Abstract
Accurate characterization of particulate matter (PM) exposure in young children is difficult, because personal samplers are often too heavy, bulky or impractical to be used. The Pretoddler Inhalable Particulate Environmental Robotic (PIPER) sampler was developed to help address this problem. In this study, we measured inhalable PM exposures in 2-year-olds via a lightweight personal sampler worn in a small backpack and evaluated the use of a robotic sampler with an identical sampling train for estimating PM exposure in this age group. PM mass concentrations measured by the personal sampler ranged from 100 to almost 1,200 μg/m3, with a median value of 331 μg/m3. PM concentrations measured by PIPER were considerably lower, ranging from 14 to 513 μg/m3 with a median value of 56 μg/m3. Floor cleaning habits and activity patterns of the 2-year-olds varied widely by home; vigorous play and recent floor cleaning were most associated with higher personal exposure. Our findings highlight the need for additional characterization of children’s activity patterns and their effect on personal exposures.
Keywords: activity patterns, children’s exposures to PM, floor type, Pretoddler Inhalable Particulate Environmental Robotic sampler, resuspension, robotic sampling platform
INTRODUCTION
Personal exposure to particulate matter (PM) is an area of concern, owing to its role in the development and exacerbation of asthma, wheezing, and other respiratory problems.1–5 Children in the United States may spend as much as 90% of their time indoors,6 making accurate characterization of indoor PM exposure an important component of estimating total exposure. Indoor PM has many sources, including resuspension of settled dust from the floor as people walk.7 For very young children who spend much of their time playing on the floor and, consequently, have their breathing zones close to the floor, resuspension of particles due to movement is likely to be an especially important contributor to overall PM exposure. Higher concentrations of resuspended particles have been shown to be associated with increased asthma levels as well.8
Personal PM monitoring is considered a better method to estimate actual exposure than stationary monitoring9–11 and has been applied to adults and older children.12 However, personal monitoring with young children (<4 years old) is difficult due to the weight of the sampling equipment and tendency of very young children (<2 years old) to try to put the sampling equipment in their mouths. The Pretoddler Inhalable Particulate Environmental Robotic (PIPER) sampler was developed to provide a more robust estimate of PM exposure in children.13,14 PIPER is an autonomous robot that moves at different speeds to simulate crawling, walking, and running by young children, and it can be equipped with various air sampling equipment. Instrument inlets are attached to a lift, which changes height according to a prescribed program to simulate breathing zone changes in children of different age groups and gender as they play.
Previous work13,15 has shown that PIPER consistently observes higher levels of PM mass and number concentration compared with a stationary sampler due to the resuspension of particles from the floor and closer proximity of its samplers to the floor. However, PIPER’s measurements have not previously been compared directly with exposure measurements by a personal sampler, for the practical reason that placing sampling equipment on young children can be challenging. In this study, we sought to compare PIPER’s PM measurements directly with short-term (2 h) personal sampling measurements and to explore the following questions:
How accurately can PIPER estimate PM exposure of young children?
How do the playing patterns of children affect their personal exposure to PM?
How do floor characteristics influence personal exposure?
METHODS
Two-year-old children were recruited from a pediatric pulmonology clinic and a general pediatric clinic. The Rutgers University Institutional Review Board reviewed and approved the study, and each parent signed an informed consent form before sampling began. The study was designed to have adequate power with 50 homes sampled to be able to detect a 10% difference in the measured mean between PIPER and personal sampler with a β of 90% and an α of 5.0%. The sample size calculation was performed using STATA Version 11.2 (STATA, College Station, TX, USA). Sampling (~4.5 h) took place by appointment during one in-home visit. Parents were asked to do no special cleaning before the appointment but to maintain their usual cleaning routine. A summary of demographic and other relevant data for the homes can be found in Supplementary Table 1. A Button inhalable aerosol sampler (model #225–360, SKC, Eighty Four, PA, USA) with a Teflon filter (3.0 μm pore size) was attached to the front strap of a small backpack (Figure 1, left). This configuration placed the Button sampler ~15–30 cm away from the child’s mouth and nose when the backpack was worn, that is, the Button sampler was inside the child’s personal breathing zone. The Button sampler is designed to be operated at 4 l/min, to ensure even distribution of particles on the filter and conforms to inhalable sampling convention,16 that is, its aspiration efficiency is close to 100% and gradually decreases to 50% for 100 μm particles.17 A personal sampling pump (Air-Check, SKC) was enclosed in a sound-insulating jacket and placed inside the backpack, to provide airflow for the Button sampler. The backpack and sampling equipment together weighed ~0.5 kg.
Figure 1.
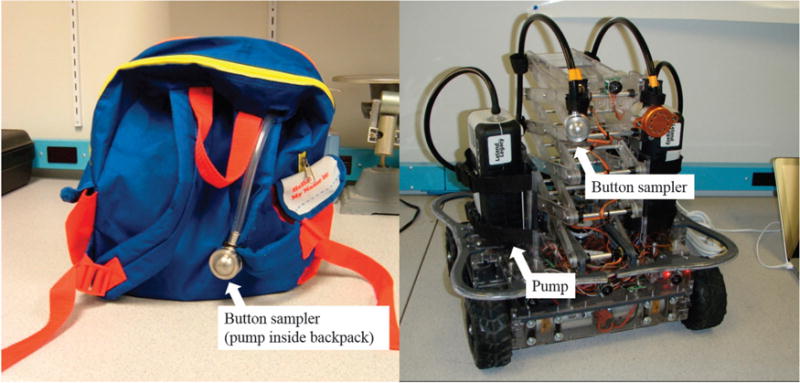
Left: age-appropriate backpack that children wore during this sampling campaign. The Button inhalable aerosol sampler is attached to the front strap and the sampling pump is inside the backpack. Right: the Pretoddler Inhalable Particulate Environmental Robotic (PIPER) sampler.
At each house, sampling took place in the room identified by the parent or caregiver as the child’s main play area. First, the child wore the backpack for 2 h while undertaking his/her normal activities. The child was encouraged to stay in the room for as much of the 2 h as possible, although short breaks outside the room were allowed. During the sampling, the parent or guardian of the child was administered a questionnaire about the child’s health, characteristics of the home’s structure and utilities, house cleaning routines, and various aspects of the child’s normal daily routine including routine play activities in the sampled room (e.g., playing with blocks, watching TV, eating, and doing gymnastics).
At the end of the play period, researchers recorded two measures of the children’s activities. First, children’s behavior was broadly categorized based on their breathing zone position relative to the floor. Researchers estimated the percentage of the time period that the child spent in three different positions while wearing the personal sampling equipment: sitting on the floor (including lying prone/supine, crawling, and kneeling), sitting off the floor (on a sofa or chair), and being upright on the floor (standing, walking, and running). Second, researchers recorded the play activities conducted by the child during sampling.
After the personal sampling was completed, the room air was sampled with PIPER (Figure 1, right). The PIPER sampling system and its features have been described previously.8,13–15,18,19 Sampling with PIPER was done separately from the personal sampling for logistical reasons; most children were uncomfortable with PIPER and would probably have altered their playing habits if PIPER was moving in the same room. During sampling by PIPER, subjects and family were asked to leave the room. Small furniture and objects were removed from the floor to create an open area for PIPER movement and sampling. Using a gender-matched movement profile for a 2-year-old (Supplementary Table 2), PIPER was then run for 2 h according to a pre-programmed motion pattern designed to approximate the level of activity and the average amount of time that 2-year-olds spend at different breathing zone heights. PIPER was equipped with two identical Button samplers (SKC) and two pumps: a standard sample was collected with an Air-Check pump (SKC) operated at 4 l/min and a high flow sample was collected with a Leland Legacy pump (SKC) operated at 10 l/min. The samplers were operated at 4 and 10 l/min to compare performance of the sampler at different sampling flow rates, potentially allowing for the use of a higher flow rate if needed. Each Button sampler was attached to PIPER’s lift, which is automatically raised and lowered according to the operating profile to simulate the changes in height of a child’s breathing zone as he or she lies down, sits, or stands.
Before sampling, filters were weighed on a microbalance after equilibrating at 21 °C and 33% relative humidity for at least 24 h. After sampling, filters were allowed to equilibrate again before being reweighed. Blank filters, which remained in the weighing room, were weighed along with the sample filters, with an average difference of ± 1.25 μg between pre- and post-sampling weighings. This is equivalent to ± 0.9% of the final net weight of the median filter among the filters placed in the personal sampler, ± 4.6% of the weight of the median filter from the 4 l/min sampler on PIPER, and ± 2.1% of the weight of the median 10 l/min filter. Based on this small difference and inconsistent sign, sample filter weights were not adjusted. All three pumps were calibrated with a flowmeter (TSI model 4199, TSI, Shoreview, MN, USA) immediately before and after sampling.
RESULTS
A total of 66 families were recruited for the study. In two homes equipment malfunctioned and in one home the child refused to wear the backpack. Data from these three homes were excluded from analysis, leaving 63 homes. Comparisons between samplers were performed with the Wilcoxon rank-sum test, which allows comparison of median values without the assumption of normally distributed data.
Comparison of Children’s Exposure Estimates by Personal Sampler and PIPER
Overall, children showed good compliance with the personal sampling. Typically, children spent no more than 1–2 min outside of the designated room during the 120-min sampling period. A summary of PM concentration data from each sampler may be found in Table 1. PM concentration values measured by the personal sampler ranged from 105 to 1,177 μg/m3, with a median value of 335 μg/m3 and an average value of 331 μg/m3. Personal exposure concentrations exceeding 1000 μg/m3 were observed in two homes, whereas other values did not exceed 600 μg/m3 and most of the observed concentrations were between 100 and 500 μg/m3. On PIPER, the standard Button sampler, operated at 4 l/min, measured a much narrower range of PM concentrations, with all but two values falling between 14 and 150 μg/m3, with a median value of 56 μg/m3 and an average value of 73 μg/m3. The two highest concentration values measured by PIPER were 214 and 513 μg/m3. The difference in medians between the two samplers was found to be significantly different (P<0.0001) from the Wilcoxon rank-sum test, suggesting that the two samplers’ medians are statistically different with a high degree of confidence. The measured mean PM exposures did not significantly depend (at α = 0.05 level) on children’s gender, sampling season, or age group (data not shown) for either the personal sampler or PIPER.
Table 1.
Summary of measured PM from personal sampler and PIPER 4 l/min sampler (n = 63).
| PM values | Personal sampler | PIPER (4 l/min) |
|---|---|---|
| Median | 335 | 56 |
| Average | 331 | 73 |
| Minimum | 105 | 14 |
| Maximum | 1177 | 513 |
| First quartile | 218 | 45 |
| Third quartile | 382 | 87 |
Abbreviations: PIPER, Pretoddler Inhalable Particulate Environmental Robotic sampler; PM, particulate matter. All PM concentrations are μg/m3.
Figure 2 presents pair-wise comparison of all PM concentrations measured by the personal sampler and the standard PIPER sampler. At all 63 homes, the personal sampler measured higher PM concentrations compared with the sampler on PIPER (P<0.0001). The median value of the ratios of the PM concentration from the personal sampler to the PM concentration from PIPER was 4.90, with a 25th percentile of 3.51 and a 75th percentile of 6.56.
Figure 2.
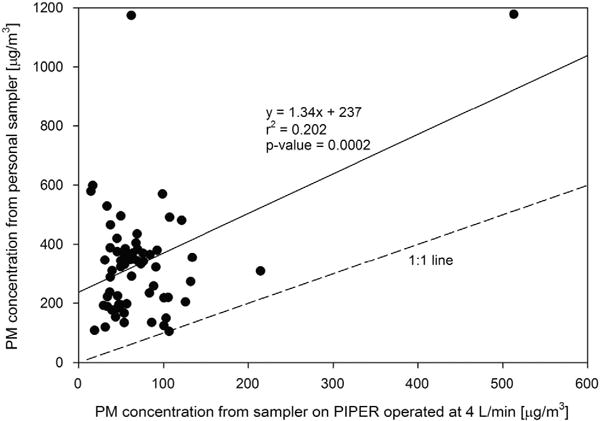
Correlation of PM concentration values from sampler on PIPER operated at 4 l/min (x axis) and personal sampler on backpack (y axis). For reference, a 1:1 line is shown along with the line of best fit.
Effects of Playing Patterns on PM Exposure
PIPER was designed to act as a child sampling surrogate by replicating young children’s movements while playing on the floor.13 However, in contrast to PIPER, no child spent the entire sampling period on the floor. The amount of time spent sitting on the floor, sitting on a sofa or chair, and standing upright (including walking and running) varied widely among the children participating in this study. Figure 3 shows the distribution of time spent in different microenvironments (different breathing zone heights) during sampling, as estimated by the researchers. Sitting on the floor and remaining upright were much more common than sitting on a sofa or chair among the subjects sampled.
Figure 3.
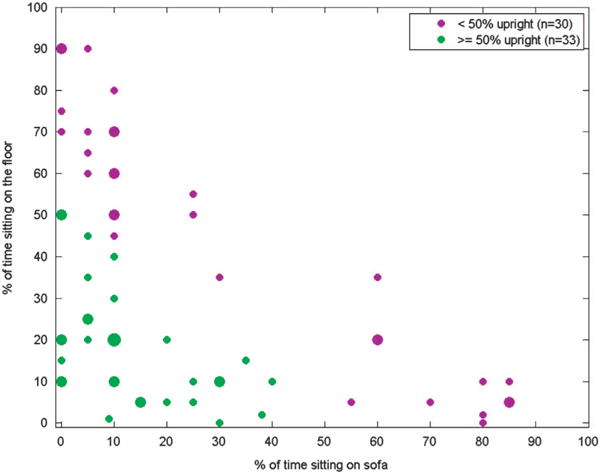
Estimated percent of time that children spent upright, sitting on the floor, and sitting on the sofa. The smallest circles indicate that one child matched a particular distribution. The largest circle (10% of time on sofa, 20% of time on floor, and 70% upright) represents four children with that particular distribution of activities; all other circles represent two children.
The amount of time a child spends upright vs sitting on the floor or on the sofa may also determine personal PM exposure. Figure 4a shows the amount of PM measured by the personal sampler stratified by floor type and then further subdivided by the amount of time children spent upright (<50% vs ≥ 50%).
Figure 4.
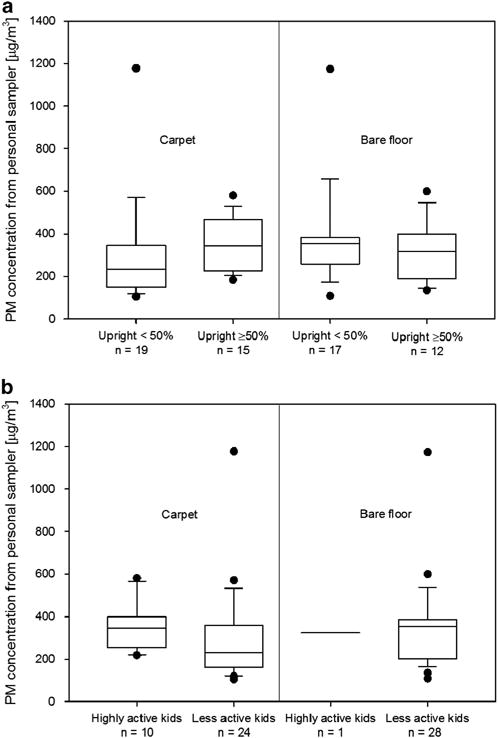
(a) PM concentrations measured by personal sampling equipment in the backpack for carpeted floors (left two boxes) and bare floors (right two boxes). Concentration values outside of the 10th and 90th percentiles are shown as black dots. (b) As in (a), but data stratified by highly active children and less active children, as defined in text.
A much higher median PM concentration (344.3 μg/m3) is seen among children in carpeted homes, who spent more time upright than the median for children who spent more time sitting (234.5 μg/m3). However, the difference was not significant at the α = 0.05 level (P = 0.06). In bare floor homes, the median PM concentration exposure values for children who spent more time on their feet and those who spent more time sitting were not as different: 316.2 and 355.5 μg/m3, respectively; this difference was not statistically significant (P = 0.49).
Some children engaged in vigorous play (roughhousing or gymnastics) during the sampling period, which may cause additional resuspension of particles from the floor into the breathing zone. A summary of the frequency of activities observed during the sampling period can be found in Supplementary Table 3. Figure 4b shows the PM measurements from highly active children (those who undertook at least one of these two activities during the sampling period) vs less active children. In homes with carpet, the median measured PM concentration among highly active kids was higher (344 vs 229 μg/m3 for less active kids), although the difference was not statistically significant (P = 0.09). No significant difference was seen among PIPER’s median measurements for carpeted floors (59 μg/m3 in homes with highly active kids vs 71 μg/m3 in homes with less active kids; P = 0.14). In bare floor homes, only one child engaged in either of the vigorous activities; hence, no comparison is possible for either sampler.
Effects of Floor Characteristics on PM Exposure
Floor type
The levels of children’s PM exposure are probably influenced to some degree by the type of flooring in the room, as particles are resuspended with different efficiency depending on flooring type.20 Unlike bare floor surfaces (e.g., tile, hard wood, and linoleum), carpeted floors act as reservoirs for PM and facilitate particle resuspension as the carpet fibers are compressed and released when they are walked on; previous studies have highlighted the increased resuspension caused by movement over carpet.13–15 We separated the homes by floor type to look for evidence of this effect as a contributor to personal exposure. We see some indication of this in PIPER’s measurements: carpeted homes had a median PM concentration of 66.1 μg/m3, while bare floor homes had a median of 54.1 μg/m3, although this difference was not significant at the α = 0.05 level (P = 0.23). No significant difference was seen in the measurements from the personal sampler as well: the median PM concentration was 280.96 μg/m3 for carpeted floors and 349.56 μg/m3 for bare floors.
Timing of most recent floor cleaning
Interestingly, the time since the last floor cleaning had a substantial effect on the PM measurements. Figure 5 shows PM concentrations as measured by the personal sampler and PIPER 4 l/min sampler stratified by floor type and time since the most recent floor cleaning (within 2 days vs more than 2 days before sampling).
Figure 5.
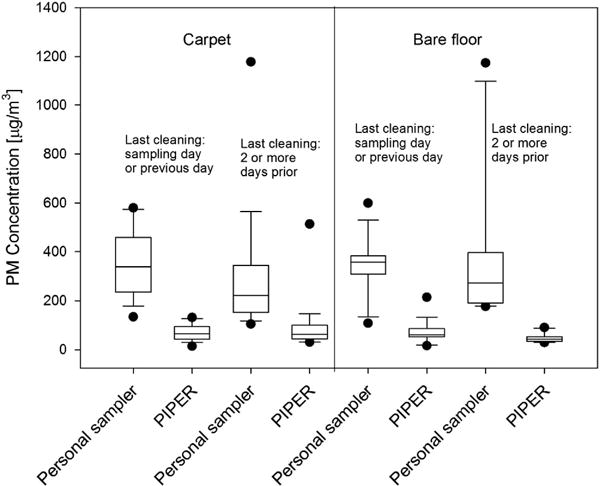
PM concentrations measured by the personal sampler and PIPER in μg/m3. The four boxes on the left are measurements from homes with carpet and the four boxes on the right are measurements from homes with bare floor. Within each floor type, homes are further subdivided by the reported date of the most recent floor cleaning as indicated on the plot. The black dots represent values that were outside of the 10th and 90th percentiles.
As can be seen, PM concentrations measured by PIPER were consistently lower than those measured by the personal sampler for both floor types and cleaning periods (Figure 5). The two samplers’ median values were significantly different (P<0.0001) when each variable was treated separately according to the Wilcoxon rank-sum test.
Parents reported that 47% (16 of 34) of carpeted homes were cleaned within 2 days of sampling, compared with 65% of bare floor homes (19 of 29). When data are not stratified by the floor type, homes reporting cleaning within 2 days (35 homes) had a higher median PM concentrations determined by the personal sampler (355.53 μg/m3) than those not cleaned within 2 days (28 homes; 223.68 μg/m3) and the difference was statistically significant (P = 0.015; the Wilcoxon rank-sum test). No significant difference was observed for PIPER data in this case (median concentration of 62.34 μg/m3 for homes cleaned within 2 days vs 49.57 μg/m3 for homes not cleaned within 2 days; P = 0.24).
When the data are stratified by the floor type, a statistically significant difference (P = 0.04) in median PM concentration was seen among PIPER’s measurements in bare floor homes: 61.8 μg/m3 among homes cleaned within 2 days of the sampling vs 42.25 μg/m3 for homes not cleaned within 2 days. There was also a difference in median PM concentration measured by the personal sampler in homes with bare floor between the two cleaning categories: 357.0 μg/m3 for homes cleaned within 2 days of sampling vs 272.4 μg/m3 for homes not cleaned within 2 days, although this difference was not significant at the α = 0.05 level (P = 0.37).
In carpeted homes, the difference in median PM concentration was not statistically significant for the personal sampler’s measurements (339.11 μg/m3 for cleaning within 2 days vs 222.31 μg/m3 for no cleaning within 2 days; P = 0.06) or PIPER’s measurements (66.1 vs 62.3 μg/m3; P = 1).
Correlation of PM concentrations between two samplers on PIPER
Figure 6 compares PM concentrations measured by the two Button samplers on PIPER operated at 4 and 10 l/min. The concentration values from the two samplers are well correlated with r2 = 0.673. The slope of the line of best fit is ~ 1.35, suggesting that, on average, the sampler operated at 4 l/min measured 35% higher PM concentration than the one operated at 10 l/min. With the removal of the anomalously high point (206 μg/m3 on the sampler operated at 10 l/min and 514 μg/m3 on the sampler operated at 4 l/min), the r2 value increases to 0.844 and the slope of the line of best fit becomes 0.90. The Wilcoxon rank-sum test suggests that the difference in medians between the two samplers (all points included) is not statistically significant at the α = 0.05 level (P = 0.0596).
Figure 6.
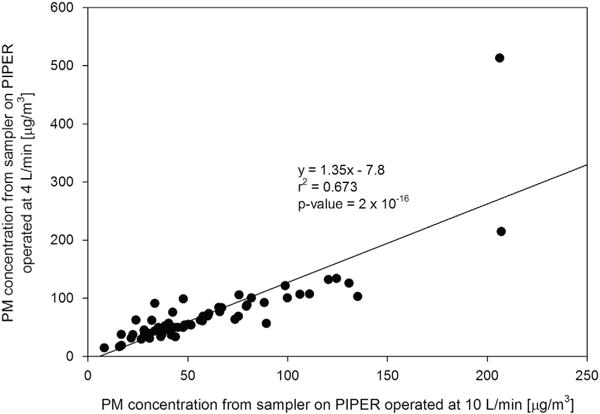
PM concentrations measured simultaneously on PIPER with samplers operated at 10 (x axis) and 4 l/min (y axis).
DISCUSSION
To the best of our knowledge, there have been no other publications of studies to measure personal PM exposure of children in this age group (24–36 months). Among 10- to 13-year-olds in Windsor, Ontario, indoor activities such as watching TV, playing, and eating were associated with personal PM2.5 exposure values of 10–30 μg/m3 as measured with a personal photometer (model pDR (ThermoScientific, Waltham, MA, USA) personal sampler).12 A study among adults in Detroit equipped with a pDR 1000AN personal sampler found that 1-min PM2.5 exposure values were <35 μg/m3 for 75% of the cases.9 Likewise, a study in North Carolina, USA, found mean personal PM2.5 exposure values of 33.3 μg/m3 among adults.10 However, the PTEAM study21 in California, USA, measured higher values and found a 24-h median PM10 exposure for adults was 130 μg/m3. In our study, both sets of measurements (the personal samples and PIPER’s samples) were taken in closer proximity to the floor, where the presence of resuspended particles, especially the coarse ones, is most pronounced; plus, our measurements included inhalable particles, which may account for some of the difference compared with other studies that measured PM2.5 or PM10. Particle resuspension from the floor has a less pronounced effect on older children and adults due to the larger distance between their breathing zone and this particle source. Thus, higher personal exposure concentrations observed in our study underscore the importance of better characterization of PM exposure among children.
As shown in Figure 2, PM concentrations measured by the personal sampler were consistently higher than those measured by PIPER. There are several potential reasons for this difference. One possibility is that particles were being generated from the child’s clothing or from the backpack itself as he or she moved around; a source that could not be duplicated on PIPER. McDonagh and Byne22 discussed the possibility of particle generation from clothing in detail and found that 27–34% of particles pre-loaded onto clothing were resuspended during high physical activity. According to our observations, most children spent only a small portion of the sampling period, if any, moving in a highly active way; hence, we deem it unlikely that particle resuspension from clothing or the backpack was the main cause of the difference between concentrations observed by PIPER and personal sampler. Another possible particle source that would not be measured by PIPER is food; some children ate a snack or meal during the sampling period, which could potentially generate new particles in the breathing zone. The median ratio of PM concentration measured by the personal sampler to that measured by PIPER was higher among children who ate a snack or meal (5.74, n = 38) than those who did not eat one (4.04, n = 25). Although this difference is not statistically significantly (P = 0.203), it nonetheless indicates that particles from food contributed to higher personal PM concentrations to some degree and may partially explain some of the higher values measured by the personal sampler.
Two homes had very high PM concentration values (Figure 2) as measured by the personal sampler. One of the homes also had a correspondingly high PM concentration from the sampler on PIPER, whereas the other did not. We have chosen to keep both homes in the data set, as there was no obvious sampling problem that could have caused these high values. The home that had high values on both samplers had a visibly dirty large area rug on which the child played and PIPER ran.
Figure 3 shows the distribution of activity patterns among the children studied. As noted, PIPER’s profiles were calculated from analysis of one cohort of 2-year-olds (n = 70) and represent the average percentage of time that children spend at each breathing zone height while on the floor. However, our results showed that 2-year-olds in this study tended to vary widely in the amount of time they spent on the floor in total and at each breathing zone height. Thus, PIPER was not always closely matching an individual child’s changes in breathing zone in this study’s cohort. Future studies using PIPER may use the Monte Carlo simulation method to better allow for variation in behavior of individual children. In addition, most children spent some portion of the sampling period on the sofa and this activity is potentially associated with increased exposure to PM through resuspension of particles from the sofa/chair, especially if children are not passively sitting but are engaged in motion, such as “flopping.” Our tests with an optical particle counter confirmed that “flopping” and sliding on a sofa produces an increase in particles larger than 2.5 μm (data not shown). PIPER, on the other hand, was not designed to account for the time children spend sitting on chairs/sofas.
In previous studies, PIPER has measured higher PM concentrations than a stationary sampler; concentrations of inhalable PM measured with Button samplers (analogous to those used in this study) were two to three times higher on PIPER than on a stationary sampler.13 Particle number concentration measured when PIPER was moving (relative to background levels) was also found to be higher as measured by PIPER than by a stationary sampler,15 highlighting the importance of resuspension of particles from the floor due to people’s movements. Given that the concentrations from the personal samplers in the current study are considerably higher than those measured by PIPER, it is apparent that PIPER’s exposure estimates do not precisely simulate personal sampling of children, while still giving higher personal exposure estimates compared with traditional stationary sampling.
It is also possible that in certain instances personal samplers might be overestimating the actual PM exposure in young children. For example, in some homes, we observed a child lay down on the floor, causing the Button sampler to come in contact with the carpet directly and act as a vacuum cleaner thus collecting more PM than was likely present in a child’s breathing zone. Thus, although personal monitoring is accepted and used as the most accurate way to measure personal exposure in adults, this approach may need modifications due to children’s particular movement patterns and contact with surfaces that act as dust reservoirs. As shown in Figure 4a, personal PM exposure for children in carpeted homes tended to be much higher among children who were upright 50% of the time or more. As the children walked or ran, they resuspended particles from the floor with greater force than they would from moving around while sitting on the floor. This may indicate that these children were not particularly active when sitting on the floor or sofa and thus not resuspending much PM from the floor or the sofa. In general, we observed that children were more likely to move around and resuspend particles, while sitting on the floor; children sitting on the sofa tended to remain mostly still and they usually sat up rather than lying down. The difference in PM concentration values between children who were upright <50% of the time and those who moved more in bare floor homes was smaller. This result may be influenced by the fact that in homes with bare floor, children are approximately equally likely to sit on the sofa and the floor, whereas in carpeted homes children are more likely to sit on the floor. In our current study, children in carpeted rooms spent an average of 32.9% of the time on the floor and 19.3% on the couch, whereas children in bare floor rooms spent an average of 28.5% of the time on the floor and 26.2% on the couch. Figure 4b illustrates the possibility of increased PM exposure via resuspension from the floor as a consequence of highly active behaviors (roughhousing and gymnastics).
We observed a clear difference in average PM concentrations measured by the personal sampler depending on the date of the most recent floor cleaning (Figure 5). Surprisingly, the higher PM concentrations were seen in homes that were reported to have been cleaned more recently (the day of the sampling or the previous day) and this difference was seen both in homes with carpet and those with bare floor. The difference in the median PM concentration from the personal sampler between carpet cleaned recently vs not cleaned recently (as previously defined) was borderline statistically significant (P = 0.056). The PM concentrations from the sampler on PIPER were also higher in the carpeted homes that were cleaned closer to the sampling time, although the difference was not statistically significant (P = 0.24). It is possible that particles not removed by cleaning were nonetheless “loosened” in the cleaning process23 and were thus more easily resuspended when the child played on the floor, as noted by Roberts et al.24 The particles resuspended during cleaning also could have potentially settled on surfaces other than the floor, such as the sofa. Resuspension of those particles from the sofa would not have been observed by PIPER. Another possible explanation is that some residents answering the questionnaire may have felt subconscious pressure to report their last cleaning as being more recent than it actually was under social desirability bias,25 thus placing homes whose floors had not been cleaned recently into the most recent cleaning category. This effect is likely to be random as we did not see higher PM concentration values from the sampler on PIPER among more recently cleaned homes. PIPER’s measurements do show a statistically significant difference in median PM value based on the most recent cleaning in bare floor homes, but not in carpeted ones. We speculate that PIPER resuspends PM more effectively from carpeted floors than from bare floors, but not as effectively as a child does in either case.
Figure 6 demonstrates that a 10-l/min air flow rate may be used with a Button sampler with relatively small difference in the measured PM concentration compared with a 4-l/min air sampling flow rate. Button samplers are designed to measure inhalable particles at a flow rate of 4 l/min. However, if higher particle mass is needed to reach above a detection limit of an analysis method, or a shorter sampling time period is desired or required for logistical reasons, our results show that PM concentration values for the Button sampler with the two flow rates are highly correlated and a 10-l/min flow rate would be acceptable. The use of a 10-l/min flow rate may be particularly useful when sampling in private residences, as this could allow for a shorter overall sampling time compared with using a 4-l/min flow rate, which may encourage participation among volunteers. However, it should be noted that the use of a flow rate that is higher than the design may affect the sampler’s adherence to the inhalability curve. Thus, further testing of the Button sampler when operated at flow rates higher than 4 l/min, including testing in a wind tunnel, would be worthwhile to help understand any effect of higher flow rates on the sampler’s inlet efficiency.
We find that personal PM monitoring on young children remains challenging. One child refused to wear the backpack and had to be excluded from the analysis. We do not believe that wearing the backpack with the personal sampling equipment changed the children’s activity patterns; when asked if their child’s play seemed typical during the sampling period, 85% of parents or guardians said “yes.” In this study, our activity assessments are based on recall by the technicians. The results suggest that children’s activities affect their PM exposures. However, both recall bias and variability among the three technicians may have influenced the data. A more rigorous assessment of children’s activities either by videorecording and/or physical activity monitor to assess activity level would be helpful in understanding how children’s activities modify their PM exposures.
The estimated PM exposures varied widely for the 2-year-old children in this study, owing to the variety of activity patterns and environmental variables encountered. Further quantification of resuspension rates due to different types of activities on the floor and from movement on a sofa may improve personal exposure estimates for young children. In addition, we demonstrate the use of PIPER as a proxy for measuring PM concentrations in a child’s breathing zone. Although further refinement is needed, PIPER is an improvement over stationary samplers due to its ability to resuspend particles from the floor as children do when they move around and the location of its samplers closer to the floor. Further work on calculating adjustment factors, which consider floor type and activity patterns, may help bring PIPER’s values more closely in line with the personal exposure estimates.
Supplementary Material
Acknowledgments
Funding for this study was provided by National Institute of Environmental Health Science (NIEHS) grants R01ES014717 and R01ES020415 (P.I.: S.L.S.) and the NIEHS funded Center for Environmental Exposure and Disease, P30ES005022 (P.I.: Zarbl H.). J.A.S. is supported by an NIEHS Training Grant in Exposure Science 1T32ES019854 (PI: Weisel, C.P.).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
References
- 1.Franck U, Herbarth O, Roder S, Schlink U, Borte M, Diez U, et al. Respiratory effects of indoor particles in young children are size dependent. Sci Total Environ. 2011;409:1621–1631. doi: 10.1016/j.scitotenv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 3.Habre R, Moshier E, Castro W, Nath A, Grunin A, Rohr A, et al. The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. J Exp Sci Environ Epidemiol. 2014;24:380–387. doi: 10.1038/jes.2014.21. [DOI] [PubMed] [Google Scholar]
- 4.Ma L, Shima M, Yoda Y, Yamamoto H, Nakai S, Tamura K, et al. Effects of airborne particulate matter on respiratory morbidity in asthmatic children. J Epidemiol. 2008;18:97–110. doi: 10.2188/jea.JE2007432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 6.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Exp Anal Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 7.Abt E, Suh HH, Catalano P, Koutrakis P. Relative contribution of outdoor and indoor particle sources to indoor concentrations. Environ Sci Technol. 2000;34:3579–3587. [Google Scholar]
- 8.Ramagopal M, Wang ZC, Black K, Hernandez M, Stambler AA, Emoekpere OH, et al. Improved exposure characterization with robotic (PIPER) sampling and association with children’s respiratory symptoms, asthma and eczema. J Exp Sci Environ Epidemiol. 2014;24:421–427. doi: 10.1038/jes.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond D, Croghan C, Shin H, Burnett R, Bard R, Brook RD, et al. Cardiovascular impacts and micro-environmental exposure factors associated with continuous personal PM2.5 monitoring. J Exp Sci Environ Epidemiol. 2014;24:337–345. doi: 10.1038/jes.2013.46. [DOI] [PubMed] [Google Scholar]
- 10.Wallace L, Williams R, Rea A, Croghan C. Continuous weeklong measurements of personal exposures and indoor concentrations of fine particles for 37 health-impaired North Carolina residents for up to four seasons. Atmos Environ. 2006;40:399–414. [Google Scholar]
- 11.Wheeler AJ, Xu XH, Kulka R, You HY, Wallace L, Mallach G, et al. Windsor, Ontario Exposure Assessment Study: design and methods validation of personal, indoor, and outdoor air pollution monitoring. J Air Waste Manag Assoc. 2011;61:324–338. [PubMed] [Google Scholar]
- 12.Van Ryswyk K, Wheeler AJ, Wallace L, Kearney J, You HY, Kulka R, et al. Impact of microenvironments and personal activities on personal PM2.5 exposures among asthmatic children. J Exp Sci Environ Epidemiol. 2014;24:260–268. doi: 10.1038/jes.2013.20. [DOI] [PubMed] [Google Scholar]
- 13.Shalat S, Stambler A, Wang Z, Mainelis G, Emoekpere O, Hernandez M, et al. Development and in-home testing of the Pretoddler Inhalable Particulate Environmental Robotic (PIPER Mk IV) sampler. Environ Sci Technol. 2011;45:2945–2950. doi: 10.1021/es1033876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalat SL, Lioy PJ, Schmeelck K, Mainelis G. Improving estimation of indoor exposure to inhalable particles for children in the first year of life. J Air Waste Manag Assoc. 2007;57:934–939. doi: 10.3155/1047-3289.57.8.934. [DOI] [PubMed] [Google Scholar]
- 15.Sagona JA, Shalat SL, Wang Z, Ramagopal M, Black K, Hernandez M, et al. Evaluation of particle resuspension in young children’s breathing zone using stationary and robotic (PIPER) aerosol samplers. J Aerosol Sci. 2015;85:30–41. doi: 10.1016/j.jaerosci.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizenberg V, Grinshpun SA, Willeke K, Smith J, Baron PA. Performance characteristics of the button personal inhalable aerosol sampler. Am Ind Hyg Assoc J. 2000;61:398–404. doi: 10.1080/15298660008984550. [DOI] [PubMed] [Google Scholar]
- 17.United States Environmental Protection Agency. Exposure Factors Handbook. United States Environmental Protection Agency; Washington, DC: 2011. [Google Scholar]
- 18.Ramagopal M, Stambler A, Wang Z, Mainelis G, Emoekpere O, Hernandez M, et al. Increased prevalence of wheeze associated with elevated levels of particulate matter (PM) measured by a child surrogate robot (PIPER) Am J Respir Crit Care Med. 2011;183 [Google Scholar]
- 19.Wang Z, Shalat S, Black K, Lioy P, Stambler A, Emoekpere O, et al. Use of a robotic sampling platform to assess young children’s exposure to indoor bioaerosols. Indoor Air. 2012;22:159–169. doi: 10.1111/j.1600-0668.2011.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian J, Ferro AR, Fowler KR. Estimating the resuspension rate and residence time of indoor particles. J Air Waste Manag Assoc. 2008;58:502–516. doi: 10.3155/1047-3289.58.4.502. [DOI] [PubMed] [Google Scholar]
- 21.Clayton CA, Perritt RL, Pellizzari ED, Thomas KW, Whitmore RW, Wallace LA, et al. Particle Total Exposure Assessment Methodology (PTEAM) Study—distributions of aerosol and elemental concentrations in personal, indoor, and outdoor air samples in a southern California community. J Exp Anal Environ Epidemiol. 1993;3:227–250. [PubMed] [Google Scholar]
- 22.McDonagh A, Byrne MA. A study of the size distribution of aerosol particles resuspended from clothing surfaces. J Aerosol Sci. 2014;75:94–103. [Google Scholar]
- 23.Ferro AR, Kopperud RJ, Hildemann LM. Source strengths for indoor human activities that resuspend particulate matter. Environ Sci Technol. 2004;38:1759–1764. doi: 10.1021/es0263893. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JW, Wallace LA, Camann DP, Dickey P, Gilbert SG, Lewis RG, et al. Monitoring and reducing exposure of infants to pollutants in house dust. In: Whitacre DM, editor. Reviews of Environmental Contamination and Toxicology. Vol. 201. Springer; New York: 2009. pp. 1–39. [DOI] [PubMed] [Google Scholar]
- 25.Tourangeau R, Yan T. Sensitive questions in surveys. Psychol Bull. 2007;133:859–883. doi: 10.1037/0033-2909.133.5.859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


