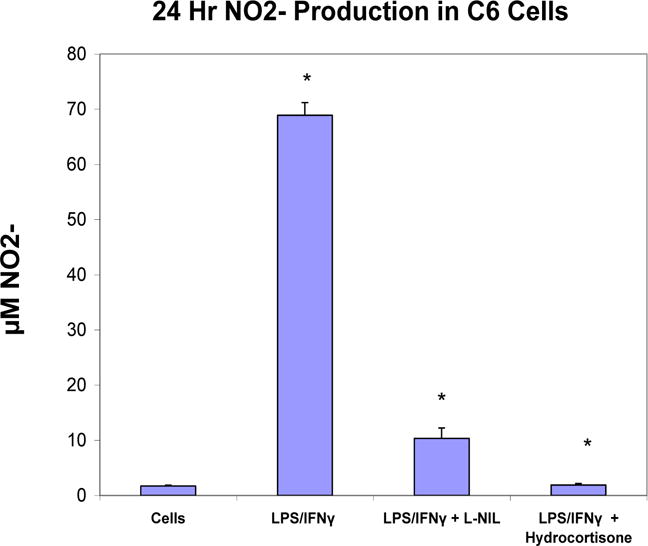
NO−2 production in resting and LPS/IFNγ treated C6 glioma cells ± selective iNOS inhibitor: L-NIL (20μg/ml) or hydrocortisone (20μg/ml). The data represent NO2− produced (μM) and are expressed as the Mean ± S. E. M., n=4. Differences between resting and activated cells were determined by a Student’s t-test (*) P<.001, and LPS vs. L-NIL and hydrocortisone treated cells [*].
Figure 2B.
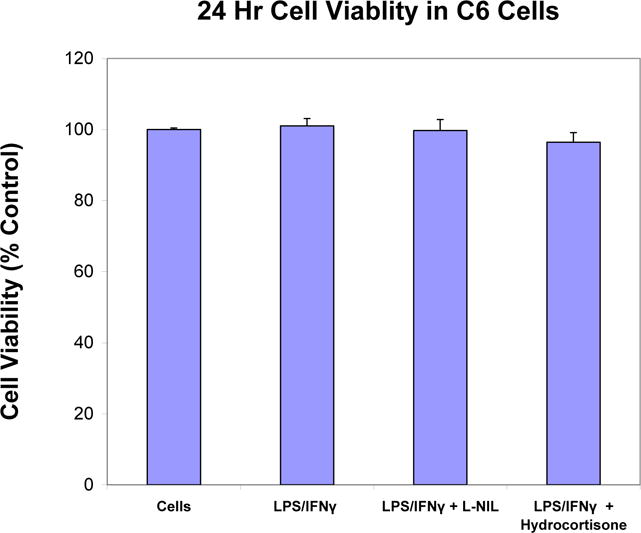
Cell viability in resting and LPS/IFNγ treated C6 glioma cells ± selective iNOS inhibitor: L-NIL (20μg/ml) and hydrocortisone (20μg/ml). The data represent cell viability as % control and are expressed as the Mean ± S. E. M., n=4. Differences between resting and activated cells were determined by a Student’s t-test (*) P<.001, and LPS vs. L-NIL and hydrocortisone treated cells [*].
Figure 2C.
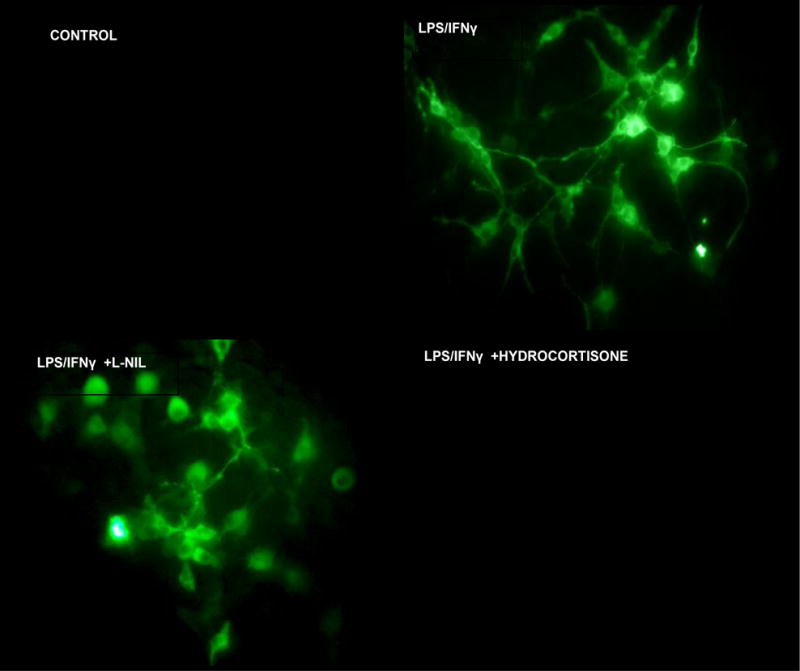
ICC imaging of iNOS expression using rabbit anti mouse iNOS/goat anti-rabbit Alexafluor 488, in fixed permeabilized: Controls: resting C6 cells, LPS/IFNγ treated, LPS/IFNγ treated + L-NIL (20μg/ml) and LPS/IFNγ + hydrocortisone (20μg/ml).
Figure 2D.
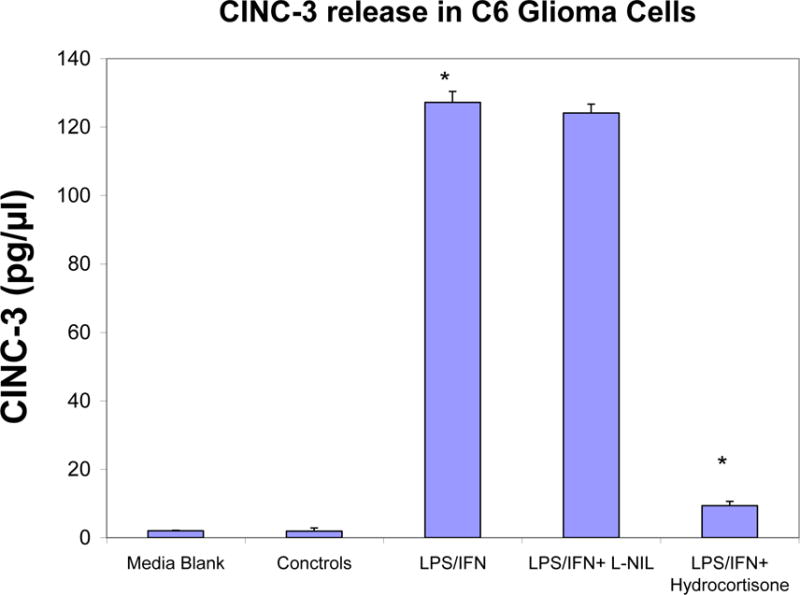
ELISA quantification of CINC-3 released in supernatant by LPS/IFNγ treated C6 glioma cells at 24 hours ± selective iNOS inhibitor: L-NIL (20μg/ml) and hydrocortisone (20μg/ml). The data represent CINC-3 (pg/μl) and expressed as the Mean ± S. E. M., n=3. Differences between resting and LPS/IFNγ activated cells were determined by a Student’s t-test (*) P<.001, and LPS/IFNγ vs. L-NIL and hydrocortisone treated cells (*) P<.001.
A high-through put screening of natural compounds consisted of product library containing ethanol extracts of 1) Plants: Seeds, Fruits, Veg etc. and herbs (commonly used Chinese, Egyptian, Indian and Organic grown in the United States) 2) Natural derived chemicals/polyphenolics 3) Metabolic Substrates: Amino Acids, Vitamins and Energy intermediates such as organic acids and 4) reference NSAID and steroidal anti-inflammatory drugs. A primary screen was conducted to which all compounds were evaluated for reduction of NO2− in LPS/IFNγ activated C6 cells. Natural plant extracts were tested at a maximum concentration of 230μg/ml and compounds at a maximum of 92μg/ml for the initial preliminary screen (Figure 3A). Substances having an IC50 for NO2− inhibition at equal to or below these levels, were re-evaluated over a minimum of 6 dose dependent concentrations where LC50 (cytotoxicity) and IC50 (NO2−) where simultaneously determined (Figure 3B) and an in vitro efficacy index (iEI) established – LC50/IC50. The higher the ratio, the greater confidence in anti-inflammatory effects, relative to influence from cytotoxicity. All iEI values are presented in Table 1, which are provided with a matching scatterplot (Figure 4). Figure 5 shows the full NO2−/viability dose response data for some of the most effective compounds, where an optimal single point n=3, reflects supernatant evaluated for CINC3 by ELISA. These findings highlight the most effective anti-inflammatory natural compounds elucidated using this study model.
Figure 3.
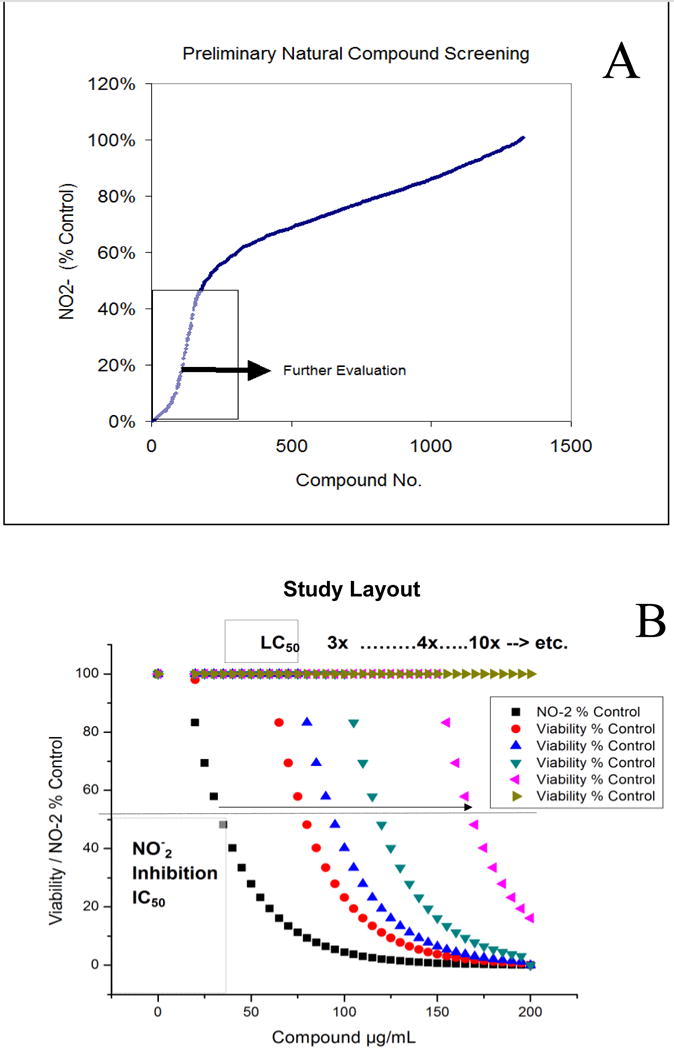
[A] The basic study layout consisted of a primary Tier 1 elimination by which all herbs were tested to reduce LPS/IFNγ induced NO-2 in C6 cells, with maximum working concentrations : 230μg/mL (herbal extracts) and <92μg/ml (metabolites, drugs and polyphenolics). [B] Compounds displaying an IC50 below 1st Tier concentrations were further evaluated where toxicity/anti-inflammatory effects were evaluated simultaneously and an in vitro efficacy index iEI differential is established (LC50/IC50).
Table 1.
Efficacy of natural anti-inflammatory products relative to toxicity in LPS/IFNγ activated C6 cells. The data represent LC50 values for toxicity and IC50 values for NO-2 reduction as determined by regression analysis, and in vitro efficacy index iEI differential is established as (LC50/IC50). < [Max concentration evaluated].
| C6 Glioma : 3μg/mL LPS + 30ng/mL IFNγ | ||||||
|---|---|---|---|---|---|---|
| Substance | Botanical Name | IC50 (μg/mL) | LC50 (μg/ml) | iEI | ||
| Ashwaganda | Withania somnifera | 11.1 | > | 1750 | > | 150.1 |
| Hydrocortisone | N/A | 1.2 | > | 90 | > | 75.7 |
| Elecampane Root | Inula Helenium | 18.9 | 1304.5 | 69 | ||
| L-N-lysine dihydrochloride | N/A | 1.2 | > | 66 | > | 54.5 |
| Feverfew | Tanacetum parthenium | 31 | 1304 | 42.1 | ||
| Quercetin | N/A | 10 | > | 363.6 | > | 36.3 |
| Turmeric Root | Curcuma Longa | 31.8 | 523.8 | 33 | ||
| Dexamethasone | N/A | 1.9 | > | 61.5 | > | 32.4 |
| Cardamonin | N/A | 1.5 | 43.9 | 29.9 | ||
| Apicidin | N/A | 0.8 | 9.8 | 12.3 | ||
| Ganthoda | N/A | 122.1 | > | 961.5 | > | 7.9 |
| Tansy Herb | Tanacetum vulgare | 175 | 1149 | 6.6 | ||
| Diclofenac | N/A | 32.3 | 184.5 | 5.7 | ||
| Maddar root | Rubia tinctorum | 34 | 186 | 5.5 | ||
| Fisetin | N/A | 10.1 | 48.4 | 4.8 | ||
| EGCG | N/A | 19.3 | > | 92 | > | 4.8 |
| Biochanin A | N/A | 78 | > | 369.2 | > | 4.7 |
| Tolfenamic Acid | N/A | 10.8 | 48 | 4.4 | ||
| Galangin | N/A | 56.5 | 246.1 | 4.4 | ||
| Red Sandlewood | Pterocarpus santalinus | 32 | 133.6 | 4.2 | ||
| Apigenin | N/A | 13.2 | 54.1 | 4.1 | ||
| Bay Leaf | Laurus nobilis | 156 | 626 | 4 | ||
| Osha Root | Ligusticum porteri | 46.4 | 183 | 3.9 | ||
| Green Tea Std Sigma T5550 | Camellia sinensis | 32 | > | 125 | > | 3.9 |
| Curcumin | N/A | 26.8 | 102.8 | 3.8 | ||
| Kalijiri Purple Fleablame | Centratherum Anthelminticum | 16.2 | 60.2 | 3.7 | ||
| Genistein | N/A | 8.7 | 31.4 | 3.6 | ||
| Glabridin | N/A | 12.7 | 45.9 | 3.6 | ||
| Ellagic Acid | N/A | 43.6 | > | 156.7 | > | 3.6 |
| Myrrh resin | Commiphora myrrha | 76.8 | 275.3 | 3.6 | ||
| Trifala | Blend | 129.4 | 453.6 | 3.5 | ||
| Amla | Phyllanthus emblica | 264 | 923.1 | 3.5 | ||
| Liquid Gold | N/A | 53.2 | 182.3 | 3.4 | ||
| Phloretin | N/A | 16.7 | 56.3 | 3.4 | ||
| Silymarin | N/A | 30 | 99.6 | 3.3 | ||
| White Sage | Salvia apiana | 52.9 | 167.9 | 3.2 | ||
| Gallic Acid | N/A | 31 | 96.7 | 3.1 | ||
| Butein | N/A | 1.8 | 5.6 | 3.1 | ||
| Gromwell Root | Lithospermum erythrorhizon | 54.1 | 156.7 | 2.9 | ||
| Yerba Santa Leaf | Eriodictyon californicum | 71.3 | 203.9 | 2.9 | ||
| Bergamottin | N/A | 86.5 | > | 246.2 | > | 2.8 |
| Sage leaf | Salvia officinalis | 241 | 683.1 | 2.8 | ||
| Chaparral | Larrea tridentata | 40.6 | 114.7 | 2.8 | ||
| Gamboic acid | N/A | 0.3 | 0.9 | 2.8 | ||
| Daidzein | N/A | 94 | > | 260 | > | 2.8 |
| Javentri | Myristica fragrans | 54.2 | 147.6 | 2.7 | ||
| Dragons Blood | Dioscorea hypoglauca rhizome | 29 | 78.4 | 2.7 | ||
| Silybinin | N/A | 32.7 | 85 | 2.6 | ||
| Ibuprofen | N/A | 100.6 | > | 260 | > | 2.6 |
| Rosemary Leaf | Rosmarinus officinalis | 174.9 | 450.2 | 2.6 | ||
| Centipeda Herb | Centipeda cunninghamii | 200.9 | 498.3 | 2.5 | ||
| Dong Ling Cao | Rabdosia rubescens | 149.5 | 361.5 | 2.4 | ||
| Herb de province | Blend | 226.1 | 541.4 | 2.4 | ||
| Resveratrol | N/A | 45.9 | 107 | 2.3 | ||
| Baicalein | N/A | 24 | 55.2 | 2.3 | ||
| Alkanet Root | Batschia canescens | 43.9 | 100.6 | 2.3 | ||
| Indomethacin | N/A | 49.4 | 109.1 | 2.2 | ||
| Cannabadiol | N/A | 4.3 | 9.5 | 2.2 | ||
| Clove Powder | Syzygium aromaticum | 420.4 | > | 923.1 | > | 2.2 |
Figure 4.
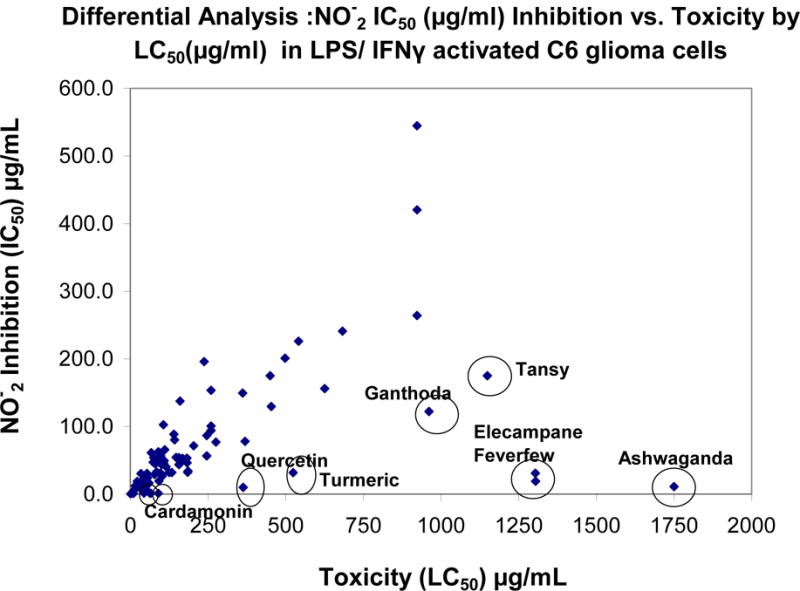
Table 1 scatter – plot showing HTP Screening Results by LC50 (μg/ml) X- Axis and IC50 (μg/ml) Y-Axis.
Figure 5.
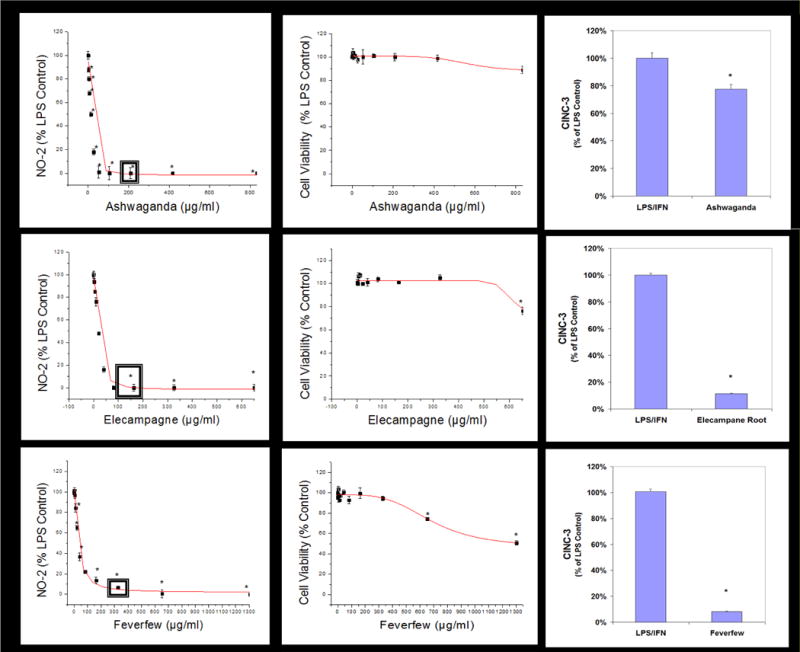
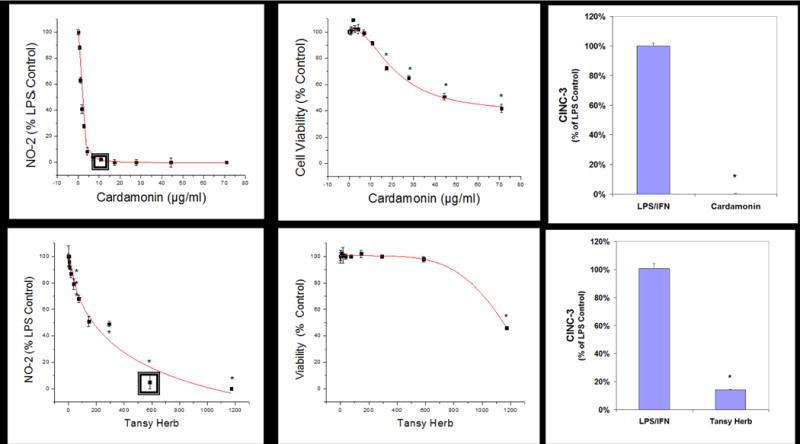
Sample linear regression profiles for dose response used to calculate LC50s (μg/ml) and IC50s (μg/ml) where sub lethal-anti-inflammatory single concentrations were used to evaluate for CINC-3 release in LPS/IFNγ glioma demarcated by a (◘). The data represents cell viability and NO2− (% LPS/IFNγ Control), and significance from controls were evaluated with a one-way ANOVA, with a Tukey Post Hoc test, (*) P<.05. The data represents CINC-3 (as % LPS/IFNγ) and expressed as the Mean ± S.E.M., n=3. Differences determined by a Student’s t-test (*) P<.05.
Discussion
In the CNS, there are numerous triggers which evoke inflammation in immunocompetent cells such as glia, with subsequent events leading to neurodegeneration as well as driving forces toward aggressive malignant glioblastoma. Outward manifestation of reactive gliosis/neuro inflammatory processes are associated with head trauma (Lopez-Rodriguez, Acaz-Fonseca, 2015), ischemia (Li, Xu, 2015c) infection (Ben Haim, Carrillo-de Sauvage, 2015a) and neuronal lesions infiltrated with protein aggregates such as amyloid β (Aβ), neurofibrillary tangles (Ben Haim, Carrillo-de Sauvage, 2015a) α-synucleinA53T (Ben Haim, Carrillo-de Sauvage, 2015a, Yang, Wang, 2015) which are common to AD and PD pathologies, respectively. Sustained neuro-inflammatory processes can be perpetuated by dying cells which release DAMPs such as high mobility group box-1 (HMGB1)/capable of activating innate immunity to remove necrotic debris, then worsening the neurodegenerative process (Frank, Adhikary, 2016, Kigerl, de Rivero Vaccari, 2014). Reactive gliosis contributes to release of toxic molecules such as NO2−, growth factors (lipocalin-2) and chemokines including the CINCs (Lee et al., 2015, Lopez-Rodriguez, Acaz-Fonseca, 2015). CINCS are reportedly elevated as a consequence to ischemic (Wang et al., 2006) and vascular injuries (Weaver, Zhang, 2010), being coexpressed with GFAP, vimentin, synemin, nestin (Hol and Pekny, 2015) and alphaB-crystallin which is present in multiple sclerosis lesions (Liu et al., 2015). In the case of glioblastoma multiforme (GBM), excessive inflammation can drive not only a genetic instabilities, but also tumor proliferation, resistance and immunological evasion (Lin, Zhang, 2013, Polyzoidis, Koletsa, 2015, Salazar-Ramiro, Ramirez-Ortega, 2016). Much of this occurs due to glioma release of chemokines such as MCP1 can traffic monocyte infiltration and docking to glioma, then capable of transforming to an M2 pro-angiogenic phenotype which propels malignant aggressively (Leung, Wong, 1997, Liang, Bollen, 2008, Lindemann, Marschall, 2015).
Drugs or natural compounds with capacities to attenuate inflammatory signaling pathways associated with reactive gliosis should theoretically mitigate CNS inflammatory or glioma advanced processes. These are known to include specific targets such as janus kinase/signal transducer and activator of transcription (JAK/STAT) (Ben Haim, Carrillo-de Sauvage, 2015a, Ben Haim et al., 2015b, Wang et al., 2015b), nuclear Factor of Kappa light polypeptide gene enhancer in B-cells (NF-κB), calcineurin, Mitogen-Activated Protein Kinases (MAPKs) (Ben Haim, Carrillo-de Sauvage, 2015a) or Notch1-STAT3-ETB (LeComte et al., 2015). These are the indirect controls account for biological events such as induction of iNOS, release of NO, MCP-1 and CINCS. (Kesanakurti et al., 2009, Lindemann, Marschall, 2015) It is likely in the future, that nutraceuticals could be utilized to both attenuate risk of CNS inflammatory processes and age-related cognitive decline (Wang et al., 2015a) as well as survival, migration and invasion of glioblastomas (Lamy et al., 2015, Leidgens et al., 2015).
The findings in the work, establish preliminary evidence showing a number of anti-inflammatory natural products, effective in the glioma model. Some of these, as in the case of green tea (Camellia sinensis) or its biologically active polyphenol: EGCG (epigallocatechin 3-gallate) are supported by a plethora of existing studies describing protection against neuroimmunological related insults such as alpha synuclein (Siddique et al., 2014) Abeta (1–42) (Lee et al., 2009) experimental hydrocephalus (Catalao et al., 2014) ischemic stroke (Liu et al., 2013) and aluminum chloride neurotoxicity. (Jelenkovic et al., 2014) Likewise, the immune modulating effects of green tea component EGCG (epigallocatechin 3-gallate) can sensitize effects of temozolomide in models of intracranially implanted human U87 or U251 glioblastoma and enhance GBM to ionizing radiation (Chen et al., 2011, McLaughlin et al., 2006). Similar effects are often reported for turmeric root (Curcuma Longa)/curcumin both in attenuation of CNS injuries (Li et al., 2015a, Li et al., 2015b, Zhu et al., 2014) as well as comprising anti-cancer modulators in general and particularly for glioblastoma. (Dhandapani et al., 2007, Karmakar et al., 2007, Perry et al., 2010)
However, for other substances reported in the current work, there is meager research available. For example, while there are a few studies showing potential for Feverfew (Tanacetum parthenium) in the treatment of migraines (Tassorelli et al., 2005) or others of benefit in ischemic injury such as Ganthoda (Valeriana wallichii) (Rehni et al., 2007) Tansy (Tanacetum vulgare) and (Vafaee et al., 2012), Bay Leaf (Laurus nobilis) (Cho et al., 2010), clearly there is a need for investigation in to glial/glioma related neuro-inflammatory effects. There is little to no research existing on therapeutic benefit of Maddar Root (Rubia tinctoria), or Red Sandlewood (Pterocarpus santalinus).
Like green tea and turmeric root, use of ashwaganda (Withania somnifera) is widely known to treat anxiety, depression and neurodegeneration (Ahmad et al., 2005, Baitharu et al., 2013, Bhattacharya et al., 2000, Jain et al., 2001), not only in research literature, but also historical documents and ancient medical texts pertaining to Ayurvedic medicine. Ashwaganda has beneficial effects on diseases of cardiovascular nature (Ravindran et al., 2015a), obesity, diabetes, infection, cancer (Choi and Kim, 2015, Khazal et al., 2014, Rai et al., 2016), arthritis (Gupta and Singh, 2014, Khan et al., 2015) and gastric inflammation (Kim et al., 2016). The major constituent in Ashwaganda, identified as Withaferin A, itself is an inhibitor of HMG-CoA, angiotensinogen-converting enzyme, (Ravindran et al., 2015b) can lower total cholesterol, triglycerides, higher HDL/LDL ratios (Shukla et al., 2014) and reduce severity or incidence of myocardial infarction, (Khalil et al., 2015) stroke (Ahmad et al., 2015, Raghavan and Shah, 2015, Sood et al., 2015) and hypertension. (Kaur et al., 2015) As far as glial cells, little has been investigated, but it has been reported that Withaferin A has potential in treatment of glioblastoma multiforme (Chang et al., 2016) with possible future therapies to target brain tumors (Kataria et al., 2015).
Elecampagne Root (Inula helenium) has also been referenced in ancient texts, referred to as elf-Dock or elfwort being used to treat digestion, nerve pain and asthma and having been sold in sweets, candies and cakes in London for aiding in respiratory health (Al-Gammal, 1998). Although future research will be required to evaluate the value of this herb for diverse neuro-inflammatory disorders, previous reports confirm its ability to attenuate inflammation in LPS-activated RAW264.7 cells where it activates p38 MAPK/Nrf2/HO-1 pathways also reducing inflammation in cecal ligation-induced sepsis in mice.(Park et al., 2013) Lastly, Ganthoda (Valeriana wallichii), is a well known sleeping aid (valerian) (Toolika et al., 2015) having historic reputations as a traditional Chinese medicine for treating insomnia, nervous disorders, epilepsy, and vision impairment. Neuroprotective activities has been demonstrated due to activity or iridoids (Zhang and Ding, 2015) which are reportedly capable of attenuating DAergic degeneration in MPTP treated mice (Sridharan et al., 2015) and cerebral injury from stroke.(Rehni, Pantlya, 2007) However, more research is needed to envelope diverse therapeutic values.
In summary, the data from this paper serve as a guide for future studies which may explore mechanism of action, constituents within and capabilities for defined natural products to prevent CNS inflammatory related degenerative injury and forces which drive aggressive malignant gliobastoma.
Highlights.
Central nervous system (CNS) inflammation involves pro-inflammatory processes within glial cells, which is often associated with neurodegenerative diseases.
C6 glioma cells were evaluated for profile changes in pro-inflammatory molecules upon treatment with lipopolysaccharide (LPS)/Interferon gamma (IFNγ) by antibody array
Differential shifts were evident for MCP-1, NO2- and cytokine-induced neutrophil chemoattractants (CINC) 1, CINC 2a and CINC 3
Over 1400 natural products were evaluated for capacity to reduce NO2- in fully viable cells and only 1.77% showed anti-inflammatory effects comparable to steroidal and non-steroidal reference drugs
Of these, many also demonstrated significant attenuation of CINC-3 determined by ELISA
Lead products included : Ashwaganda (Withania somnifera), Elecampagne Root (Inula helenium), Feverfew (Tanacetum parthenium), Green Tea (Camellia sinensis), Turmeric Root (Curcuma Longa) Ganthoda (Valeriana wallichii), Tansy (Tanacetum vulgare), Maddar Root (Rubia tinctoria), Red Sandle wood (Pterocarpus santalinus), Bay Leaf (Laurus nobilis, Lauraceae), quercetin, cardamonin, fisetin, EGCG, biochanin A, galangin, apigenin and curcumin.
Acknowledgments
This research was supported by the National Institute of Minority Health and Health Disparities of the National Institutes of Health through Grant Number 8 G12MD007582-28 and Grant Number 1P20 MD006738-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
N/A
References
- Adach-Kilon A, Swiatek-Machado K, Kaminska B, Dabrowski M. Signal transducer and activator of transcription 1 (Stat1) maintains basal mRNA expression of pro-survival stat3-target genes in glioma C6 cells. J Cell Biochem. 2011;112:3685–94. doi: 10.1002/jcb.23305. [DOI] [PubMed] [Google Scholar]
- Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, Carlos TM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir Suppl. 1998;71:104–6. doi: 10.1007/978-3-7091-6475-4_31. [DOI] [PubMed] [Google Scholar]
- Ahmad H, Khandelwal K, Samuel SS, Tripathi S, Mitra K, Sangwan RS, et al. Neuro-protective potential of a vesicular system of a standardized extract of a new chemotype of Withania somnifera Dunal (NMITLI118RT+) against cerebral stroke in rats. Drug delivery. 2015:1–12. doi: 10.3109/10717544.2015.1041579. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Saleem S, Ahmad AS, Ansari MA, Yousuf S, Hoda MN, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–47. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- Al-Gammal SY. Elecampane and Job’s disease. Bulletin of the Indian Institute of History of Medicine. 1998;28:7–11. [PubMed] [Google Scholar]
- Amirghofran Z. Herbal medicines for immunosuppression. Iranian journal of allergy, asthma, and immunology. 2012;11:111–9. [PubMed] [Google Scholar]
- Baitharu I, Jain V, Deep SN, Hota KB, Hota SK, Prasad D, et al. Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J Ethnopharmacol. 2013;145:431–41. doi: 10.1016/j.jep.2012.10.063. [DOI] [PubMed] [Google Scholar]
- Ben Haim L, Carrillo-de Sauvage MA, Ceyzeriat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Frontiers in cellular neuroscience. 2015a;9:278. doi: 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, Aubry F, Auregan G, Guillermier M, et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015b;35:2817–29. doi: 10.1523/JNEUROSCI.3516-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson’s disease. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 1999;53:141–5. doi: 10.1016/S0753-3322(99)80079-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7:463–9. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neuroscience letters. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Bodnar TS, Hill LA, Taves MD, Yu W, Soma KK, Hammond GL, et al. Colony-Specific Differences in Endocrine and Immune Responses to an Inflammatory Challenge in Female Sprague Dawley Rats. Endocrinology. 2015;156:4604–17. doi: 10.1210/en.2015-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodacki B, Staszewski J, Toczylowska B, Kozlowska E, Drela N, Chalimoniuk M, et al. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neuroscience letters. 2008;441:158–62. doi: 10.1016/j.neulet.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Catalao CH, Correa DA, Saito ST, Lopes LdaS. Camellia sinensis neuroprotective role in experimentally induced hydrocephalus in Wistar rats. Childs Nerv Syst. 2014;30:591–7. doi: 10.1007/s00381-013-2262-x. [DOI] [PubMed] [Google Scholar]
- Cendan JC, Topping DL, Pruitt J, Snowdy S, Copeland EM, 3rd, Lind DS. Inflammatory mediators stimulate arginine transport and arginine-derived nitric oxide production in a murine breast cancer cell line. The Journal of surgical research. 1996;60:284–8. doi: 10.1006/jsre.1996.0044. [DOI] [PubMed] [Google Scholar]
- Chang E, Pohling C, Natarajan A, Witney TH, Kaur J, Xu L, et al. AshwaMAX and Withaferin A inhibits gliomas in cellular and murine orthotopic models. Journal of neuro-oncology. 2016;126:253–64. doi: 10.1007/s11060-015-1972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Wang W, Golden EB, Thomas S, Sivakumar W, Hofman FM, et al. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011;302:100–8. doi: 10.1016/j.canlet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Cho EY, Lee SJ, Nam KW, Shin J, Oh KB, Kim KH, et al. Amelioration of oxygen and glucose deprivation-induced neuronal death by chloroform fraction of bay leaves (Laurus nobilis) Biosci Biotechnol Biochem. 2010;74:2029–35. doi: 10.1271/bbb.100301. [DOI] [PubMed] [Google Scholar]
- Choi BY, Kim BW. Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity. Journal of cancer prevention. 2015;20:185–92. doi: 10.15430/JCP.2015.20.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs CS, Whisenhunt TR, Wesemann DR, Harkins LE, Van Meir EG, Samanta M. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 2003;63:8670–3. [PubMed] [Google Scholar]
- de Souza DF, Wartchow K, Hansen F, Lunardi P, Guerra MC, Nardin P, et al. Interleukin-6-induced S100B secretion is inhibited by haloperidol and risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:14–22. doi: 10.1016/j.pnpbp.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–38. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- di Patti MC, Persichini T, Mazzone V, Polticelli F, Colasanti M, Musci G. Interleukin-1beta up-regulates iron efflux in rat C6 glioma cells through modulation of ceruloplasmin and ferroportin-1 synthesis. Neurosci Lett. 2004;363:182–6. doi: 10.1016/j.neulet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- dos Santos AQ, Nardin P, Funchal C, de Almeida LM, Jacques-Silva MC, Wofchuk ST, et al. Resveratrol increases glutamate uptake and glutamine synthetase activity in C6 glioma cells. Arch Biochem Biophys. 2006;453:161–7. doi: 10.1016/j.abb.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Doyle CJ, Fitzsimmons TR, Marchant C, Dharmapatni AA, Hirsch R, Bartold PM. Azithromycin suppresses P. gingivalis LPS-induced pro-inflammatory cytokine and chemokine production by human gingival fibroblasts in vitro. Clinical oral investigations. 2015;19:221–7. doi: 10.1007/s00784-014-1249-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Casartelli A, Herreros E, Minnick DT, Day C, George E, et al. Development of a high throughput in vitro toxicity screen predictive of high acute in vivo toxic potential. Toxicology in vitro : an international journal published in association with BIBRA. 2001;15:579–84. doi: 10.1016/s0887-2333(01)00064-9. [DOI] [PubMed] [Google Scholar]
- Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. 2016;51:99–108. doi: 10.1016/j.bbi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JJ, Carvajal-Gil J, Guerrero-Bonmatty R. Altered release of chemokines by phagocytes from fibromyalgia patients: a pilot study. Innate immunity. 2016;22:3–8. doi: 10.1177/1753425915602959. [DOI] [PubMed] [Google Scholar]
- Gupta A, Singh S. Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharmaceutical biology. 2014;52:308–20. doi: 10.3109/13880209.2013.835325. [DOI] [PubMed] [Google Scholar]
- Hammond TR, McEllin B, Morton PD, Raymond M, Dupree J, Gallo V. Endothelin-B Receptor Activation in Astrocytes Regulates the Rate of Oligodendrocyte Regeneration during Remyelination. Cell reports. 2015;13:2090–7. doi: 10.1016/j.celrep.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Harzheim M, Altenschmidt M, Heneka MT, Schroder R, Klockgether T, Schmidt S. IFN-beta1a (Rebif) modifies the expression of microfilament-associated cell-cell contacts in C6 glioma cells. J Interferon Cytokine Res. 2003;23:83–9. doi: 10.1089/107999003321455471. [DOI] [PubMed] [Google Scholar]
- Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Current opinion in cell biology. 2015;32:121–30. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Huang WC, Hsu RM, Chi LM, Leu YL, Chang YS, Yu JS. Selective downregulation of EGF receptor and downstream MAPK pathway in human cancer cell lines by active components partially purified from the seeds of Livistona chinensis R. Brown. Cancer letters. 2007;248:137–46. doi: 10.1016/j.canlet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Jain S, Shukla SD, Sharma K, Bhatnagar M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15:544–8. doi: 10.1002/ptr.802. [DOI] [PubMed] [Google Scholar]
- Jelenkovic A, Jovanovic MD, Stevanovic I, Petronijevic N, Bokonjic D, Zivkovic J, et al. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother Res. 2014;28:82–7. doi: 10.1002/ptr.4962. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Ray SK. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32:2103–13. doi: 10.1007/s11064-007-9376-z. [DOI] [PubMed] [Google Scholar]
- Kataria H, Kumar S, Chaudhary H, Kaur G. Withania somnifera Suppresses Tumor Growth of Intracranial Allograft of Glioma Cells. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9320-1. [DOI] [PubMed] [Google Scholar]
- Kaur G, Singh N, Samuel SS, Bora HK, Sharma S, Pachauri SD, et al. Withania somnifera shows a protective effect in monocrotaline-induced pulmonary hypertension. Pharmaceutical biology. 2015;53:147–57. doi: 10.3109/13880209.2014.912240. [DOI] [PubMed] [Google Scholar]
- Kawashima A, Harada T, Imada K, Yano T, Mizuguchi K. Eicosapentaenoic acid inhibits interleukin-6 production in interleukin-1beta-stimulated C6 glioma cells through peroxisome proliferator-activated receptor-gamma. Prostaglandins Leukot Essent Fatty Acids. 2008;79:59–65. doi: 10.1016/j.plefa.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Kesanakurti D, Sareddy GR, Babu PP, Kirti PB. Mustard NPR1, a mammalian IkappaB homologue inhibits NF-kappaB activation in human GBM cell lines. Biochem Biophys Res Commun. 2009;390:427–33. doi: 10.1016/j.bbrc.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Khalil MI, Ahmmed I, Ahmed R, Tanvir EM, Afroz R, Paul S, et al. Amelioration of Isoproterenol-Induced Oxidative Damage in Rat Myocardium by Withania somnifera Leaf Extract. BioMed research international. 2015;2015:624159. doi: 10.1155/2015/624159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Subramaneyaan M, Arora VK, Banerjee BD, Ahmed RS. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. Journal of complementary & integrative medicine. 2015;12:117–25. doi: 10.1515/jcim-2014-0075. [DOI] [PubMed] [Google Scholar]
- Khazal KF, Hill DL, Grubbs CJ. Effect of Withania somnifera root extract on spontaneous estrogen receptor-negative mammary cancer in MMTV/Neu mice. Anticancer research. 2014;34:6327–32. [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kim TH, Kang MJ, Choi JA, Pack DY, Lee IR, et al. Inhibitory effect of withaferin A on Helicobacter pyloriinduced IL8 production and NFkappaB activation in gastric epithelial cells. Mol Med Rep. 2016;13:967–72. doi: 10.3892/mmr.2015.4602. [DOI] [PubMed] [Google Scholar]
- Lamy S, Moldovan PL, Ben Saad A, Annabi B. Biphasic effects of luteolin on interleukin-1beta-induced cyclooxygenase-2 expression in glioblastoma cells. Biochim Biophys Acta. 2015;1853:126–35. doi: 10.1016/j.bbamcr.2014.10.010. [DOI] [PubMed] [Google Scholar]
- LeComte MD, Shimada IS, Sherwin C, Spees JL. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8726–31. doi: 10.1073/pnas.1501029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, et al. Green tea (−)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr. 2009;139:1987–93. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- Lee S, Jha MK, Suk K. Lipocalin-2 in the Inflammatory Activation of Brain Astrocytes. Critical reviews in immunology. 2015;35:77–84. doi: 10.1615/critrevimmunol.2015012127. [DOI] [PubMed] [Google Scholar]
- Leidgens V, Seliger C, Jachnik B, Welz T, Leukel P, Vollmann-Zwerenz A, et al. Ibuprofen and Diclofenac Restrict Migration and Proliferation of Human Glioma Cells by Distinct Molecular Mechanisms. PLoS One. 2015;10:e0140613. doi: 10.1371/journal.pone.0140613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–27. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- Li L, Li H, Li M. Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med. 2015a;8:14985–91. [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu Y. Aging-related gene signature regulated by Nlrp3 predicts glioma progression. Am J Cancer Res. 2015;5:442–9. [PMC free article] [PubMed] [Google Scholar]
- Li M, Cui Y, Li X, Guo Y, Wang B, Zhang J, et al. Functional Changes of Dendritic Cells in C6 Glioma-Bearing Rats That Underwent Combined Argon-Helium Cryotherapy and IL-12 Treatment. Technol Cancer Res Treat. 2016;15:618–24. doi: 10.1177/1533034615606322. [DOI] [PubMed] [Google Scholar]
- Li Y, Li J, Li S, Wang X, Liu B, Fu Q, et al. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol. 2015b;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu XL, Zhao D, Pan LN, Huang CW, Guo LJ, et al. TLR3 ligand Poly IC Attenuates Reactive Astrogliosis and Improves Recovery of Rats after Focal Cerebral Ischemia. CNS neuroscience & therapeutics. 2015c;21:905–13. doi: 10.1111/cns.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Bollen AW, Gupta N. CC chemokine receptor-2A is frequently overexpressed in glioblastoma. J Neurooncol. 2008;86:153–63. doi: 10.1007/s11060-007-9463-7. [DOI] [PubMed] [Google Scholar]
- Liau LM, Fakhrai H, Black KL. Prolonged survival of rats with intracranial C6 gliomas by treatment with TGF-beta antisense gene. Neurol Res. 1998;20:742–7. doi: 10.1080/01616412.1998.11740594. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhang G, Zhang J, Gao G, Li M, Chen Y, et al. A panel of four cytokines predicts the prognosis of patients with malignant gliomas. J Neurooncol. 2013;114:199–208. doi: 10.1007/s11060-013-1171-x. [DOI] [PubMed] [Google Scholar]
- Lindemann C, Marschall V, Weigert A, Klingebiel T, Fulda S. Smac Mimetic-Induced Upregulation of CCL2/MCP-1 Triggers Migration and Invasion of Glioblastoma Cells and Influences the Tumor Microenvironment in a Paracrine Manner. Neoplasia. 2015;17:481–9. doi: 10.1016/j.neo.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Z, Wang P, Yu B, Liu Y, Xue Y. Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement Altern Med. 2013;13:187. doi: 10.1186/1472-6882-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu Y, Zhou Q, Tang M, Fu N, Shao W, Zhang S, et al. Upregulation of alphaB-crystallin expression in the substantia nigra of patients with Parkinson’s disease. Neurobiology of aging. 2015;36:1686–91. doi: 10.1016/j.neurobiolaging.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, Garcia-Segura LM. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PloS one. 2015;10:e0128782. doi: 10.1371/journal.pone.0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykhmus O, Voytenko L, Koval L, Mykhalskiy S, Kholin V, Peschana K, et al. alpha7 Nicotinic acetylcholine receptor-specific antibody induces inflammation and amyloid beta42 accumulation in the mouse brain to impair memory. PLoS One. 2015;10:e0122706. doi: 10.1371/journal.pone.0122706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin N, Annabi B, Bouzeghrane M, Temme A, Bahary JP, Moumdjian R, et al. The Survivin-mediated radioresistant phenotype of glioblastomas is regulated by RhoA and inhibited by the green tea polyphenol (−)-epigallocatechin-3-gallate. Brain Res. 2006;1071:1–9. doi: 10.1016/j.brainres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Mohn TC, Koob AO. Adult Astrogenesis and the Etiology of Cortical Neurodegeneration. Journal of experimental neuroscience. 2015;9:25–34. doi: 10.4137/JEN.S25520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Fernandez MA, Fresno M. Involvement of nitric oxide on the cytokine induced growth of glial cell. Biochem Biophys Res Commun. 1993;194:319–25. doi: 10.1006/bbrc.1993.1822. [DOI] [PubMed] [Google Scholar]
- Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK, Zhao WJ. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochemistry international. 2015;80:60–71. doi: 10.1016/j.neuint.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Park EJ, Kim YM, Park SW, Kim HJ, Lee JH, Lee DU, et al. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;55:386–95. doi: 10.1016/j.fct.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Perry MC, Demeule M, Regina A, Moumdjian R, Beliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res. 2010;54:1192–201. doi: 10.1002/mnfr.200900277. [DOI] [PubMed] [Google Scholar]
- Pineda B, Estrada-Parra S, Pedraza-Medina B, Rodriguez-Ropon A, Perez R, Arrieta O. Interstitial transfer factor as adjuvant immunotherapy for experimental glioma. J Exp Clin Cancer Res. 2005;24:575–83. [PubMed] [Google Scholar]
- Polyzoidis S, Koletsa T, Panagiotidou S, Ashkan K, Theoharides TC. Mast cells in meningiomas and brain inflammation. J Neuroinflammation. 2015;12:170. doi: 10.1186/s12974-015-0388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan A, Shah ZA. Withania somnifera Improves Ischemic Stroke Outcomes by Attenuating PARP1-AIF-Mediated Caspase-Independent Apoptosis. Mol Neurobiol. 2015;52:1093–105. doi: 10.1007/s12035-014-8907-2. [DOI] [PubMed] [Google Scholar]
- Rai M, Jogee PS, Agarkar G, Santos CA. Anticancer activities of Withania somnifera: Current research, formulations, and future perspectives. Pharmaceutical biology. 2016;54:189–97. doi: 10.3109/13880209.2015.1027778. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV Kielian T. Astrocytes and lysosomal storage diseases. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Sharma N, Roy S, Thakur AR, Ganesh S, Kumar S, et al. Interaction Studies of Withania Somnifera’s Key Metabolite Withaferin A with Different Receptors Assoociated with Cardiovascular Disease. Current computer-aided drug design. 2015a;11:212–21. doi: 10.2174/1573409912666151106115848. [DOI] [PubMed] [Google Scholar]
- Ravindran R, Sharma N, Roy S, Thakur AR, Subhadra G, Sriram K, et al. Interaction studies of Withania somnifera’s key metabolite Withaferin A with different receptors associated with cardiovascular disease. Current computer-aided drug design. 2015b doi: 10.2174/1573409912666151106115848. [DOI] [PubMed] [Google Scholar]
- Rehni AK, Pantlya HS, Shri R, Singh M. Effect of chlorophyll and aqueous extracts of Bacopa monniera and Valeriana wallichii on ischaemia and reperfusion-induced cerebral injury in mice. Indian J Exp Biol. 2007;45:764–9. [PubMed] [Google Scholar]
- Salazar-Ramiro A, Ramirez-Ortega D, Perez de la Cruz V, Hernandez-Pedro NY, Gonzalez-Esquivel DF, Sotelo J, et al. Role of Redox Status in Development of Glioblastoma. Front Immunol. 2016;7:156. doi: 10.3389/fimmu.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla K, Dikshit P, Shukla R, Sharma S, Gambhir JK. Hypolipidemic and antioxidant activity of aqueous extract of fruit of Withania coagulans (Stocks) Dunal in cholesterol-fed hyperlipidemic rabbit model. Indian journal of experimental biology. 2014;52:870–5. [PubMed] [Google Scholar]
- Siddique YH, Jyoti S, Naz F. Effect of epicatechin gallate dietary supplementation on transgenic Drosophila model of Parkinson’s disease. J Diet Suppl. 2014;11:121–30. doi: 10.3109/19390211.2013.859207. [DOI] [PubMed] [Google Scholar]
- Sood A, Kumar A, Dhawan DK, Sandhir R. Propensity of Withania somnifera to Attenuate Behavioural, Biochemical, and Histological Alterations in Experimental Model of Stroke. Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan S, Mohankumar K, Jeepipalli SP, Sankaramourthy D, Ronsard L, Subramanian K, et al. Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology. 2015;51:172–83. doi: 10.1016/j.neuro.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Greco R, Morazzoni P, Riva A, Sandrini G, Nappi G. Parthenolide is the component of tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: studies in an animal model of migraine. Cephalalgia. 2005;25:612–21. doi: 10.1111/j.1468-2982.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- Toolika E, Bhat NP, Shetty SK. A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia) Ayu. 2015;36:46–9. doi: 10.4103/0974-8520.169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee F, Zangiabadi N, Pour FM, Dehghanian F, Asadi-Shekaari M, Afshar HK. Neuroprotective effects of the immunomodulatory drug Setarud on cerebral ischemia in male rats. Neural Regen Res. 2012;7:2085–91. doi: 10.3969/j.issn.1673-5374.2012.27.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang J, Lu P, Cai Y, Wang Y, Hong L, et al. Local delivery of FTY720 in PCL membrane improves SCI functional recovery by reducing reactive astrogliosis. Biomaterials. 2015a;62:76–87. doi: 10.1016/j.biomaterials.2015.04.060. [DOI] [PubMed] [Google Scholar]
- Wang T, Yuan W, Liu Y, Zhang Y, Wang Z, Zhou X, et al. The role of the JAK-STAT pathway in neural stem cells, neural progenitor cells and reactive astrocytes after spinal cord injury. Biomedical reports. 2015b;3:141–6. doi: 10.3892/br.2014.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Luo W, Stricker R, Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J Neurochem. 2006;98:1046–60. doi: 10.1111/j.1471-4159.2006.03950.x. [DOI] [PubMed] [Google Scholar]
- Weaver JL, Zhang J, Knapton A, Miller T, Espandiari P, Smith R, et al. Early events in vascular injury in the rat induced by the phosphodiesterase IV inhibitor SCH 351591. Toxicol Pathol. 2010;38:738–44. doi: 10.1177/0192623310374331. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang G, Wang CE, Guo X, Yin P, Gao J, et al. Mutant alpha-synuclein causes age-dependent neuropathology in monkey brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:8345–58. doi: 10.1523/JNEUROSCI.0772-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WL, Lu DY, Liou HC, Fu WM. A forward loop between glioma and microglia: glioma-derived extracellular matrix-activated microglia secrete IL-18 to enhance the migration of glioma cells. J Cell Physiol. 2012;227:558–68. doi: 10.1002/jcp.22746. [DOI] [PubMed] [Google Scholar]
- Zhang NN, Ding GZ. Development and research advances of iridoids from Valeriana jatamansi and their bioactivity. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2015;40:1893–7. [PubMed] [Google Scholar]
- Zhang X, Luo W, Zhao W, Lu J, Chen X. Isocryptotanshinone Induced Apoptosis and Activated MAPK Signaling in Human Breast Cancer MCF-7 Cells. Journal of breast cancer. 2015;18:112–8. doi: 10.4048/jbc.2015.18.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, et al. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014;11:59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]


