Abstract
During the immune response to pathogens and autoantigens, CD8 T cells are exposed to numerous inflammatory agents including the cytokine IL-12. Previous studies have focused on how IL-12 regulates T cell functions when present during or after the activation of the T cell receptor (TCR). However, recent studies suggest that prior exposure to IL-12 also alters the TCR responsiveness of murine T cells. Whether similar phenomena occur in human activated CD8 T cells and the mechanisms mediating these effects remain unexplored. In this study, we observed that pretreatment of human activated CD8 T cells with IL-12 results in increased cytokine mRNA and protein production following subsequent TCR challenge. The potentiation of TCR-mediated cytokine release was transient and required low doses of IL-12 for at least 24 hours. Mechanistically, prior exposure to IL-12 increased the TCR induced activation of select MAPKs and AKT without altering the activation of more proximal TCR signaling molecules, suggesting that the IL-12 mediated changes in TCR signaling are responsible for the increased production of cytokines. Our data suggest that prior treatment with IL-12 potentiates human CD8 T cell responses at sites of infection and inflammation, expanding our understanding of the function of this clinically important cytokine.
Keywords: Human T cells, CD8 T cells, IL-12, TCR signaling
1. Introduction
Activated effector or memory CD8 T cells are constantly bombarded with different inflammatory signals that regulate their function. One of these signals is the proinflammatory cytokine IL-12 [1]. IL-12 is quickly produced by antigen presenting cells following induction with products from various microorganisms, including bacteria, fungi, intracellular parasites, double stranded RNA, bacterial DNA, and CpG-containing oligonucleotides [2, 3]. IL-12 is also clinically important, where it is found at sites of inflammation in a number of human disorders [2, 4-7]. T cell subsets vary in their responsive to IL-12 based on the expression of the IL-12 receptor. Resting CD8 T cells have undetectable levels of the IL-12 receptor [8]. However, the receptor is upregulated in activated CD8 T cells, allowing them to respond to IL-12 signals [2, 3, 8].
Many groups have examined the effects of IL-12 in altering T cell responses. The presence of IL-12 during the priming of CD4 T cells promotes the differentiation of naïve CD4 T cells into type 1 T helper (Th1) cells [2, 3, 9]. Also, the presence of IL-12 during priming of CD8 T cells in secondary lymphoid organs has been shown to promote strong effector functions and memory development [10]. Finally, IL-12 enhances TCR-induced proliferation, IFN-γ production and cytotoxicity of T cells 14-16[1-3, 11-13]. Although highly informative, this work has largely examined the effects of IL-12 if it's present during or following TCR activation. However, there are common situations during the immune response to infection where T cells will encounter IL-12 after TCR stimulation. Upon infection, naïve CD8 T cells will be TCR-stimulated in secondary lymphoid organs via antigen-bound MHC class I present on activated antigen presenting cells. After expansion and differentiation, these primed CD8 T cells will migrate out of the secondary lymphoid organs, where they will be exposed to IL-12 in the absence of TCR stimulation as they travel through the blood or lymph. These activated CD8 T cells will then be further activated through the TCR in infected and inflamed tissue. Thus, during the normal response to infection, previously activated CD8 T cells will be stimulated via IL-12 before subsequently receiving further TCR stimulation at sites of infection. Unfortunately, how prior exposure to IL-12 alters activated CD8 T cell responses to subsequent TCR activation is not well understood.
Several recent studies have shown the prior exposure to inflammatory signals enhances subsequent T cell responsiveness. To this end, prior exposure of human T cells to IL-7, IL-15, or the TLR5 ligand flagellin increases the responsiveness of these cells to TCR stimulation [14, 15]. Similarly, we have recently found that short exposure of human CD4 T cells to IL-12 enhances the TCR-induced production of a range of cytokines [16]. In addition, exposure to pathogen-induced inflammation alters the responsiveness of murine effector/memory CD8 T cells and secondary effector CD4 T cells to subsequent activation through the TCR [17-19]. From the multiple cytokines which compose the inflammatory environment, this response was attributed to IL-12 and type I interferons [17, 18]. Collectively, these studies demonstrate that cytokines and/or inflammatory signals alter the function of T cells if they are present before further TCR stimulation, suggesting a new role for IL-12 in the regulation of T cell responses. Although these studies are informative, there are still key questions unanswered. First, whether similar biology will occur in human activated CD8 T cells remains to be addressed. Furthermore, the precise mechanisms by which IL-12 signals could alter T cell function if they are present before TCR activation remains to be fully elucidated. Addressing these knowledge gaps will increase our understanding of the basic properties of human CD8 T cells, which is crucial for clinical applications due to an increasing awareness that human and mice have subtly different mechanisms driving immune function that markedly alter clinical outcomes [20-22]. Furthermore, since IL-12 is currently been tested as a therapy for infections and human cancer; a better understanding of how IL-12 regulates human T cell functions could provide insights for improving the current uses of IL-12 therapeutics.
2. Materials and Methods
2.1 Study approval
Blood donors at the DeGowin Blood Center at the University of Iowa Hospitals and Clinics provided written informed consent for cells not used for transfusion to be used for research. The consent process and document was approved by the Institutional Review Board (IRB) for the University of Iowa. The peripheral blood mononuclear cells were removed using leukocyte reduction systems (LRS) and the LRS cones were then provided to our laboratory. Our laboratory was not provided with information regarding the gender, age, or health status of these individual donors. However, we requested that donors were 18-55 years of age and that they were not taking any anti-viral or anti-bacterial medications at the time of donation. All human subject studies were in compliance with the Declaration of Helsinki.
2.2 Isolation of human activated CD8 T cells and cytokine pretreatments
Peripheral blood mononuclear cells (PMBCs) were obtained from anonymous donors as previously described [23]. The CD8 T cells were isolated by negative selection using an enrichment kit for CD8 T cells (Stem Cell Technologies). These isolations yielded cells that were consistently >98% positive for CD8 T cells (data not shown). CD8 T cells were activated for 5 days with magnetic Dynabeads (Invitrogen) bound with anti-CD3 (OKT3, BioLegend) and anti-CD28 (CD28.2, BioLegend) antibodies in the presence of 100 U/mL IL-2. Following activation and expansion, the stimulatory anti-CD3/anti-CD28 beads and IL-2 were removed, and the cells were rested for 24 h in complete RPMI (RPMI 1640 supplemented with 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM l-glutamine (Gibco)) before further experimentation. Activated CD8 T cells were split into different flasks and exposed to different doses of recombinant cytokines (R&D Systems) or exposed to IL-12 for different times. Media was not changed in between the times. Cells were then washed three times in complete RPMI to remove the recombinant cytokines.
2.3 Cytokine production measured by ELISA
After the pretreatments, activated CD8 T cells were stimulated with different doses of plate-bound anti-CD3 for 24 h. For the rest experiments, pretreated cells were rested for various times before anti-CD3 stimulation. Media was not changed in between the resting times. The cell culture supernatants were then collected, and the amount of cytokines present in the cultures was measured in triplicate using standard TMB-based ELISA. The absorbance at 490 nm was measured using the Epoch plate reader (Biotek). The cut-off sensitivity of the assays was 0.7 ng/mL for IFN-γ and 0.2 ng/mL for TNF-α.
2.4 Flow cytometry for IL-12R β1 and IL-12R β2 expression
Activated CD8 T cells pretreated with IL-12 or media alone were washed with FACS buffer (PBS, 10% FBS, and 0.05% sodium azide) and stained with anti-IL-12 Rβ2 (R&D Systems), anti-IL-12Rβ1 (BD Biosciences), and isotype controls for 30 minutes on ice. Cells were washed, resuspended in FACS buffer, and collected using the Accuri C6 flow cytometer (BD Biosciences). Live lymphocyte gate was set based on forward and side scatter and then 50,000 events were collected inside the live gate. The median fluoresce intensities (Median FI) of the expression of the IL-12 receptor subunits were then determined using the BD Accuri C6 software.
2.5 Cytokine production measured by intracellular staining
After pretreatments, activated CD8 T cells were resuspended in complete RPMI and stimulated with or without 1 μg/mL of plate-bound anti-CD3 for 6 and 18 h. Brefeldin A (BioLegend) was added during the last 5 h of the stimulation. Cells were then washed with FACS buffer, fixed, and permeabilized. Intracellular cytokines were then examined in IL-12 pretreated cells and control cells stimulated with or without anti-CD3 by staining with FITC anti-IFN-γ (4S.B3), APC anti-TNF-α (Mab11), or isotype controls per the manufacturer's instructions (BioLegend). Cells were collected using the Accuri C6 flow cytometer (BD Biosciences). Live lymphocyte gate was set based on forward and side scatter and then 50,000 events were collected inside the live gate (Supplementary Fig. 2A). To examine the intracellular levels of the cytokines, quadrants were set so the baseline cytokine production of non-TCR stimulated cells was less than 1% (Supplementary Fig. 2B). Similar results were found when quadrants were set using isotype controls therefore baseline cytokine production was set simply using non–TCR stimulated cells (Supplementary Fig. 2C). The frequencies and median fluorescence intensities of cytokine expression were then determined using the BD Accuri C6 software.
2.6 Quantitative Real-time PCR
Total RNA was isolated with an RNeasy Kit (Qiagen) from IL-12 pretreated and untreated cells at 6 and 18 hours following stimulation with 1 μg/mL of plate-bound anti-CD3 (anti-CD3). Single-strand cDNA was then synthesized from 1μg of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time RT-PCR was performed on an Applied Biosystems Model 7000 using SYBR Green PCR master mix (Applied Biosystems) and primers according to the manufacturer's instructions. The expression of mRNA was normalized to that of mRNA encoding β-actin and quantification of fold induction of treated vs untreated was analyzed by the 2−ΔΔCT method [24]. The primers used for these studies were IFN-γ forward (TCGGTAACTGACTTGAATGTCCA), IFN-γ reverse (TCGCTTCCCTGTTTTAGCTGC), TNF-α forward (GGAGAAGGGTGACCGACTCA) and TNF-α reverse (CTGCCCAGACTCGGCAA).
2.7 Immunoblotting
To examine STAT4 levels and phosphorylation, activated CD8 T cells were treated with IL-12 (50 ng/mL) for different times and then lysed with the addition of two-fold excess of hot lysis buffer (20mM Tris pH8.0, 2mM EDTA, 2mM Na3VO4, 20mM DTT, 2% SDS and 20% glycerol). Lysates were then heated to 95 °C for 4 min and sonicated to reduced viscosity. Immunoblotting was then performed. Cellular lysates were loaded onto a 4-15% precast Criterion polyacrylamide gel (Biorad) and proteins were separated using SDS-PAGE. Membranes were then blocked using 50% (v/v) SEA BLOCK buffer (Thermo Scientific) diluted in PBS. Membranes were then incubated with two primary antibodies of different species overnight at 4°C; One towards the protein of interest and another one for glyceraldehyde 3-phosphate dehydrogenase GAPDH (used as a loading control). Then membranes were washed 2X using PBST (PBS pH 7.2 and 0.1% Tween 20) and incubated with DyLight 680- and DyLight 800-conjugated secondary antibodies for 45 minutes at room temperature. Subsequently, the membranes were washed once with PBST containing 0.05% SDS and twice with PBST alone. The immunoblots were visualized using the LICOR Odyssey Infrared Imager. The intensity of the immunoblotting bands was determined using the Licor Odyssey v3.0 software. The protein intensity was normalized to the expression of GAPDH using the following formulas:
-
(1)
Normalized GAPDH = Raw intensity of GAPDH of time point ÷ raw intensity of lowest GAPDH value.
-
(2)
Normalized intensity at time point = Raw intensity of phospho-protein at time point ÷ Normalized GAPDH value at time point.
-
(3)
% of the control maximum = (Normalized intensity at time point ÷ Normalized intensity of maximum control value) × 100%
The normalized values were then averaged and expressed as the mean ± s.e.m. as indicated in each figure legend. The loading controls shown for each representative figure correspond to at least one of the blots shown. We do not show loading controls for all blots, simply because of space issues. However, for the quantification each blot was quantified with its respective control.
To examine TCR signaling molecules, activated CD8 T cells were treated with IL-12 (50 ng/mL) for 24 h and washed. After a short incubation on ice, 3 μg/mL of anti-CD3 was added, and the cells were incubated on ice for 30 more minutes. Then, the cells were warmed at 37°C for 10 minutes and stimulated with 25 μg/mL of mouse anti-IgG antibody (Southern Biotech) for various times. This method results in a minimal, yet detectable, level of signaling compared to cells not incubated with anti-CD3 antibodies. Samples were lysed with the addition of two-fold excess of hot lysis buffer, heated to 95 °C for 4 min, and sonicated to reduced viscosity. Then, immunoblotting was performed as described above. Normalization of the phospho-protein intensity to the GAPDH intensity was conducted as described above. The total protein expression of signaling molecules was calculated as follows: the average of the protein intensities of the different time points =protein intensity at each time point ÷ total number of time points.
2.8 Antibodies
Antibodies used for immunoblotting, cell-surface, and intracellular stains were purchased from commercial sources. The anti-LAT Y191 (C305) was from Millipore. The anti-LCK pY505 (4/Lck (pY505), anti-SLP-76 pY128 (J141-668.36.58), and anti-IL-12Rβ1 (114) were from BD Biosciences. The anti-PLC-γ1 pY783 (polyclonal), anti-p38 pT180/Y182 (3D7), anti-p38 (polyclonal), anti-AKT pT308 (244F9), anti-ZAP-70 pY319 (polyclonal), anti-SRC pY416 (polyclonal), anti-STAT4 (2A2), anti-JNK T183/Y185 (polyclonal), anti-MKK3/MKK6 pS189/S207 (D8E9), and anti-STAT4 pY693 (D2E4) antibodies were purchased from Cell Signaling Technologies. The anti-ERK1/2 pTpY185/187 (polyclonal) was from Invitrogen. The anti-SOS1 (polyclonal) and anti-CD3-ζ (6B10.2) from Santa Cruz Biotechnology. The anti-GAPDH was from Meridian Life Science. The DyLight 800 and DyLight 680 labeled secondary antibodies were obtained from Thermo Scientific. The FITC anti-IFN-γ (4S.B3), APC anti-TNF-α (Mab11), anti-CD3 (OKT3), anti-CD28 (CD28.2), anti-CD2 (RPA-2.10), anti-CD49d (9F10), anti-CD11a (HI111) from BioLegend. Anti-IL-12 Rβ2 (305719) was purchased from R&D Systems. The anti-mouse IgG was from Southern Biotech.
2.9 Statistical analysis
Statistical analysis between the groups was assessed using GraphPad Prism. Specific tests for statistical significance are indicated in the figure legends. Differences were considered significant when p values were below 0.05.
3. Results
3.1 Conditioning human activated CD8 T cells with IL-12 leads to increased production of IFN-γ and TNF-α upon TCR stimulation
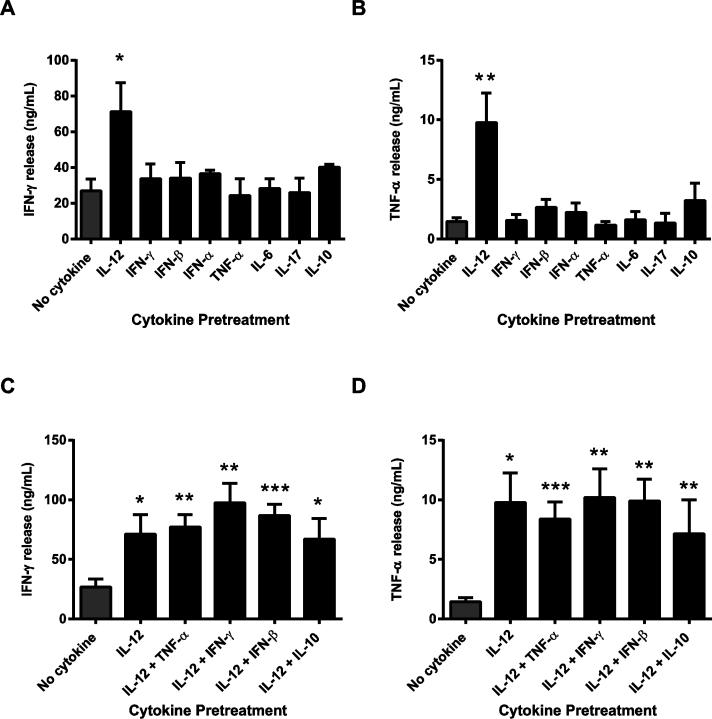
Previous studies have shown that prior exposure to inflammatory cytokines alters the responses of murine CD4 and CD8 T cells to TCR stimulation [17, 19]. However, whether similar effects occur in human activated CD8 T cells, and which cytokines are important in mediating these effects, remain to be examined. To address this knowledge gap, human activated CD8 T cells were exposed to different recombinant cytokines or media alone, washed to remove the cytokines, and stimulated through the TCR complex. Cytokine production was then used as a read out for T cell activation. No other additional costimulatory signals were provided in order to determine the effects of the inflammatory cytokine on secondary TCR-induced T cell activation alone. We found that prior exposure to IFN-γ, IFN-β, IFN-α, TNF-α, IL-6, IL-17, or IL-10 did not alter the TCR-induced production of IFN-γ and TNF-α in comparison to cells treated in media alone (Fig. 1A and B). In contrast, pretreatment with IL-12 significantly increased the TCR-induced production of IFN-γ and TNF-α compared to control cells (Fig. 1A and B). Importantly, this effect was not due to IL-12-driven cytokine production, since IL-12 pretreated cells had undetectable levels of cytokine release in the absence of TCR stimulation (Supplementary Fig.1A and B). Since the cytokine production assay required 24 hours of stimulation, it was possible that the IL-12 mediated increase in cytokine production was a consequence of increased proliferation/survival of the activated CD8 T cells. To control for this, cell viability was determined in human activated CD8 T cells untreated or pretreated with IL-12 or other cytokines before and after 24 h TCR stimulation. Similar viable cell numbers were found in IL-12 pretreated and controls cells before and after TCR stimulation (Supplementary Fig.1C), suggesting that the IL-12 effects on cytokine production were not due to altered cell viability between the groups.
Figure 1. Human activated CD8 T cells pretreated with IL-12 have increased IFN-γ and TNF-α production following TCR stimulation.
(A-D) Human activated CD8 T cells were exposed to media alone (no cytokine) or to different recombinant cytokines (50 ng/mL) for 24 h, washed, and then stimulated with 1 μg/mL of plate bound anti-CD3 antibodies for 24 h. IFN-γ and TNF-α production were determined in the cell culture supernatants by ELISA. Graphs are shown as the mean ±SEM of values from three to seven different donors. Data were statistically compared to no cytokine cells using a two-tail Student's t test. *p<0.05; **p<0.01; ***p<0.001; no symbol=not significant.
During a normal in vivo immune response, human activated CD8 T cells are exposed to a combination of cytokines. Therefore, we then examined whether the IL-12 potentiation of cytokine production was altered when other cytokines were present during pretreatment. As expected, IL-12 pretreatment significantly increased the TCR-induced production of IFN-γ and TNF-α in comparison to control cells. However, pretreatment with IL-12 in combination with other cytokines did not have any agonistic or antagonistic effects on cytokine production (Fig. 1C and D). Collectively, our data suggest that human activated CD8 T cells pretreated with IL-12 have increased cytokine production following subsequent TCR challenge. These effects appear to be specific for IL-12, since pretreatment with other pro- and anti-inflammatory cytokines did not alter human CD8 T cell responses to TCR stimulation.
3.2 Characterizing the IL-12 mediated priming of cytokine production
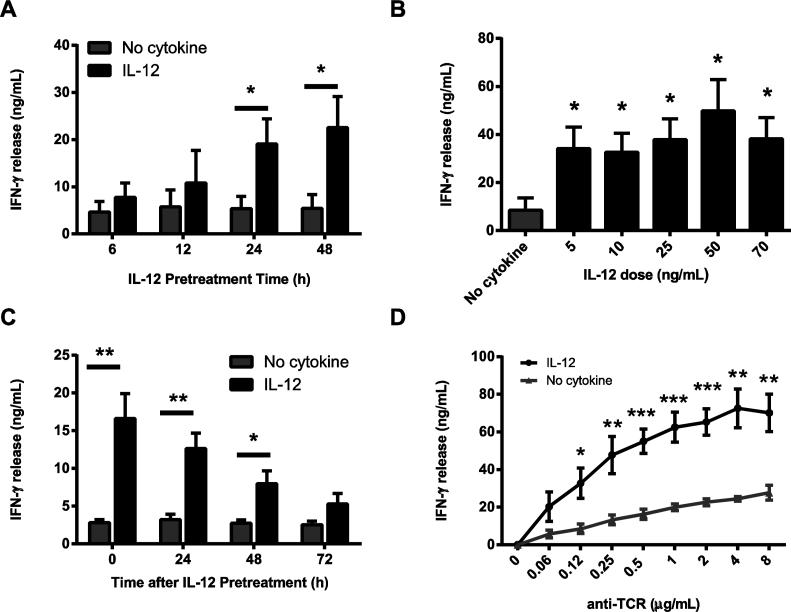
To further characterize these effects, we first examined the length of IL-12 pretreatment needed to alter human activated CD8 T cell responses. To address this question, human activated CD8 T cells were pretreated with IL-12 for different times, washed, and stimulated through the TCR. We found that exposing the cells to IL-12 for 6 and 12 h resulted in a consistent, but not statistically significant, increase in the production of IFN-γ following TCR stimulation in comparison to cells treated in media alone (Fig. 2A). In contrast, cells pretreated for 24 and 48 h had significantly increased amounts of IFN-γ production compared to control cells (Fig. 2A). We next examined the responsiveness of human activated CD8 T cells to various doses of IL-12. Human activated CD8 T cells were pretreated with a range of physiological doses of IL-12 [25-27], washed, and stimulated through the TCR. Following TCR stimulation, we found that pretreatment with doses of 5-70 ng/mL of IL-12 results in augmented TCR–induced IFN-γ production in comparison to cells treated in media alone; however, there were no significant differences in the potentiation of IFN-γ production between any of the doses (Fig. 2B and data not shown). Next, we examined the duration of the effects after removal of IL-12. Following IL-12 pretreatment, human activated CD8 T cells were rested for various times before being challenged through the TCR. As seen in Fig. 2C, IL-12 pretreated cells stimulated through the TCR immediately after IL-12 pretreatment had a significant increase in the production of IFN-γ in comparison to cells treated in media alone. We also observed that the ability of IL-12 to enhance TCR-induced IFN-γ production lasted for 48-72 h. Finally, the effects of IL-12 pretreatment were examined following challenge with different doses of TCR. To explore this, IL-12 pretreated or untreated cells were stimulated with titrating doses of anti-CD3. As expected, TCR stimulation with low doses of anti-CD3 increased IFN-γ production in untreated cells (Fig. 2D). Interestingly, we saw that the IL-12-pretreated cells stimulated with different doses of anti-CD3 had a dose dependent increase in the production of IFN-γ (Fig. 2D). The IL-12-mediated increase in IFN-γ production was significant for all doses of anti-CD3 higher than 0.12 μg/mL (Fig. 2D). Collectively these data demonstrate that prior exposure to physiologically-relevant doses of IL-12 for at least 24 h transiently potentiates the TCR-induced production of IFN-γ.
Figure 2. The potentiation of TCR-mediated cytokine production by human activated CD8 T cells is transient and requires low physiological doses of IL-12 for at least 24 hours.
Human activated CD8 T cells were left untreated (no cytokine) or (A) treated for various times with IL-12 (50 ng/mL), (B) exposed for 24 h to various doses of IL-12, or (C) treated with IL-12 for 24 h (50 ng/mL), washed and rested for various times. After treatment, the cells were washed and stimulated with 1 μg/mL of plate bound anti-CD3 antibodies for 24 hours. Then IFN-γ production was determined by ELISA. (D) Human activated CD8 T cells were left untreated or treated with IL-12 for 24 h (50 ng/mL), washed and immediately stimulated with different doses of plate bound anti-CD3 antibodies for 24 hours. IFN-γ production was then determined by ELISA. Graphs are shown as the mean ±SEM of values from three to five different donors. Data were statistically compared to no cytokine cells using a two-tail Student's t test. *p<0.05; **p<0.01; ***p<0.001; n.s. or no symbol=not significant.
3.3 Prior exposure to IL-12 increases the frequency of cells capable of producing IFN-γ and TNF-α upon TCR stimulation
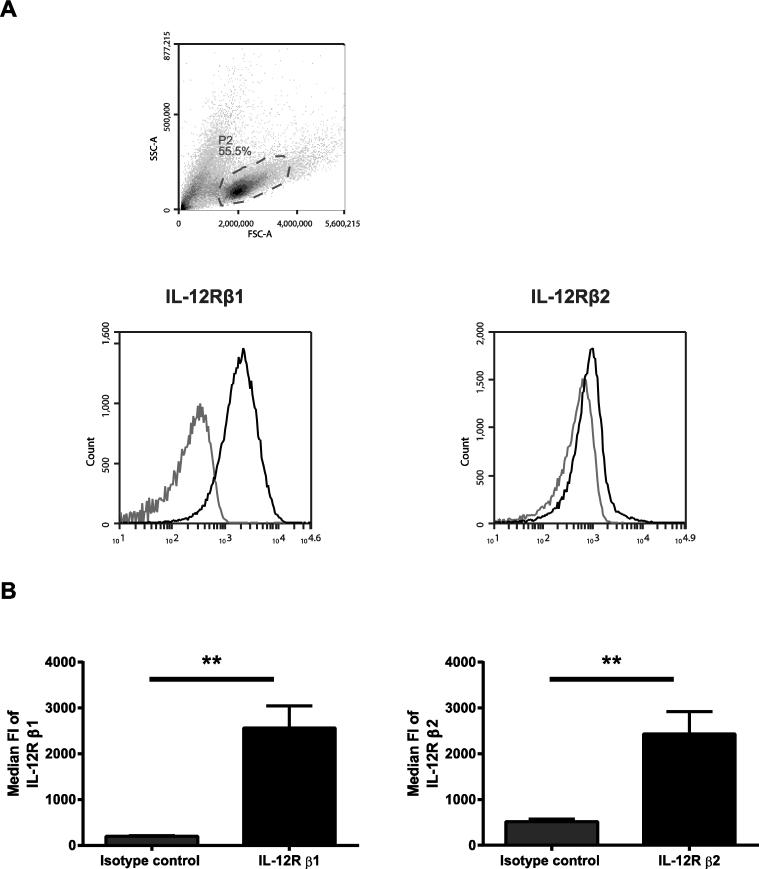
The IL-12 receptor consists of two subunits, IL-12R β1 and β2 [2, 28]. The expression of these subunits in T cells is highly regulated by TCR stimulation and cytokines [2, 28]. Previous literature suggests that resting T cells do not express detectable levels of the receptor, but the subunits are upregulated following TCR activation [28]. However, no studies to date have examined the expression of the IL-12 receptor on human activated CD8 T cells. In order to examine the cells capable of responding to IL-12 in our system, we sought to determine the expression of the IL-12 receptor subunits on these cells using flow cytometry. We found that a substantial proportion of human activated CD8 T cells expressed IL-12R β1above the level of the isotype stained control, suggesting that nearly all the CD8 T cells express surface levels the IL-12R β1 (Fig. 3A). In addition, we found that a small fraction of the human activated CD8 T cells expressed the IL-12R β2 above the level of the isotype stained control (Fig. 3A). Furthermore, we determined that the median fluorescence intensity (Median FI) of both the IL-12R β1 and β2 were significantly higher than isotype controls on human activated CD8 T cells (Fig. 3B). These data indicate that the human activated CD8 T cells used in our system are able to respond to IL-12 signals.
Figure 3. Human activated CD8 T cells have variable surface expression of IL-12R β1 and β2.
Human activated CD8 T cells were stained with anti-IL-12 R β1 or anti-IL-12R β2 and isotype controls and the expression of the IL-12R was then analyzed using flow cytometry. (A) Representative histograms show the expression of IL-12 receptor β1 or β2 following gating on live lymphocytes (based on forward and side scatter). Black line represents staining with IL-12R antibody and gray line represents staining with isotype controls. (B) The median fluoresce intensities (Median FI) of the expression of the IL-12 receptor subunits were determined and plotted as Mean±SD of three separate donors. Data were analyzed with two-tail, unpaired Student's t test. *p<0.05; **p<0.01; ***p<0.001
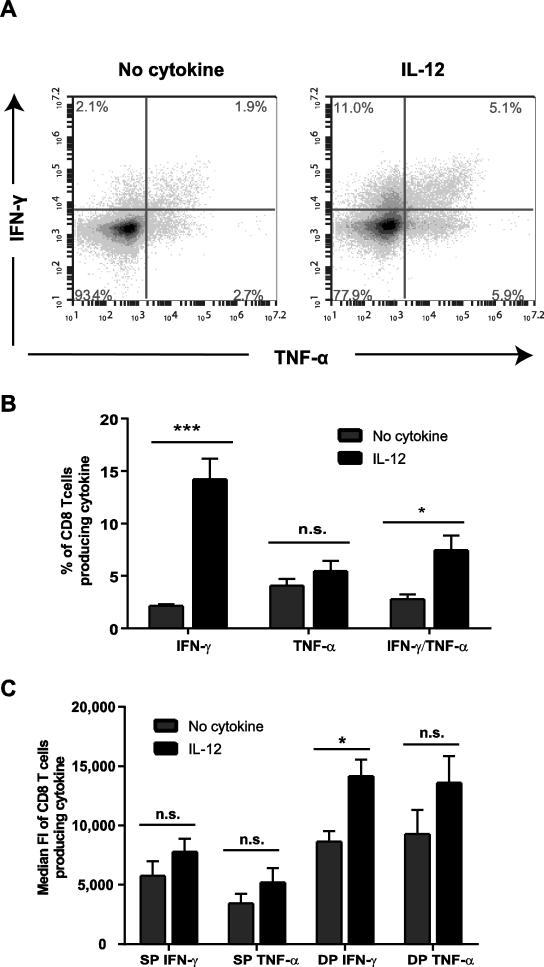
We next wanted to determine if IL-12 pretreatment potentiated the production of IFN-γ and TNF-α by increasing the frequency of cells capable of producing cytokine or by increasing the amount of cytokine produced on a per-cell basis. To explore this, human activated CD8 T cells were left untreated or pretreated with IL-12, and then the intracellular levels of IFN-γ and TNF-α were determined after 6 h of TCR stimulation. At 6 h post TCR stimulation, both IL-12 pretreated and untreated cells had similar frequencies of cells producing IFN-γ and TNF-α (Supplementary Fig. 2A). We then examined the effects of IL-12 pretreatment on cytokine production at later points of TCR stimulation (18 h). Exposure to IL-12 before TCR stimulation for 18 h resulted in a significant increase in the frequency of cells producing IFN-γ alone or IFN-γ/TNF-α together in comparison to cells not treated with IL-12 (Fig. 4A and B). There was no significant difference between cell treated with or without IL-12 in the percentage of cell producing TNF-α alone (Fig. 4B). On a per-cell basis, IL-12 pretreatment resulted in significantly increased IFN-γ median fluorescence intensity (median FI) in double producing cells compared to cells treated in media alone (Fig. 4A and 4C). IL-12 pretreatment also induced a trend towards increased median FI for IFN-γ and TNF-α in single producing T cells and TNF-α in double producing T cells in comparison to cells treated in media alone, but these effects did not reached statistical significance (Fig. 4C). Interestingly, the median FI for both IFN-γ and TNF-α was consistently higher in double producing T cells compared to signal producing T cells, irrespective of cytokine treatment (Fig. 4C). Overall, these data demonstrate that prior exposure to IL-12 increases the proportion of cells capable of producing cytokines upon TCR re-stimulation.
Figure 4. Prior exposure to IL-12 increases the frequency of human activated CD8 T cells making IFN-γ and TNF-α upon TCR stimulation.
Human activated CD8 T cells were left untreated or pretreated with IL-12 for 24 h (50 ng/mL). Cells were then washed and stimulated with 1 μg/mL of plate bound anti-CD3 antibodies for 18 h with BFA added for the last 5 h. Intracellular protein levels of IFN-γ and TNF-α were determined by flow cytometry. Live lymphocytes were gated based on forward and side scatter. Quadrants were set so the baseline cytokine production of non-TCR stimulated cells was less than 1%. The frequencies and median fluorescence intensities of cytokine expression for IFN-γ and TNF-α single producing cells and IFN-γ/TNF-α double producing cells were then determined. Data are shown as (A) representative plots, (B) percentage of T cells producing IFN-γ or TNF-α alone or IFN-γ and TNF-α together, or (C) median fluorescent intensity of IFN-γ or TNF-α in single (SP) and double (DP) producing cells. Each data set is shown as the mean ± SEM of four separate donors. Data were analyzed with two-tail, unpaired Student's t test. *p<0.05; **p<0.01; ***p<0.001; n.s.=not significant.
3.4 The IL-12-mediated increase of cytokine production is a consequence of increased transcription of cytokines
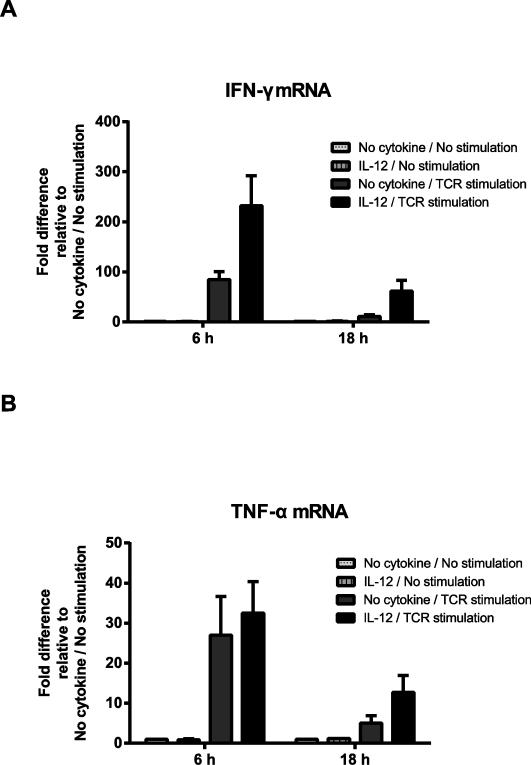
We next determined whether the IL-12-mediated increase in IFN-γ and TNF-α was a consequence of enhanced transcription of cytokine mRNA. To explore this possibility, the mRNA levels of IFN-γ and TNF-α were examined in IL-12 pretreated and untreated cells at 6 and 18 h after TCR stimulation. We found that, in the absence of TCR stimulation, both untreated and IL-12 pretreated cells had similar mRNA levels of IFN-γ and TNF-α (Fig. 5A and B). This is consistent with our results showing that IL-12 pretreatment alone has no effect on cytokine production (Supplementary Fig.1A and B). As expected, in cells pretreated with media alone, TCR stimulation for 6 and 18 h increased mRNA levels of IFN-γ and TNF-α (Fig. 5A and B). However, when cells were pretreated with IL-12, we found that they had substantially increased mRNA levels of IFN-γ compared to media treated cells at both 6 and 18 h after TCR stimulation (Fig. 5A and B). The TCR-mediated increase in TNF-α mRNA expression at 6 h was not altered in IL-12 pretreated cells in comparison to untreated cells (Fig. 5B). In contrast, IL-12 pretreatment consistently led to a 2.5-fold increase in the mRNA levels of TNF-α at 18 h after TCR stimulation (Fig. 5B). Overall, these data show that IL-12 pretreatment increases TCR-induced IFN-γ and TNF-α mRNA expression.
Figure 5. The IL-12 pretreatment increases IFN-γ and TNF-α mRNA expression after TCR stimulation in human activated CD8 T cells.
Human activated CD8 T cells were left untreated or pretreated with IL-12 for 24 h (50 ng/mL), washed, and stimulated with or without 1 μg/mL of plate bound anti-CD3 antibodies for 6 and 18 h. The expression of IFN-γ and TNF-α mRNA were then determined by qPCR at these times. Data were normalized to that of mRNA encoding β-actin and presented relative to that of no cytokine cells. Results are shown as mean ± SEM of three to four separate donors.
3.5 The IL-12 mediated enhancement of cytokine production is not due to residual STAT4 synergizing with TCR stimulation signals
To explore the mechanisms by which IL-12 pretreatment alters the responses of human activated CD8 T cells to TCR stimulation, we first examined whether IL-12 exposure was changing the expression of surface molecules involved in T cell activation. We found that IL-12 exposure does not substantially alter the expression of TCR, CD2, CD49d, or CD11a (data not shown). These results suggest that IL-12 is not mediating its effects via changes in TCR expression or expression of the major adhesion proteins.
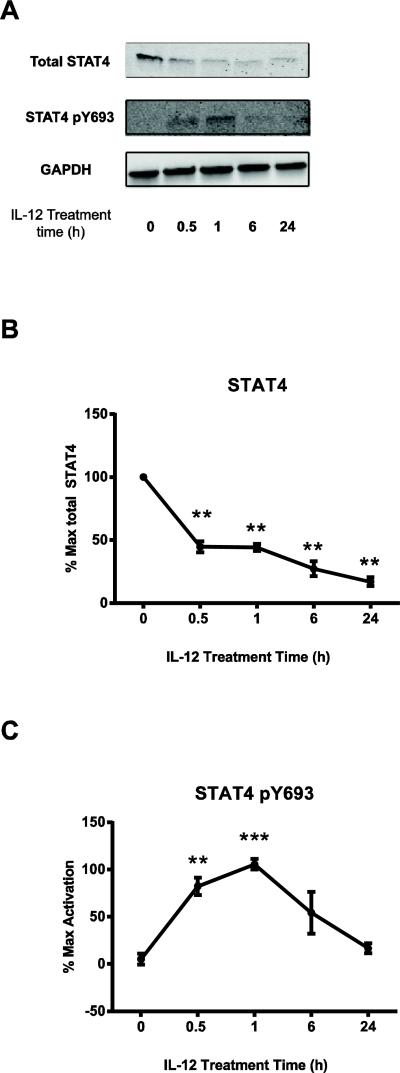
We next explored whether IL-12 pretreatment was altering intracellular signaling events. IL-12 stimulation is known to induce STAT4 phosphorylation, which allows STAT4 to translocate into the nucleus and promote IFNG transcription [2, 29]. Moreover, STAT4 is one of the critical mediators of the canonical IL-12 effects, because STAT4-knockout mice have impaired Th1 differentiation and IFN-γ production [28]. Since IL-12 pretreatment enhanced the transcription and protein levels of IFN-γ and TNF-α after TCR stimulation, we tested the hypothesis that long-term STAT4 phosphorylation following IL-12 pretreatment synergizes with TCR-induced signaling pathways to promote these effects. To test our hypothesis, we examined the total expression and the phosphorylation levels of STAT4 in untreated and IL-12-pretreated cells; in these experiments, no TCR stimulation was provided. STAT4 protein levels were reduced over time following IL-12 pretreatment (Fig. 6A and B), while there was a transient increase in STAT4 phosphorylation that peaked at 1 h after exposure and returned to basal levels by 24 h (Fig. 6A and C). The phosphorylation of STAT4 did not appear to be due to residual IFN-γ release by the cells during the time course of the experiment, since IL-12-treated cells without TCR stimulation had no detectable production of IFN-γ (Fig. 2D). The lack of overt STAT4 phosphorylation after 24 hours of treatment suggests that the IL-12-mediated increase of IFN-γ after TCR stimulation is not due to residual STAT4 synergizing with TCR signals.
Figure 6. The IL-12 mediated enhancement of cytokine production in human activated CD8 T cells is not due to residual STAT4 synergizing with TCR signals.
Human activated CD8 T cells were left untreated or pretreated with 50 ng/mL of IL-12 for different times. At the indicated times, cells were lysed and immunoblotting for total and phosphorylated STAT4 was performed in whole cell lysates as described in the materials and methods section. Results were normalized to GAPDH and the maximal level of activation of no cytokine cells. Data are shown as representative blots (A) and (B-C) mean ± SEM of normalized results of three separate donors. Data were analyzed with two-tail, unpaired Student's t test. *p<0.05; **p<0.01; ***p<0.001; n.s.=not significant.
3.6 IL-12 pretreatment enhances the TCR-induced activation of select MAPK and AKT without altering the activation of more proximal TCR signaling molecules
TCR stimulation results in a coordinated activation of a series of signaling molecules that eventually promote the transcription of cytokine genes. In murine CD4 and CD8 T cells, it was shown that exposure to pathogen-induced inflammation enhances T cell functions by increasing the activation of TCR signaling molecules [17, 18]. In addition, pretreatment of human T cells with IL-12, IL-7 and IL-15, or the TLR5 ligand bacterial flagellin, which all potentiated TCR-mediated functions, altered the activation of distinct sets of molecules downstream of the TCR [14-16]). Therefore, inflammatory stimuli that enhance subsequent TCR-induced downstream functions all regulate TCR signaling through distinct mechanisms. Because of this, we explored whether IL-12 pretreatment alters the activation of TCR signaling molecules in human activated CD8 T cells.
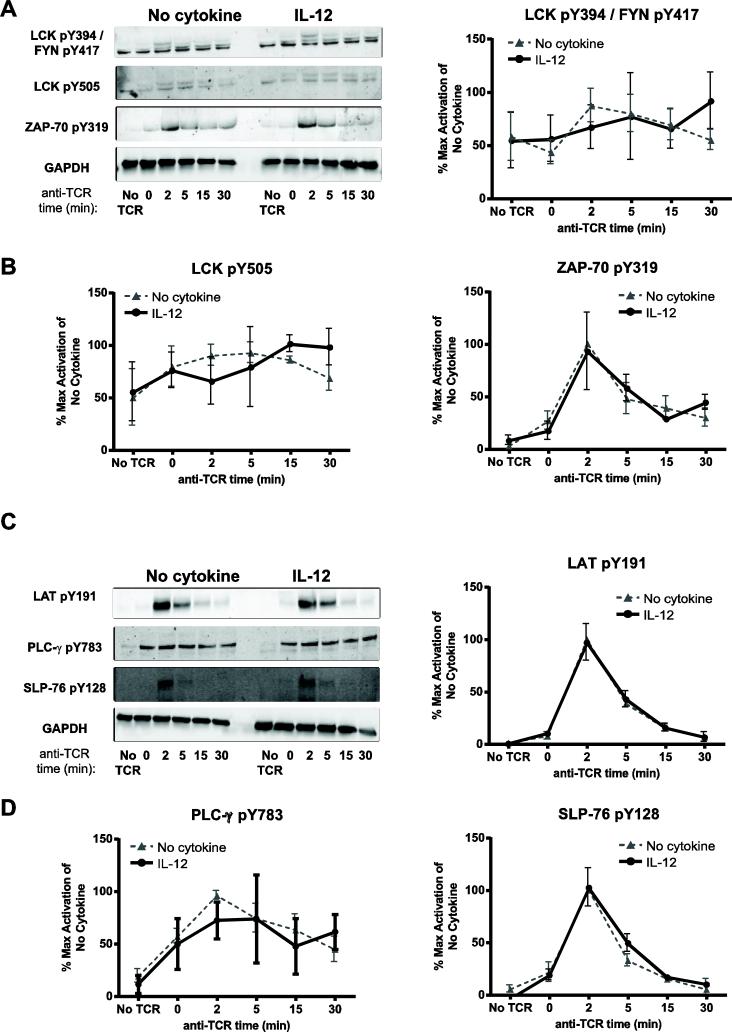
To test this possibility, human activated CD8 T cells were left untreated or exposed to IL-12, washed, and then stimulated through the TCR for various times. Then, changes in the phosphorylation and total expression of TCR-induced signaling molecules were measured using quantitative immunoblotting. The earliest events detected following TCR stimulation are the activation of the kinases, LCK and FYN. The activities of these kinases are positively regulated by the phosphorylation of tyrosine residues (LCK Y394 and FYN Y417) in their kinase domains and negatively regulated by the phosphorylation on tyrosine residues on their C-terminal tail (LCK Y505) [30]. To detect changes in the activation of LCK Y394 and FYN Y417, we used an anti-SRC pY416 antibody, which recognizes all SRC kinases, including LCK and FYN, when they are phosphorylated on their activating sites. We found that cells treated in media alone had substantial phosphorylation of activating tyrosine kinases at basal state, and the phosphorylation of these sites remained unaltered following TCR stimulation (Fig. 7A and B). We also found that the TCR-induced phosphorylation of LCK Y394 and FYN Y417 was similar between IL-12 pretreated and untreated cells (Fig. 7A and B). We observed that, in cells treated in media alone, the phosphorylation of LCK at its inhibitory residue (LCK Y505) was observable at basal state and was modestly increased following TCR stimulation (Fig. 7A and 7B). Again, IL-12 pretreatment had no effect on the phosphorylation of LCK at its inhibitory site Y505 after TCR stimulation (Fig. 7A and B). This suggests that IL-12 pretreatment does not alter the TCR-induced activation of LCK or FYN.
Figure 7. Prior exposure to IL-12 does not alter the activation of proximal TCR signaling molecules in human activated CD8 T cells.
Human activated CD8 T cells were left untreated or pretreated with IL-12 for 24 h (50 ng/mL). Cells were then washed and stimulated for various times using 3 μg/mL of crosslinked anti-CD3. Then the phosphorylation of signaling molecules was assessed in whole cell lysates by immunoblotting as described in the materials and methods section. Results were normalized to GAPDH and the maximal level of activation of no cytokine cells. Data are shown as (A and C) representative blots and (B and D) mean ± SEM of normalized results of three separate donors. Data were analyzed with two-tail, unpaired Student's t test. *p<0.05; **p<0.01; ***p<0.001; n.s.=not significant.
LCK and FYN phosphorylate ITAMs on the CD3 subunits of the TCR. The full phosphorylation of the ITAMs promotes the recruitment of ZAP-70 to the TCR, where it is activated and phosphorylated by LCK on Y319 [31]. Activated ZAP-70 then phosphorylates the adaptor proteins LAT and SLP-76, inducing downstream signaling events. LAT is phosphorylated on four key tyrosine residues that serve as docking sites for several molecules, such as the phospholipase PLC-γ [31-33]. Once recruited to LAT, PLC-γ is phosphorylated on Y783, leading to its activation and the subsequent release of intracellular calcium and the activation of the protein kinase C (PKC) and MAPK pathways [31, 34]. TCR stimulation also promotes the phosphorylation of SLP-76 on several tyrosine residues [35]. Similarly to LCK and FYN, the TCR-induced phosphorylation of the activating tyrosine on ZAP-70 Y319 was comparable between IL-12 treated and untreated cells (Fig. 7A and B). We also observed that IL-12 pretreated cells and untreated cells had no difference in the kinetics of phosphorylation of TCR-induced LAT Y191, SLP-76 Y128, or PLC-γ Y783 (Fig. 7C and D). Together, these data show that prior exposure to IL-12 does not alter the TCR-induced activation of proximal signaling.
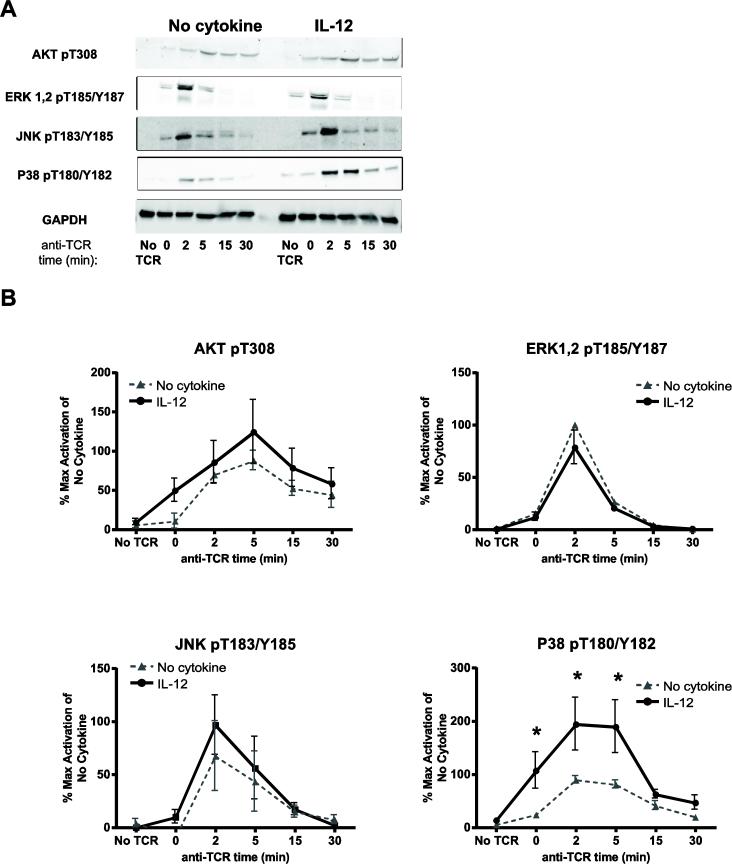
Phosphorylated SLP-76 directs the recruitment and activation of PI3K, which is an important regulator of downstream molecules like AKT [31, 36, 37]. AKT is phosphorylated on T308 and the phosphorylation of this residue is correlated with its activity [38]. We observed that IL-12 pretreated cells had a trend towards increased phosphorylation of AKT T308 in comparison to untreated cells (Fig. 8A and B). However, the slight IL-12-mediated increase of AKT T308 phosphorylation was variable between donors and it did not reach statistical significance after compiling the data from four different donors (Fig. 8A and B).
Figure 8. IL-12 pretreatment enhances the TCR-induced activation of select MAP kinases and AKT in human activated CD8 T cells.
Human activated CD8 T cells were left untreated or pretreated with IL-12 for 24 h (50 ng/mL). Cells were then washed and stimulated for various times using 3 μg/mL of crosslinked anti-CD3. Then, the phosphorylation of signaling molecules was assessed in whole cell lysates by immunoblotting as described in the materials and methods section. Results were normalized to GAPDH and the maximal level of activation of no cytokine cells. Data are shown as (A) representative blots and (B) mean ± SEM of normalized results of three to seven separate donors. Data were analyzed with two-tail, unpaired Student's t test. *p<0.05; **p<0.01; ***p<0.001; n.s.=not significant.
Downstream of the LAT and SLP-76 complexes is the activation of the MAP kinase pathways. This family is comprised of three groups of kinases: ERK1/ERK2, JNK, and P38. These kinases have multiple substrates that play key roles in a variety of cellular functions, including cytokine secretion [31]. We found that IL-12 pretreated and untreated cells had no difference in the TCR-induced phosphorylation of ERK1/ERK2 pT187/pY187 (Fig. 8A and B). In addition, we observed that IL-12 pretreated samples had a slight increase in the levels of JNK T183/Y185 phosphorylation compared to control samples that were not significant after compiling multiple donors (Fig. 8A and B). Finally, we saw that IL-12 pretreated had an overall increase in the TCR-induced phosphorylation kinetics of P38 pT180/T182 in comparison to untreated cells. The basal phosphorylation of P38 pT180/T182 was similar in both control and IL-12 pretreated cells. However, the TCR-induced phosphorylation of P38 pT180/T182 in IL-12 pretreated cells was significantly increased at 0, 2, and 5 minutes in comparison to untreated cells. The fact that both untreated and IL-12 pretreated cells have similar P38 activation at basal state suggests that IL-12 is not directly activating the P38 pathway after 24 hours of stimulation. As shown in Supplemental Fig. 2B, the increased activation of P38 was not due to altered total expression of the protein. Collectively, these findings suggest that IL-12 signals significantly increase the TCR-induced activation of P38, and to a lesser extent JNK and AKT, without altering the activation of other more proximal TCR signals.
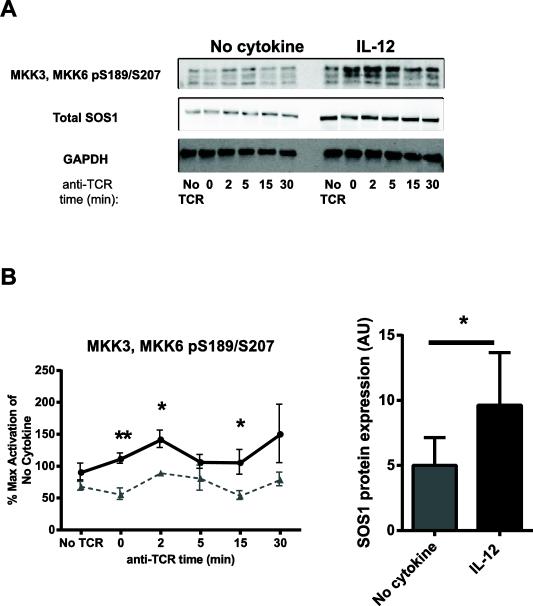
3.8 IL-12 pretreatment enhances the levels of SOS1 and increases the activation of MKK3/MKK6 downstream of the TCR
Our previous data suggests that IL-12 signals alter the expression or activation of a positive or negative regulator of P38 activation. Although the exact mechanism regulating the activation of MAPK kinases following TCR stimulation remain unclear, TCR activation recruits effector molecules to LAT, such as the son-of-sevenless (SOS) family proteins [31]. The GRB2/SOS1 axis has recently been shown by us and others to control the activation of P38 in human T cells [39, 40]. In addition, the kinases MKK3/MKK6 are known to be direct upstream activators of P38 [41]. To further investigate the crosstalk between IL-12 signals and P38 activation, we determined whether IL-12 pretreatment altered the activation of MKK3/MKK6 and/or the total expression of SOS1. We found that cells treated in media alone had substantial basal phosphorylation of MKK3/MKK6, and this was remained relatively unaltered upon TCR stimulation (Fig. 9A and B). We also we observed that that upon TCR stimulation, IL-12 pretreated cells had increased phosphorylation of MKK3/MKK6 compared to the control cells that was significant at several time points (Fig. 9A and B). The IL-12 mediated enhancement of MKK3/MKK6 was significant at 0, 2, and 15 min after TCR stimulation (Fig. 9A and B). We also found that the IL-12 pretreated cells had significantly increased levels of SOS1 before and after TCR stimulation in comparison to control cells (Fig. 9C). Together, these data suggest that IL-12 pretreated cells have increased levels of SOS1, which upon TCR stimulation, results in increased activation of MKK3/MKK6 and P38.
Figure 9. IL-12 pretreatment increases the expression of SOS1 and the phosphorylation of MKK3/MKK6 downstream of the TCR.
Human activated CD8 T cells were left untreated or pretreated with IL-12 for 24 h (50 ng/mL). Cells were then washed and stimulated for various times using 3 μg/mL of crosslinked anti-CD3. The phosphorylation or total expression of signaling molecules was assessed in whole cell lysates by immunoblotting and the results were normalized to GAPDH. Upon normalization, the average of the total expression of SOS1 at each time point was calculated. Data are shown as (A) representative blots and (B-C) mean ± SEM of normalized results of three to seven separate donors. Data were analyzed with two-tail, unpaired Student's t test. *p<0.05; **p<0.01; ***p<0.001; n.s.=not significant.
4. Discussion
Numerous studies have examined the effects of IL-12 in regulating T cell responses when it's present during and after TCR stimulation. IL-12 present during priming of CD4 and CD8 T cells plays key roles in the regulation of the responses of these cells [2, 10]. Furthermore, IL-12 acts as a costimulatory signal by enhancing TCR-induced proliferation, IFN-γ production and cytotoxicity [2]. However, we have only recently begun to appreciate that exposure to inflammatory signals like IL-12 could alter how T cells respond to subsequent TCR stimulation. In this regard, Richer et al. and Kim et al. showed that murine effector/memory CD8 T cells or secondary effector CD4 T cells exposed to pathogen induced-inflammation, primarily driven by IL-12 and type I interferons, have enhanced ability to respond to TCR stimulation [17, 18]. Similarly, Raue and colleagues showed that murine memory CD8 T cells conditioned with IL-12 and IL-18 in vitro have enhanced cytokine production and cytotoxic activity upon TCR re-challenge [19]. Finally, we have recently found that IL-12 pretreatment in human CD4 T cells enhances the production of a range of cytokines following TCR induction [16]. Collectively, these studies suggest that the regulation of T cell responses by IL-12 is more complex than previously appreciated. Beyond its well-studied co-stimulatory effects, prior exposure to IL-12 alters the responsiveness of murine CD4 and CD8 T cells to TCR challenge and enhances the production of multiple cytokines in human activated CD4 T cells. The results reported in this study extend our expanding understanding of the effects of IL-12 on antigen experienced T cells to activated human CD8 T cells.
During the response to infection, naïve or central memory CD8 T cells will be TCR activated in secondary lymphoid organs. These activated effector or memory CD8 T cells will then be released from the secondary lymphoid organs where they will encounter inflammatory signals in the absence of TCR stimulation in the blood and lymph as they migrate from the lymph nodes into sites of infection. In the site of infection, the activated CD8 T cells exposed to inflammatory stimuli receive secondary antigen and/or inflammatory signals that impact their activation. In this study, we mimicked this physiological setting in vitro using human peripheral blood CD8 T cells that have been activated for 5 days with anti-CD3 /anti-CD28 antibodies and recombinant IL-2, removed from the priming stimuli, and transiently pretreated with inflammatory signals before being restimulated via the TCR. Interestingly, our data suggest that among different pro and anti-inflammatory cytokines, only IL-12 alters the responses of human CD8 T cell responses to subsequent TCR stimulation. In contrast to the previous findings from murine CD8 and CD4 T cells, we found that pretreatment with type I interferons (IFN-α or IFN-β) has no effect on subsequent human activated CD8 T cell responses. This could be attributed to differences between human and mouse T cell responses, but could also be derived from differences in the experimental setup. In the murine studies, the receptor for type I interferons was shown to be important to alter the T cell responses following pathogen-induced inflammation, which consists of multiple cytokines. In contrast, our studies tested only key inflammatory cytokines to allow for mechanistic insight into these processes. Our results provide novel evidence that prior exposure specifically to IL-12 increases the responses of CD8 T cells to TCR stimulation and that there is no synergistic or antagonist effects seen when IL-12 is present in combination with other cytokines.
It was possible that the increased release of IFN-γ and TNF-α in the IL-12-pretreated cells was due to residual IL-12 present during TCR stimulation. However, the IL-12-mediated increase of human activated CD8 T cells responses to further TCR stimulation required pretreatments with IL-12 of at least 24 h. These results suggest that the observed effects are not because residual IL-12 is providing co-stimulatory signals upon TCR stimulation, since any potential effects would be observed at all times of pretreatment. The requirement for at least 24 hours of treatment also suggests that effects of IL-12 require transcriptional alterations. In addition, IL-12 pretreatment transiently increased the TCR-induced production of cytokines for 48-72 h. We speculate that the effects of IL-12 are short lived in order to minimize the risk of immunopathology that would occur with long term enhancement of CD8 T cell function. We also observed that IL-12 pretreatment resulted in increased mRNA levels of IFNG and TNF following TCR stimulation. Interestingly, examining the intracellular levels of the cytokines, we found that IL-12 pretreatment also increased the frequency of cells producing IFN-γ and TNF-α, both alone and co-producing both cytokines, without altering the amount of cytokines produced on a per cell basis. These results suggest that the increased transcriptional activity of IFNG and TNF detected was because more cells were making cytokine mRNA and not because each cell had increased transcription of the cytokines.
We found that the effects of prior exposure to IL-12 regulated human CD4 and CD8 T cells in distinct ways. In human CD8 T cells, IL-12 exposure for at least 24 h or longer amplified subsequent TCR-induced functions. In contrast, human CD4 T cells required only 1.5 h of IL-12 pretreatment to observe significant alterations in TCR function [16]. Furthermore, we observed that in human activated CD4 T cells the effects of IL-12 pretreatment in enhancing TCR-induced functions lasted for 3-6 hours [16]. In contrast, in human activated CD8 T cells the IL-12 mediated potentiation of TCR-induced responses lasted for 48-72 h. In addition, we found that, in human activated CD8 T cells, IL-12 pretreatment increases the mRNA expression of IFNG and TNF and the intracellular levels of these cytokines after 18 h of TCR stimulation. In contrast, with the IL-12 pretreated human activated CD4 T cells, we found that TCR stimulation for 6-8 and 18 h increased the mRNA expression of IFNG without altering the mRNA expression of TNF [16]. Finally, on the IL-12 pretreated human CD4 T cells, we saw that TCR stimulation increased the intracellular levels of IFN-γ without altering the intracellular levels of TNF-α. On the other hand, on the IL-12 pretreated human CD8 T cells, we found that TCR stimulation increased the intracellular levels of IFN-γ and TNF-α, especially in T cells that produce both cytokines. We speculate that the different responses to IL-12 exposure in human CD4 and CD8 T cells are because of the different functions that these cells play during immune responses. The mechanism for these differences is unknown and needs to be explored more fully.
IL-12 signals are known to promote STAT4 activation, which is thought to mediate IFN-γ transcription. Therefore, it was tempting to predict that following IL-12 pretreatment high levels of phosphorylated STAT4 would synergize with TCR-mediated signaling pathways to enhance the production of cytokines following TCR stimulation. Contrary to this hypothesis, we found that IL-12 pretreated cells had little detectable phosphorylation levels of STAT4 after 24 hours of treatment, which is the time when TCR stimulation occurred in the majority of our experiments. Thus, there is minimal phosphorylated, active STAT4 in T cells upon restimulation via the TCR. Interestingly, we found that STAT4 expression was reduced following IL-12 exposure, suggesting that STAT4 is degraded following IL-12 exposure. Our findings are supported by previous studies showing that IL-12 signaling results in STAT4 degradation [42]. Even though there was no overt STAT4 phosphorylation after 24 hours of treatment, it is still possible that a minor proportion of STAT4 bound to DNA and capable of synergizing with TCR signals. However, the fact that IL-12 pretreatment required at least 24 h in order to alter how cells respond to TCR stimulation and that IL-12 pretreated cells had increased expression of the TCR signaling protein SOS1, suggest that large scale STAT4 signaling is not synergizing with TCR-induced pathways. Instead, IL-12-induced pathways, including STAT4 and other signaling proteins, appear to increase the transcription of positive or negative regulators of TCR signaling molecules such as SOS1. It should be noted that type I interferons are known to activate STAT4 in human T cells [43], and pretreatments with type I interferons did not alter how our human CD8 T cells responded to TCR stimulation. This suggests that STAT4 is not solely mediating the ability of IL-12 pretreatment to increase the responses of human activated CD8 T cells to TCR stimulation.
Our results indicate that IL-12 could be mediating its effects by rewiring TCR signaling pathways. We found that IL-12 signals significantly increase the TCR-induced activation of P38, and to a lesser extent JNK and AKT, without altering the activation of other more proximal TCR signals. Interestingly, P38 activation has been shown to have multiple roles on T cell responses, including cytokine release. Even though the effects of IL-12 in altering the activation of AKT and JNK were not statistically significant, it is possible these minor alterations have substantial biological relevance. Several recent reviews have suggested that relatively small perturbations in the activation of proximal signaling proteins can result in extensive changes in downstream functions [44, 45]. Thus, the subtle changes we observe in transient proximal signaling events are capable of altering subsequent cellular function.
Our results fit well with previous literature from us and others demonstrating that prior exposure to inflammatory signals change the responses of T cells to subsequent TCR stimulation by altering TCR-mediated signaling. We have recently showed that in human activated CD4 T cells, IL-12 exposure selectively increased the TCR-induced activation of LCK, AKT, and P38 without altering the activation of other signaling molecules [16]. In contrast, pathogen-induced inflammation increased proximal TCR-induced ZAP-70, PLC-γ, ERK1/2 and JNK1/2 phosphorylation without altering the activation of P38 in murine effector/memory CD8 T cells and increased ZAP-70 and ERK1/2 phosphorylation in murine secondary effector CD4 T cells [17, 18]. In addition, in human T cells, IL-7 and IL-15 mediated their effects by increasing the activation of ERK1/2 following TCR stimulation [14]. Our laboratory also observed that prior activation of T cells with a TLR5 ligand enhances TCR-mediated AKT activation, while simultaneously reducing LCK and LAT phosphorylation [15]. This suggests that individual inflammatory signals that alter subsequent T cell functions mediate this phenomenon by regulating TCR-mediated signaling through distinct mechanisms. Although based on the current dogma it might seem unexpected to see an increase in the activation of downstream signals in the absence of increased activation of more proximal signals, this disconnect has been described before. We have shown that prior TLR5 ligand (flagellin) induction enhanced TCR-mediated activation of AKT without increasing the activation of LCK or LAT [15]. Furthermore, we have shown that following TCR stimulation, GRB2-deficient T cells have enhanced LCK, ZAP-70, and SLP-76 activation and decreased or unaltered ERK, P38 and JNK activation [46].
While examining the mechanism for the increase in TCR-induced P38 activation, we found that IL-12 pretreatment resulted in increased activation of the direct upstream activators of P38 (MKK3/MKK6) and SOS1. Even though the GRB2/SOS1 axis has recently been shown by us and others to control the activation of P38 [39, 40], the mechanism by which this is happening remains unexplored. SOS1 is one of the two Ras guanine nucleotide exchange factors present on T cells that play a key role in regulating Ras activity [31]. Ras in turn is canonically known to mediate the activation of ERK1/ERK2. However, recent studies suggest that SOS1 may mediate the activation of the P38 pathway independently of the enzymatic function of SOS1 [40]. Therefore, we speculate that the IL-12 mediated increased levels of SOS1 could be leading to the increased activation of P38 by an unknown mechanism. Overall, our results suggest that the IL-12 mediated increase in mRNA and protein levels of IFN-γ and TNF-α are mediated by increasing the TCR-induced activation of select MAPK and AKT without altering more proximal TCR signals.
5. Conclusion
In this study, we examined how exposure to different cytokines alters the responses of human activated CD8 T cells to TCR stimulation. Our studies are the first to show that prior exposure specifically to IL-12 results in increased cytokine production following subsequent TCR challenge. Furthermore, we found that IL-12 pretreatment increased the TCR-induced activation of select MAPKs and AKT without altering the activation of more proximal TCR signaling molecules. Our data expands our understanding of the functions of IL-12 in regulating the responses of human activated CD8 T cells and provides insights into the mechanisms by which IL-12 is mediating these effects.
Supplementary Material
Highlights.
Prior exposure to IL-12 enhances TCR-induced cytokine release in human CD8 T cells
IL-12 pretreatment increases the TCR-induced activation of select signaling pathways
Primed human CD8 T cells are regulated by IL-12 via an unappreciated mechanism
Acknowledgements
We thank Mahmood Bilal, Mikaela Tremblay, and Michael Zhang for helpful discussions and careful reading of the manuscript. The JCDH laboratory was supported by the National Cancer Institute at the National Institutes of Health (CA136729). NMC was supported by the American Heart Association Predoctoral (11PRE7390070) and the NIH Predoctoral Training Grant in Immunology (T32 AI007485).
Abbreviations
- TCR
T cell receptor
- MAPK
Mitogen-activated protein kinases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. The Journal of experimental medicine. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature reviews. Immunology. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 3.Zundler S, Neurath MF. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–568. doi: 10.1016/j.cytogfr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 5.el-Shabrawi Y, Livir-Rallatos C, Christen W, Baltatzis S, Foster CS. High levels of interleukin-12 in the aqueous humor and vitreous of patients with uveitis. Ophthalmology. 1998;105:1659–1663. doi: 10.1016/S0161-6420(98)99035-2. [DOI] [PubMed] [Google Scholar]
- 6.Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. Journal of neuroimmunology. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- 7.Pope RM, Shahrara S. Possible roles of IL-12-family cytokines in rheumatoid arthritis. Nature reviews. Rheumatology. 2013;9:252–256. doi: 10.1038/nrrheum.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. Journal of immunology. 1992;148:3125–3132. [PubMed] [Google Scholar]
- 9.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. The Journal of experimental medicine. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perussia B, Chan SH, D'Andrea A, Tsuji K, Santoli D, Pospisil M, Young D, Wolf SF, Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. Journal of immunology. 1992;149:3495–3502. [PubMed] [Google Scholar]
- 12.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. The Journal of experimental medicine. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. The Journal of experimental medicine. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshpande P, Cavanagh MM, Le Saux S, Singh K, Weyand CM, Goronzy JJ. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. Journal of immunology. 2013;190:1416–1423. doi: 10.4049/jimmunol.1201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremblay MM, Bilal MY, Houtman JC. Prior TLR5 induction in human T cells results in a transient potentiation of subsequent TCR-induced cytokine production. Mol Immunol. 2014;57:161–170. doi: 10.1016/j.molimm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vacaflores A, Chapman NM, Harty JT, Richer MJ, Houtman JC. Exposure of Human CD4 T Cells to IL-12 Results in Enhanced TCR-Induced Cytokine Production, Altered TCR Signaling, and Increased Oxidative Metabolism. PLoS One. 2016;11:e0157175. doi: 10.1371/journal.pone.0157175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity. 2013;38:140–152. doi: 10.1016/j.immuni.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C, Jay DC, Williams MA. Dynamic functional modulation of CD4+ T cell recall responses is dependent on the inflammatory environment of the secondary stimulus. PLoS pathogens. 2014;10:e1004137. doi: 10.1371/journal.ppat.1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raue HP, Beadling C, Haun J, Slifka MK. Cytokine-mediated programmed proliferation of virus-specific CD8(+) memory T cells. Immunity. 2013;38:131–139. doi: 10.1016/j.immuni.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leist M, Hartung T. Inflammatory findings on species extrapolations: humans are definitely no 70-kg mice. Archives of toxicology. 2013;87:563–567. doi: 10.1007/s00204-013-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation LSCRP. Host Response to Injury, Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay MM, Houtman JC. TCR-mediated functions are enhanced in activated peripheral blood T cells isolated from leucocyte reduction systems. J Immunol Methods. 2015;416:137–145. doi: 10.1016/j.jim.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Madhumitha H, Mohan V, Deepa M, Babu S, Aravindhan V. Increased Th1 and suppressed Th2 serum cytokine levels in subjects with diabetic coronary artery disease. Cardiovascular diabetology. 2014;13:1. doi: 10.1186/1475-2840-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen KB, Biron CA. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. Journal of immunology. 1999;162:5238–5246. [PubMed] [Google Scholar]
- 27.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infection and immunity. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunological reviews. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 29.Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. International journal of molecular sciences. 2010;11:789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunological reviews. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houtman JC, Barda-Saad M, Samelson LE. Examining multiprotein signaling complexes from all angles. The FEBS journal. 2005;272:5426–5435. doi: 10.1111/j.1742-4658.2005.04972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annual review of immunology. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 34.Irvin BJ, Williams BL, Nilson AE, Maynor HO, Abraham RT. Pleiotropic contributions of phospholipase C-gamma1 (PLC-gamma1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-gamma1-deficient Jurkat T-cell line. Molecular and cellular biology. 2000;20:9149–9161. doi: 10.1128/mcb.20.24.9149-9161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu JN, Koretzky GA. The SLP-76 family of adapter proteins. Semin Immunol. 2004;16:379–393. doi: 10.1016/j.smim.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Shim EK, Jung SH, Lee JR. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. Journal of immunology. 2011;186:2926–2935. doi: 10.4049/jimmunol.1001785. [DOI] [PubMed] [Google Scholar]
- 37.Chapman NM, Yoder AN, Barbon KM, Bilal MY, Connolly SF, Houtman JC. Proline-rich tyrosine kinase 2 controls PI3-kinase activation downstream of the T cell antigen receptor in human T cells. Journal of leukocyte biology. 2015;97:285–296. doi: 10.1189/jlb.2A1013-568RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. American journal of translational research. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 39.Bilal MY, Houtman JCD. GRB2 nucleates T cell receptor-mediated LAT clusters that control PLC-gamma 1 activation and cytokine production. Frontiers in immunology. 2015;6 doi: 10.3389/fimmu.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun JE, Rubio I, Roose JP. Regulation of ras exchange factors and cellular localization of ras activation by lipid messengers in T cells. Frontiers in immunology. 2013;4:239. doi: 10.3389/fimmu.2013.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodeller F, Schulze-Koops H. The p38 mitogen-activated protein kinase signaling cascade in CD4 T cells. Arthritis Res Ther. 2006;8 doi: 10.1186/ar1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang KS, Zorn E, Ritz J. Specific down-regulation of interleukin-12 signaling through induction of phospho-STAT4 protein degradation. Blood. 2001;97:3860–3866. doi: 10.1182/blood.v97.12.3860. [DOI] [PubMed] [Google Scholar]
- 43.Huber JP, Farrar JD. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132:466–474. doi: 10.1111/j.1365-2567.2011.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zikherman J, Au-Yeung B. The role of T cell receptor signaling thresholds in guiding T cell fate decisions. Curr Opin Immunol. 2015;33:43–48. doi: 10.1016/j.coi.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nature reviews. Immunology. 2013;13:257–269. doi: 10.1038/nri3403. [DOI] [PubMed] [Google Scholar]
- 46.Bilal MY, Houtman JC. GRB2 Nucleates T Cell Receptor-Mediated LAT Clusters That Control PLC-gamma1 Activation and Cytokine Production. Frontiers in immunology. 2015;6:141. doi: 10.3389/fimmu.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.