Abstract
Oseltamivir is contraindicated for people aged 10–19 in principle in Japan, due to concern about abnormal behaviours. Sudden death is another concern. This review examines growing evidence of their association and discusses underlying mechanisms of these sudden‐onset type reactions to oseltamivir. First, the importance of animal models and the concept of human equivalent dose (HED) is summarized. Second, the specific condition for oseltamivir use, influenza infection, is reviewed. Third, findings from toxicity studies conducted prior to and after the marketing of oseltamivir are reported on to provide context on the observation of a possible causal association. Fourth, similarity and consistency of toxicity in humans with that in other animals is described. Finally, coherence of toxicokinetic and molecular level of evidence (channels, receptors and enzymes), including differences from the toxicity of other neuraminidase inhibitors, is reviewed. It is concluded that unchanged oseltamivir has various effects on the central nervous system (CNS) that may be related to clinical findings including hypothermia, abnormal behaviours including with fatal outcome, and sudden death. Among receptors and enzymes related to CNS action, it is known that oseltamivir inhibits nicotinic acetylcholine receptors, which are closely related to hypothermia, as well as human monoamine oxidase‐A (MAO‐A), which is closely related to abnormal or excitatory behaviours. Receptors such as GABAA, GABAB and NMDA and their related receptors/channels including Na+ and Ca2+ channels are thought to be other candidates for investigation related to respiratory suppression followed by sudden death and psychotic reactions (both acute and chronic), respectively.
Keywords: abnormal behaviour, monoamine oxidase‐A, neuropsychiatric adverse effects, nicotinic acetylcholine receptor, oseltamivir, respiratory arrest, sudden death
1. Introduction
Oseltamivir and zanamivir are neuraminidase inhibitors (NIs). These are stockpiled and recommended to use for the treatment of seasonal and pandemic influenza, especially in high‐risk people.1, 2 Oseltamivir was used world widely for the treatment of 2009/2010 H1N1 influenza and is included in the model list of essential medicines of the World Health Organization (WHO).3 In Japan, oseltamivir is contraindicated for children and adolescents of ages 10–19 years in principle since March 2007, due to concerns that it may cause abnormal behaviours.4 Sudden death is another concern.5, 6, 7 A causal association between oseltamivir use and abnormal behaviours or sudden death has not been established and it is considered negative1, 2 based on retrospective observational studies,8, 9, 10, 11, 12 systematic reviews of retrospective observational studies13, 14 and a systematic review and meta‐analysis of randomized controlled trials of oseltamivir treatment for influenza of adults.15
However, serious neuropsychiatric adverse reactions to oseltamivir, including sudden death and abnormal behaviours leading to accidental death, were reported soon after the drug was introduced as a medicine.5, 6, 7 Prospective cohort studies16, 17, 18, 19, 20 and a systematic review of the prospective cohort studies21 indicate an association between abnormal behaviours and oseltamivir use. A recent systematic review and meta‐analysis of both treatment and prophylaxis randomized controlled trials including adults and children shows that oseltamivir increased risk of nausea; vomiting; headaches; psychiatric, renal, diabetic/hyperglycaemic events; and pain in the limbs. However, zanamivir did not.22 According to the prospective cohort studies,16, 17, 18, 19, 20, 21 NNTH (number needed to treat to harm) of oseltamivir for abnormal behaviours is estimated about 25 (95% CI: 19–35). Oseltamivir was prescribed to 2.85 million people during the 2013/2014 winter season in Japan.23 If oseltamivir causes abnormal behaviours, more than 100 thousand persons could experience additional oseltamivir‐associated abnormal behaviours. While sudden deaths or very early deteriorations leading to death were specifically reported after taking oseltamivir, none have been reported for zanamivir.24 Among 10 million people who were prescribed with oseltamivir during 2009/2010 season in Japan, 38 patients deteriorated within 12 hours after taking oseltamivir before death.24 On the other hand, among 61 sudden death cases that were reported to FDA and health Canada including reports from Japan during 2004–2014, only four sudden death cases were reported during 2009/2010 influenza season.25 If oseltamivir causes sudden death, there may be substantial unreported sudden death cases in the world.
Opinion on causal association between oseltamivir use and serious adverse events including sudden death and abnormal behaviours remains controversial.
This review describes growing evidence from non‐clinical studies that contradicts the widely held opinion that there is no association between oseltamivir use and abnormal behaviour or sudden death.
We first comment on the important role that animal models play in predicting human toxicities and the concept of human equivalent dose (HED) is summarized, for better understanding of evidence from animal toxicity studies. Second, the specific condition under which oseltamivir is used to treat patients infected with influenza is described. Third, findings from toxicity studies conducted prior to and after the marketing of oseltamivir are reviewed to consider the strength and consistency of causal association. Fourth, similarity and consistency of toxicity findings in humans with that in other animals is reported. Finally, coherence of toxicokinetic and molecular level of evidence (channels, receptors and enzymes), including differences from the toxicity of other neuraminidase inhibitors, is reviewed.
2. Principles of Animal Toxicity Tests
2.1. Objective: to predict toxicity in humans
Zbinden, a researcher at Roche Pharmaceutical, writes as follows about the objectives of the animal toxicity tests.
It would be very desirable if the harmful effects of newly developed compounds could be recognized in animals before any damage is done to human subjects. For that reason it has become a standard practice to subject every drug to extensive trials in laboratory animals prior to their release for human use.26
He writes about principles of animal toxicity studies as follows:
In any toxicity experiment, animals are treated with drugs and observed for toxic manifestations. In order to increase the chances of recognizing possible toxic properties, the dose is raised above the therapeutically useful range, the duration of treatment is often lengthened, and the drug is administered not only to one individual animal but to animal groups. Thus, the toxicity experiment tries to imitate the clinical use of the drug and although bold exaggerations with respect to dose and duration of treatment are common and permissible, it is important that the future therapeutic applications in man guide the planning of a toxicity study. 26
This implies that animal toxicity studies are conducted in order to learn what toxic effects may be predicted and to prevent harmful outcome in humans. Toxicity studies are conducted in exaggerated doses and periods of treatment in order to predict rarely occurring reactions in humans using a small number of animals. Ideally, they are conducted in conditions similar to clinical use. Exploratory toxicity studies are conducted to learn what toxicities may possibly be produced, while confirmatory toxicity studies are conducted to verify a hypothesis of the “presence” or “absence” of certain toxicity.27, 28
2.2. Human equivalent dose
To accurately understand animal toxicity data, the conversion factor for converting animal dose to human equivalent dose (HED), described in the “Guidance for Industry” for a person weighing 60 kg,29 is important. According to the Guidance, conversion factors for HED for mouse, rat, ferret and marmoset monkey are 12.3, 6.2, 5.3, 6.2, respectively. Hence, if the “no observed adverse effect level” (NOAEL) is 114 mg/kg in a rat oral toxicity test, the HED of NOAEL should be estimated as 18 mg/kg (114/6.2), if conversion by systemic levels or exposure (i.e. AUC or Cmax) is not available.
2.3. Summary of this section
We discussed the importance of animal models mimicking clinical use and bold exaggerations with respect to clinical dose for predicting human toxicities and the importance of human equivalent dose (HED) concept for better understanding of evidence from animal toxicity studies.
3. Influenza Infection, a Specific Condition for Oseltamivir Use
3.1. Action of oseltamivir and pharmacokinetics in non‐infectious state
Oseltamivir phosphate (OP) is an ethyl ester prodrug requiring ester hydrolysis for conversion to the active form of the neuraminidase inhibitor oseltamivir carboxylate (OC). The branded OP capsule (Tamiflu capsule) contains 75 mg of oseltamivir expressed as free base (OT), which is compatible with 98.6 mg of OP. OP dissociates in the gastrointestinal tract to form OT, which is absorbed and metabolized into OC by hepatic carboxylesterase (hCE).
In healthy volunteers, the area under the curve (AUC) of OP is 3%–5% that of OC. OP is restricted (<10%) to penetrate across the blood–brain barrier (BBB) by P‐glycoprotein (P‐gp) in mature non‐infected animals.30, 31, 33
When healthy adult volunteers were administered with 75 mg of OT (equivalent to 98.6 mg of OP), approximate average Cmax (ng/mL), Tmax (hours), AUC (ng.h/mL) and elimination half‐life (t1/2:hours) of OP were as follows, respectively: 60 ng/mL, 0.7–2 hours, 150–200 ng h/mL and 1.2–1.9 hours. For OC, these values are 200–300 ng/mL, 4–5 hours, 3000–4000 ng h/mL and 5–10 hours.34 The pharmacokinetic (PK) parameters are not so different in healthy children aged 3 years or older from what they are in adults.35
Almost all OC is secreted in urine and dose adjustment is necessary if a patient's creatinine clearance is <30 mL/min.34, 37, 39
Suzaki et al.40 reported one subject who showed an approximately ten‐fold higher peak concentration (Cmax) and area under the concentration–time curve (AUC) of oseltamivir than the mean values of 29 other healthy Japanese males and females administered a single 75 mg dose of oseltamivir. The ratios of Cmax and AUC of this person to those of the person with the lowest values were 25 times and 38 times, respectively. Watangoon reported that one of eight healthy adult Thai volunteers showed an AUC six times greater than the average of the other subjects.41 These differences are derived from difference in the activity of carboxylesterase which metabolizes OT to OC.40, 41
One of the peculiar pharmacokinetic characters of oseltamivir is the bimodal peak of plasma OT concentration.40 This is probably related to the bimodal abnormal behaviours with dyspnoea in a 14‐year‐old boy (case 6),6 and deaths during 8–24 hours after one dose of oseltamivir in rat toxicity test may also be related as discussed in the section 2.2 (or section for toxicity and toxicokinetic studies).
3.2. Action and pharmacokinetics of oseltamivir in infected patients or animal models
3.2.1. Influenza and pro‐inflammatory cytokines
Oseltamivir is taken primarily for treatment of influenza. In influenza‐infected individuals, as with other infections, pro‐inflammatory cytokines such as interleukin‐6 (IL‐6), tumour necrosis factor‐α (TNF‐α) and interferon‐γ (IFN‐γ) are induced.42
3.2.2. Infectious state and carboxylesterase
Treatment with IL‐6 in vitro decreased the expression of human hCE1 and hCE2 by as much as 60%. The decreased expression occurred at both mRNA and protein levels and was confirmed by enzymatic assay.43
Pharmacokinetic parameters of oseltamivir in plasma during influenza infection were not provided. However, it can be assumed that the activity of hCE is reduced during the early phase or exacerbated state of influenza infection and that subsequently, concentration of unchanged OT may increase in plasma.
3.2.3. Infectious state and P‐glycoprotein (P‐gp)
P‐glycoprotein is a critical efflux transporter at the blood–brain barrier (BBB) where its luminal location and substrate promiscuity limit the brain distribution of numerous therapeutics. P‐gp is highly regulated by inflammatory mediators.44
Inflammation and infection produce dynamic changes in P‐gp expression and activity in the blood–brain barrier. In vitro, BBB P‐gp activity is downregulated after short‐term exposure to inflammatory mediators,45, 46 while its activity is upregulated following more prolonged exposure,47 or in experimental model of chronic, peripheral inflammatory pain in rats.43, 46
Pharmacokinetic parameters during influenza infection were not provided in the brain either. However, it can be assumed that the activity of P‐gp is reduced during the early phase or exacerbated state of influenza infection and that subsequently, brain concentration of unchanged OT may increase.
3.2.4. Hepatic carboxylesterase (hCE) and P‐gp in immaturity
The esterase (hCE) activity is low in infants less than one year old and in immature animals.35, 48 P‐gp expression in the mouse brain increases with maturation.49 Hence, it can be assumed that plasma and brain concentrations of unchanged OT may increase in immature animals, including humans.
3.2.5. Animal models of influenza infection
Animal toxicity (Tx) tests and toxicokinetic (TK) tests on juvenile rats before weaning (usually 7‐day‐old pups, weighing about 11–23 g), on mature rats using intraduodenal injection by catheter and intravenous administration which produces high plasma level and brain level of oseltamivir, provide good animal models for detecting toxicities in human influenza in the absence of pharmacokinetic data on influenza infection in humans.34, 35, 36, 37, 38, 50, 51, 52
In fact, the brain‐to‐plasma concentration ratio of oseltamivir is significantly higher in newborn rats than in mature rats, which is consistent with the ontogenetic expression profile of P‐gp.49
3.2.6. Spontaneous recovery of hCE and P‐gp in the course of influenza
During the acute phase of influenza, pro‐inflammatory cytokines increase; the plasma oseltamivir level may increase due to decreased hCE activity; and the brain/plasma ratio of oseltamivir level may increase due to downregulated BBB P‐gp activity. It can be speculated that during the convalescent period, the activity of hCE and BBB P‐gp may recover as pro‐inflammatory cytokine level decreases. In the same way, activity of hCE and BBB P‐gp may increase with growth.
3.2.7. Brain/plasma concentration of oseltamivir in pregnant mothers and newborns
Patterns of expression of cytokines in the foetal membranes and decidua suggest that inflammatory activation occurs modestly with term labour, but much more robustly in preterm delivery, particularly in the presence of intrauterine infection.53Therefore, the brain‐to‐plasma ratio of Cmax undoubtedly increases more in cases of abortion and preterm delivery than during term labour, particularly in the presence of intrauterine infection.
Maternal plasma oseltamivir may readily pass the placenta, and plasma concentration in foetus may be half that of the maternal plasma concentration.34, 35 Carboxylesterase48 and brain P‐gp49 of newborns are particularly immature, and newborn infants are at very high risk of brain exposure of oseltamivir. The topic of adverse effects on pregnant mothers, foetuses and newborns will be discussed elsewhere, as space in this review is limited.
3.3. Summary of this section
Cmax and AUC of oseltamivir in non‐infectious state vary greatly, and bimodal peak concentration is sometimes observed. In influenza infection to which oseltamivir is used and in immaturity, plasma and brain concentrations of OT increase due to inhibition of carboxylesterase in the liver and P‐gp in the BBB, respectively. Hence, newborn animals are the good models for predicting and confirming the toxicity of oseltamivir.
4. Toxicity and Toxicokinetic Studies in Juvenile Rats: An Excellent Animal Model of Human Influenza Infection
As we do not have pharmacokinetic (PK) parameters for the human infectious state of influenza, toxicity studies (Tx studies) and toxicokinetic (TK) parameters from juvenile rats, a good animal model of infection, provide the navigation chart for estimating what toxicity effects might appear in humans.
4.1. Mortality from toxicity studies before and after approval of oseltamivir
The drug's manufacturer conducted a series of acute Tx studies using 7‐day‐old rats (before weaning). At least seven such tests are known: (i) a dose‐finding preliminary Tx study (dose‐finding Tx); (ii) a Tx study, along with (iii) a TK study performed before approval of oseltamivir for children (pre‐1 studies) [(i)~(iii)];35 (iv) a Tx study, along with (v) a TK study performed before approval of oseltamivir capsule for prophylaxis (pre‐2 study) [(iv),(v)];36 (vi) a post‐marketing Tx study (post‐M Tx);50, 51, 52 and (vii) a post‐marketing TK study (post‐M TK).50, 51, 52
Studies (vi) and (vii) were ordered by the Japanese Ministry of Health, Labour and Welfare (MHLW) in April 2007 to reassess (verify) the causality of fatal adverse effects after the “contra‐indication in principle for children and adolescents aged 10–19 years old” regulation was established (in March 2007).32
Figure 1 shows the relationship between dose and probability of death using the data from all toxicity and TK studies. The numbers of rat pups that died and the denominators of each study are shown in Table 1. In the TK studies (e.g. in the post‐marketing TK study), each group started with 96 pups, and six pups (three of each sex) were sacrificed at 0.25, 0.5, 1, 1.5, 2, 4 and 8 hours for determination of blood and brain concentration before the end of the study (24 hours). Hence, the observed total rat‐days were estimated at 58.3 (In the case that one rat was observed for 8 hours, it is calculated 8/24=0.33 rat‐days). The dose–mortality relationship is very clear (chi‐square value for linear trend=432.1, P<.0001). The results show that for each additional dose of 100 mg/kg, the odds of death was more than double (OR=2.26, 95% CI: 2.01–2.54, P<.0001).
Figure 1.
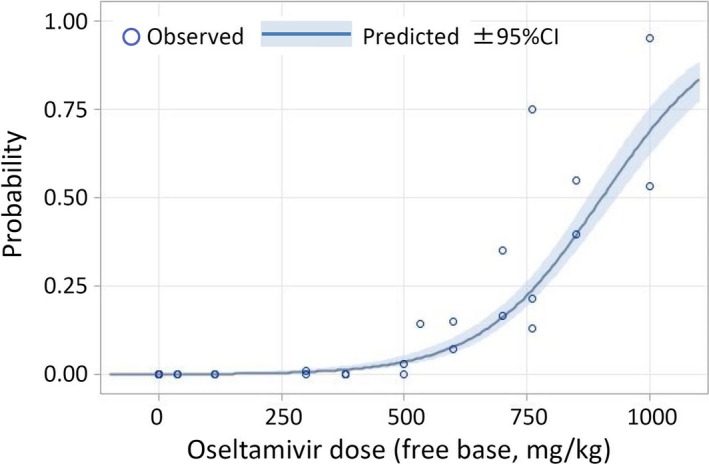
Dose of oseltamivir and mortality trend in 7‐day‐old rats. Data from Refs. 35, 36, 50, 51, 52. In the TK tests, the proportion of deaths is underestimated because rats were withdrawn for determination of plasma and brain concentration (see the footnote of Table 1). The results show that for each additional dose of 100 mg/kg, the odds of death more than double (OR=2.26, 95% CI: 2.01–2.54, P<.0001)
Table 1.
Mortality of 7‐day‐old rats from toxicity tests (Tx) and toxicokinetic (TK) tests
| Oseltamivir (free base) dose (mg/kg) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Rate of death (total) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose‐finding | Pre‐1 Txa | Pre‐1 TK | Pre‐2 Tx | Pre‐2 TK b | Post‐M Txc | Post‐M TKc | ||||||||||||||||||
| Rds | d | Rate | Rds | d | Rate | Rds | d | Rate | Rds | d | Rate | Rds | d | Rate | Rds | d | Rate | Rds | d | Rate | Rds | d | Rate | |
| 0 | 24 | 0 | 0 | 48 | 0 | 0 | 48 | 0 | 0 | 14 | 0 | 0 | 20 | 0 | 0 | 96 | 0 | 0 | 250 | 0 | 0 | |||
| 38 | 24 | 0 | 0 | 48 | 0 | 0 | 30.6 | 0 | 0 | 102.6 | 0 | 0 | ||||||||||||
| 114 | 24 | 0 | 0 | 48 | 0 | 0 | 30.6 | 0 | 0 | 102.6 | 0 | 0 | ||||||||||||
| 300 | 20 | 0 | 0 | 58.3 | 1 | 1.7 | 78.3 | 1 | 1 | |||||||||||||||
| 380 | 24 | 0 | 0 | 48 | 0 | 0 | 30.6 | 0 | 0 | 14 | 0 | 0 | 116.6 | 0 | 0 | |||||||||
| 500 | 20 | 0 | 0 | 58.3 | 3 | 5 | 78.3 | 3 | 4 | |||||||||||||||
| 533 | 14 | 2 | 14 | 14 | 2 | 14 | ||||||||||||||||||
| 600 | 20 | 3 | 15 | 58.3 | 7 | 12 | 78.3 | 10 | 13 | |||||||||||||||
| 700 | 20 | 7 | 35 | 58.3 | 16 | 27 | 78.3 | 23 | 29 | |||||||||||||||
| 761d | 24 | 18 | 75 | 14 | 3 | 21 | 34.8 | 7 | 20 | 72.8 | 28 | 38 | ||||||||||||
| 850 | 20 | 11 | 55 | 58.3 | 38 | 65 | 78.3 | 49 | 63 | |||||||||||||||
| 1000 | 20 | 19 | 95 | 58.3 | 51 | 87 | 78.3 | 70 | 89 | |||||||||||||||
Data from Refs. 34, 35, 50, 51, 52. “Rate” or “rate of death” is expressed as number of deaths (d) per 100 rat‐days (Rds). d: number of rats that died.
For the TK tests, denominators were expressed as rat‐days (Rds), calculated using the number of rats sacrificed for determination of plasma and brain concentrations at each time point. For example, in study 7 (Post‐M TK), six rats each were withdrawn at 0.25, 0.5, 1, 1.5, 2, 4 and 8 hours before the end of observation and the last measurement. Chi‐square value for linear trend=432.1, P<.0001.
Dose of oseltamivir and proportion of death in 7‐day‐old rats using the number of rats at the start of the studies as denominators are shown in Fig. 1.
Pre‐1:before approval of oseltamivir for children,
Pre‐2: before approval of oseltamivir capsule for prophylaxis.
Post‐M: post‐marketing toxicity study. Tx: toxicity study. TK: toxicokinetic study.
High mortality may be due to the rats having lower body weight. Although their weights were not shown, the rats used in the Pre‐1 Tx and TK studies weighed less (11.5–18.9 g) than those in studies 4 and 5 (14.0–26.0), which means they were less grown. Body weight of the rats in post‐marketing Tx and post‐marketing TK studies were not shown.
It is noted that among 40 pups died within 24 hours after a single dose oseltamivir, 15 died within 4 hours and 25 died after 8 hours post‐dosing.51 This may be related to the bimodal peak concentration of OT.40
In a juvenile rat toxicity study, a single dose of 114 mg/kg oseltamivir (expressed as free base; the same applies below unless otherwise explained) was the “no observable adverse effect level” (NOAEL) for death (also called NODEL: no observable death effect level). As the PK parameters for influenza‐infected humans are not available, the human equivalent dose (HED) was calculated using the following formula:29
Animal weights were 11–23 g (approximate average=17 g). Hence, for a human weight of 15 kg, the HED of 114 mg/kg (for juvenile rat) is 12.2 mg/kg. This dose is about six times higher than the single dose and three times higher than the daily dose for oseltamivir. For an adult weighing 60 kg, the HED of NODEL is 7.7 mg/kg; this is 7.7 and 3.8 times higher than the single dose (1 mg/kg) and daily (2 mg/kg) adult dose, respectively. (The HEDs below are expressed as free base for an infant weighing 15 kg, unless explained otherwise).
4.2. Suppression of central nervous system known before marketing
Respiratory suppression is one of the most serious reactions to suppressants of the central nervous system, including barbiturates and benzodiazepines, because respiratory suppression is followed by cardiac arrest and sudden death.54
Suppression of the central nervous system, including respiratory suppression, hypoactivity or sleep (low arousal) followed by sudden death, was observed in at least seven of eight animal toxicity studies of oseltamivir. At least five of these studies were conducted and submitted to the regulators for approval,34, 35, 36 and at least two were conducted after approval by the pharmaceutical industry for confirmation of the causality.50, 52
Signs of central nervous system disorders, such as abnormal gait with shallow respiration and seizure, were observed in a surviving mouse administered a single intravenous dose of 190 mg/kg (HED=20 mg/kg).34 The “no observable adverse effects level” (NOAEL) of OT was 38 mg/kg (HED=4.1 mg/kg).
In a one‐week oral toxicity study, all four marmoset monkeys treated with 1522 mg/kg of oseltamivir as free base were sacrificed due to impending death within 4 days (one on day 2, the others on day 4), with manifestations of hypoactivity, sleep and collapse before sacrifice. The NOAEL of OT in this study was 76 mg/kg (HED=12 mg/kg).34
In a dose‐finding toxicity study on 7‐day‐old rats (juvenile rats weighing 11.5–18.9 g), 18 of 24 treated with doses of 761 mg/kg (HED=78 mg/kg) died within seven hours after treatment. Lung oedema was observed in nine of the 18 dead rats on histological examination. Lung oedema is very often observed in cases of sudden death during sleep in both infants and adults.6 No deaths were observed in 114 mg/kg (HED=12.2 mg/kg) or lower dose groups, including the vehicle group35 (Table 1).
In the Tx studies on 7‐day‐old rats35, CNS suppression symptoms including hypothermia, hypoactivity and slow and/or irregular breathing, with some deaths, were observed in 43% (6/14) of the 533 mg/kg (HED=57 mg/kg) dose group and 86% (12/14) of the 761 mg/kg (HED=81 mg/kg) dose group (Fig. 2).
Figure 2.
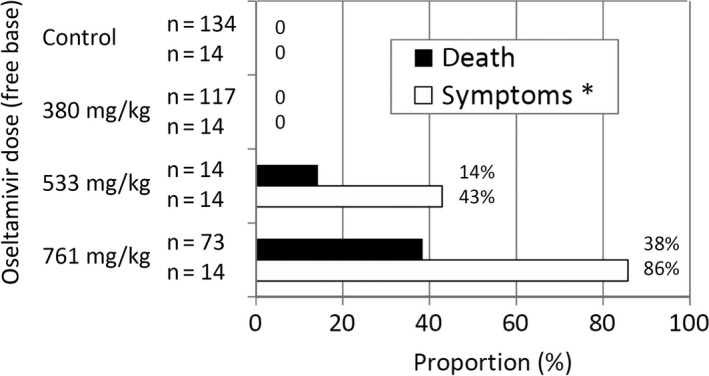
Dose‐response (death/symptoms) relationship in 7‐day‐old rats. Data from refs. 35 and 36. OT: oseltamivir. Chi Square for linear trend: for symptoms: 28.88, P<.0001, for death: 87.76, P<.0001. *Symptoms: decreased temperature, decreased locomotion, slow/irregular respiration and/or death
In a series of TK studies, 28 seven‐day‐old rats of both sexes were treated with a single dose of 761 mg/kg (HED=81 mg/kg). Symptoms such as hypothermia, paleness and hypoactivity were observed before death.36
Symptoms such as dose‐related increases in hypothermia, hypoactivity and slow/irregular breathing before death, and frequent findings of lung oedema at autopsy, suggest that the major cause of death is probably respiratory arrest due to central nervous system suppression. These are findings that were known from the Tx studies prior to approval.
4.3. Adverse effects on central nervous system known after marketing
4.3.1. Hypothermia, sensory, cognitive, behavioural and conscious impairment
Hypothermia
Ono et al. found that oseltamivir administered orally and intraperitoneally induced hypothermia, but the zanamivir did not.55, 56 OT and OC administered intracerebroventricularly (i.c.v.) also induced hypothermia dose‐dependently, but zanamivir did not.55 A study by Roche Pharmaceutical57 criticizes the Ono et al. study.55 However, the Roche study itself57 found a non‐significant dose–hypothermic relationship (P=.0546) at one hour after a single oral dose of OP in one experiment. Ono et al.55, 56 found significant dose‐dependent effects of hypothermia in ten different experiments repeatedly, including dosing of purified OP (without additives),56 which is the main point of criticism by Roche.57
Muraki et al.58 showed that oseltamivir blocks nicotinic acetylcholine receptors (nAchRs) at more than 3 μ mol/L, presumably via binding to a site in the channel pore. There was little effect on nAchRs at oseltamivir concentrations of 0.3 and 1 μ mol/L, but at concentrations 3, 10, 30 and 100 μ mol/L, nAchRs were inhibited by 30%, 40%, 75% and 90%, respectively.
Sensory, cognitive, behavioural and conscious impairment
A post‐marketing toxicology phase study (Tx study) conducted by Roche to verify the causality showed that oseltamivir induced many symptoms which the manufacturer considered “item‐related”: alterations in respiration including decreased respiratory rate/gasping and altered mucous membrane/skin colour (paleness) prior to death.52
Although the manufacturer denied causality50 symptoms at 2 hours after administration that showed dose‐related increases included lack of olfactory orientation (Fig. 3), lack of cliff aversion and low or very low arousal (Fig. 4). Among 58 pups in the 600 mg/kg or higher groups that were alive at 2 hours without cliff aversion 24 (41%) were later found dead, dose‐dependently (see footnote of Fig. 4).52 Among 17 animals that were alive with low or very low arousal in the 600 mg/kg or higher groups, 14 (82%) died thereafter within 24 hours. Among 51 animals that were alive without low arousal in the 600 mg/kg or higher groups, 14 (27%) died thereafter. Odd ratio for subsequent death with low arousal compared with no low arousal was 12.33 (95% CI: 3.07, 49.53, P<.0001). Among 60 animals in the control, 300 and 500 mg/kg group, none showed low arousal at 2 hours and none died within 24 hours.52
Figure 3.
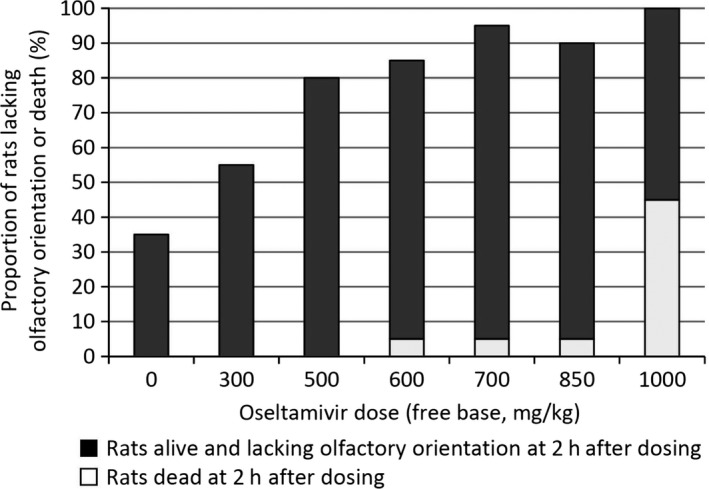
Oseltamivir dose and proportion of rats lacking olfactory orientation or died at 2 hours after dosing. Data from Refs. 50, 51. For each dose group (including control), 20 rats were used. Proportion of rats lacking olfactory orientation or death 2 hours after dosing were 35%, 55%, 80%, 80%, 95%, 90% and 100% for control, 300, 500, 600, 700, 850, 1000 mg/kg group respectively. Odds of lacking olfactory orientation in 500 mg/kg group is higher than control group: OR=7.43 (95%CI:1.78–31.04, P=.004). Dose‐response is clear.(Chi² for linear trend: 31.08, P<.0001)
Figure 4.
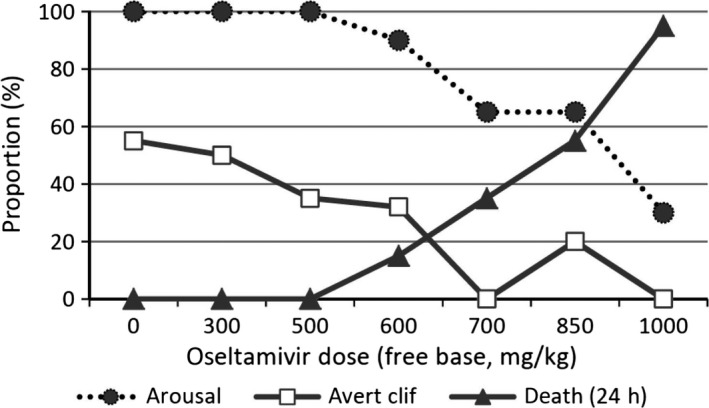
Cliff aversion, arousal at 2 hours after dosing and proportion of death at 24 hours. Data from Refs. 50, 51. (i) “Arousal” means “rats without low or very low arousal”. They decreased dose dependently in the 600 mg/kg or more groups. Most animals with low arousal were found dead by 24 hours after dosing. Odds of dead rats were significantly higher in rats with low arousal than without: pooled odds ratio (random effects) was 7.31 (95%CI: 1.88, 28.50, P=.0041). (ii) More than a half in control group averted cliff but proportion decreased dose dependently (Chi square for linear trend: 24.4, P<.0001). (iii) All rats that could avert cliff at 2 hours did not die within 24 hours except four in OT 600 mg/kg or higher group. Thirty two rats lacking cliff aversion at 2 hours in control, 300 and 500 mg/kg of OT groups did not die at all within 24 hours. However, rats lacking aversion at 2 hours in the OT group administered 600 mg/kg or more died dose dependently: 8%, 32%, 47% and 91% for 600, 700, 850 and 1000 mg/kg group respectively. Chi square for linear trend=32.5, P<.0001
These findings are consistent with clinically observed psychiatric symptoms in post‐marketing spontaneous reports6 (in particular, many cases in which sleep was followed by sudden death or abnormal behaviours with fatal outcome), some prospective cohort studies16, 17, 18, 19, 20 and a systematic review of the cohort studies21 and a systematic review of RCTs.22
Izumi et al. reported that systemic injection of oseltamivir (50 mg/kg i.p.) following non‐sedating dose of ethanol injection in rats significantly altered the duration of loss of righting reflex.59 They also reported diminished behavioural activity and poor locomotion by measuring the number of arm entries in the Y‐maze, representing a form of anxious or fearful behaviour as manifest by altered exploration.60 Ethanol injection in the presence of oseltamivir also resulted in enhanced hypothermia.59 Izumi et al. also reported that combination of oseltamivir with other neurostimulants altered synaptic plasticity and this may contribute to behavioural changes associated with the drug.60 They discussed that the neuraminidase, the enzyme targeted by OC, can modulate synaptic function and possibly alter propagation of signals based on the fact that many ion channels are glycosylated.59
Very low level of OC can affect the excitability of nociceptive neurons immediately after the administration of oseltamivir by reducing endogenous GM1 ganglioside.61 QT prolongation is closely and immediately related to the plasma concentration of OC.34, 62 These findings suggest that if the concentration of OC in the brain increased and affected the excitability of the excitatory and/or inhibitory neurons and affected neuroplasticity, oseltamivir may contribute to behavioural changes, especially to the delayed‐onset and prolonged type of psychiatric symptoms such as the case 8 in the case series.6 However, the acute behavioural changes observed in the in vivo experiment by Izumi et al.59, 60 may be induced rather by OT considering all other findings.
4.3.2. Respiratory suppression and respiratory arrest followed by cardiac arrest
Kimura et al.63 reported that intravenous oseltamivir (30–200 mg/kg expressed as free base) administered to mature rats caused dose‐dependent hypopnoea followed by ventilatory arrest at 200 mg/kg. This approximate lethal dose is compatible with an approximate lethal dose in mice: of two mice administered 190 mg/kg of oseltamivir as free base intravenously, one mouse survived exhibiting abnormal gait, shallow respiration and seizure, and one mouse died of seizure.34
Intraduodenal oseltamivir (500–1000 mg/kg) provoked similar ventilatory arrest 72–218 minutes after injection, followed by cardiac arrest,63 while oseltamivir carboxylate (OC: 100–200 mg/kg i.v.) had no significant effect on ventilation and blood pressure. The researchers concluded that oseltamivir causes central suppression of the respiratory function in rats, suggesting a relationship between oseltamivir‐induced cardiorespiratory arrest and sudden death observed in influenza patients after taking oseltamivir.63
4.3.3. Stimulatory or excitatory effects of oseltamivir on CNS
Suzuki et al.64 reported that oseltamivir sialylates a serum glycolipid and that this modified glycolipid induced jump‐down behaviour via stimulation of dopamine D2 receptors; they suggest that this mechanism may be involved in the abnormal behaviour seen in some children taking oseltamivir.
Hiasa et al.,65 through behavioural monitoring using automated video analysis, revealed that intraventricular administration of very small doses of oseltamivir, but not oseltamivir carboxylate, significantly enhanced spontaneous behavioural activities in mice, such as jumping, rearing, sniffing, turning and walking. Hiasa et al. also found that oseltamivir, but not oseltamivir carboxylate, competitively and selectively inhibited human MAO‐A. The estimated Ki value was 25–28 μ mol/L determined by two methods that are comparable with the Km values of native substrates of MAO‐A. Docking simulations in silico based on the tertiary structure of MAO‐A suggested that oseltamivir could fit into the inner pocket of the enzyme. IC50 was shown to be between 50 and 100 μ mol/L in their paper.65
4.4. Summary of this section
Many toxicity studies conducted by the pharmaceutical company prior and after approval, and those by other investigators indicate clear dose‐related increase of hypothermia, abnormal behaviours (inhibitory and excitatory), sensory, cognitive, conscious, respiratory suppression and mortality within 24 hours after single administration of oseltamivir. Lung oedema may only be the pathological findings in the dead animal within 24 hours. It is concluded that these CNS depressive action of oseltamivir is related to OT (unchanged oseltamivir). However, excitatory action of oseltamivir is related to inhibition of MAO‐A. Behavioural change, especially the delayed‐onset and prolonged ones, may be related to OC with affected neuroplasticity.
5. Similarity of Spectrum of Reactions in Humans and Other Animals
Clinical adverse reactions reported following oseltamivir administration include sudden‐onset type, delayed‐onset type and others.5, 6, 62 Similarities between sudden‐onset type reactions in humans and those in other animals are summarized as follows.
Sudden‐onset type reactions include nausea, vomiting, hypothermia and neuropsychiatric reactions such as abnormal behaviours, hallucination, psychotic symptoms and sudden respiratory arrest followed by cardiac arrest and death. These appear very shortly (from less than one hour up to 12 hours at most) after the first dose of oseltamivir and disappear rapidly unless respiratory arrest and sudden death or sequelae are induced.5, 6 These reactions may disappear even if oseltamivir is continuously taken, although symptoms may reappear several times after subsequent intake. The underlying mechanism is discussed below in section 3.
6. Coherence in Toxicokinetic and Molecular Level of Evidence
6.1. Toxicokinetics: very high estimated concentrations in the brains of dead rats
The average maximum concentration (Cmax) in the brain after administration of 761 mg/kg of oral oseltamivir as free base was 144 μ mol/L for surviving 7‐day‐old rats, compared with only 2.3 μ mol/L for adult rats (all survived).36 Ministry of Health, Labour and Welfare (Japan) ordered the pharmaceutical company to conduct toxicity and TK studies in order to reassess the causality of serious adverse effects on central nervous system of oseltamivir after the ministry contraindicated oseltamivir for children and adolescents aged 10–19 years.
Maximum concentration (Cmax) in the brain of surviving 7‐day‐old rats was estimated to be 64 times higher than that of mature (42‐day‐old) rats.6, 36 This estimate was derived from the product of 6.4 times the difference in plasma Cmax (due to immaturity of CES135) and 10 times the difference in brain‐to‐plasma ratio due to immaturity of P‐gp in the BBB.30, 31, 33
However, this difference is underestimated, because it can be assumed that the average level of Cmax of juvenile rats that died may be higher than that of surviving rats. Hence, the ratio of brain Cmax of juvenile rats that died to that of mature rats may be higher. If the Cmax of oseltamivir in the brain of the died rat was two or three times higher than the average Cmax of survived rats, it could be 300, 400 μ mol/L or more. In fact, average Cmax of deceased juvenile rats in the 300–600 mg/kg group was estimated to be about 10 times higher than for surviving rats. Thus, concentrations could be 200 or more times greater in the brains of surviving 7‐day‐old rats than in the mature brains of adult rats, based on the data of post‐marketing TK studies50, 51, 52 (Table 2).
Table 2.
Estimated plasma and brain concentrations (Cmax) of oseltamivir (as free base) for mature, surviving juvenile and deceased juvenile rats, and juvenile/mature ratio by survival state (calculated using the data from post‐marketing TK test)
| Dose (mg/kg) | Number of rats that died | Concentration (μ mol/L) | Ratio (juvenile/mature)e | Ratio (died/alive) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mature rats (estimated)a | Juvenile rats | ||||||||||||||
| Aliveb | Allc | Diedd | Plasma | Brain | Plasma | Brain | |||||||||
| Plasma | Brain | Plasma | Brain | Plasma | Brain | Plasma | Brain | Alive | Died | Alive | Died | ||||
| 300 | 1 | 11.0 | 1.2 | 136 | 34 | 159 | 40.9 | 1509 | 416 | 12 | 137 | 29 | 346 | 11 | 12 |
| 500 | 3 | 18.4 | 2.0 | 186 | 52 | 265 | 68.2 | 1732 | 365 | 10 | 94 | 26 | 182 | 9 | 7 |
| 600 | 5 | 22.1 | 2.5 | 225 | 45 | 318 | 81.9 | 1316 | 474 | 10 | 60 | 18 | 190 | 6 | 11 |
| 700 | 12 | 25.8 | 2.9 | 266 | 60 | 371 | 95.5 | 778 | 234 | 10 | 30 | 21 | 81 | 3 | 4 |
| 850 | 33 | 31.3 | 3.5 | 292 | 56 | 451 | 116.0 | 573 | 162 | 9 | 18 | 16 | 46 | 2 | 3 |
| 1000 | 40 | 36.8 | 4.1 | 275 | 47 | 530 | 136.5 | 647 | 177 | 8 | 18 | 11 | 43 | 2 | 4 |
Plasma and brain concentrations in mature rats for each dose group are estimated based on the concentration of the 1000 mg/kg group, which was proportionally allotted for dose.
Plasma and brain concentrations of surviving rats are the averages of two rats.
Calculated average for all animals including surviving and deceased.
Calculated average for deceased only.
Note that proportionality of juvenile/mature ratio breaks down for deceased rats. The reasons may include: (i) death occurred at a certain brain concentration level, making further increase impossible; (ii) sampling methods may not have been adequate.
6.2. Potential target receptors and enzymes for sudden‐onset reactions
Roche (Lindeman et al.)66 reported that the binding assays to 155 receptors and other molecular targets, including those related to mood, cognition and behaviour, did not show any pharmacological activities by oseltamivir phosphate and oseltamivir carboxylate at 3 and 30 μ mol/L of concentration. However, the concentration in their study (3 and 30 μ mol/L) may not be sufficient for the binding assays in view of the levels noted in the brains of surviving juvenile rats (144 μ mol/L)6, 36 and of rats that died (possibly 300 μ mol/L or more: Table 2), according to estimates using the data reported in the post‐marketing toxicokinetic study.52
6.2.1. For hypothermia and excitatory abnormal behaviours
Muraki et al.58 showed that oseltamivir blocks nicotinic acetylcholine receptors (nAchRs: α3β4) dose‐dependently at concentrations of more than 3 μ mol/L: by 30%, 40%, 75% and 90% at concentrations of 3, 10, 30 and 100 μ mol/L, respectively. Concentrations of 0.3 and 1 μ mol/L had little effect. IC50 is estimated to be between 10 and 30 μ mol/L. They also discussed the results by Lindeman et al.66 stating “30 μ mol/L of oseltamivir inhibited Na+ and Ca2+ channel binding of their respective blockers by 38% and 41%, respectively, suggesting the possibility that oseltamivir blocks Na+ and Ca2+ channels.”58
As noted above, Hiasa et al.65 found that oseltamivir, but not oseltamivir carboxylate, competitively and selectively inhibited human MAO‐A. They estimated the Ki value to be 25–28 μ mol/L, and IC50 was shown to be between 50 and 100 μ mol/L in their paper.
Hypothermia may be closely related to inhibition of nAch receptor, and abnormal behaviour of excitatory manner may be closely related to inhibition of MAO‐A.
6.2.2. For sensory, cognitive and respiratory impairment
However, a receptors, channels and/or enzymes related to impairment of sensory, cognition and respiratory suppression, respiratory arrest followed by cardiac arrest and death have not been known yet.
Among many agents acting on the central nervous system, those that induce all symptoms including hypothermia, excitatory abnormal behaviour, psychosis (both acute and chronic), sensory/cognitive/consciousness disturbance and respiratory arrest may be rare.
Central nervous system suppressants such as barbiturates, benzodiazepines and ketamine may induce disinhibition or dyscontrol leading to psychiatric reactions and respiratory arrest at higher dose.54 It is rather difficult to explain delayed‐onset and prolonged psychotic reactions only by GABAergic action. Ketamine, a non‐competitive antagonist of NMDA/glutamate receptor and an agonist of certain subtype of GABAA,54, 67 may better explain such delayed‐onset psychotic reactions.6, 7 Among the NMDA antagonists, ketamine is the most similar to the action/reaction of oseltamivir, because ketamine, but not phencyclidine, produces dose‐dependent hypothermia68 and respiratory depression leading to death in very high doses.54 It induces acute schizophrenic symptoms, including hallucination, especially during recovery from anaesthesia.54 Chronic low doses of ketamine also induce schizophreniform reactions in animals and humans.69, 70, 71, 72 Ketamine, but not phencyclidine (PCP), modulates α6β2δ and α6β3δ receptors. 50% effective concentrations (EC50) for α6β2δ and α6β3δ subtype were 570 and 245 μ mol/L, although human anaesthetic concentration of ketamine is thought to be 1 μg/mL (3.6 μ mol/L), and that for respiratory arrest is thought to be 20 μ mol/L. Hence, far higher concentrations than 30 μ mol/L and various subtypes are necessary to determine EC50 of ketamine to GABAA which is related to respiratory arrest by ketamine.
In determining inhibition (%) of GABAA receptor by oseltamivir, Lindemann et al. 66 used only one subtype (α1β2γ2) among many subtypes of GABAA receptors, with a concentration up to 30 μ mol/L, which is far lower than the average brain concentration among surviving juvenile rats (144 μ mol/L). The concentration of 30 μ mol/L is extremely low compared with the EC50 of ketamine on some subtypes of GABAA (570 or 245 μ mol/L).
Among many receptors that Roche reported, several receptors were apparently inhibited dose‐dependently by oseltamivir (Table 3). It should also be born in mind that EC50 and/or IC50 may differ greatly according to the substrate used.
Table 3.
Activity of OP against molecular targets of high relevance for mood, cognition and behaviour in binding or functional assays
| Target/assay | Inhibition (% control)Concentration of OP | |
|---|---|---|
| 3 μ mol/L | 30 μ mol/L | |
| L‐type Ca2+ channel (diltiazem site) | 14 | 41 |
| Na+ channel (site 2) | 11 | 38 |
| NMDA‐type glutamate receptor (PCP) | 14 | 23 |
| AMPA‐type glutamate receptor | 4 | 17 |
| Kainate‐type glutamate receptor | 0 | 14 |
| Serotonin 5‐HT2A receptor | 4 | 13 |
| Serotonin 5‐HT4E receptor | 0 | 13 |
Extracted from Ref. 64.
Note that maximum concentration of OP tested was 30 μ mol/L. Hence, these, especially Ca2+ channel, Na+ channel and NMDA‐type glutamate receptor, could be affected by oseltamivir.
OP, oseltamivir phosphate; OT, free form of unmetabolized oseltamivir.
Neuropsychiatric symptoms occurring in very early phases of treatment, such as acute behavioural change and respiratory depression leading to death, may be due to effects on the central nervous system (CNS) of unchanged oseltamivir phosphate (OP).
If oseltamivir has affinity to NMDA receptors, as suggested by the data reported in Lindeman et al.,66 and if oseltamivir is used for a long period of time, it could induce psychiatric reactions including psychosis, confusion and depression in humans, as reported in the RCTs for prophylaxis of influenza.22, 62
Gabapentin is an agent with anticonvulsant, antinociceptive and anxiolytic properties. It is a structural analogue of GABA or baclofen (a GABAB agonist), but it had been considered to have no direct or apparent effects on GABAA and GABAB receptors, although it has been reported to bind with nanomolar affinity to the auxiliary α2‐δ subunit of voltage‐dependent calcium channels (VD‐CCs),73 before the study by Ng et al.74, 75 They reported that gabapentin is an agonist at the GABAB gb1a‐gb2 heterodimer.74 Selective gabapentin activation of GABAB receptors negatively coupled to VD‐CCs may account for gabapentin actions.75
On the other hand, VD‐CCs, predominately the L‐type channel, are proposed as a direct target of gabapentin, as it inhibits L‐type calcium currents.76
Baclofen, a GABAB agonist induces respiratory arrest (apnoea) in high dose in both animals77 and humans.78
Voltage‐dependent calcium channels that were investigated by Lindeman et al.66 are three L‐type (DHP site, diltiazem site and verapamil site) and “N‐type” Ca2+ channels. Of these, L‐type Ca2+ channel (diltiazem site) was dose‐dependently inhibited by unchanged oseltamivir (14% and 41% at 3 and 30 μ mol/L, respectively: Table 3). However, α2‐δ subunit of VD‐CC was not investigated by Lindeman et al.66
It should also be considered that apnoea can be caused by apneusis that is induced by NMDA receptor blockade.79
6.3. Mode of common adverse reactions: headache, nausea and vomiting
Nausea and vomiting were demonstrated as adverse reactions to oseltamivir in treatment trials involving both adults and children22 and are the established adverse reactions to oseltamivir. Dose‐dependent increase of headache was demonstrated only in prophylaxis trials, because common symptoms of influenza including headache were not considered adverse event in the treatment trials.22 One of the definitions of adverse events of the trials was “any adverse change from the subject's baseline (pretreatment) condition, which occurred during the course of the study after treatment had started, whether considered related to treatment or not”.22 Nausea and vomiting may be symptoms of gastrointestinal system dysfunction but could also be induced by central nervous system disturbance, such as increased intracranial pressure. Increased intracranial pressure induces headache except in infants <1 year old, in whom it may induce bulging of fontanelle.
A typical case with increased intracranial pressure was reported as a spontaneous adverse reaction case reported from Japan and disclosed to the Food and Drug Administration.80 A 5‐month‐old male infant who was treated with oseltamivir for prophylaxis vomited one and a half hours after receiving the drug. His mother noticed that his fontanelle bulged repeatedly throughout the treatment period of 8 days.
Vomiting as an effect of neurological disturbance is consistent with the findings that vomiting in children increases only on day 1 of treatment but not thereafter35 and that neuropsychiatric adverse reactions such as abnormal behaviour, delirium, unconsciousness16, 17, 18 and respiratory arrest leading to death5, 6, 7, 24 occur in the very early phase of oseltamivir treatment.
6.4. Summary of this section
The maximum concentration (30 μ mol/L) in the study by the pharmaceutical company was insufficient for the binding assays compared with the brain levels of surviving juvenile rats (144 μ mol/L) and is far lower than that estimated in the dead rats (possibly 300 μ mol/L or more). Unchanged oseltamivir has various effects on the CNS that may be related to clinical findings including hypothermia, abnormal behaviours including with fatal outcome, and sudden death. Known and potential targets related to inhibitory and excitatory reactions to oseltamivir were discussed as in the following Summary and conclusions.
7. Summary and Conclusions
Unchanged oseltamivir (OT) has CNS actions which are related to sudden‐onset type reactions such as sudden death from respiratory arrest and abnormal behaviours with fatal outcome.
To date, it is known that unchanged oseltamivir inhibits nAchR and MAO‐A, which are related to hypothermia and abnormal behaviours. However, the receptors related to sudden respiratory arrest are not yet known. Among many agents, ketamine has the effect most similar to oseltamivir, as it induces hypothermia, acute and chronic psychosis, and respiratory arrest at high doses. Ketamine acts on both NMDA and GABA receptors. NMDA and GABA receptors and their related receptors/channels including Na+ and Ca2+ channels should be extensively investigated in relation to sudden respiratory arrest.
Conflict of Interest
Rokuro Hama was a corecipient of a UK National Institute for Health Research grant (HTA 10/80/01, Update and amalgamation of two Cochrane reviews: neuraminidase inhibitors for preventing and treating influenza in healthy adults and children (www.nets.nihr.ac.uk/projects/hta/108001). RH wrote two books published in 2008 about the harm of oseltamivir and antipyretics. He provided scientific opinions and expert testimony on 14 adverse reaction cases related to oseltamivir for the applications by their families for adverse reaction relief by PMDA (Pharmaceuticals and Medical Devices Agency) and in the lawsuits for revocation of the PMDA's decision concerning with these reactions. Most of the cases were reported in: IJRSM 2008:20: 5‐36.6
Charles Bennett is a recipient of grants from the South Carolina Center of Economic Excellence Center to conduct studies of medication safety initiative, the National Cancer Institute (1R01CA165609‐01A1) and a philanthropic grant from Doris Levkoff Meddin to the South Carolina Center for Medication Safety and Efficacy.
Acknowledgement
Statistical analysis for Fig. 1 by Dr Mark A Jones (School of Population Health, The University of Queensland, Australia) is gratefully acknowledged.
Hama, R. and Bennett, C. L. (2017), The mechanisms of sudden‐onset type adverse reactions to oseltamivir. Acta Neurologica Scandinavica, 135: 148–160. doi: 10.1111/ane.12629
References
- 1. Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza— recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 2. Antiviral Drugs‐Information for Health Care professionals (for the 2015–2016 influenza season). http://www.cdc.gov/flu/professionals/antivirals/index.htm. Accessed October 09, 2015.
- 3. World Health Organization . WHO Model List of Essential Medicines.19th edition, 2015. http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf?ua=1. Accessed May 27, 2015.
- 4. Maxwell SR. Tamiflu and neuropsychiatric disturbance in adolescents. BMJ. 2007;334:1232–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hama R. Discussion of the causal relationship between oseltamivir phosphate (Tamiflu), and sudden death and death from abnormal behaviour (presentation at a session of Japanese Society for Pediatric Infectious Diseases in Tsu, Mie Prefecture November 12, 2005), 2005. (in English). http://www.npojip.org/english/no59.html. Accessed June 05, 2015.
- 6. Hama R. Fatal neuropsychiatric adverse reactions to oseltamivir: case series and overview of causal relationships. Int J Risk Saf Med. 2008;20:5–36. http://www.npojip.org/sokuho/no105.pdf. (errata: http://www.npojip.org/sokuho/107seigohyo-e.pdf). Accessed May 27, 2015. [Google Scholar]
- 7. Hama R. Oseltamivir's adverse reactions: Fifty sudden deaths may be related to central suppression. BMJ. 2007;335:59 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1914480/. Accessed June 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blumentals WA, Song X. The safety of oseltamivir in patients with influenza: analysis of healthcare claims data from six influenza seasons. MedGenMed. 2007;9:23. [PMC free article] [PubMed] [Google Scholar]
- 9. Toovey S, Rayner C, Prinssen E, et al. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: a comprehensive review. Drug Saf. 2008;31:1097114. [DOI] [PubMed] [Google Scholar]
- 10. Tamura D, Miura T, Kikuchi Y. Oseltamivir phosphate in infants under 1 year of age with influenza infection. Pediatr Int. 2005;47:484. [DOI] [PubMed] [Google Scholar]
- 11. Shalabi M, Abughali N, Abzug M, et al., Safety of oseltamivir vs. amantadine of rimantadine in children under 1 year of age [Abstract]. Presented at the 45th annual meeting of the Infectious Diseases Society of America, October 47, 2007; San Diego, California.
- 12. Okamoto S, Kamiya I, Kishida K, et al. Experience with oseltamivir for infants younger than 1 year old in Japan. Pediatr Infect Dis J. 2005;24:5756. [DOI] [PubMed] [Google Scholar]
- 13. Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta‐analysis of observational studies. Ann Intern Med. 2012;156(7):512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muthuri SG, Myles PR, Venkatesan S, et al. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009‐10 influenza A(H1N1) pandemic: a systematic review and metaanalysis in hospitalized patients. J Infect Dis. 2013;207:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta‐analysis of randomised controlled trials. Lancet. 2015;385:1729–1737. [DOI] [PubMed] [Google Scholar]
- 16. Yokota S, Fujita T, Mori M, et al. Epidemiologic survey of influenza‐associated complications‐ I. Clinical assessment of symptoms and signs, and medication. J Japan Pediatr Soc, 2007;111:1545–1558. [Google Scholar]
- 17. Fujita T, Mori M, Yokota S., et al. Epidemiologic survey of influenza‐associated complications‐ II. A statistical analysis of symptoms and signs, and medication. J Japan Pediatr Soc, 2007;111:1559–1567. [Google Scholar]
- 18. Fujita T, Fujii Y, Watanabe Y, Mori M, Yokota S. A pharmacoepidemiological study on the relationship between neuropsychiatric symptoms and therapeutic drugs after influenza infection. Jpn J Pharmacoepidemiol 2010;15:73–92. http://www.jstage.jst.go.jp/article/jjpe/15/2/73/_pdf. [Google Scholar]
- 19. Yorifuji T, Suzuki E, Tsuda T. Oseltamivir and abnormal behaviors: true or not? Epidemiology. 2009;20:619–621. [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara F, Ikushima S, Hibi N, et al. An observational study to assess risk factors of abnormal behaviours during influenza. Poster and oral presentation at the 40th annual meeting of Japanese Society for Pediatric Infectious Diseases held on 15 and 16 Nov. 2008.
- 21. Hama R, Jones M, Hayashi K, Yanagi T, Sakaguchi K. Oseltamivir: A systematic review and meta‐analysis of adverse effects in prospective cohort studies (a preliminary report was presented at the 16th JSPE and 5th ACPE joint meeting Tokyo 29–30th 2010 Tokyo).
- 22. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews 2014. [DOI] [PMC free article] [PubMed]
- 23. MHLW . Documents for the 2014 advisory Panel on Drug Safety (held on 29/Oct/2015): Use of drugs for influenza. http://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000063406.pdf. Accessed October 10, 2015.
- 24. Hama R, Jones M, Hayashi K, Sakaguchi K. Oseltamivir and early deterioration leading to death. Int J Risk Saf Med 2011;23:201–215. http://content.iospress.com/articles/international-journal-of-risk-and-safety-in-medicine/jrs545. Accessed June 23, 2016. (note, this is not a proportional mortality study but comparative mortality study). [DOI] [PubMed] [Google Scholar]
- 25. Rxisk.org. http://rxisk.org/drugs-a-z/. Accessed June 21, 2016.
- 26. Zbinden G. Experimental and Clinical Aspects of Drug Toxicity. Adv Pharmacol. 1963;2:1–112. [Google Scholar]
- 27. Festing MF, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 2002;43:244–258. Erratum in: ILAR J. 2005;46(3):320. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto K, Yoshimura I. Toxicity study and selection of statistical methods in Yoshimura and Ohashi ed Statistical analysis for toxicity study data p1‐11. Chijinn‐shokan, 1992. (in Japanese).
- 29. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) . Guidance for Industry‐Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, 2005. http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf. Accessed June 09, 2015.
- 30. Morimoto K, Nakakariya M, Shirasaka Y, et al. Oseltamivir (Tamiflu) efflux transport at the blood‐brain barrier via P‐glycoprotein. Drug Metab Dispos. 2008;36:6–9. [DOI] [PubMed] [Google Scholar]
- 31. Ose A, Kusuhara H, Yamatsugu K, et al. P‐glycoprotein restricts the penetration of oseltamivir across the blood‐brain barrier. Drug Metab Dispos. 2008;36:427–434. [DOI] [PubMed] [Google Scholar]
- 32. MHLW . Documents, advisory Panel on Drug Safety (4 April 2007, in Japanese). http://www.mhlw.go.jp/shingi/2007/04/s0404-2.html. Accessed June 09, 2015.
- 33. MHLW . Documents, advisory Panel on Drug Safety (25 Dec. 2007, in Japanese). http://www.mhlw.go.jp/shingi/2007/12/s1225-7.html. Accessed June 09, 2015.
- 34. Chugai Pharm Co (then Roche Japan). Summary basis of approval (SBA) of Tamiflu, 2000. http://www.pmda.go.jp/drugs/2000/g001202/index.html. Accessed June 09, 2015.
- 35. Chugai Pharm Co . SBA of Tamiflu dry syrup, 2002. http://www.pmda.go.jp/drugs/2002/P200200011/index.html. Accessed June 09, 2015.
- 36. Chugai Pharm Co . SBA of Tamiflu capsule 75 for prophylaxis, 2004. http://www.pmda.go.jp/drugs/2004/P200400015/index.html. Accessed June 09, 2015.
- 37. Examination results by PMDA . Tamiflu capsule. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/6250021M1. Accessed June 09, 2015.
- 38. Examination results by PMDA . Tamiflu dry syrup. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/6250021R1. Accessed June 09, 2015.
- 39. Full prescription information of oseltamivir (revised in April 2010). http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021087s07lbl.pdf. Accessed June 09, 2015.
- 40. Suzaki Y, Uemura N, Takada M, et al. The effect of carboxylesterase 1 (CES1) polymorphisms on the pharmacokinetics of oseltamivir in humans. Eur J Clin Pharmacol. 2013;69:21–30. [DOI] [PubMed] [Google Scholar]
- 41. Wattanagoon Y, Stepniewska K, Lindegårdh N, et al. Pharmacokinetics of high‐dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother. 2009;53:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. [DOI] [PubMed] [Google Scholar]
- 43. Yang D, Song X, Yan B. Interleukin‐6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007;72:686–694. [DOI] [PubMed] [Google Scholar]
- 44. Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P‐glycoprotein. J Neurochem. 2007;102:1677–1690. [DOI] [PubMed] [Google Scholar]
- 45. Théron D, Barraud de Lagerie S, Tardivel S, et al. Influence of tumor necrosis factor‐alpha on the expression and function of P‐glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochem Pharmacol. 2003;66:579–587. [DOI] [PubMed] [Google Scholar]
- 46. Roberts DJ, Goralski KB. A critical overview of the influence of inflammation and infection on P‐glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol. 2008;4:1245–1264. [DOI] [PubMed] [Google Scholar]
- 47. Miller DS, Bauer B, Hartz AM. Modulation of P‐glycoprotein at the blood‐brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang D, Pearce RE, Wang X, Gaedigk R, Wan YJ, Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter‐individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsai CE, Daood MJ, Lane RH, Hansen TW, Gruetzmacher EM, Watchko JF. P‐glycoprotein expression in mouse brain increases with maturation. Biol Neonate. 2002;81:58–64. [DOI] [PubMed] [Google Scholar]
- 50. Freichel C, Prinssen E, Hoffmann G, et al. Oseltamivir is devoid of specific behavioral and other central nervous system effects in juvenile rats at supratherapeutic oral doses. Int J Virol. 2009;5:119–130. [Google Scholar]
- 51. F. Hofman‐La‐Roche, Ltd . An oral (gavage) toxicity study of Tamiflu (oseltamivir phosphate) in juvenile rats: disclosed via FOIA; 2007.
- 52. F. Hofman‐La‐Roche, Ltd . Toxicokinetic phase report for the final study report of Tamiflu (oseltamivir phosphate) in juvenile rats: disclosed via FOIA; 2007.
- 53. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition‐a review. Placenta 2003;24(Suppl A):S33–S46. [DOI] [PubMed] [Google Scholar]
- 54. Hardeman JG, Limbird LN, Gilman AG. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 10th edition New York: McGraw‐Hill; 2001. [Google Scholar]
- 55. Ono H, Nagano Y, Matsunami N, Sugiyama S, Yamamoto S, Tanabe M. Oseltamivir, an anti‐influenza virus drug, produces hypothermia in mice. Biol Pharm Bull. 2008;31:638–642. [DOI] [PubMed] [Google Scholar]
- 56. Ono H, Iwajima Y, Nagano Y, et al. Reduction in sympathetic nerve activity as a possible mechanism for the hypothermic effect of oseltamivir, an anti‐influenza virus drug, in normal mice. Basic Clin Pharmacol Toxicol. 2013;113:25–30. [DOI] [PubMed] [Google Scholar]
- 57. Freichel C, Breidenbach A, Hoffmann G, et al. Absence of central nervous system and hypothermic effects after single oral administration of high doses of oseltamivir in the rat. Basic Clin Pharmacol Toxicol. 2012;111:50–57. [DOI] [PubMed] [Google Scholar]
- 58. Muraki K, Hatano N, Suzuki H, et al. Oseltamivir Blocks Human Neuronal Nicotinic Acetylcholine Receptor‐Mediated Currents. Basic Clin Pharmacol Toxicol. 2015;116:87–95. [DOI] [PubMed] [Google Scholar]
- 59. Izumi Y, Tokuda K, O'dell KA, Zorumski CF, Narahashi T. Neuroexcitatory actions of Tamiflu and its carboxylate metabolite. Neurosci Lett. 2007;426:54–58. Epub 2007 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Izumi Y, Tokuda K, O'Dell K, Zorumski C, Narahashi T. Synaptic and behavioral interactions of oseltamivir (Tamiflu) with neurostimulants. Hum Exp Toxicol. 2008;27:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crain SM, Shen KF. Neuraminidase inhibitor, oseltamivir blocks GM1 ganglioside‐regulated excitatory opioid receptor‐mediated hyperalgesia, enhances opioid analgesia and attenuates tolerance in mice. Brain Res. 2004;995:260–266. [DOI] [PubMed] [Google Scholar]
- 62. Hama R. The mechanisms of delayed onset type adverse reactions to oseltamivir. Infect Dis. 2016. DOI: 10.1080/23744235.2016.1189592. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kimura S, Niwa Y, Iwajima Y, et al. High doses of oseltamivir phosphate induce acute respiratory arrest in anaesthetized rats. Basic Clin Pharmacol Toxicol. 2012;111:232–239. [DOI] [PubMed] [Google Scholar]
- 64. Suzuki M, Masuda Y. Effect of a neuraminidase inhibitor (oseltamivir) on mouse jump‐down behavior via stimulation of dopamine receptors. Biomed Res. 2008;29:233–238. [DOI] [PubMed] [Google Scholar]
- 65. Hiasa M, Isoda Y, Kishimoto Y, et al. Inhibition of MAO‐A and stimulation of behavioural activities in mice by the inactive prodrug form of the anti‐influenza agent oseltamivir. Br J Pharmacol. 2013;169:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lindemann L, Jacobsen H, Schuhbauer D, et al. In vitro pharmacological selectivity profile of oseltamivir prodrug (Tamiflu) and active metabolite. Eur J Pharmacol. 2010;628:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hevers W, Hadley SH, Lüddens H, Amin J. Ketamine, but not phencyclidine, selectively modulates cerebellar GABA(A) receptors containing alpha6 and delta subunits. J Neurosci. 2008;28:5383–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fahim I, Ismail M, Osman OH. Role of 5‐hydroxytryptamine in ketamine‐induced hypothermia in the rat. Br J Pharmacol. 1973;48:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aalto S, Ihalainen J, Hirvonen J, et al. Cortical glutamate‐dopamine interaction and ketamine‐induced psychotic symptoms in man. Psychopharmacology. 2005;182:375–383. Epub 2005 Oct 19. [DOI] [PubMed] [Google Scholar]
- 70. Freeman TP, Morgan CJ, Klaassen E, et al. Superstitious conditioning as a model of delusion formation following chronic but not acute ketamine in humans. Psychopharmacology. 2009;206:563–573. [DOI] [PubMed] [Google Scholar]
- 71. Malhotra AK, Pinals DA, Adler CM., et al. Ketamine‐induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic‐free schizophrenics. Neuropsychopharmacology 1997;17:141–150. [DOI] [PubMed] [Google Scholar]
- 72. Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose‐response study. Psychopharmacology. 2004;172:298–308. [DOI] [PubMed] [Google Scholar]
- 73. Gee NS, Brown JP, Disanayake VUK, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. [DOI] [PubMed] [Google Scholar]
- 74. Ng GY, Bertrand S, Sullivan R, et al. Gamma‐aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentinaction. Mol Pharmacol. 2001;59(1):144–152. [PubMed] [Google Scholar]
- 75. Bertrand S, Ng GY, Purisai MG, et al. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain gamma‐aminobutyric acid type B receptors negatively coupled to voltage‐dependent calcium channels. J Pharmacol Exp Ther. 2001;298:15–24. [PubMed] [Google Scholar]
- 76. Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. [DOI] [PubMed] [Google Scholar]
- 77. Hey JA, Mingo G, Bolser DC, Kreutner W, Krobatsch D, Chapman RW. Respiratory effects of baclofen and 3‐aminopropylphosphinic acid in guinea‐pigs. Br J Pharmacol. 1995;114:735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Perry HE, Wright RO, Shannon MW, Woolf AD. Baclofen overdose: drug experimentation in a group of adolescents. Pediatrics. 1998;101:1045–1048. [DOI] [PubMed] [Google Scholar]
- 79. Feldman JL, Windhorst U, Anders K, Richter DW. Synaptic interaction between medullary respiratory neurones during apneusis induced by NMDA‐receptor blockade in cat. J Physiol. 1992;450:303–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Edwards Et, Truffa MM. (Division of Drug Risk Evaluation: DDRE) One‐Year Post Pediatric Exclusivity Postmarketing Adverse Events Review (Drug: Oseltamivir phosphate): Department of Health and Human Services, Public Health Services, Food and Drug administration: Center for Drug Evaluation and Research=FDA CDER, 2005. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4180b_06_01_Tamiflu%20AE_reviewed.pdf. Accessed May 28, 2015.


