Abstract
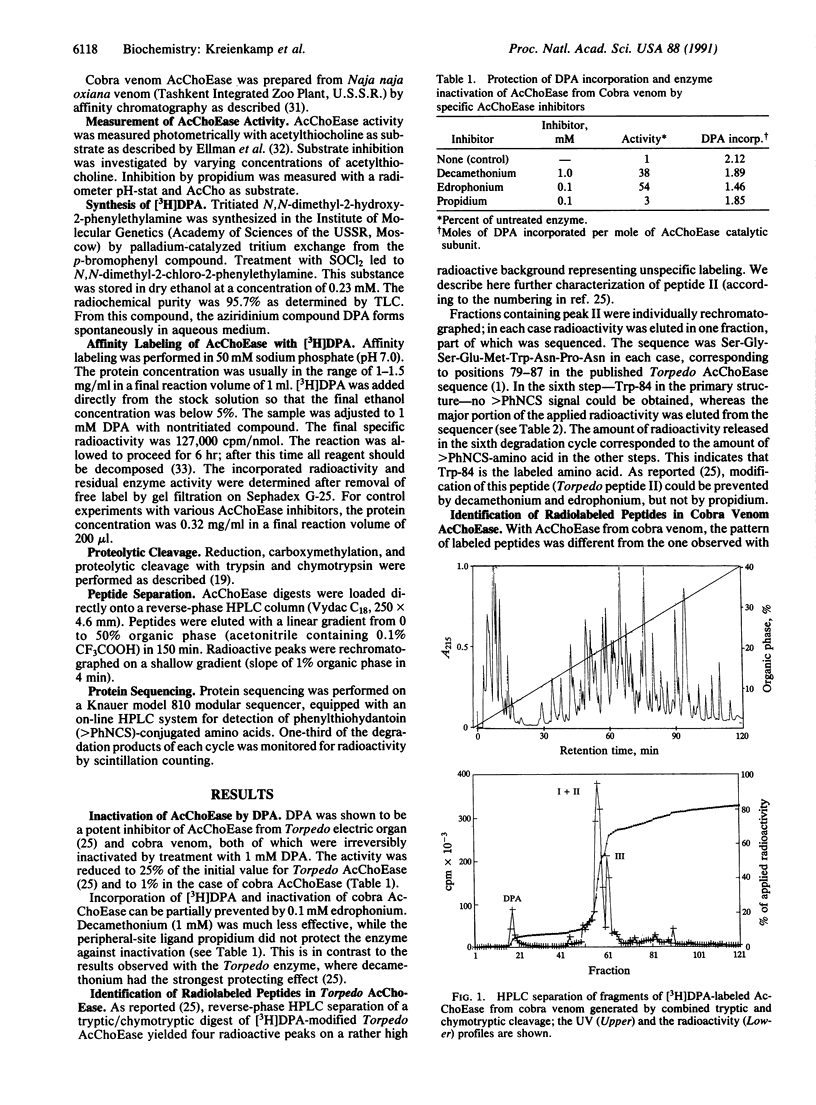
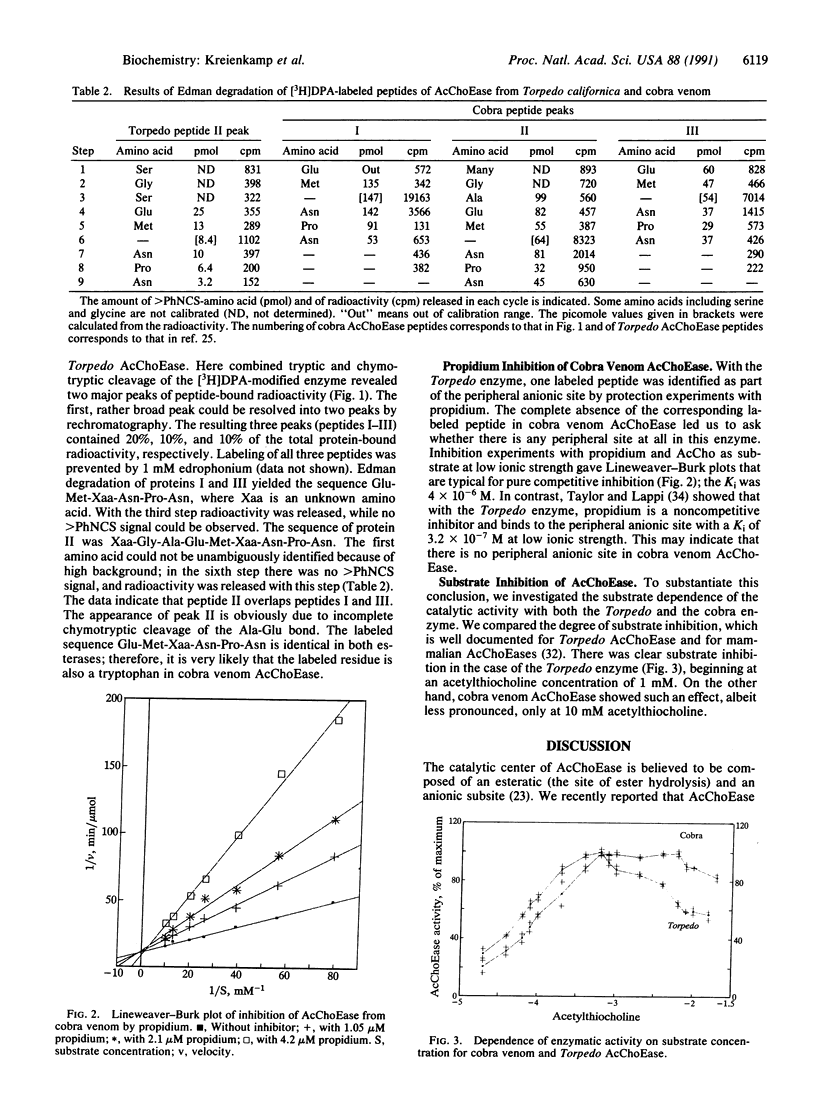
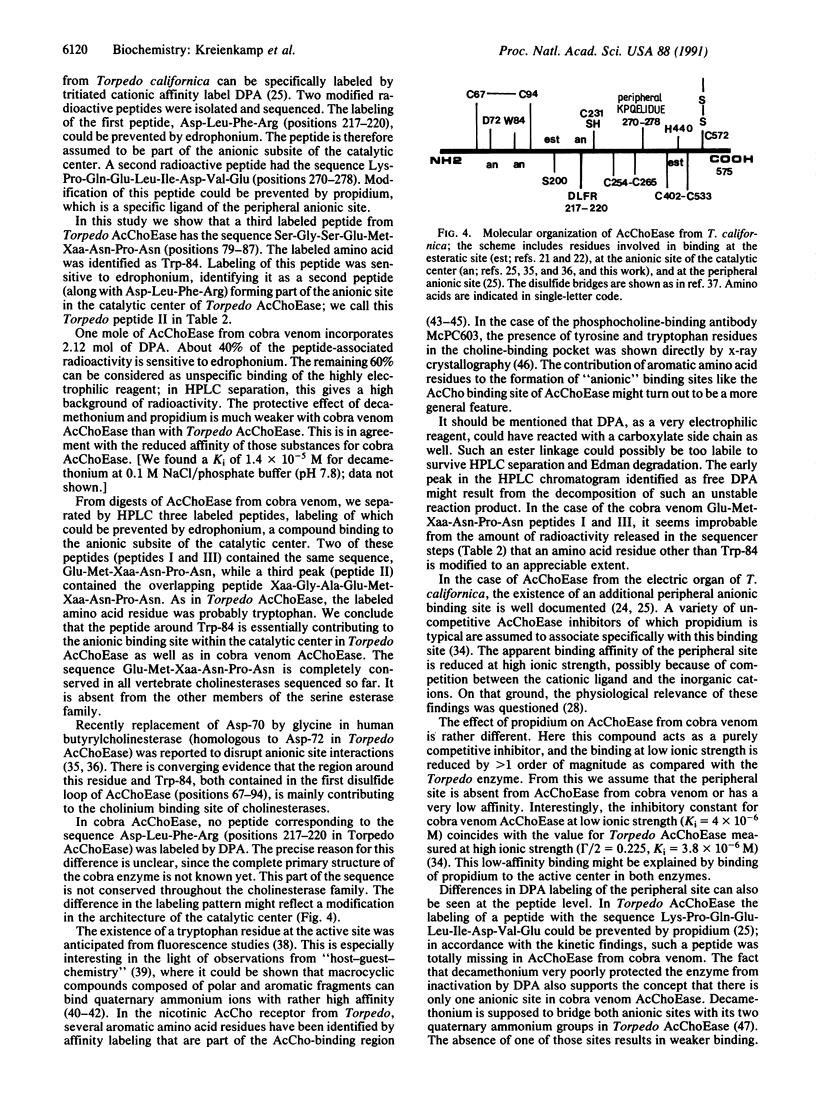
A peptide of acetylcholinesterase (AcChoEase; acetylcholine acetylhydrolase, EC 3.1.1.7) from the venom of the cobra Naja naja oxiana labeled by the affinity reagent N,N-dimethyl-2-phenylaziridinium (DPA) has been identified. The sequence is Gly-Ala-Glu-Met-Trp-Asn-Pro-Asn. In AcChoEase from Torpedo californica, a homologous peptide was labeled and isolated. Its sequence is Ser-Gly-Ser-Glu-Met-Trp-Asn-Pro-Asn, representing positions 79 through 87. In both cases labeling can be prevented by 0.1 mM edrophonium, indicating that the respective peptides form part of the anionic subsite of the catalytic center. The modified residue was tryptophan (Trp-84 in Torpedo AcChoEase) in both enzymes. In contrast to AcChoEase from Torpedo, the enzyme from cobra venom does not contain a peripheral anionic binding site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. N., Li Y., Culver P., Taylor P. An analog of lophotoxin reacts covalently with Tyr190 in the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1989 Jul 25;264(21):12666–12672. [PubMed] [Google Scholar]
- Belleau B., Tani H. A novel irreversible inhibitor of acetylcholinesterase specifically directed at the anionic binding site: structure-activity relationships. Mol Pharmacol. 1966 Sep;2(5):411–422. [PubMed] [Google Scholar]
- Bomblies L., Biegelmann E., Döring V., Gerisch G., Krafft-Czepa H., Noegel A. A., Schleicher M., Humbel B. M. Membrane-enclosed crystals in Dictyostelium discoideum cells, consisting of developmentally regulated proteins with sequence similarities to known esterases. J Cell Biol. 1990 Mar;110(3):669–679. doi: 10.1083/jcb.110.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Lazure C., Chrétien M., Changeux J. P. Amino acids of the Torpedo marmorata acetylcholine receptor alpha subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry. 1988 Apr 5;27(7):2346–2357. doi: 10.1021/bi00407a016. [DOI] [PubMed] [Google Scholar]
- Doctor B. P., Chapman T. C., Christner C. E., Deal C. D., De La Hoz D. M., Gentry M. K., Ogert R. A., Rush R. S., Smyth K. K., Wolfe A. D. Complete amino acid sequence of fetal bovine serum acetylcholinesterase and its comparison in various regions with other cholinesterases. FEBS Lett. 1990 Jun 18;266(1-2):123–127. doi: 10.1016/0014-5793(90)81522-p. [DOI] [PubMed] [Google Scholar]
- Dougherty D. A., Stauffer D. A. Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science. 1990 Dec 14;250(4987):1558–1560. doi: 10.1126/science.2274786. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I., Shinitzky M., Blumberg S. Purification by affinity chromatography of the molecular forms of acetylcholinesterase present in fresh electric-organ tissue of electric eel. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2400–2403. doi: 10.1073/pnas.69.9.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Epstein D. J., Berman H. A., Taylor P. Ligand-induced conformational changes in acetylcholinesterase investigated with fluorescent phosphonates. Biochemistry. 1979 Oct 16;18(21):4749–4754. doi: 10.1021/bi00588a040. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Black D., Goeldner M., Hirth C., Changeux J. P. Identification of a novel amino acid alpha-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. J Biol Chem. 1990 Jun 25;265(18):10430–10437. [PubMed] [Google Scholar]
- Gibney G., Camp S., Dionne M., MacPhee-Quigley K., Taylor P. Mutagenesis of essential functional residues in acetylcholinesterase. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7546–7550. doi: 10.1073/pnas.87.19.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5' leader. EMBO J. 1986 Nov;5(11):2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hanzlik T. N., Abdel-Aal Y. A., Harshman L. G., Hammock B. D. Isolation and sequencing of cDNA clones coding for juvenile hormone esterase from Heliothis virescens. Evidence for a catalytic mechanism for the serine carboxylesterases different from that of the serine proteases. J Biol Chem. 1989 Jul 25;264(21):12419–12425. [PubMed] [Google Scholar]
- Korza G., Ozols J. Complete covalent structure of 60-kDa esterase isolated from 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced rabbit liver microsomes. J Biol Chem. 1988 Mar 5;263(7):3486–3495. [PubMed] [Google Scholar]
- Krupka R. M. Hydrolysis of neutral substrates by acetylcholinesterase. Biochemistry. 1966 Jun;5(6):1983–1988. doi: 10.1021/bi00870a028. [DOI] [PubMed] [Google Scholar]
- Kupke T., Lechner M., Kaim G., Götz F. Improved purification and biochemical properties of phosphatidylinositol-specific phospholipase C from Bacillus thuringiensis. Eur J Biochem. 1989 Oct 20;185(1):151–155. doi: 10.1111/j.1432-1033.1989.tb15096.x. [DOI] [PubMed] [Google Scholar]
- Kyger E. M., Wiegand R. C., Lange L. G. Cloning of the bovine pancreatic cholesterol esterase/lysophospholipase. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1302–1309. doi: 10.1016/0006-291x(89)91811-1. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Taylor P., Taylor S. Primary structures of the catalytic subunits from two molecular forms of acetylcholinesterase. A comparison of NH2-terminal and active center sequences. J Biol Chem. 1985 Oct 5;260(22):12185–12189. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Vedvick T. S., Taylor P., Taylor S. S. Profile of the disulfide bonds in acetylcholinesterase. J Biol Chem. 1986 Oct 15;261(29):13565–13570. [PubMed] [Google Scholar]
- McGuire M. C., Nogueira C. P., Bartels C. F., Lightstone H., Hajra A., Van der Spek A. F., Lockridge O., La Du B. N. Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase. Proc Natl Acad Sci U S A. 1989 Feb;86(3):953–957. doi: 10.1073/pnas.86.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Myers M., Richmond R. C., Oakeshott J. G. On the origins of esterases. Mol Biol Evol. 1988 Mar;5(2):113–119. doi: 10.1093/oxfordjournals.molbev.a040485. [DOI] [PubMed] [Google Scholar]
- NACHMANSOHN D., WILSON I. B. The enzymic hydrolysis and synthesis of acetylcholine. Adv Enzymol Relat Subj Biochem. 1951;12:259–339. doi: 10.1002/9780470122570.ch5. [DOI] [PubMed] [Google Scholar]
- Neville L. F., Gnatt A., Padan R., Seidman S., Soreq H. Anionic site interactions in human butyrylcholinesterase disrupted by two single point mutations. J Biol Chem. 1990 Dec 5;265(34):20735–20738. [PubMed] [Google Scholar]
- Oakeshott J. G., Collet C., Phillis R. W., Nielsen K. M., Russell R. J., Chambers G. K., Ross V., Richmond R. C. Molecular cloning and characterization of esterase-6, a serine hydrolase of Drosophila. Proc Natl Acad Sci U S A. 1987 May;84(10):3359–3363. doi: 10.1073/pnas.84.10.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba R., Aaviksaar A., Raba M., Siigur J. Cobra venom acetylcholinesterase. Purification and molecular properties. Eur J Biochem. 1979 May 2;96(1):151–158. doi: 10.1111/j.1432-1033.1979.tb13024.x. [DOI] [PubMed] [Google Scholar]
- Rachinsky T. L., Camp S., Li Y., Ekström T. J., Newton M., Taylor P. Molecular cloning of mouse acetylcholinesterase: tissue distribution of alternatively spliced mRNA species. Neuron. 1990 Sep;5(3):317–327. doi: 10.1016/0896-6273(90)90168-f. [DOI] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Shinitzky Meir, Dudai Yadin, Silman Israel. Spectral evidence for the presence of tryptophan in the binding site of acetylcholinesterase. FEBS Lett. 1973 Feb 15;30(1):125–128. doi: 10.1016/0014-5793(73)80633-7. [DOI] [PubMed] [Google Scholar]
- Sikorav J. L., Krejci E., Massoulié J. cDNA sequences of Torpedo marmorata acetylcholinesterase: primary structure of the precursor of a catalytic subunit; existence of multiple 5'-untranslated regions. EMBO J. 1987 Jul;6(7):1865–1873. doi: 10.1002/j.1460-2075.1987.tb02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Varon L., Toker L., Futerman A. H., Silman I. Purification and crystallization of a dimeric form of acetylcholinesterase from Torpedo californica subsequent to solubilization with phosphatidylinositol-specific phospholipase C. J Mol Biol. 1988 Oct 5;203(3):821–823. doi: 10.1016/0022-2836(88)90213-6. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B. Interaction of selected cholinergic effector molecules with acetylcholinesterase in physiological eel Ringer's solution. Mol Pharmacol. 1973 Jul;9(4):561–570. [PubMed] [Google Scholar]
- Taylor P., Lappi S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry. 1975 May 6;14(9):1989–1997. doi: 10.1021/bi00680a029. [DOI] [PubMed] [Google Scholar]
- Weise C., Kreienkamp H. J., Raba R., Aaviksaar A., Hucho F. The active site and partial sequence of cobra venom acetylcholinesterase. J Protein Chem. 1990 Feb;9(1):53–57. doi: 10.1007/BF01024984. [DOI] [PubMed] [Google Scholar]
- Weise C., Kreienkamp H. J., Raba R., Pedak A., Aaviksaar A., Hucho F. Anionic subsites of the acetylcholinesterase from Torpedo californica: affinity labelling with the cationic reagent N,N-dimethyl-2-phenyl-aziridinium. EMBO J. 1990 Dec;9(12):3885–3888. doi: 10.1002/j.1460-2075.1990.tb07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller A. E. Snake venom action: are enzymes involved in it? Experientia. 1977 Feb 15;33(2):143–150. doi: 10.1007/BF02124033. [DOI] [PubMed] [Google Scholar]
- de la Escalera S., Bockamp E. O., Moya F., Piovant M., Jiménez F. Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 1990 Nov;9(11):3593–3601. doi: 10.1002/j.1460-2075.1990.tb07570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



