Abstract
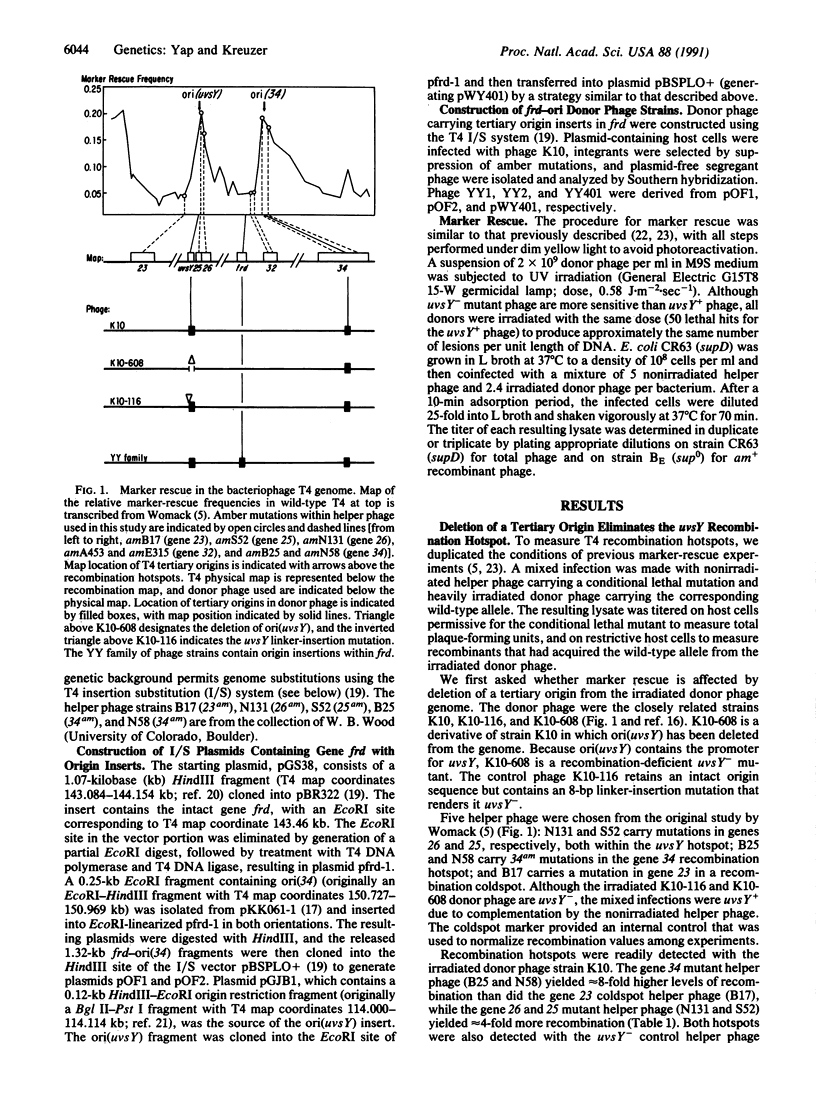
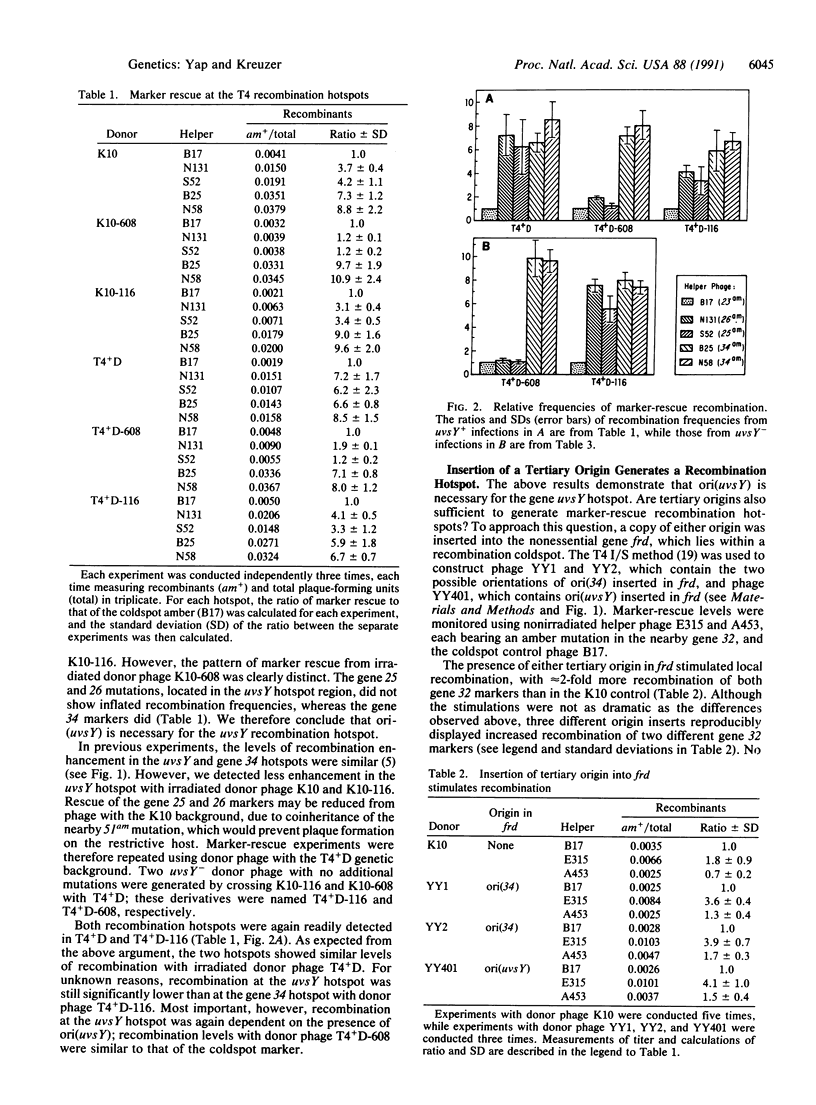
Bacteriophage T4 recombination "hotspots" were first detected by the rescue of genetic markers from UV-irradiated phage particles. These hotspots have since been detected following treatments that yield other forms of DNA damage, and at least one is active in the absence of damage. The previous mapping of phage replication origins near the peaks of two recombination hotspots suggested that the origins cause the localized enhancement of recombination. Here we show that deletion of one origin eliminates the corresponding recombination hotspot, as judged by rescue of markers from UV-irradiated phage. Furthermore, insertion of either origin into a recombination "coldspot" enhances rescue of nearby markers. We conclude that these origins are necessary, and very likely sufficient, for the generation of recombination hotspots. We also show that the hotspots are active in the absence of both phage-encoded UvsY and host-encoded RecA proteins, suggesting that some of the stimulated recombination occurs by a synaptase-independent mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckendorf S. K., Wilson J. H. A recombination gradient in bacteriophage T4 gene 34. Virology. 1972 Nov;50(2):315–321. doi: 10.1016/0042-6822(72)90382-0. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Burck K. B., Miller R. C., Jr Marker rescue and partial replication of bacteriophage T7 DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6144–6148. doi: 10.1073/pnas.75.12.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. P., Berger H. Mutations affecting genetic recombination in bacteriophage T4D. I. Pathway analysis. Virology. 1977 Jul 1;80(1):67–82. doi: 10.1016/0042-6822(77)90381-6. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Berger H. Mutations affecting genetic recombination in bacteriophage T4D. II. Genetic properties. Virology. 1978 Jul 1;88(1):62–70. doi: 10.1016/0042-6822(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., James A. A., Kolodner R. recA-independent general genetic recombination of plasmids. Nature. 1981 Nov 12;294(5837):184–186. doi: 10.1038/294184a0. [DOI] [PubMed] [Google Scholar]
- Formosa T., Alberts B. M. DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986 Dec 5;47(5):793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Mattson T., Kozinski A. W. Origins of phage T4 DNA replication as revealed by hybridization to cloned genes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6137–6141. doi: 10.1073/pnas.76.12.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett N. V., Berger H. Mutations altering genetic recombination and repair of DNA in bacteriophage T4. Virology. 1975 Feb;63(2):539–567. doi: 10.1016/0042-6822(75)90326-8. [DOI] [PubMed] [Google Scholar]
- Harris L. D., Griffith J. D. UvsY protein of bacteriophage T4 is an accessory protein for in vitro catalysis of strand exchange. J Mol Biol. 1989 Mar 5;206(1):19–27. doi: 10.1016/0022-2836(89)90520-2. [DOI] [PubMed] [Google Scholar]
- Hoess R., Wierzbicki A., Abremski K. Isolation and characterization of intermediates in site-specific recombination. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6840–6844. doi: 10.1073/pnas.84.19.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodadek T., Gan D. C., Stemke-Hale K. The phage T4 uvs Y recombination protein stabilizes presynaptic filaments. J Biol Chem. 1989 Oct 5;264(28):16451–16457. [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. A defective phage system reveals bacteriophage T4 replication origins that coincide with recombination hot spots. Proc Natl Acad Sci U S A. 1985 May;82(10):3345–3349. doi: 10.1073/pnas.82.10.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. Characterization of a defective phage system for the analysis of bacteriophage T4 DNA replication origins. J Mol Biol. 1986 Mar 20;188(2):185–198. doi: 10.1016/0022-2836(86)90303-7. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Engman H. W., Yap W. Y. Tertiary initiation of replication in bacteriophage T4. Deletion of the overlapping uvsY promoter/replication origin from the phage genome. J Biol Chem. 1988 Aug 15;263(23):11348–11357. [PubMed] [Google Scholar]
- Kreuzer K. N., Yap W. Y., Menkens A. E., Engman H. W. Recombination-dependent replication of plasmids during bacteriophage T4 infection. J Biol Chem. 1988 Aug 15;263(23):11366–11373. [PubMed] [Google Scholar]
- Kuempel P. L., Pelletier A. J., Hill T. M. Tus and the terminators: the arrest of replication in prokaryotes. Cell. 1989 Nov 17;59(4):581–583. doi: 10.1016/0092-8674(89)90001-9. [DOI] [PubMed] [Google Scholar]
- Levy J. N. Effects of radiophosphorus decay in bacteriophage T4D. II. The mechanism of marker rescue. Virology. 1975 Nov;68(1):14–26. doi: 10.1016/0042-6822(75)90143-9. [DOI] [PubMed] [Google Scholar]
- Levy J. N., Goldberg E. B. Region-specific recombination in phage T4. I. A special glucosyl-dependent recombination system. Genetics. 1980 Mar;94(3):519–530. doi: 10.1093/genetics/94.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luder A., Mosig G. Two alternative mechanisms for initiation of DNA replication forks in bacteriophage T4: priming by RNA polymerase and by recombination. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1101–1105. doi: 10.1073/pnas.79.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkens A. E., Kreuzer K. N. Deletion analysis of bacteriophage T4 tertiary origins. A promoter sequence is required for a rifampicin-resistant replication origin. J Biol Chem. 1988 Aug 15;263(23):11358–11365. [PubMed] [Google Scholar]
- Mosig G. A map of distances along the DNA molecule of phage T4. Genetics. 1968 Jun;59(2):137–151. doi: 10.1093/genetics/59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. Distances separating genetic markers in T4 DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1177–1183. doi: 10.1073/pnas.56.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. The essential role of recombination in phage T4 growth. Annu Rev Genet. 1987;21:347–371. doi: 10.1146/annurev.ge.21.120187.002023. [DOI] [PubMed] [Google Scholar]
- Rayssiguier C., Kozinski A. W., Doermann A. H. Partial replicas of UV-irradiated bacteriophage T4 genomes and their role in multiplicity reactivation. J Virol. 1980 Aug;35(2):451–465. doi: 10.1128/jvi.35.2.451-465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayssiguier C., Vigier P. R. Genetic evidence for the existence of partial replicas of T4 genomes inactivated by irradiation under ultraviolet light.?*ULTRAVIOLET RAYS. Virology. 1977 May 15;78(2):442–452. doi: 10.1016/0042-6822(77)90121-0. [DOI] [PubMed] [Google Scholar]
- Selick H. E., Kreuzer K. N., Alberts B. M. The bacteriophage T4 insertion/substitution vector system. A method for introducing site-specific mutations into the virus chromosome. J Biol Chem. 1988 Aug 15;263(23):11336–11347. [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Womack F. C. Cross-reactivation differences in bacteriophage T4D. Virology. 1965 Aug;26(4):758–760. [PubMed] [Google Scholar]
- Yee J. K., Marsh R. C. Locations of bacteriophage T4 origins of replication. J Virol. 1985 May;54(2):271–277. doi: 10.1128/jvi.54.2.271-277.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonesaki T., Minagawa T. Synergistic action of three recombination gene products of bacteriophage T4, uvsX, uvsY, and gene 32 proteins. J Biol Chem. 1989 May 15;264(14):7814–7820. [PubMed] [Google Scholar]



