Abstract
Objectives
To propose approaches tailored to the specific needs of neonates, such as structured product development programmes, with the ultimate goal to improve the safe and effective use of analgosedatives in these fragile patients.
Key findings
The feasibility and relevance of a structured product development programme in neonates (optimal study design based on preliminary data; model development; internal, external and prospective evaluation; an individualized dosing regimen; long-term safety; pharmacogenetics) are illustrated for the use of morphine. Based on changes in clinical practices, similar development plans are in progress for short-acting analgosedatives such as propofol, but are in need of tailored pharmacodynamic tools to assess and quantify effects. Furthermore, for drugs like paracetamol where there is already sufficient clinical pharmacology knowledge, attention needs to be given to long-term safety aspects. Finally, new covariates such as pharmacogenetics might further improve neonatal pain management, but clearly need to be integrated with other well-established covariates like age or weight.
Summary
Product development programmes for analgosedatives in neonates are needed. These programmes should be tailored to their specific needs (short-acting sedation, pain relief), should include long-term safety and should incorporate the exploration of newer covariates like pharmacogenetics.
Keywords: clinical pharmacology, newborn, pain, pharmacodynamics, pharmacogenetics
Introduction
Anand et al. showed that the assumption that prematurity or immaturity protects neonates from pain and its negative effects was incorrect when he documented that untreated perioperative pain resulted in increased morbidity (e.g. stress response, inflammation) and mortality in these young infants. These negative outcome variables were also observed in later paediatric life.[1,2] Similar short-term and long-term outcome observations have been reported following repeated or prolonged medical or procedural pain.[3]
The benefits of opioids in neonatal pain largely depend on the clinical indication: there is strong evidence for postoperative analgesia, while the evidence in support of opioids during medical treatment or procedural pain is much less robust.[2,4] In the latest meta-analysis on opioids in ventilated preterm neonates, Bellu et al. concluded that there is insufficient evidence to recommend routine opioid administration. She suggested that opioids should be used selectively, when indicated by clinical evaluation of pain indicators to avoid the side effects of stress or pain.[4]
Irrespective of the indication, the administration of opioids has its own set of side effects. Opioids are narcotics that stimulate opioid receptors within as well as outside the central nervous system (CNS). This explains effects (analgesia, sedation), but also side effects (paralytic ileus, respiratory depression, hypotension) that may in part be more pronounced in (pre)term neonates compared to children or adults. Morphine delayed the attainment of full enteral feeds with 3 days in the NEOPAIN trial,[5] and respiratory depression resulted in prolonged duration of ventilation with 1 day).[6] Hypotension is another clinically relevant side effect, and the available data reported in different studies likely reflect differences in morphine doses used.[7,8] Pre-emptive morphine (100 μg/kg loading, 10–30 μg/kg per hour maintenance), additional morphine administration and lower gestational age were associated with hypotension in the NEOPAIN study.[7] In contrast, Simons et al.[8] were unable to document differences in blood pressure, the need for inotropic agents or blood pressure variability associated with morphine infusion (100 μg/kg loading, 10 μg/kg per hour maintenance). The threefold differences in morphine maintenance doses used between the NEOPAIN and Dutch study hereby nicely illustrate the off-knowledge dosing practices for morphine at that time.[7,8]
Since these studies (2000–2001), additional relevant knowledge about the clinical pharmacology of morphine in (pre)term neonates has been generated. This includes the development of a maturational pharmacokinetic model, with subsequent validation of dosing regimens and also covers long-term safety outcome observations. This process and its morphine-specific outcome data will be discussed. Interestingly, the stepwise approach used to generate more knowledge on morphine may also serve as a model for any product development plan for an analgosedative (pharmacokinetics, dose finding, modelling, confirmatory studies, long-term safety) like propofol or paracetamol.
Both compounds have been introduced more recently in the clinical care of newborns. For propofol, we will describe an ongoing effort to describe the maturational pharmacokinetics and dynamics of short-lasting analgosedation with propofol (pharmacokinetics, dose finding, methods to assess pharmacodynamics) to perform the insure (intubation, surfactant administration, extubation) procedure in preterm neonates. We hereby applied a similar approach to the morphine maturational PK model. Interestingly, the currently available knowledge on opioids also covers data on long-term outcome following neonatal exposure. We will summarize this information to illustrate the feasibility to collect this kind of data and will discuss the currently available association studies on long-term outcome after paracetamol exposure to illustrate the need for similar studies on paracetamol (Figure 1).
Figure 1.
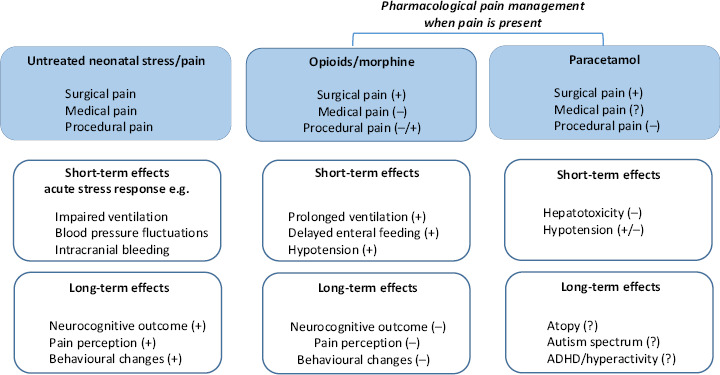
Schematic illustration of the available data on short-term and long-term outcome effects of untreated neonatal pain/stress, or exposure to opioids or paracetamol in (pre)term neonates.
Finally and irrespective of specific compounds, new covariates have been suggested to be of relevance on analgesia. This includes the impact of pharmacogenetics on the clinical pharmacology of analgesics. However, when these new covariates are considered in early infancy, this has to be added to the already existing covariates that are used and have been already validated to describe the impact of developmental pharmacology.
Towards evidence-based dosing for morphine in neonates
Morphine is likely the most commonly administered analgesic in neonates. Morphine is a narcotic analgesic that stimulates opioid receptors, both within and outside the CNS. This explains effects (sedation, analgesia, miosis) and side effects (bladder retention, paralytic ileus, respiratory depression). This obviously necessitates appropriate clinical monitoring (cardiorespiratory, sedation) during and following morphine exposure. It has been suggested that pain relief necessitates a morphine plasma level of 120 ng/ml, while adverse effects appear at levels > 300 ng/ml.[9] Despite the fact that morphine has been used for at least three decades, important progress in the maturational clinical pharmacology of morphine in neonates has only been made more recently.
As mentioned in the introduction, we want to describe the methodological approach taken to make this progress, as we do believe that this serves as an illustration for any product development plan in neonates, including for analgesics. Development of an evidence-based individual dosing regimen through population pharmacokinetic/pharmacodynamics (PK/PD) modelling is optimally achieved using a multistep approach.[10,11]
optimal study design based on preliminary data, or in the absence of such data, mechanism or physiology based modelling
development and internal validation of PK/PD model
external validation of the PK/PD model
prospective validation in a clinical study
a proposed individualized dosing regimen
Based on these consecutive steps,[11] such an approach has been followed for morphine in neonates.
Using pooling of earlier published pharmacokinetic observations on morphine disposition, model-based dosing regimens were developed and validated by internal validation procedures. The authors hereby suggested a loading dose (μg/kg) and a maintenance dose (μg/kg1.5 per hour), with an additional reduction (−50%) of the maintenance dose in neonates younger than 10 days because of lower metabolic clearance to morphine glucuronides. This approach was predicted to result in a narrow range of morphine and its metabolites (step a+b).[12] These simulations were subsequently validated on their pharmacokinetic predictability using additional, external data sets of other already published morphine observations in neonates (step c).[13] These pharmacokinetic models have subsequently been used to validate or reject the above suggested pharmacodynamic levels (120 and 300 ng/ml thresholds).[9] Using the model-based dosing regimens, an evidence-based dosing regimen was developed, which was prospectively validated in a double-blinded clinical controlled trial (step d). The authors hereby evaluated the PK/PD aspects of both paracetamol and morphine after non-cardiac surgery in neonates and young infants.[14] In this clinical study, patients postoperatively received a morphine loading dose followed by either paracetamol or morphine infusion. Morphine dosing in both the morphine treatment arm and rescue medication was given according to the individual dosing regimen. This included a 50–75% lower maintenance dose in the first 10 days of life than predicted in the PK-PD models. In case of pain, morphine rescue doses were given in both treatment arms.[14]
Interestingly, while morphine concentrations were comparable within the cohort, rescue morphine doses were more frequently administered to older infants, reflecting a deviation from the prediction. This may reflect differences in either active morphine metabolite disposition in infancy (maturational PK) or differences in opioid receptor activity (maturational PD). Morphine is converted into two glucuronide metabolites (PK, morphine-3-glucuronide and morphine-6-glucuronides), and these metabolites are subsequently eliminated by renal route. While morphine-3-glucuronide is an antagonist to the effects of morphine, morphine-6-glucuronide also has an analgesic and a respiratory depressant effect.[15] The available knowledge on maturational opioid receptor expression and activity (maturational PD) is very limited, but this aspect will be discussed in the section (Sec 12) on the relevance of pharmacogenetics on neonatal analgosedation. This newly acquired (PK and PD) knowledge should result in individualized dosing regimens, based on maturational PK and PD (step e).
Besides the clinical relevance for morphine itself, we would like to stress the relevance to extrapolate the approach taken to document the developmental pharmacology of morphine to other, more recently introduced analgosedatives. Short-acting analgosedatives emerged in neonatal intensive care, because there is a shift towards less invasive neonatal care – e.g. insure procedure instead of prolonged mechanical ventilation. We subsequently will illustrate the relevance to collect long-term safety data, using paracetamol as example (Figure 1), as well as the need to integrate new covariates in neonatal clinical pharmacology, illustrated by pharmacogenetics.
Propofol pharmacokinetic/pharmacodynamics studies of the ‘insure’ procedure in neonates
One of the short-acting analgosedatives more commonly administered to neonates is propofol, a highly lipophilic anaesthetic. In addition to its short-acting effect, propofol also has the advantage to maintain spontaneous breathing, making it suitable for the ‘insure’ procedure.[16] In line with many other drugs in neonates, its prescription is off-label, and the compound is on the latest European Medicines Agency priority list (2013) for neonatal drug research.
Clinical relevance of pharmacokinetic/pharmacodynamics research during the ‘insure’ procedure
Adequate sedation during endotracheal intubation is needed for patient comfort, to avoid trauma and to facilitate intubation. In contrast to pre-intubation sedation with primary outcome ‘successful intubation’[16] or ‘good intubation conditions’,[17] a combined outcome has to be taken into account in case of an insure procedure. This is because both successful intubation and subsequent extubation are prerequisites to classify the procedure as successful. The search for the most optimal propofol dose in neonates in this clinical context is challenging and needs a tailored PK/PD approach. PK studies of an intravenous propofol bolus up to 3 mg/kg in neonates (non-insure conditions) documented reduced propofol clearance compared to older children with extensive interindividual clearance variability within the neonatal population, in part explained by postmenstrual age and postnatal age.[18]
Pharmacodynamics data on propofol bolus for endotracheal intubation in neonates are still limited.[16,19–21] These observations confirm the clinically short-acting sedative effect compared to, e.g. an morphine + atropine + suxamethonium regimen,[16] but are conflicting concerning the presence (39–100%) or extent of blood pressure decrease as side effect,[19–21] and a fixed but variable dose (1–3 mg/kg) instead of dose-seeking study design was used.[16,19–21]
This is of relevance since changes in blood pressure can result in cerebral blood flow alterations. Significant hypotension (defined as mean arterial blood pressure <25 mmHg using oscillometric measurement) was documented by Welzing et al. investigating the use of 1 mg/kg propofol in preterm neonates undergoing insure. This resulted in early termination of their study, although this decision has been questioned, because definitions of hypotension in preterm neonates remain controversial.[20,22] Furthermore, it is questionable if the observed hypotension is a propofol-specific or rather a procedure-related effect. Lakkundi et al.[23] reported on the impact of the insure procedure itself on transitional hemodynamics in preterm neonates within 30 min after birth (‘prophylactic surfactant administration’) without premedication. A lower incidence of both hypotension and impaired circulation was observed compared to studies where mechanical ventilation was applied during transition. The authors speculate that altered ventilation strategies (i.e. avoidance of mechanical ventilation) contributed to this difference.[23,24]
Based on the above-mentioned propofol PK and PD data, a propofol dose-finding study searching for the optimal dose for (semi-)elective endotracheal intubation (mainly in insure cases) in neonates has recently been finalized (NCT01621373, Neoprop study) (Figure 2). Once this propofol dose-finding study has been reported, these dosing suggestions should subsequently be validated and randomized trials with other short-acting sedatives (e.g. remifentanil) for insure can be considered.[25]
Figure 2.
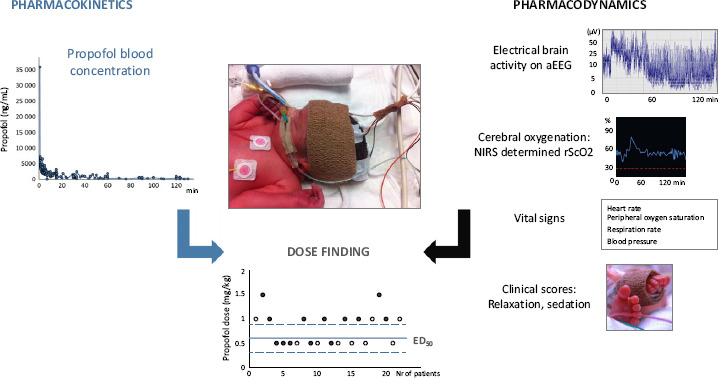
Propofol dose-finding approach, based on pharmacokinetic and pharmacodynamic data, during insure (intubation – surfactant administration – extubation) procedure. aEEG: amplitude-integrated electroencephalography, NIRS: near infrared spectroscopy, rScO2: regional cerebral oxygen saturation, ED50: effective dose in 50% of patients.
Tailored pharmacodynamic monitoring tools
To assess the clinical effects of propofol during insure in neonates, published scoring systems for relaxation and sedation are available.[26] Even if validated, these instruments have their limitations. Scoring systems reflecting short-term phenotypic effects will not inform us on the long-term drug effects, they remain indirect and (inter)subjective, and may not reflect all physiological or neurobiological effects occurring after drug administration. Consequently, we strongly suggest to consider additional monitoring techniques to assess drug effects on vital signs (cardiorespiratory monitor), cerebral oxygenation (NIRS) and electrical brain activity (aEEG) (Figure 2).
The interpretation of these physiological characteristics to assess the propofol-induced pharmacodynamics in an insure setting remains difficult, as this also includes the co-administration of surfactant and the intubation procedure itself. Evidence on the effect of surfactant administration on cerebral oxygenation is conflicting. Dorrepaal et al.[27] documented a short-lasting decrease in cerebral oxygenation, related to the surfactant volume administered. In contrast, Van den Berg et al.[28] found no important perturbation of cerebral oxygen delivery and extraction due to insure. Also the reported duration of brain activity depression (aEEG) after surfactant administration varies from 10 min up to 24 h.[28,29] Finally, even if physiological observations are collected, we should be aware that continuous registration of these data also include any minor or major fluctuations, reflecting the clinical setting of a sick newborn in need for intubation and surfactant, subsequently exposed to a drug-like propofol. To facilitate the evaluation of pharmacodynamics, we suggest to collect data over a sufficiently relevant period of time (e.g. up to 12 h after propofol) and also to include baseline, pre-exposure observations. Although useful, the duration of such pre-exposure observations is often limited in duration and difficult to collect because of the clinical setting. The lack of validated reference values may further limit the interpretation of the data.
The morphine long-term outcome studies and why we need similar data after paracetamol exposure
The morphine long-term outcome studies
A growing body of animal and laboratory evidence suggests a link between long-term side effects and exposure to opioids in early neonatal life (Figure 1).[30] These observations also initiated clinical research in the human setting. For neurocognitive outcome after neonatal exposure, there seems to be an age-dependent trend when we combine the results of the NEOPAIN and Rotterdam study.[31,32]
In a cohort of 137 preterm neonates, Grunau et al. documented an association of poorer cognition at the corrected ages of 8 and 18 months with a higher number of skin-breaking procedures independent of early illness severity, overall intravenous morphine exposure and exposure to postnatal steroids. When she focused on morphine exposure, an association with poorer motor development was documented at 8 months, but no longer at 18 months corrected age.[33]
For the NEOPAIN study, there is only a small pilot (n = 19) study looking at the effect of pre-emptive morphine administration at the age of 5–7 years. Based on observations in cases, the authors concluded that weight was lower (−4%), head circumference was smaller (−7%) and social behaviour problems were more common (effect size −0.83). Response latencies were slower (+40%) and task completion was lower (−27%) while IQ tests were similar.[34] The Rotterdam group also reported on the outcome of their larger cohort (n = 90) exposed to morphine (49/90) (placebo-controlled) at the age of 5 years and observed only some minor differences in specific subtests (visual analysis) of the IQ tests.[35] At the age of 8–9 years, there was no longer an association between morphine exposure and poorer neurocognitive outcome in the same cohort (89/90).[36] In contrast, Van den Bosch et al.[37] documented in a small subgroup of 19 of these former preterm neonates that the global brain volume (quantified through structural imaging analysis of MRI images) was significantly associated with prematurity (r = 0.76), the number of painful procedures (r = −0.67) and the extent of opioid exposure (r = −0.47) at the age of 10 years. Despite the differences in brain volume, morphine exposure itself had no effect on neurocognitive development and children scored ‘average’ by Dutch reference norms. In a cohort of 101 former preterm (<32 weeks) neonates, the number of skin-breaking procedures and parenting stress were related to greater internalizing behaviour in non-ventilated cases. In ventilated cases, morphine exposure was related to greater internalizing behaviour, but both covariates obviously reflect disease severity.[38] Based on the above-provided information, it seems that in contrast to specific short-term neurological outcomes, long-term neurodevelopmental outcome in human neonates is not affected by morphine.[39]
Why we need similar data after paracetamol exposure
As mentioned earlier, adequate management of pain in neonates is a major issue in contemporary neonatal care.[2] Paracetamol, N-acetyl-p-aminophenol, is the most commonly administered drug to treat mild-to-moderate pain in neonates and can be administered by different (oral, rectal, i.v.) routes. Although intravenous paracetamol is still off-label for specific subpopulations (e.g. preterm or extreme preterm neonates), these formulations are increasingly used in (pre)term neonates in an attempt to reduce or even avoid opioid administration.[40,41]
Similar to the available evidence of opioids, we should be aware of the differences in currently available evidence to support the use of paracetamol for procedural vs postoperative pain in neonates. In essence, the available data suggest a poor – if any – analgesic effect of paracetamol for procedural pain relief.[41] In contrast, there is published data on the morphine sparing effect of paracetamol in neonates and young infants following non-cardiac surgery or during stay in the NICU. Paracetamol has a very relevant opioid sparing effect (−66%) after major non-cardiac surgery in neonates. Following recruitment of 71 neonates and infants undergoing major non-cardiac surgery in a randomized placebo-controlled setting, co-administration of intravenous paracetamol resulted in a significant reduction (−66%) in morphine exposure.[14] More recently, a similar magnitude of effect has been suggested in preterm neonates (−54% cumulative morphine exposure) with medical conditions.[42] In a cohort of 108 preterm neonates (<32 weeks gestational age), paracetamol (loading dose 20 mg/kg, followed by 7.5 mg/kg q6h) was administered in early (<72 h) neonatal life and resulted in a reduction of 54% compared to a historical control group from the same unit and using the same pain assessment tool (neonatal infant acute pain assessment scale).[42] In contrast, there is only a very poor analgesic effect of paracetamol when used for procedural (e.g. heel lancing) pain relief.[41]
Undesired short-term side effects of paracetamol described in other populations mainly relate to hemodynamics or hepatotoxicity and have mainly been documented in (pre)term neonates > 32 weeks, with only limited data in extreme preterm neonates. The hemodynamic side effects of intravenous paracetamol in neonates are very modest and likely explained by a transient reduction in systemic vascular resistance.[43,44] Prospective data suggest good hepatic tolerance.[45] Besides these short-term outcome side effects, recent epidemiological data also show a possible link between (over)exposure to paracetamol in pregnancy and infancy and an emergence of different kind of pathologies throughout childhood (neurodevelopmental impairment, immune deviations). As these studies describe associations, causality remains questionable and certainly not yet proven. At least, further pharmacovigilance is warranted to unveil the complex, potential causal, association between atopy and paracetamol exposure.[46,47]
Epidemiological associations also suggest a link between paracetamol exposure and adverse effects (autism spectrum disorders, ADHD) in the developing CNS. Using a sibling-control study design, Brandlistuen et al.[48] explored the impact of prenatal paracetamol exposure and observed an impact on gross motor development, communication and behaviour (externalizing and internalizing). Bauer and Kriebel[47] described a synchronous rise in perinatal paracetamol exposure and autism spectrum disorder prevalence and hereby suggested an ecological link between both. Frisch and Simonsen confirmed the association between neonatal circumcision (linked to paracetamol exposure, 3347 cases) and autism spectrum disorder (4986/342 877 cases) with a Hazard Risk of 1.46 (95% CI 1.11–1.93).[49] A similar link has been suggested between perinatal paracetamol exposure and ADHD (13–37% increase).[50] Thompson et al.[51] also described an association between ADHD symptoms at the age of 7–11 years and paracetamol intake during pregnancy (in 49.8% of pregnancies) in a study population of 871 infants. Assessment was hereby based on validated questionnaires and similar associations were not documented for maternal intake of anti-acid drugs or antibiotics. Finally, in a Danish National Birth Cohort (n = 64 322 children), hyperkinetic disorders (hazard ratio 1.37) and ADHD (risk ratio 1.13) were associated with maternal prenatal paracetamol use.[52] These epidemiological data are supported by mechanism based observations (cyclo-oxygenase inhibition in the CNS).[47,53,54]
There is an exponential increase in the frequency of immune deviations in young children. Besides its central action, paracetamol also has a non-selective inhibitory action on peripheral cyclo-oxygenase 2 activity. This inhibition only relates to low arachidonic acid concentrations and explains the difference between ibuprofen and paracetamol in anti-inflammatory effects. In a recent meta-analysis, a link between paracetamol exposure and the risk (odd's ratio 1.2–1.3) to develop asthma in young infants has been suggested.[46] This may at least in part be explained by confounding by indication, i.e. antipyretic intake because of respiratory tract infections during infancy. Sordillo et al.[55] documented that adjustment for respiratory tract infections in early life substantially diminished, but not completely removed the association between paracetamol and early childhood asthma (paracetamol adjusted Odd Ratio 1.21 and 1.35 to 1.03).
Pharmacogenetics and neonatal analgosedation
Pharmacogenetics reflects the notion that a given (side) effect is not at random distributed and holds the promise of personalized pharmacotherapy. Pharmacogenetics contributes to the PK/PD variability of analgesics in adults.[56] This is likely also the case in young infants, but only when combined with more established covariates such as age, size or weight reflecting maturation (Figure 3), and after the expression or activity of the enzyme, transporter or target involved is already sufficiently (genotype/phenotype concordance) active to show a potential impact of a genetic variant. Data on genotype/phenotype concordance for phase I (cytochrome P450 enzyme 2D6, CYP2D6) and phase II (UDP-glucuronosyltransferase 2B7, UGT2B7) enzymes have been reported.[57,58]
Figure 3.
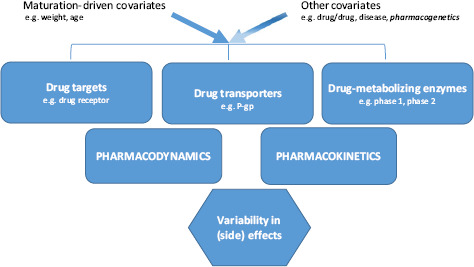
Developmental pharmacology in neonates is mainly driven by maturational covariates such as weight or age, with additional effects of other covariates, including pharmacogenetics.
In adults, phenotypic O-desmethyltramadol (M1) formation depends on CYP2D6 polymorphisms, quantified by the CYP2D6 activity score. In young infants, variability in M1 disposition has been linked to maturational changes (weight, age), comedication (e.g. drug–drug interactions), comorbidity (e.g. renal impairment) and CYP2D6 polymorphisms.[57] This is likely of clinical relevance as tramadol only has a weak μ-opioid receptor affinity, while M1 has a much higher affinity for this receptor. Similarly, Matic et al.[58] recently documented the impact of UGT2B7 polymorphisms on the morphine-3-glucuronide/morphine ratio after a single intravenous morphine (0.3 mg/kg) bolus in preterm neonates. Compared to the knowledge on the ontogeny of drug-metabolizing enzymes, data on the maturation of human drug transporter and target expression and activity are still much more limited. Mooij et al.[59] recently suggested exploring the presence of pharmacogenetic concordance in early infancy and other age cohorts to learn more about their ontogeny.
The impact of pharmacogenetics should not be limited to specific polymorphisms initially linked to specific PK/PD observations (phenotype/genotype concordance) in adults and subsequently explored in neonates. In addition to such an ‘adult-driven’ approach, there may also be age-specific concordances between genotype and phenotype that are only present in perinatal life. Pharmacogenetics hereby is a predicting covariate limited to periods during development in which genotype–phenotype concordance for a given outcome variable still exists and subsequently even may disappear (population-related genotype–phenotype concordance).[60] Likely, such analyses should also focus on links between polymorphisms and perinatal outcome variables, different from the outcome variables described in other populations. The recently reported observations on the impact of pharmacogenetics (Figure 3) on neonatal abstinence syndrome (NAS) and breastfeeding-related neonatal opioid CNS sedation were summarized to illustrate the potential clinical relevance of PG on drug transporters and targets in perinatal life.
In addition to polymorphisms in placental enzymes (e.g. CYP3A, CYP2D6), P-glycoprotein (P-gp) polymorphisms affect maternal, foetal and neonatal exposure and effects from opioids, including the incidence and extent of NAS.[61] Catechol-O-methyltransferase (COMT) and μ-opioid receptor (OPRM1) polymorphisms or epigenetic changes (extent of methylation) also affect the severity of NAS, the number of drugs needed to control NAS and the length of stay.[62,63] Similarly, the combination of a COMT/OPRM1 ‘high-risk’ genotype was associated with a higher risk to need rescue morphine in a cohort of ventilated preterm neonates.[64] In the specific setting of breastfeeding, maternal CYP2D6 ultrafast metabolizer status – especially when combined with UGT2B7 *2/*2 polymorphism results in higher exposure and an increased risk for CNS depression for both newborn and mother during codeine intake.[65] To further illustrate the complex interaction between different covariates, Sistonen et al.[66] documented that a genetic model combining the maternal risk genotypes in CYP2D6 and P-gp was significantly associated with CNS depression in both mothers (OR 2.74) and their infants (OR 2.68) during maternal codeine intake in the early postpartum period.
Very intriguingly, polymorphisms of the same OPRM1 receptor have also been linked to infant/mother attachment in primate studies, suggesting a role for OPRM1 polymorphisms in the ontogeny and expression of attachment.[67,68] Similarly, COMT polymorphisms are also associated with relevant outcome variables like early-onset antisocial behaviour in children with attention-deficit/hyperactivity disorder, foetal growth restriction, the morphometry of the corpus callosum after very preterm (<33 weeks gestational age) birth and the risk for spontaneous preterm delivery in African Americans.[69–72] These kinds of observations may guide clinical researchers to develop focused pharmacovigilance studies for compounds that interact with these receptor or drug targets.
A similar approach can be considered if we want to explore long-term neurodevelopmental outcome after COX-inhibitor exposure in perinatal life. The inducible form of cyclo-oxygenase 2 (COX2) gene is polymorphic, and the C allele variant (associated with reduced COX2 activity) was associated with poorer cognitive outcome in a cohort of 207 former preterm neonates at the age of 2–5 years.[53] This suggests that the phenotypic cyclo-oxygenase activity may be linked to neurocognitive outcome and may hereby provide a pathophysiological link between perinatal paracetamol exposure and long-term neurobehavioural outcome.[53,54]
Discussion: on clinical practice and research directions, in search of ‘a new deal’
Effective pain management remains a very relevant indicator of the care provided to (pre)term neonates. New techniques (insure), compounds (paracetamol, propofol) and knowledge on covariates (pharmacogenetics) force caregivers to reconsider the clinical and research aspects of effective and safe pain management in neonates.[3] Our goals were to illustrate how to study analgesics in neonates, using the morphine studies (morphine PK/PD and long-term safety outcome data) as a framework that can also be applied to study other analgosedatives (propofol and paracetamol). Finally, new covariates such as pharmacogenetics might further improve neonatal pain management, but clearly need to be integrated with other well-established covariates such as age or weight. The output of key papers on neonatal analgosedation is summarized in Table 1.
Table 1.
Summary of output of key papers on neonatal analgosedation
| Krekels et al.[13] |
| Morphine paediatric dosing algorithms corrected for pharmacokinetic differences alone yield effective doses that prevent overdosing for neonates younger than 10 days. Neonates and infants with a postnatal age beyond 10 days still required rescue medication. This warrants additional pharmacokinetic and pharmacodynamic studies to further optimize the dosing algorithm for these patients |
| Ceelie et al.[14] |
| Among infants – including neonates – undergoing major non-cardiac surgery, postoperative use of intermittent intravenous paracetamol compared with continuous morphine results in a lower cumulative morphine dose over 48 h (maintenance dose was reduced by 75%) |
| Anand et al.[31] and Simons et al.[32] |
| Pre-emptive morphine infusions do not reduce the frequency of severe intraventricular haemorrhage, periventricular leucomalacia or death in ventilated preterm neonates. Moreover, intermittent boluses of open-label morphine were associated with an increased rate of this composite outcome. The morphine doses used in this study decrease clinical signs of pain, but can cause significant adverse effects in ventilated preterm neonates. These adverse effects include prolonged respiratory support, hypotension and delayed attainment of full enteral feeding.[5–8] |
| de Graaf et al.[36] |
| Using the cohort initially described by Simons et al.,[32] this study demonstrated that continuous morphine infusion (10 μg/kg per hour) during the neonatal period does not harm general functioning at 8–9 years. There may even be a positive influence on executive functions at 8–9 years |
| Härma et al.[42] |
| Introduction of intravenous paracetamol was associated with a reduced need for morphine in a cohort of preterm neonates (<32 weeks gestational age) |
| Matic et al.[64] |
| Re-analysing earlier data sets ([32]), the need for rescue morphine in preterm neonates was associated with specific pharmacogenetic polymorphisms (μ-opioid receptor, cathechol O-methyltransferase gene variant) |
Although we hereby stress the use of analgosedatives in neonates, we should be aware that adequate pain management is not limited to drugs only. Effective and safe pharmacotherapy can only be achieved if integrated in a structured approach on pain management. Such an approach should be based (i) on preventive strategies, including the decrease of environmental stress and number of painful procedures, (ii) be driven by systematic assessment of pain based on validated assessment tools and (iii) followed by treatment through titrated administration of the best fitted intervention (pharmacological or non-pharmacological) with subsequent reassessment.[2,73]
Preventive strategies and non-pharmacological interventions (sucrose, swaddling) stress the fact that not only the procedure matters, but also the way we perform painful procedures will affect the pain response.[74] The relevance of this integrated prevention–assessment–treatment concept is further supported by the observation that behavioural outcome in former preterm infants was associated with the level of both developmental care and pain management.[75] Validated and robust assessment of pain in neonates remains difficult, as it is obvious that there is a difference between pain expression and nociception. Unfortunately, all currently available pain assessment tools are driven by expression of distress or pain.[76]
As reflected in the section on the insure procedure and the propofol studies, there are also shifts in our clinical practices and subsequent shifts in pharmacotherapy. Although the use of less invasive surfactant administration techniques (insure) and avoidance of mechanical ventilation has been introduced, the percentage of VLBW infants who receive analgesia and/or sedation has remained unchanged in Germany in recent years (German Neonatal network, 2003–2010), but with shifts towards the use of novel drugs such as paracetamol, sufentanil or propofol.[77]
In conclusion, we illustrated the feasibility and relevance of pharmacological studies on analgesics, including long-term safety and pharmacogenetics, and provided some suggestions on how to further improve the knowledge on safe and effective pharmacotherapy for analgosedation in neonates (product development plan, PK/PD of propofol in neonates). Based on these data, it is clear that there are still important issues on the clinical pharmacology of analgosedatives in neonates that deserve further evaluation, especially for the newer compounds that are used in neonates off-label and without sufficient validation. As reflected in the section on long-term outcomes, this is even the case for the use of paracetamol.
Declarations
Acknowledgements
The clinical research of K Allegaert was supported by the Fund for Scientific Research, Flanders (fundamental clinical investigatorship 1800214N) and the research activity is further facilitated by the agency for innovation by Science and Technology in Flanders (IWT) through the SAFEPEDRUG project (IWT/SBO 130033). J van den Anker is supported by NIH (K24DA027992, R01HD060543, U54HD071601, T32HD087969).
References
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med 1987; 317: 1321–1329. [DOI] [PubMed] [Google Scholar]
- Hall RW, Anand KJ. Pain management in newborns. Clin Perinatol 2014; 41: 895–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellieni C, Buonocore G. Improve the struggle against babies’ pain. Lancet 2011; 377: 1315–1316. [DOI] [PubMed] [Google Scholar]
- Bellu R et al. Opioids for neonates receiving mechanical ventilation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2010; 95: F241–F251. [DOI] [PubMed] [Google Scholar]
- Menon G et al. Morphine analgesia and gastrointestinal morbidity in preterm infants: secondary results from the NEOPAIN trial. Arch Dis Child Fetal Neonatal Ed 2008; 93: F362–F367. [DOI] [PubMed] [Google Scholar]
- Bhandari V et al. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics 2005; 116: 352–359. [DOI] [PubMed] [Google Scholar]
- Hall RW et al. Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics 2005; 115: 1351–1359. [DOI] [PubMed] [Google Scholar]
- Simons SH et al. Morphine in ventilated neonates: its effects on arterial blood pressure. Arch Dis Child Fetal Neonatal Ed 2006; 91: F46–F51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkola KT et al. Clinical pharmacokinetics and pharmacodynamics of opioid analgesics in infants and children. Clin Pharmacokinet 1995; 28: 385–404. [DOI] [PubMed] [Google Scholar]
- De Cock RF et al. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol 2011; 67(Suppl 1): 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admiraal R et al. Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child 2014; 99: 267–272. [DOI] [PubMed] [Google Scholar]
- Knibbe CA et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet 2009; 48: 371–385. [DOI] [PubMed] [Google Scholar]
- Krekels EH et al. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet 2014; 53: 553–563. [DOI] [PubMed] [Google Scholar]
- Ceelie I et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA 2013; 309: 149–154. [DOI] [PubMed] [Google Scholar]
- Taylor J et al. The relationship between age an morphine infusion rate in children. Paediatr Anaesth 2013; 23: 40–44. [DOI] [PubMed] [Google Scholar]
- Ghanta S et al. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics 2007; 119: e1248–e1255. [DOI] [PubMed] [Google Scholar]
- Norman E et al. Rapid sequence induction is superior to morphine for intubation of preterm infants: a randomized controlled trial. J Pediatr 2011; 159: 893–899. [DOI] [PubMed] [Google Scholar]
- Allegaert K et al. Inter-individual variability in propofol pharmacokinetics in preterm and term neonates. Br J Anaesth 2007; 99: 864–870. [DOI] [PubMed] [Google Scholar]
- Simons SH et al. Clinical evaluation of propofol as sedative for endotracheal intubation in neonates. Acta Paediatr 2013; 102: e487–e492. [DOI] [PubMed] [Google Scholar]
- Welzing L et al. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr Anaesth 2010; 20: 605–611. [DOI] [PubMed] [Google Scholar]
- Nauta M et al. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm infants. Paediatr Anaesth 2011; 21: 711–712. [DOI] [PubMed] [Google Scholar]
- Lerman J et al. Neonatal tracheal intubation: an imbroglio unresolved. Paediatr Anaesth 2010; 20: 585–590. [DOI] [PubMed] [Google Scholar]
- Lakkundi A et al. Transitional hemodynamics in preterm infants with a respiratory management strategy directed at avoidance of mechanical ventilation. Early Hum Dev 2014; 90: 409–412. [DOI] [PubMed] [Google Scholar]
- Aguar M et al. Administration of surfactant using less invasive techniques as a part of a non-aggressive paradigm towards preterm infants. Early Hum Dev 2014; 90(Suppl 2): S57–S59. [DOI] [PubMed] [Google Scholar]
- van den Anker JN. Timing of dose-finding studies: before or after completion of a randomized clinical trial? Pediatrics 2007; 120: 691–692. [DOI] [PubMed] [Google Scholar]
- Naulaers G et al. Use of methohexital for elective intubation in neonates. Arch Dis Child Fetal Neonatal Ed 1997; 77: F61–F64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrepaal CA et al. Cerebral hemodynamics and oxygenation in preterm infants after low-vs. high-dose surfactant replacement therapy. Biol Neonate 1993; 64: 193–200. [DOI] [PubMed] [Google Scholar]
- van den Berg E et al. Effect of the “InSurE” procedure on cerebral oxygenation and electrical brain activity of the preterm infant. Arch Dis Child Fetal Neonatal Ed 2010; 95: F53–F58. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Westas L et al. Cerebroelectrical depression following surfactant treatment in preterm neonates. Pediatrics 1992; 89: 643–647. [PubMed] [Google Scholar]
- Attarian S et al. The neurodevelopmental impact of neonatal morphine administration. Brain Sci 2014; 4: 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004; 363: 1673–1682. [DOI] [PubMed] [Google Scholar]
- Simons SH et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003; 290: 2419–2427. [DOI] [PubMed] [Google Scholar]
- Grunau RE et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 2009; 143: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA et al. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol 2012; 34: 47–55. [DOI] [PubMed] [Google Scholar]
- de Graaf J et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain 2011; 152: 1391–1397. [DOI] [PubMed] [Google Scholar]
- de Graaf J et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age ? Pain 2013; 154: 449–458. [DOI] [PubMed] [Google Scholar]
- van den Bosch GE et al. Prematurity, opioid exposure and neonatal pain: do they affect the developing brain? Neonatology 2015; 108: 8–15. [DOI] [PubMed] [Google Scholar]
- Ranger M et al. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur J Pain 2014; 18: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans J et al. Neonatal morphine in extremely and very preterm neonates: its effect on the developing brain – a review. J Matern Fetal Neonatal Med 2015; 28: 222–228. [DOI] [PubMed] [Google Scholar]
- van den Anker JN, Allegaert K. Treating pain in preterm infants: moving from opioids to acetaminophen. J Pediatr 2016; 168: 13–15. [DOI] [PubMed] [Google Scholar]
- Cuzzolin L et al. Paracetamol (acetaminophen) efficacy and safety in the newborn. Curr Drug Metab 2013; 14: 178–185. [PubMed] [Google Scholar]
- Härmä A et al. Intravenous paracetamol decreases requirements of morphine in very preterm infants. J Pediatr 2016; 168: 36–40. [DOI] [PubMed] [Google Scholar]
- Allegaert K, Naulaers G. Haemodynamics of intravenous paracetamol in neonates. Eur J Clin Pharmacol 2010; 66: 855–858. [DOI] [PubMed] [Google Scholar]
- Chiam E et al. The haemodynamic effects of intravenous paracetamol (acetaminophen) in healthy volunteers: a double-blinded, randomized, triple crossover trial. Br J Clin Pharmacol 2016; 81: 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegaert K et al. Hepatic tolerance of repeated intravenous paracetamol administration in neonates. Paediatr Anaesth 2008; 18: 388–392. [DOI] [PubMed] [Google Scholar]
- Dick S et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open 2014; 4: e006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AZ, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environ Health 2013; 12: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandlistuen RE et al. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol 2013; 42: 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M, Simonsen J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year old boys: national cohort study in Denmark. J R Soc Med 2015; 108: 266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser JA, Allan GM. Acetaminophen in pregnancy and future risk of ADHD in offspring. Can Fam Physician 2014; 60: 642. [PMC free article] [PubMed] [Google Scholar]
- Thompson JM et al. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS One 2014; 9: e108210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z et al. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr 2014; 168: 313–320. [DOI] [PubMed] [Google Scholar]
- Harding DR et al. Cognitive outcome and cyclo-oxygenase-2 gene (-765 G/C) variation in the preterm infant. Arch Dis Child Fetal Neonatal Ed 2007; 92: F108–F112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H et al. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol Sci 2014; 138: 139–147. [DOI] [PubMed] [Google Scholar]
- Sordillo JE et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol 2015; 135: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg R et al. Pharmacogenetics of analgesic drugs. Br J Pain 2013; 7: 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegaert K et al. Postmenstrual age and CYP2D6 polymorphisms determine tramadol O-demethylation in critically ill neonates and infants. Pediatr Res 2008; 63: 674–679. [DOI] [PubMed] [Google Scholar]
- Matic M et al. Effect of UGT2B7 -900G>A (-842G>A; rs 7438135) on morphine glucuronidation in preterm newborns: results from a pilot cohort. Pharmacogenomics 2014; 15: 1589–1597. [DOI] [PubMed] [Google Scholar]
- Mooij MG et al. Development of human membrane transporters: drug disposition and pharmacogenetics. Clin Pharmacokinet 2016; 55: 507–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegaert K, van den Anker J. Neonatal drug therapy: the first frontier of therapeutics for children. Clin Pharmacol Ther 2015; 98: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T et al. Genetic determinants of fetal opiate exposure and risk of neonatal abstinence syndrome: knowledge deficits and prospects for future research. Clin Pharmacol Ther 2015; 98: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman EM et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 2013; 309: 1821–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman EM et al. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J Pediatr 2014; 165: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic M et al. Rescue morphine in mechanically ventilated newborns associated with combined OPRM1 and COMT genotype. Pharmacogenomics 2014; 15: 1287–1295. [DOI] [PubMed] [Google Scholar]
- Madadi P et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin Pharmacol Ther 2009; 85: 31–35. [DOI] [PubMed] [Google Scholar]
- Sistonen J et al. Prediction of codeine toxicity in infants and their mothers using a novel combination of maternal genetic markers. Clin Pharmacol Ther 2012; 91: 692–699. [DOI] [PubMed] [Google Scholar]
- Curley JP. The mu-opioid receptor and the evolution of mother-infant attachment: theoretical comment on Higham et al.. Behav Neurosci 2011;125:273–278. [DOI] [PubMed] [Google Scholar]
- Barr CS et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA 2008; 105: 5277–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A et al. Catechol O-methyltransferase gene variant and birth weight predict early-onset antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2005; 62: 1275–1278. [DOI] [PubMed] [Google Scholar]
- Sata F et al. Functional maternal catechol-O-methyltransferase polymorphism and fetal growth restriction. Pharmacogenet Genomics 2006; 16: 775–781. [DOI] [PubMed] [Google Scholar]
- Dutt A et al. COMT gene polymorphism and corpus callosum morphometry in preterm born adults. NeuroImage 2011; 54: 148–153. [DOI] [PubMed] [Google Scholar]
- Thota C et al. A single-nucleotide polymorphism in the fetal catechol-O-methyltransferase gene is associated with spontaneous preterm birth in African Americans. Reprod Sci 2012; 19: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk M, Tibboel D. Update on pain assessment in sick neonates and infants. Pediatr Clin North Am 2012; 59: 1167–1181. [DOI] [PubMed] [Google Scholar]
- Cignacco E et al. The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. Eur J Pain 2007; 11: 139–152. [DOI] [PubMed] [Google Scholar]
- Montirosso R et al. Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics 2012; 129: e1129–e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berde CB et al. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics 2012; 129: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler K et al. Use of analgesic and sedative drugs in VLBW infants in German NICUs from 2003–2010. Eur J Pediatr 2013; 172: 1633–1639. [DOI] [PubMed] [Google Scholar]


