Abstract
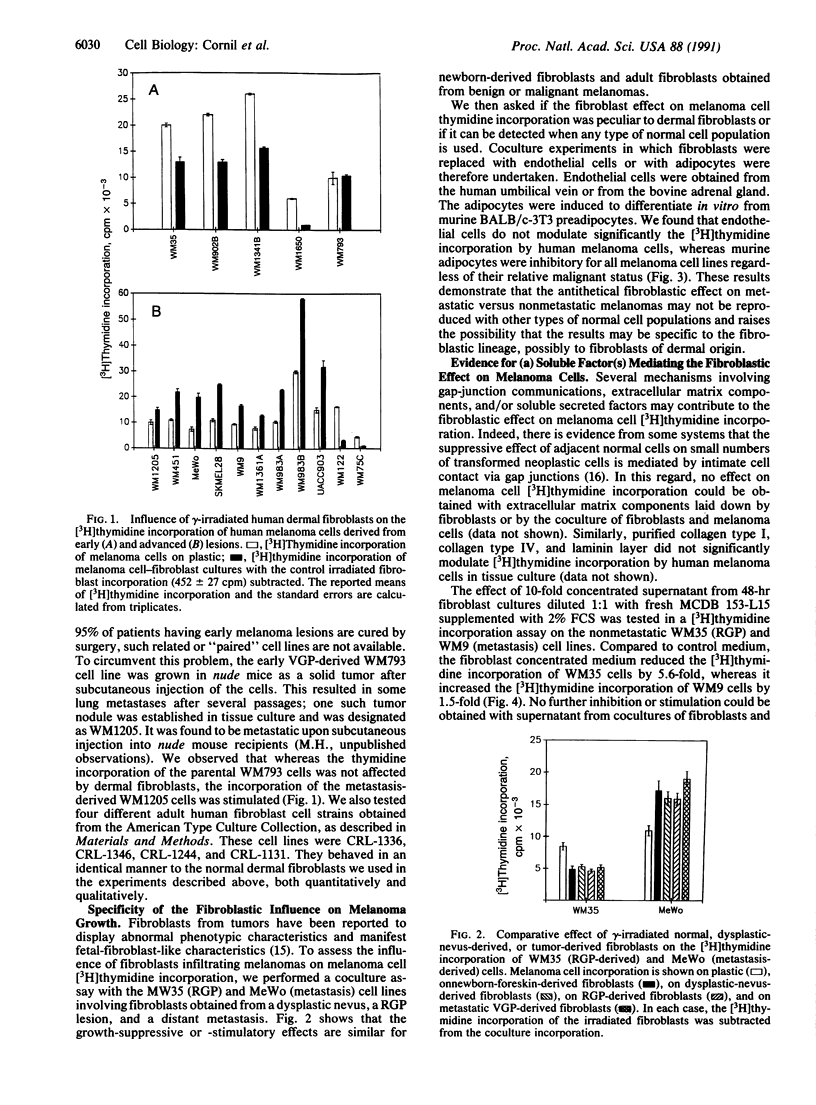
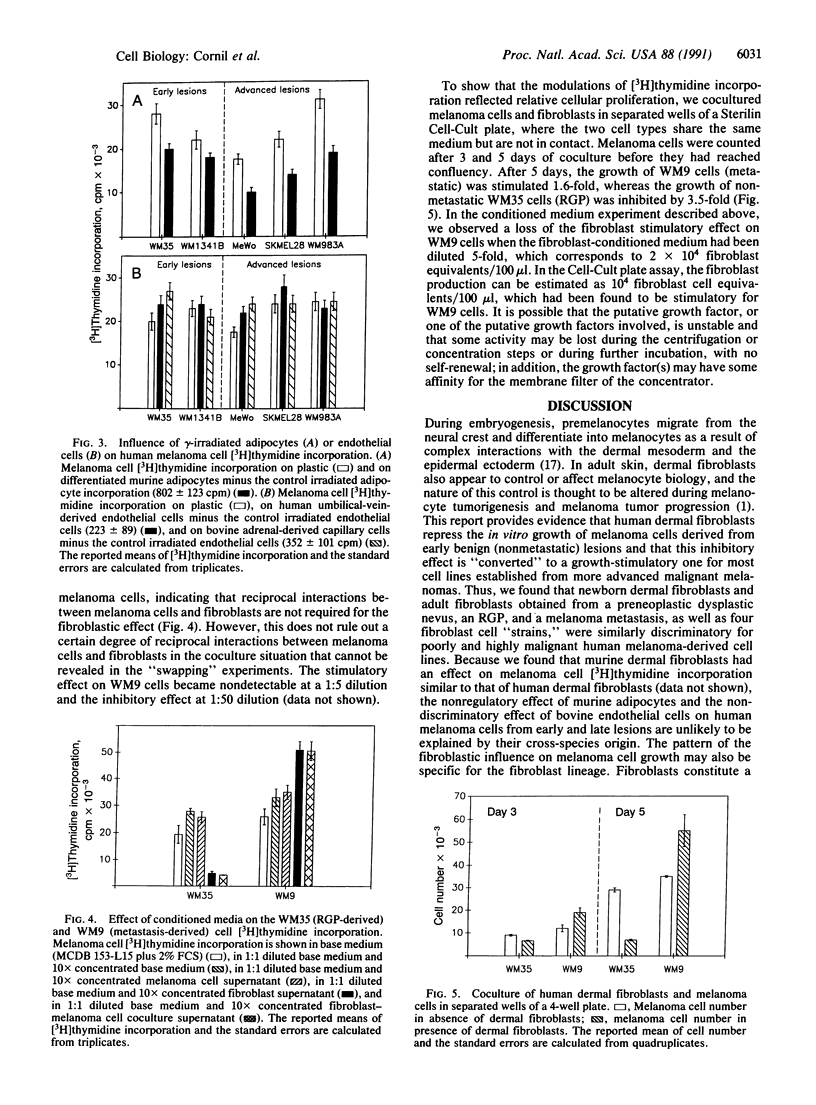
It is known from a variety of experimental systems that the ability of tumor cells to grow locally and metastasize can be affected by the presence of adjacent normal tissues and cells, particularly mesenchymally derived stromal cells such as fibroblasts. However, the comparative influence of such normal cell-tumor cell interactions on tumor behavior has not been thoroughly investigated from the perspective of different stages of tumor progression. To address this question we assessed the influence of normal dermal fibroblasts on the growth of human melanoma cells obtained from different stages of tumor progression. We found that the in vitro growth of most (4 out of 5) melanoma cell lines derived from early-stage radial growth phase or vertical growth phase metastatically incompetent primary lesions is repressed by coculture with normal dermal fibroblasts, suggesting that negative homeostatic growth controls are still operative on melanoma cells from early stages of disease. On the other hand, 9 out of 11 melanoma cell lines derived from advanced metastatically competent vertical growth phase primary lesions, or from distant metastases, were found to be consistently stimulated to grow in the presence of dermal fibroblasts. Evidence was obtained to show that this discriminatory fibroblastic influence is mediated by soluble inhibitory and stimulatory growth factor(s). Taken together, these results indicate that fibroblast-derived signals can have antithetical growth effects on metastatic versus metastatically incompetent tumor subpopulations. This resultant conversion in responsiveness to host tissue environmental factors may confer upon small numbers of metastatically competent cells a growth advantage, allowing them to escape local growth constraints both in the primary tumor site and at distant ectopic tissue sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blat C., Chatelain G., Desauty G., Harel L. Inhibitory diffusible factor IDF45, a G1 phase inhibitor. FEBS Lett. 1986 Jul 28;203(2):175–180. doi: 10.1016/0014-5793(86)80737-2. [DOI] [PubMed] [Google Scholar]
- Chadwick D. E., Lagarde A. E. Coincidental acquisition of growth autonomy and metastatic potential during the malignant transformation of factor-dependent CCL39 lung fibroblasts. J Natl Cancer Inst. 1988 May 4;80(5):318–325. doi: 10.1093/jnci/80.5.318. [DOI] [PubMed] [Google Scholar]
- Cornil I., Man S., Fernandez B., Kerbel R. S. Enhanced tumorigenicity, melanogenesis, and metastases of a human malignant melanoma after subdermal implantation in nude mice. J Natl Cancer Inst. 1989 Jun 21;81(12):938–944. doi: 10.1093/jnci/81.12.938. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Bigsby R. M., Cooke P. S., Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985 Sep;17(3):137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Moellmann G., Ghosh S., Edwards M., Halaban R. Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotype in a reconstituted cutaneous environment. J Cell Biol. 1989 Dec;109(6 Pt 1):3115–3128. doi: 10.1083/jcb.109.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Weinberg R. A., Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6389–6393. doi: 10.1073/pnas.85.17.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Garin-Chesa P., Old L. J., Rettig W. J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson M., Graves K., Carson S. D., Wells R. S., Pierce G. B. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Kath R., Williams N., Valyi-Nagy I., Rodeck U. Growth-regulatory factors for normal, premalignant, and malignant human cells in vitro. Adv Cancer Res. 1990;54:213–234. doi: 10.1016/s0065-230x(08)60812-x. [DOI] [PubMed] [Google Scholar]
- Hsu Y. M., Wang J. L. Growth control in cultured 3T3 fibroblasts. V. Purification of an Mr 13,000 polypeptide responsible for growth inhibitory activity. J Cell Biol. 1986 Feb;102(2):362–369. doi: 10.1083/jcb.102.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S. Growth dominance of the metastatic cancer cell: cellular and molecular aspects. Adv Cancer Res. 1990;55:87–132. doi: 10.1016/s0065-230x(08)60469-8. [DOI] [PubMed] [Google Scholar]
- Klein G. Multistep emancipation of tumors from growth control: can it be curbed in a single step? Bioessays. 1990 Jul;12(7):347–350. doi: 10.1002/bies.950120708. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Vogel F., Funa K., Heldin C. H., Grosse R. Developmental regulation of mammary-derived growth inhibitor expression in bovine mammary tissue. J Cell Biol. 1990 May;110(5):1779–1789. doi: 10.1083/jcb.110.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rocca S. A., Grossi M., Falcone G., Alemà S., Tatò F. Interaction with normal cells suppresses the transformed phenotype of v-myc-transformed quail muscle cells. Cell. 1989 Jul 14;58(1):123–131. doi: 10.1016/0092-8674(89)90409-1. [DOI] [PubMed] [Google Scholar]
- Mayer T. C. Site of gene action in steel mice: analysis of the pigment defect by mesoderm-ectoderm recombinations. J Exp Zool. 1973 Jun;184(3):345–352. doi: 10.1002/jez.1401840308. [DOI] [PubMed] [Google Scholar]
- Miller F. R., Medina D., Heppner G. H. Preferential growth of mammary tumors in intact mammary fatpads. Cancer Res. 1981 Oct;41(10):3863–3867. [PubMed] [Google Scholar]
- Montesano R., Mossaz A., Ryser J. E., Orci L., Vassalli P. Leukocyte interleukins induce cultured endothelial cells to produce a highly organized, glycosaminoglycan-rich pericellular matrix. J Cell Biol. 1984 Nov;99(5):1706–1715. doi: 10.1083/jcb.99.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett T. R., Wang J. K., Jones P. A. Mesenchymal-epithelial interactions between normal and transformed human bladder cells. Cancer Res. 1989 May 15;49(10):2750–2754. [PubMed] [Google Scholar]
- Rodeck U., Herlyn M., Menssen H. D., Furlanetto R. W., Koprowsk H. Metastatic but not primary melanoma cell lines grow in vitro independently of exogenous growth factors. Int J Cancer. 1987 Nov 15;40(5):687–690. doi: 10.1002/ijc.2910400520. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M. Clonal heterogeneity in fibroblast phenotype: implications for the control of epithelial-mesenchymal interactions. Bioessays. 1987 Nov;7(5):200–204. doi: 10.1002/bies.950070503. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Durning P., Rushton G. Skin fibroblasts obtained from cancer patients display foetal-like migratory behaviour on collagen gels. J Cell Sci. 1985 Feb;73:235–244. doi: 10.1242/jcs.73.1.235. [DOI] [PubMed] [Google Scholar]
- Schwarz L. C., Gingras M. C., Goldberg G., Greenberg A. H., Wright J. A. Loss of growth factor dependence and conversion of transforming growth factor-beta 1 inhibition to stimulation in metastatic H-ras-transformed murine fibroblasts. Cancer Res. 1988 Dec 15;48(24 Pt 1):6999–7003. [PubMed] [Google Scholar]
- Swope V. B., Abdel-Malek Z., Kassem L. M., Nordlund J. J. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991 Feb;96(2):180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- Whicher J. T., Evans S. W. Cytokines in disease. Clin Chem. 1990 Jul;36(7):1269–1281. [PubMed] [Google Scholar]



