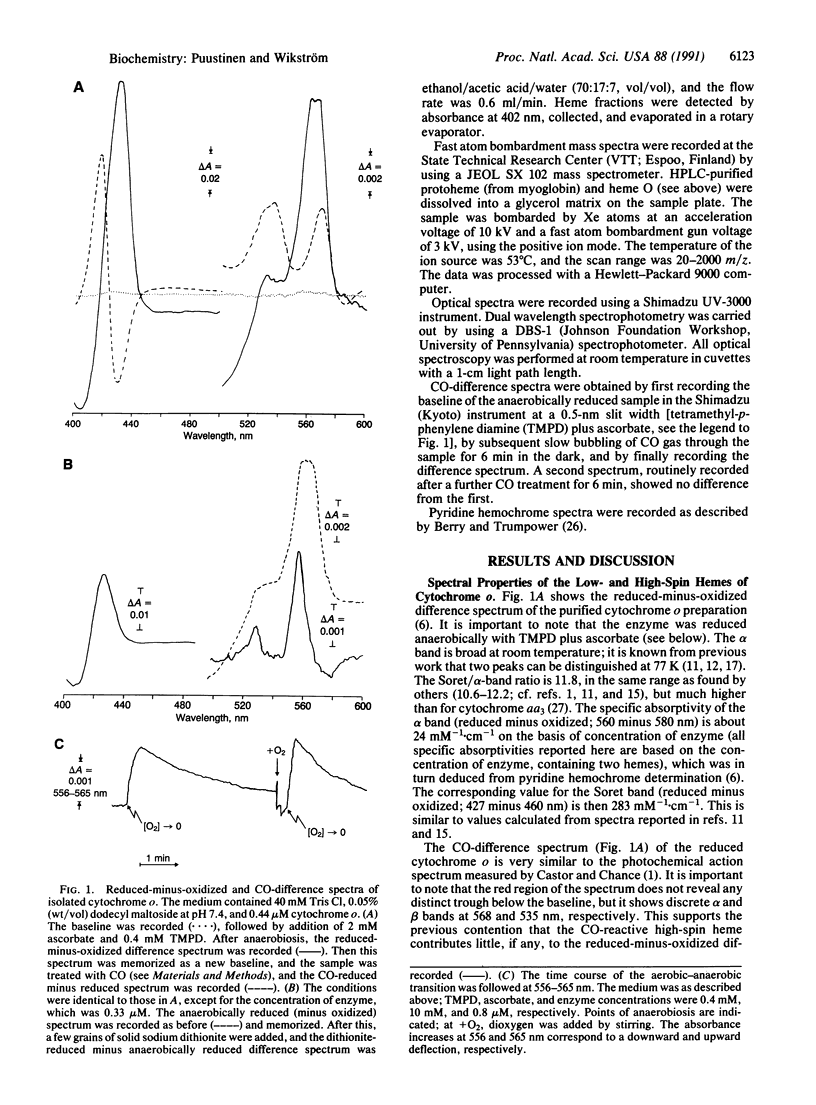
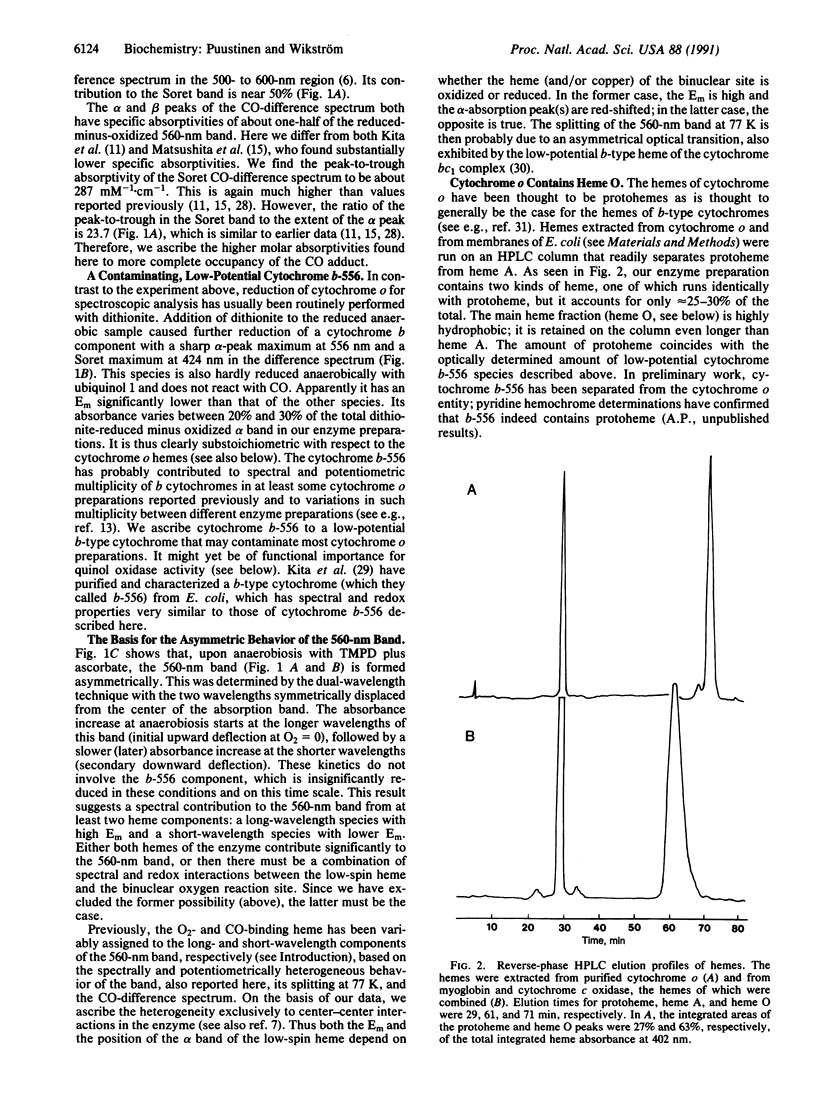
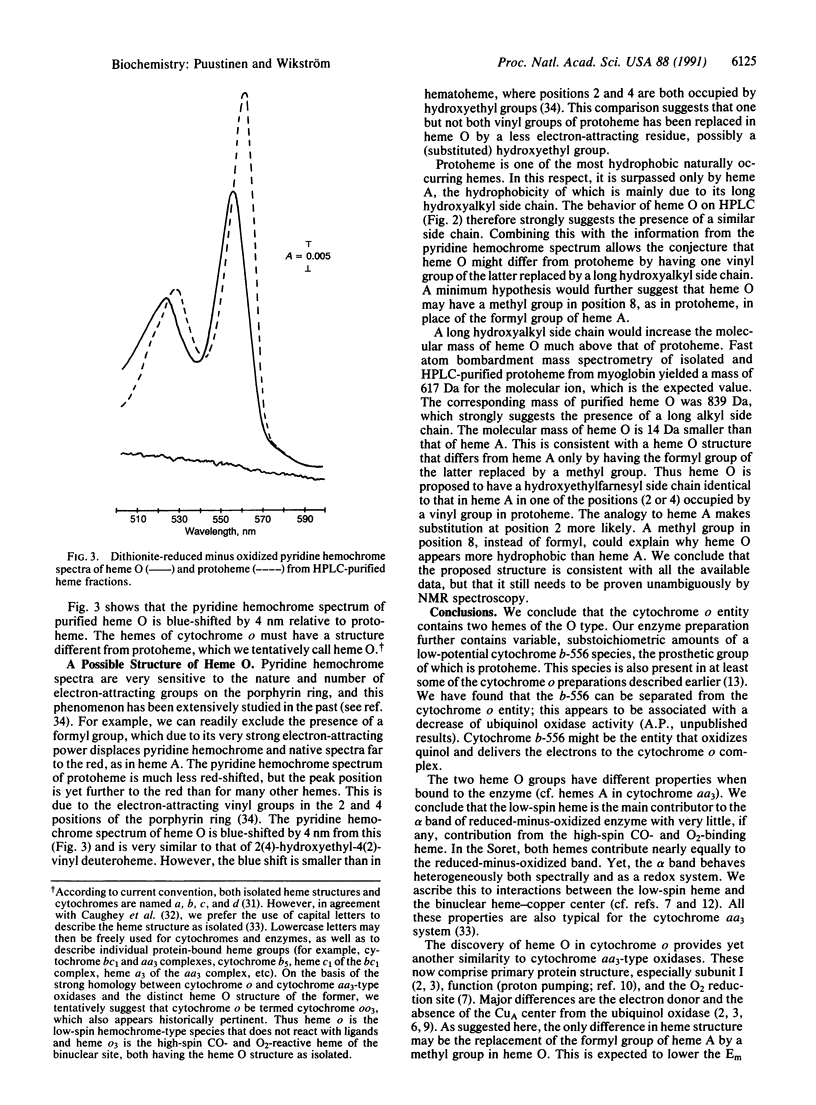
Abstract
Cytochrome o, one of the two terminal ubiquinol oxidases of Escherichia coli, is structurally and functionally related to cytochrome c oxidase of mitochondria and some bacteria. It has two heme groups, one of which binds CO and forms a binuclear oxygen reaction center with copper. The other heme is unreactive toward ligands, exhibits strong interactions with the binuclear center, and is mainly responsible for the reduced-minus-oxidized alpha band. Protoheme has been thought to be the prosthetic group of b-type cytochromes, including cytochrome o. However, the hemes of cytochrome o are of a different kind, for which we propose the name heme O. Its pyridine hemochrome spectrum is blue-shifted by 4 nm relative to that of protoheme, and chromatographic behavior showed that it is much more hydrophobic than protoheme. Fast atom bombardment mass spectrometry yielded a molecular mass of 839 Da. Heme O is proposed to be a heme A-like molecule, containing a 17-carbon hydroxyethylfarnesyl side chain, but with a methyl residue replacing the formyl group.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au D. C., Gennis R. B. Cloning of the cyo locus encoding the cytochrome o terminal oxidase complex of Escherichia coli. J Bacteriol. 1987 Jul;169(7):3237–3242. doi: 10.1128/jb.169.7.3237-3242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987 Feb 15;161(1):1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Caughey W. S., Smythe G. A., O'Keeffe D. H., Maskasky J. E., Smith M. I. Heme A of cytochrome c oxicase. Structure and properties: comparisons with hemes B, C, and S and derivatives. J Biol Chem. 1975 Oct 10;250(19):7602–7622. [PubMed] [Google Scholar]
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Hata A., Kirino Y., Matsuura K., Itoh S., Hiyama T., Konishi K., Kita K., Anraku Y. Assignment of ESR signals of Escherichia coli terminal oxidase complexes. Biochim Biophys Acta. 1985 Oct 29;810(1):62–72. doi: 10.1016/0005-2728(85)90206-3. [DOI] [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. I. Purification and properties of cytochrome b562-o complex from cells in the early exponential phase of aerobic growth. J Biol Chem. 1984 Mar 10;259(5):3368–3374. [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984 Mar 10;259(5):3375–3381. [PubMed] [Google Scholar]
- Kita K., Yamato I., Anraku Y. Purification and properties of cytochrome b556 in the respiratory chain of aerobically grown Escherichia coli K12. J Biol Chem. 1978 Dec 25;253(24):8910–8915. [PubMed] [Google Scholar]
- Ludwig B. Cytochrome c oxidase from Paracoccus denitrificans. Methods Enzymol. 1986;126:153–159. doi: 10.1016/s0076-6879(86)26017-6. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Patel L., Kaback H. R. Cytochrome o type oxidase from Escherichia coli. Characterization of the enzyme and mechanism of electrochemical proton gradient generation. Biochemistry. 1984 Sep 25;23(20):4703–4714. doi: 10.1021/bi00315a028. [DOI] [PubMed] [Google Scholar]
- Minagawa J., Nakamura H., Yamato I., Mogi T., Anraku Y. Transcriptional regulation of the cytochrome b562-o complex in Escherichia coli. Gene expression and molecular characterization of the promoter. J Biol Chem. 1990 Jul 5;265(19):11198–11203. [PubMed] [Google Scholar]
- Nakamura H., Yamato I., Anraku Y., Lemieux L., Gennis R. B. Expression of cyoA and cyoB demonstrates that the CO-binding heme component of the Escherichia coli cytochrome o complex is in subunit I. J Biol Chem. 1990 Jul 5;265(19):11193–11197. [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Finel M., Haltia T., Gennis R. B., Wikström M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 1991 Apr 23;30(16):3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Finel M., Virkki M., Wikström M. Cytochrome o (bo) is a proton pump in Paracoccus denitrificans and Escherichia coli. FEBS Lett. 1989 Jun 5;249(2):163–167. doi: 10.1016/0014-5793(89)80616-7. [DOI] [PubMed] [Google Scholar]
- Salerno J. C., Bolgiano B., Ingledew W. J. Potentiometric titration of cytochrome-bo type quinol oxidase of Escherichia coli: evidence for heme-heme and copper-heme interaction. FEBS Lett. 1989 Apr 10;247(1):101–105. doi: 10.1016/0014-5793(89)81249-9. [DOI] [PubMed] [Google Scholar]
- Salerno J. C., Bolgiano B., Poole R. K., Gennis R. B., Ingledew W. J. Heme-copper and heme-heme interactions in the cytochrome bo-containing quinol oxidase of Escherichia coli. J Biol Chem. 1990 Mar 15;265(8):4364–4368. [PubMed] [Google Scholar]
- Saraste M., Raitio M., Jalli T., Chepuri V., Lemieux L., Gennis R. B. Cytochrome o from Escherichia coli is structurally related to cytochrome aa3. Ann N Y Acad Sci. 1988;550:314–324. doi: 10.1111/j.1749-6632.1988.tb35346.x. [DOI] [PubMed] [Google Scholar]
- Sato N., Wilson D. F., Chance B. The spectral properties of the b cytochromes in intact mitochondria. Biochim Biophys Acta. 1971 Nov 2;253(1):88–97. doi: 10.1016/0005-2728(71)90236-2. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]
- Weinstein J. D., Branchaud R., Beale S. I., Bement W. J., Sinclair P. R. Biosynthesis of the farnesyl moiety of heme a from exogenous mevalonic acid by cultured chick liver cells. Arch Biochem Biophys. 1986 Feb 15;245(1):44–50. doi: 10.1016/0003-9861(86)90188-8. [DOI] [PubMed] [Google Scholar]
- Withers H. K., Bragg P. D. Potentiometric and spectroscopic properties of the cytochrome o complex of Escherichia coli. Biochem Cell Biol. 1990 Jan;68(1):83–90. doi: 10.1139/o90-010. [DOI] [PubMed] [Google Scholar]
- Yang T. Y., Jurtshuk P., Jr Studies on the red oxidase (cytochrome o) of Azotobacter vinelandii. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1032–1039. doi: 10.1016/0006-291x(78)91454-7. [DOI] [PubMed] [Google Scholar]
- Yu C., Yu L., King T. E. Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J Biol Chem. 1975 Feb 25;250(4):1383–1392. [PubMed] [Google Scholar]



