Abstract
Medial artery calcification develops in diabetes, chronic kidney disease, and as part of the aging process. It is associated with increased morbidity and mortality in vascular patients. Bone morphogenetic proteins (BMPs) have previously been implicated in the initiation and progression of vascular calcification. We thus evaluated whether DMH1, a highly specific BMP inhibitor, could attenuate vascular calcification in vitro and in an organ culture model of medial calcification.
Methods
Confluent human aortic smooth muscle cells (HASMCs) were cultured in calcification medium containing 3.0 mM inorganic phosphate (Pi) for 7 days with or without DMH1. Medial calcification was assessed using an aortic organ culture model. Calcification was visualized by Alizarin Red S staining, and calcium concentration was assessed by an o-cresolphthalein complexone calcium assay. Osteogenic cell and vascular smooth muscle cell (SMC) markers were determined by Western blot, qRT-PCR and immunohistochemistry staining.
Results
DMH1 reduced Pi-induced calcium deposition in human SMCs. It also antagonized human recombinant BMP2-induced calcium accumulation. Western blot further revealed that DMH1 was able to block Pi-mediated up-regulation of osteoblast markers including osterix and alkaline phosphatase (ALP), and down-regulation of SMC markers such as smooth muscle cell markers myosin heavy chain (SM-MHC) and SM22α, as well as P-Smad1/5/8, suggesting that DMH1 may regulate SMC osteogenic differentiation via the BMP/Smad1/5/8 signal pathway. Finally, utilizing an ex vivo aortic ring organ culture model, we observed that DMH1 has an ability to reduce Pi-induced aortic medial calcification.
Conclusions
The selective BMP inhibitor DMH1 can inhibit calcium accumulation in vascular smooth muscle cells and arterial segments exposed to elevated phosphate levels. Such small molecules may have clinical utility in reducing medial artery calcification in our vascular patient population.
Introduction
Arterial calcification is associated with increased morbidity and mortality in patients with cardiovascular diseases.1, 2 It predicts amputation in a manner that is independent of the ankle-brachial index (ABI) and atherosclerosis risk factors.3 Higher scores are seen in patients with foot ulcers even after adjusting for the amount of occlusive disease.4 And lesion calcification is associated with higher restenosis rates and decreased patency after endovascular superficial femoral artery intervention in patients with diabetes.5 Arterial calcification occurs in two basic forms that correlate with its location within the arterial wall. It is seen in the intima in association with atherosclerotic plaques, and in the media where it involves elastin and develops through mechanisms associated the metabolic disturbances.6–8
Previously, calcification of arteries was thought to be a passive process that involved precipitation of calcium phosphate crystals onto plaque. It is now known, however, to be a tightly regulated process controlled by an array of stimulators and inhibitors. 9, 10. Recent experimental findings suggest that a variety of cellular mechanisms involving microRNAs 11, endoplasmic reticulum (ER) stress 12, the inflammasome 13, and autophagy 14 are also involved in its regulation. For these reasons, it is now considered to be an independent biological process that contributes to poor outcomes in patients with cardiovascular diseases.
Bone morphogenetic proteins (BMPs) provide critical signals for determining cell fate and function, and they are implicated in the development of vascular calcification.15, 16 BMP2 is enriched in calcified arteries, and elevation of smooth muscle cell-specific BMP2 accelerates vascular calcification in hyperlipidemic mice, suggesting that BMP2 plays a crucial role in the pathogenesis of vascular smooth muscle cell calcification.17, 18 These studies suggest that the BMP signaling pathway is a promising target for interrupting pathologic arterial calcification.
Recently, a highly selective second generation of BMP inhibitor has been developed that specifically target BMP signaling but not VEGF and angiogenesis. 19 Dorsomorphin homologue 1 (DMH1), is one such highly selective molecule that has been shown to reduce BMP signaling in cell culture and in vitro models. 19 The effect of DMH1 on smooth muscle cells grown under calcifying conditions and on whole arterial segments, however, has not been studied. In the present study, we investigated the effect of DMH1, a highly selective small molecule inhibitor of the BMP type 1 receptor, on vascular SMC in vitro and medial artery calcification in an organ culture model system.
Material and methods
Reagents
Human recombinant BMP2 protein was obtained from Prospec tech (Ness-ziona, Israel). DMH1 was purchased from the Vanderbilt University Chemical Synthesis Core (Nashville, TN). For controls, the vehicle dimethyl sulfoxide (DMSO) was used in similar amounts and concentrations as treated groups. All chemicals were purchased from Sigma Aldrich (St. Louis, MO).
Vascular smooth muscle cell culture
Human aortic smooth muscle cells (HASMCs) were obtained from ATCC (Manassas, VA). HASMCs were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% FBS at 37°C in a humidified, 5% CO2 incubator. To induce calcification, confluent HASMCs were cultured in a calcification medium containing 3.0 mM inorganic phosphate (Pi) for 7 days.
Aortic ring organ culture
Animal experiments were performed in accordance with guidelines of Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center. Thoracic aortas were harvested from Sprague Dawley rats (250 g), gently cleared of surrounding tissues, and cut into 0.5 cm-long segments. Arterial calcification was induced as previously described 20. Briefly, aortic segments were incubated in calcification culture medium (DMEM with 100 unit/ml penicillin and 100μg/ml streptomycin) containing 3.8 mM Pi in the absence or presence of BMP inhibitor DMH1 for 7 days at 37°C in a humidified 5% CO2 incubator.
Calcium Assay
For HASMCs culture, at the end of the experiment, cells were washed with PBS and directly decalcified in 300 μL 0.6 N HCl for 24 h. For aortic ring organ culture, after 7 days, the segments were dried and weighed, and then decalcified in 200 μL 0.6 N HCl for 24 h. Calcium content was measured using the o-Cresolphthalein Complexone method 21. Results were expressed as μg/mg protein (HASMCs) and μg/mg dry tissue (organ culture).
RNA Isolation and qRT-PCR
Total RNA was isolated from HASMCs by using RNeasy mini kit (Qiagen, USA). The first strand cDNA was synthesized from 1μg total RNA using a iScript cDNA Synthesis kit (Bio-Rad Laboratories, USA), following the manufacturer’s protocols. The Qpcr was performed using an AB 7500 Fast Real-time PCR System (Applied Biosystem, USA). The primers for BMP4 (Forward: 5′-GGACTACATGCGGGATCTTTAC-3′; Reverse: 5′-AGCAGAGTTTTCACTGGTCC-3′); BMP2 (Forward: 5′-ATAATCCACTCTGCTGACTTTC-3′; Reverse: 5′-TCAGCAATGTCTGGTTCTTATC-3′); ALK2 (Forward: 5′-ACCAAGAACGCCTCAATCC-3′; Reverse: 5′-CAGAGCCACTTCCTGATGTAC-3′); ALK3 (Forward: 5′-AAAACCACTTCCAGCCCTAC-3′; Reverse: 5′-ACACAACCTCACGCATATCTTC-3′); GAPDH (Forward: 5′-ACATCAAGAAGGTGGTGAAG-3′; Reverse: 5′-TGACAAAGTGGTCGTTGAG-3′). Primers were synthesized by IDT DNA Technologies (Iowa, US). The relative expression level of target genes was calculated by the 2−ΔΔCt method. The values were presented as fold-change compare to control group.
Western blot
Western blot was performed as previously described 22. Briefly, lysates were prepared in RIPA buffer with protease inhibitor cocktail (Roche Applied Science, Germany). The concentrations of total proteins were measured by BCA protein assay (ThermoFisher Scientific, USA). Total lysates were loaded on SDS-PAGE, electrotransfered into nitrocellulose membrane. The primary antibodies against SM-MHC (Biomedical technology, BT-562), SM22α (Abcam, ab14106), Osterix (Abcam, ab94744), Alkaline Phosphatase (Santa Cruz, sc-137213), p-Smad 1/5/8 (Cell signaling, 9511s), Smad (Santa Cruz, sc-7153) and horseradish peroxidase-coupled secondary antibodies were used. The signals were visualized using ECL reagents (ThermoFisher Scientific, USA). The density of protein expression was quantified by using Image J.
Histology and Immunohistochemistry
After organ culture for 7 days, thoracic aorta segments were washed with PBS and fixed with 10% neutral buffer formalin. The specimens were then embedded in paraffin, and 5μm cross-sections were cut. The sections were deparaffinized and H&E staining was performed. Arterial medial calcification was visualized using Alizarin Red S staining as previously described 23. For immunohistochemistry, the sections were deparaffinized, followed by treatment with citrate buffer for antigen retrieval and 3% H2O2. The sections were blocked with Dako serum-free blocking solution (Dako North America, USA) and incubated with primary antibody for overnight at 4°C. The primary antibodies included SM22α (Abcam, ab14106), Osterix (Abcam, ab94744). Subsequently, the sections were incubated with biotinylated secondary antibodies for 30 min at room temperature. Avidin-biotinylated enzyme complex (Vector Laboratories, USA) and a diaminobenzidine substrate chromogen system (Dako North America, USA) were used for detection. Sections were counterstained with hematoxylin. The images were captured by Olympus DP260.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6.0 software. Differences were analyzed by Student’s t-test. P values less than 0.05 were considered significant.
Results
DMH1 suppresses phosphate-induced calcification in vascular smooth muscle cells
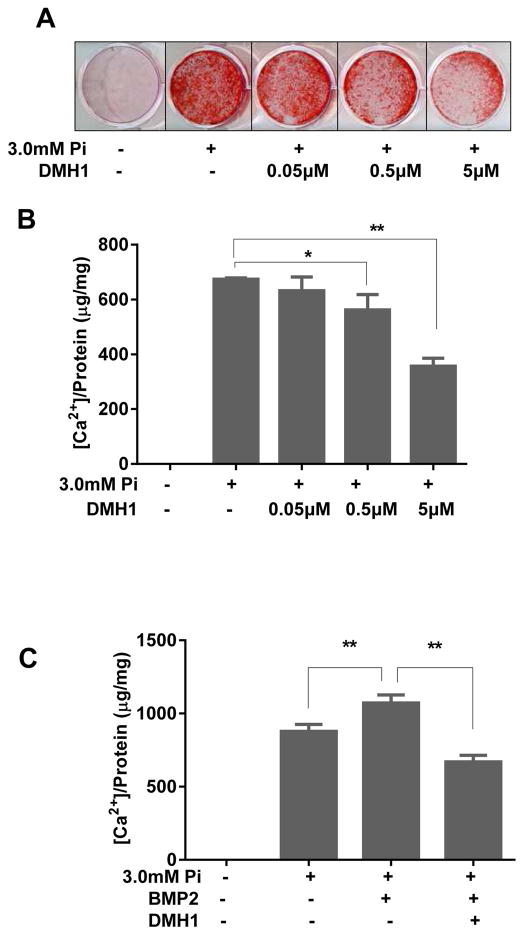
To study the effects of BMP receptor inhibition on vascular SMC calcification in vitro, confluent human aortic smooth muscle cells (HASMCs) were induced to calcify by culturing in medium containing 3.0 mM inorganic phosphate (Pi) for 7 days. As shown in Figure 1A, treatment with 3.0 mM Pi resulted in extensive Alizarin Red S staining compared with normal phosphate levels, indicating that inorganic phosphate induces calcification. However, in the presence of DMH1, the effect of calcification was suppressed, and 5 μM DMH1 markedly reduced Pi-induced calcium deposition. Quantification of calcium accumulation by use of the o-cresolphthalein complexone method showed that DMH1 dose-dependently attenuated Pi-induced calcium accumulation in HASMCs, and 5 μM DMH1 caused a 50% reduction of calcium accumulation. (Figure 1B)
Figure 1. DMH1 suppresses phosphate-induced SMC calcification.
Confluent HASMCs grown in 3.0 mM Pi were treated for 7 days without or with increasing doses of DMH1. (A) Alizarin red histological staining showing effects of DMH1 on Pi-induced calcium deposition. (B) Effects of DMH1 on calcium levels in Pi treated SMCs as measured using the o-cresolphthalein complexone method. (C) Effects of DMH1 on calcium levels in SMCs cultured with Pi and BMP2. Values are mean ± SD (n=3). *P<0.05, **P<0.01 vs. Pi but without DMH1.
To investigate whether DMH1 could reduce calcification in the setting of increased BMP2 protein levels, recombinant BMP2 was added to calcification culture medium. As shown in Figure 1C, the addition of BMP2 further elevated Pi-induced calcium accumulation, but DMH1 blocked it. These data suggest that blocking BMP signaling can suppress Pi-induced SMC calcification.
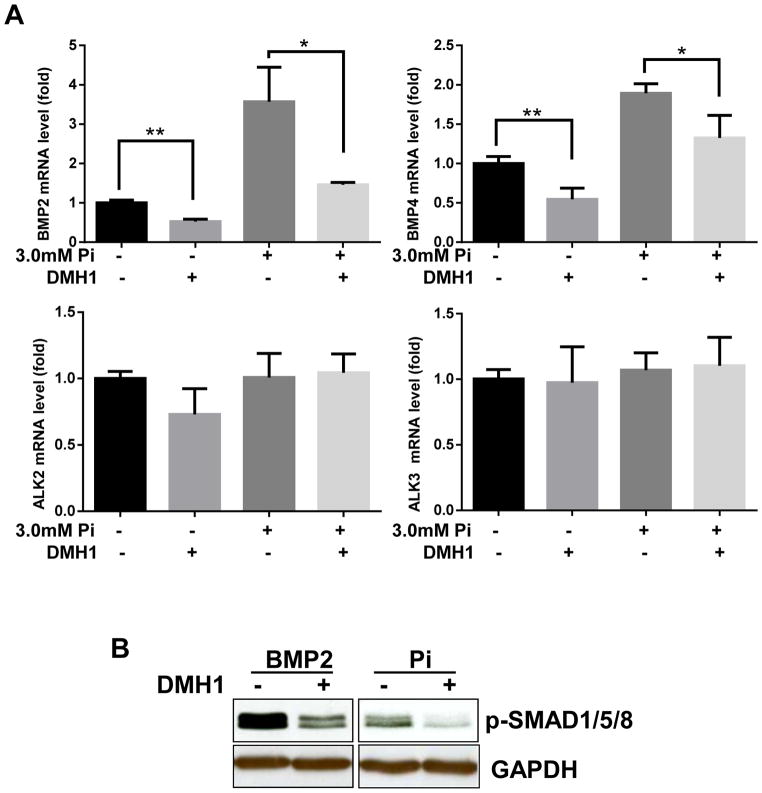
We next assessed the effects of Pi on the expression of BMPs and their receptors using qPCR. In HASMCs, the mRNA levels of BMP2 and BMP4 were significantly increased after treatment with 3 mM Pi compared to control, whereas levels of the BMP receptors 1A (ALK3) and activin A receptor type1 (ALK2) were not altered. (Figure 2A) We also found that DMH1 could decrease the mRNA expression of BMP2 and BMP4, but not of ALK2 or ALK3.(Figure 2A) To investigate whether DMH1 is able to suppress BMP-receptor activation, its down-stream signaling molecule Smad1/5/8 was assessed by western blot. HASMCs were treated with either recombinant BMP2 or Pi in the presence or absence of DMH1 for 24 h. DMH1 markedly decreased p-Smad1/5/8 levels in BMP2-treated HASMCs (Figure 2B, left panel). DMH1 also decreased p-Smad1/5/8 expression in Pi-treated HASMCs (Figure 2B, right panel).
Figure 2. DMH1 suppresses BMP expression and signaling.
(A) mRNA levels of BMP2, BMP4, ALK2 and ALK3 were analyzed by qPCR. Data represent the fold-change relative to expression of transcript in control cells calculated using the 2−ΔΔCt method. (B) Western blot showing effects of DMH1 on p-Smad1/5/8 levels in cells treated with recombinant BMP2 or Pi. Values are mean ± SD (n=3). **P<0.01.vs. control.
DMH1 reduces phosphate-induced osteogenic differentiation
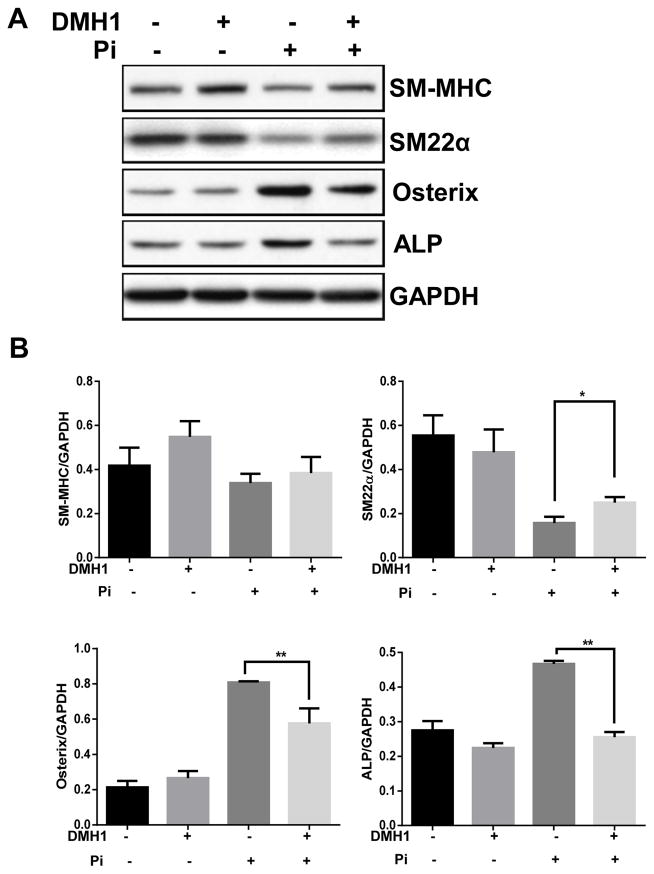
Vascular smooth muscle cells are able to undergo osteogenic transformation, a change in phenotype to a more bone-like cell, when exposed to elevated phosphate levels.24 Such cellular changes are thought to play a critical role in calcification. To determine whether DMH1 affects osteogenic differentiation in vascular smooth muscle cells, confluent HASMCs were cultured in high Pi (3mM) calcification medium for 7 days, and osteoblast markers were examined by western blot. As shown in Figure 3A and 3B, the osteoblast markers Osterix and alkaline phosphatase (ALP) were significantly induced in Pi-treated SMCs compared with control. However, this effect was reduced by treatment with DMH1. In contrast, SM-MHC and SM22-α were decreased by Pi and partially restored by DMH1. These data suggest that blocking BMP receptor activation can inhibit calcification by influencing osteogenic transformation of SMCs.
Figure 3. DMH1 reduces Pi induced osteogenic differentiation of HASMCs.
(A) Western blot showing effect of DMH1 on osteoblast markers Osterix and ALP, and SMC markers SM-MHC and SM22α. (B) Quantitative analysis of western blot data. Values are mean ± SD (n=3). **P<0.01. vs. Pi but without DMH1
DMH1 attenuates medial artery calcification
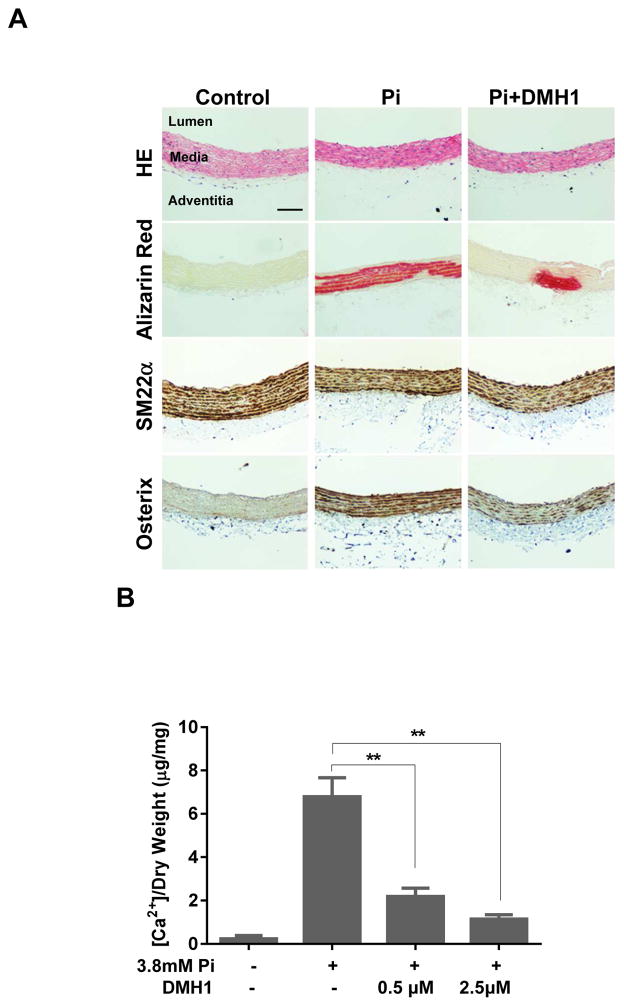
To study the effects of DMH1 in whole artery segments, we used an organ culture system that results in mineral deposition within the media. In this model, calcium hydroxyapatite is deposited on medial elastin in a manner analogous to the calcification seen in patients with diabetes and chronic renal failure. This method has been validated recently as a model of medial calcification that is sensitive to age and diabetes similar to humans.25 Harvested thoracic aortas from Sprague-Dawley rats were cultured in calcification medium containing 3.8 mM Pi with or without indicated concentrations of DMH1 for 7 days. Alizarin Red S staining was used to visualize the extent of calcium deposition. As shown in Figure 4A, in untreated control aortas, calcification is almost undetectable. After treatment with 3.8 mM phosphate for 7 days, aortas showed extensive calcification within the smooth muscle medial layer as revealed by Alizarin Red S staining. However, this effect was largely diminished by the treatment with DMH1. Accordingly, the osteogenic marker osterix was increased by Pi, whereas it was suppressed by DMH1. Consistent with the histological findings, treatment with DMH1 also significantly reduced calcium concentration in the Pi-treated aortas as determined by calcium assay (Figure 4B).
Figure 4. DMH1 reduces medial calcification in an organ culture model of arterial calcification.
Thoracic aortas were harvested form Sprague Dawley rats, cut into 0.5 cm rings, then incubated in calcification medium with or without DMH1 for 7 days. (A) Chemical (H&E and Alizarin Red S) and immunohistochemical staining for markers of SMC (SM22α) and osteogenic differentiation (osterix). (Scale bar: 100μm). (B) DMH1 significantly suppressed Pi-induced calcium accumulation in aortic rings. Values are mean ± SD (n=4). **P<0.01 vs. Pi alone.
Discussion
In the present study, we demonstrate that DMH1, a highly selective BMP inhibitor, reduces SMC calcification and osteogenic transformation. We further show that it inhibits activation of downstream signaling molecules in vitro, and elastin mineralization in an organ culture model of medial artery calcification. Our data suggest that inhibition of BMP signaling using synthetic, small molecule inhibitors may be a useful strategy to reduce medial artery calcification in patients.
Arterial calcification has emerged as a critical and independent predictor of morbidity and mortality in patients with cardiovascular disease 9, 26. It is particularly important in patients with diabetes and chronic kidney disease 1, 7. A driving mechanism in patients with end-stage kidney disease is the associated disturbances of phosphate metabolism 1, 27. To date, no effective therapeutic strategies have become available.
Vascular smooth muscle cells exposed to elevated phosphate levels develop bone-cell-like characteristics including the ability to secrete matrix vesicles 28. They also demonstrate increased expression of osteogenic markers including the transcription factors BMP2, Cbfa1/Runx2, Msx2, alkaline phosphatase (ALP), and osteopontin (OPN), while markers of the SMC phenotype including SM-MHC and SM22α are decreased 28. Considerable data support the concept that BMP signaling is involved in the vascular calcification that develops in CKD 29, 30. Pi increases BMP-2 expression at both the mRNA and protein levels 31. SMC treatment with BMP2 further enhances Pi-induced osteo-chondrogenic modulation and SMC calcification, and Pit-1 plays a crucial role in BMP-2-regulated SMC calcification 16. In the present series of studies, we show that BMP2 and BMP4 are increased by the addition of Pi to culture medium. DMH1 treatment suppressed Pi-induced up-regulation of BMP2 and BMP4 mRNA. The mechanisms behind this effect warrant further investigation. We further demonstrate that addition of the small molecule BMP inhibitor can suppress SMC dedifferentiation while reducing activation of the BMP receptor pathway. Our data appear to suggest that there are effects of Pi on SMC de-differentiation that are independent BMP signaling because DMH1 failed to completely restore SMC markers. We believe this is because Pi triggers osteogenic transformation of SMC through multiple mechanisms although it is also possible that Pi-induced osteogenic transformation occurs earlier than the decrease in SMC markers expression. Although the mechanisms of BMP-induced calcification are not fully understood, our present findings suggest that DMH1 attenuates SMC and aortic medial calcification via inhibition of BMP-receptor/Smad1/5/8 signaling.
Several strategies for manipulating BMP signaling have been established. These include the use of endogenous antagonists such as noggin and chordin, soluble co-receptors, and neutralizing antibodies 32–35. Dorsomorphin was identified as the first inhibitor of BMP signaling and has been demonstrated to selectively inhibit the BMP type I receptors ALK2, ALK3 and ALK6. It can block BMP-induced Smad1/5/8 phosphorylation and attenuate BMP4 and BMP6-induced osteoblastic differentiation 36. LDN-193189, a dorsomorphin analog, potently suppresses BMP4-induced Smad1/5/8 activation and inhibits transcriptional activity of the BMP type I receptors ALK2 and ALK3. It is also able to inhibit vascular calcification and atherosclerosis through its negative effects on BMP2 signaling in LDLR−/− mice fed with a high-fat diet. 18 LDN-193189 prevents endothelial dysfunction and SMC osteogenic differentiation in CKD mice. 29 Utilizing LDN-193189, Malhotra et al demonstrated that BMP signaling plays critical roles in the development of vascular calcification in MGP-deficient mice 37. However, recent studies have revealed that Dorsomorphin has significant off-target effects including inhibition of the vascular endothelial growth factor (VEGF) type-2 receptor (Flk1/KDR) and angiogenesis 19. For these reasons, use of a more selective BMP inhibitor may provide benefit and offer greater clinical applicability.
A highly selective second generation of BMP inhibitors has been developed recently based on structure-activity relationship (SAR) study of dorsomorphin analogues on live zebrafish embryos. These newer molecules specifically target BMP signaling but not VEGF and angiogenesis 19. DMH1 has been shown to promote cardiac differentiation in mouse embryonic stem cells 38 and facilitate neurogenesis of human-induced pluripotent stem cells39, and inhibit ovarian cancer cell growth40. In this study, we found that DMH1 significantly inhibited Pi and BMP2-induced calcium deposition in HASMCs as revealed by Alizarin Red S staining and calcium assay. It also blocked Pi-induced osteogenic transformation of SMC from a contractile to an osteoblast phenotype. Moreover, we showed that DMH1 treatment was able to reduce Pi-induced p-SMAD1/5/8, a key downstream signaling complex involved in BMP signaling, and medial calcification in arterial ring segments. Accumulating data suggest that the aorta organ culture model has become an ideal platform to understand basic mechanisms of medial artery calcification because it maintains arterial architecture.20, 25, 27 In this study, we show that DMH1 is also capable of reducing elastin calcification in arterial specimens. When we performed immunohistochemical staining for osterix in our aortic specimens, we saw diffusely increased expression that did not correspond to areas of calcification. This is likely because SMC transformation precedes calcium deposition in this model. We did not see intimal hyperplasia in our aortic ring specimens likely because no serum is added to the medium used in this model. Future investigations involving alternative blocking strategies such as siRNA or work with BMP or receptor specific knockout cells may allow for more precise identification of the relevant calcification-related pathways. Additional in vivo studies using specific BMP receptor deficient mice may provide added impetus for future clinical investigations.
In conclusion, we demonstrate that the BMP inhibitor DMH1 attenuates SMC calcification and osteogenic differentiation, and it prevents mineralization in an aortic organ culture model. These findings add to the growing evidence suggesting a prominent role for BMPs in arterial calcification and present the possibility of improving outcomes by reducing calcification in our vascular patient population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. European heart journal. 2014;35(23):1515–25. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. Journal of the American College of Cardiology. 2006;47(5):921–9. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 3.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. Journal of the American College of Cardiology. 2008;51(20):1967–74. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman RJ, Bian A, Shintani A, Stein CM. Association of foot ulcer with tibial artery calcification is independent of peripheral occlusive disease in type 2 diabetes. Diabetes research and clinical practice. 2013;99(3):281–6. doi: 10.1016/j.diabres.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakken AM, Palchik E, Hart JP, Rhodes JM, Saad WE, Davies MG. Impact of diabetes mellitus on outcomes of superficial femoral artery endoluminal interventions. J Vasc Surg. 2007;46(5):946–58. doi: 10.1016/j.jvs.2007.06.047. discussion 58. [DOI] [PubMed] [Google Scholar]
- 6.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 7.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Mechanisms of medial arterial calcification in diabetes. Current pharmaceutical design. 2014;20(37):5870–83. doi: 10.2174/1381612820666140212210451. [DOI] [PubMed] [Google Scholar]
- 8.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nature reviews Cardiology. 2010;7(9):528–36. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leopold JA. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends in cardiovascular medicine. 2015;25(4):267–74. doi: 10.1016/j.tcm.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goettsch C, Hutcheson JD, Aikawa E. MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circulation research. 2013;112(7):1073–84. doi: 10.1161/CIRCRESAHA.113.300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan X, Zhou Y, Teng X, Tang C, Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochemical and biophysical research communications. 2009;387(4):694–9. doi: 10.1016/j.bbrc.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 13.Wen C, Yang X, Yan Z, Zhao M, Yue X, Cheng X, et al. Nalp3 inflammasome is activated and required for vascular smooth muscle cell calcification. International journal of cardiology. 2013;168(3):2242–7. doi: 10.1016/j.ijcard.2013.01.211. [DOI] [PubMed] [Google Scholar]
- 14.Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney international. 2013;83(6):1042–51. doi: 10.1038/ki.2012.482. [DOI] [PubMed] [Google Scholar]
- 15.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & growth factor reviews. 2005;16(3):251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199(2):271–7. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circulation research. 2010;107(4):485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, et al. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(3):613–22. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS chemical biology. 2010;5(2):245–53. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(7):1510–6. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 21.Connerty HV, Briggs AR. Determination of serum calcium by means of orthocresolphthalein complexone. American journal of clinical pathology. 1966;45(3):290–6. doi: 10.1093/ajcp/45.3.290. [DOI] [PubMed] [Google Scholar]
- 22.Cai Y, Nagel DJ, Zhou Q, Cygnar KD, Zhao H, Li F, et al. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circulation research. 2015;116(7):1120–32. doi: 10.1161/CIRCRESAHA.116.304408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha CM, Park S, Choi YK, Jeong JY, Oh CJ, Bae KH, et al. Activation of Nrf2 by dimethyl fumarate improves vascular calcification. Vascular pharmacology. 2014;63(1):29–36. doi: 10.1016/j.vph.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circulation research. 2000;87(7):E10–7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 25.Akiyoshi T, Ota H, Iijima K, Son BK, Kahyo T, Setou M, et al. A novel organ culture model of aorta for vascular calcification. Atherosclerosis. 2015;244:51–8. doi: 10.1016/j.atherosclerosis.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(7):1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 27.Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. Journal of the American Society of Nephrology : JASN. 2013;24(2):179–89. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 28.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney international. 2009;75(9):890–7. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kajimoto H, Kai H, Aoki H, Uchiwa H, Aoki Y, Yasuoka S, et al. BMP type I receptor inhibition attenuates endothelial dysfunction in mice with chronic kidney disease. Kidney international. 2015;87(1):128–36. doi: 10.1038/ki.2014.223. [DOI] [PubMed] [Google Scholar]
- 30.Lomashvili KA, Wang X, Wallin R, O’Neill WC. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. The Journal of biological chemistry. 2011;286(33):28715–22. doi: 10.1074/jbc.M111.251462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tada H, Nemoto E, Foster BL, Somerman MJ, Shimauchi H. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone. 2011;48(6):1409–16. doi: 10.1016/j.bone.2011.03.675. [DOI] [PubMed] [Google Scholar]
- 32.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, et al. Structural basis of BMP signaling inhibition by Noggin, a novel twelve-membered cystine knot protein. The Journal of bone and joint surgery American volume. 2003;85-A(Suppl 3):52–8. doi: 10.2106/00004623-200300003-00010. [DOI] [PubMed] [Google Scholar]
- 33.Dudaric L, Cvek SZ, Cvijanovic O, Santic V, Maric I, Crncevic-Orlic Z, et al. Expression of the BMP-2, -4 and -7 and their antagonists gremlin, chordin, noggin and follistatin during ectopic osteogenesis. Collegium antropologicum. 2013;37(4):1291–8. [PubMed] [Google Scholar]
- 34.Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127(4):821–30. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocrine reviews. 2003;24(2):218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 36.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nature chemical biology. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra R, Burke MF, Martyn T, Shakartzi HR, Thayer TE, O’Rourke C, et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PloS one. 2015;10(1):e0117098. doi: 10.1371/journal.pone.0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ao A, Hao J, Hopkins CR, Hong CC. DMH1, a novel BMP small molecule inhibitor, increases cardiomyocyte progenitors and promotes cardiac differentiation in mouse embryonic stem cells. PloS one. 2012;7(7):e41627. doi: 10.1371/journal.pone.0041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neely MD, Litt MJ, Tidball AM, Li GG, Aboud AA, Hopkins CR, et al. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and SOX1 expression during neural induction. ACS chemical neuroscience. 2012;3(6):482–91. doi: 10.1021/cn300029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hover LD, Young CD, Bhola NE, Wilson AJ, Khabele D, Hong CC, et al. Small molecule inhibitor of the bone morphogenetic protein pathway DMH1 reduces ovarian cancer cell growth. Cancer letters. 2015;368(1):79–87. doi: 10.1016/j.canlet.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]