ABSTRACT
In the livers of C57BL/6 mice, gamma interferon (IFN-γ) controls intracellular Leishmania donovani infection and the efficacy of antimony (Sb) chemotherapy. Since both responses usually correlate with granulomatous inflammation, we tested six prominently expressed, IFN-γ-regulated chemokines—CXCL9, CXCL10, CXCL13, CXCL16, CCL2, and CCL5—for their roles in (i) mononuclear cell recruitment and granuloma assembly and maturation, (ii) initial control of infection and self-cure, and (iii) responsiveness to Sb treatment. Together, the results for the L. donovani-infected livers of chemokine-deficient mice (CXCR6−/− mice were used as CXCL16-deficient surrogates) indicated that individual IFN-γ-induced chemokines have diverse affects and (i) may be entirely dispensable (CXCL13, CXCL16), (ii) may promote (CXCL10, CCL2, CCL5) or downregulate (CXCL9) initial granuloma assembly, (iii) may enhance (CCL2, CCL5) or hinder (CXCL10) early parasite control, (iv) may promote granuloma maturation (CCL2, CCL5), (v) may exert a granuloma-independent action that enables self-cure (CCL5), and (vi) may have no role in responsiveness to chemotherapy. Despite the near absence of tissue inflammation in early-stage infection, parasite replication could be controlled (in CXCL10−/− mice) and Sb was fully active (in CXCL10−/−, CCL2−/−, and CCL5−/− mice). These results characterize chemokine action in the response to L. donovani and also reemphasize that (i) recruited mononuclear cells and granulomas are not required to control infection or respond to Sb chemotherapy, (ii) granuloma assembly, control of infection, and Sb's efficacy are not invariably linked expressions of the same T cell-dependent, cytokine-mediated antileishmanial mechanism, and (iii) granulomas are not necessarily hallmarks of protective antileishmanial immunity.
KEYWORDS: chemokines, visceral leishmaniasis, Leishmania donovani, granuloma, pentavalent antimony
INTRODUCTION
In visceral leishmaniasis, a disseminated protozoal infection, tissue macrophages in the liver, spleen, and bone marrow are targeted and support intracellular parasite replication. In the susceptible host, experimental Leishmania donovani infection in the liver does not come under control nor are parasites killed until either chemotherapy is given or T cell-dependent, multi-cytokine-driven mechanisms emerge and macrophage activation is induced. In the livers of infected wild-type (WT) C57BL/6 (B6) and BALB/c mice, this immunoinflammatory response is usually associated with mononuclear cell recruitment to and granuloma assembly at parasitized Kupffer cells (1–8). In the granulomatous environment, this T cell- and cytokine-mediated response also directs self-cure in initially susceptible B6 and BALB/c mice and, in addition, regulates the intracellular efficacy of conventional antileishmanial chemotherapy, pentavalent antimony (Sb) (2, 4, 6, 7, 9–11).
Gamma interferon (IFN-γ) plays a particularly prominent role in the preceding responses in L. donovani infection in the liver. In its absence, there is little T cell or monocyte influx or granuloma formation and no evidence of macrophage activation, since intracellular infection is unrestrained and Sb fails to exert its leishmanicidal effect (3, 4, 12–14). How IFN-γ initiates the inflammatory environment in the liver, in which recruited mononuclear cells, activated macrophages, and, if given, chemotherapy successfully interdigitate to eradicate L. donovani, has not been well clarified. In the study described here, we focused on selected chemokines induced by L. donovani infection in WT mice and regulated by IFN-γ. Using deficient mice, we tested chemokine roles in granuloma assembly, initial control, the outcome of infection, and the response to chemotherapy.
RESULTS
IFN-γ-regulated chemokine and chemokine receptor expression.
Microarray gene expression analysis was performed using liver tissue from WT and IFN-γ−/− mice infected for 2 or 3 weeks. Enhanced expression was arbitrarily defined as a ≥4-fold increase in gene expression compared with the results in uninfected mice. As shown in Table S2 in the supplemental material, WT mice demonstrated enhanced expression of 11 chemokines and eight chemokine receptors. In contrast, infected IFN-γ−/− mice showed enhanced gene expression of only 1 of the 11 chemokines (CCL8 [monocyte chemoattractant protein 2 {MCP-2}]) and only one of the eight receptors (CXCR6).
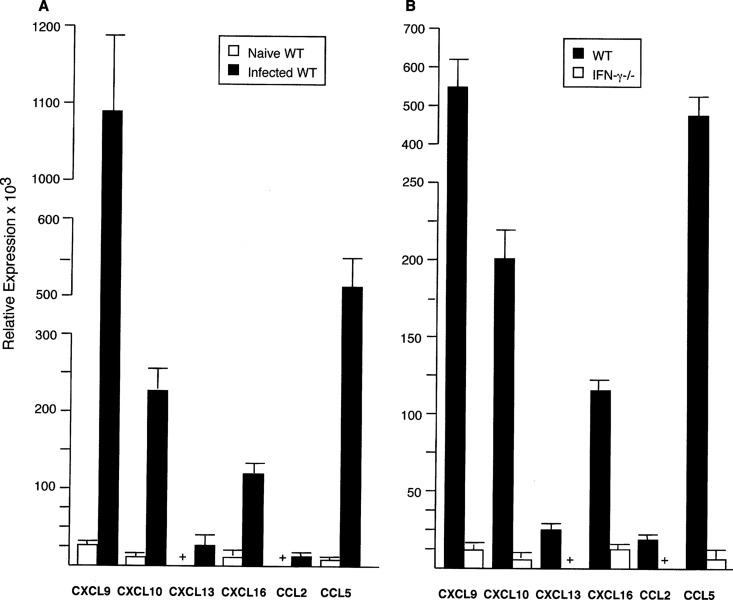
To select chemokines for our analysis, two additional criteria were applied: (i) prominent gene expression in infected WT mouse livers (defined as a ≥10-fold increase on day 21 compared with that in uninfected mice) and (ii) IFN-γ regulation (defined as a ratio of ≥10 in the day 21 fold increase in expression in WT mice compared with that in IFN-γ−/− mice). This winnowing identified six candidates for study (Table S2): chemokines which primarily attract T cells (CXCL9, CXCL10, CXCL16), monocytes (CCL2), monocytes and T cells (CCL5), or B and T cells (CXCL13) (15–19). Reverse transcription-PCR (RT-PCR) testing using livers from WT and IFN-γ−/− mice that had been infected for 3 weeks confirmed the findings for the six selected chemokines (Fig. 1).
FIG 1.
Relative mRNA expression of CXCL9, CXCL10, CXCL13, CXCL16, CCL2, and CCL5 3 weeks after infection in the livers of WT (A) and WT versus IFN-γ−/− (B) mice. RT-PCR results (mean ± SEM) are from 2 experiments (A) (8 mice per group) and from 1 experiment (B) (4 mice per group). In panel A, P was <0.05 for all results for expression in the livers of infected WT mice versus expression in the livers of 8 naive (uninfected) WT mice, and the + symbols for uninfected mice for CXCL13 and CCL2 indicate values of 3.6 ± 1.6 and 0.33 ± 0.9 units, respectively. In panel B, P was <0.05 for all results for infected WT mice versus infected IFN-γ−/− mice, and the + symbols for IFN-γ−/− mice indicate values for CXCL13 and CCL2 of 1.5 ± 0.6 and 1.3 ± 0.3 units, respectively.
Among receptors for the six chemokines, expression of the genes for CXCR3 (the ligand for CXCL9 and CXCL10), CXCR6 (the ligand for CXCL16), CCR2 (the ligand for CCL2), and CCR3 and CCR5 (the ligands for CCL5) at day 14 or day 21 was also prominent and IFN-γ regulated (Table S2). Expression of CXCR5, the ligand for CXCL13, was not detected in the microarray analysis.
Kinetics and outcome of L. donovani liver infection in chemokine-deficient and chemokine-treated mice.
We used IFN-γ−/− and WT mice as opposing benchmarks with which to compare the responses in chemokine- and chemokine receptor-deficient mice. As anticipated, infection was unrestrained in IFN-γ−/− mice and controlled in initially susceptible WT mice (Fig. S1). The latter demonstrated parasite killing at week 4 and a phenotype of a near cure by week 8.
(i) C-X-C chemokines.
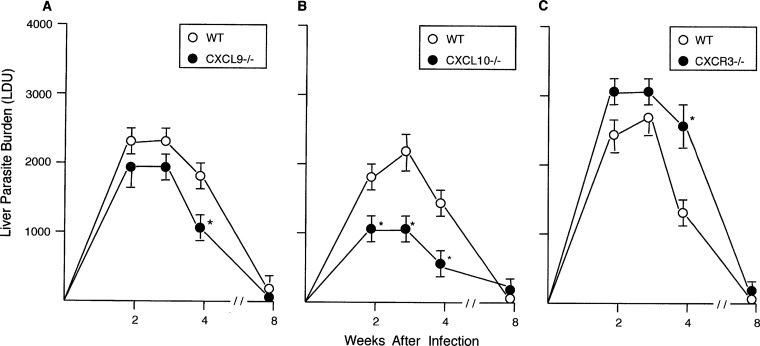
Mice deficient in CXCL9, CXCL10, or CXCL13 or deficient in CXCR6 (surrogates for CXCL16−/− mice [16, 20]) controlled early-stage liver infection and showed parasite killing (week 4) and self-cure (week 8) (Fig. 2 and S2). The initially increased parasite burdens in CXCR6−/− mice were not significantly different from those in WT mice. Thus, by themselves, the selected C-X-C chemokines, CXCL9, CXCL10, CXCL13, and, by inference, CXCL16, played no apparent host defense role in the response to L. donovani in the liver.
FIG 2.
Course of L. donovani infection in the livers of WT, CXCL9−/−, CXCL10−/−, and CXCR3−/− mice. Results from 2 experiments (A and C) and 3 experiments (B), indicated as mean ± SEM values, are for 8 to 9 mice (A), 12 mice (B), and 7 mice (C) at each time point. *, P < 0.05 versus the WT liver parasite burden (LDU).
Paradoxically, however, CXCL10−/− mice (and, at one time point, CXCL9−/− mice) expressed enhanced antileishmanial activity (Fig. 2), prompting us to test the effect of treating WT mice with anti-CXCL10. Given from the outset of infection, anti-CXCL10 injections enabled parasite killing (Fig. S3), suggesting that, along with providing enhanced control in gene-deficient mice, CXCL10 can act to promote parasite replication. Although mice deficient in CXCR3, the receptor for both CXCL10 and CXCL9, are not more susceptible to a different strain of L. donovani (21), we also tested CXCR3−/− mice in our model, anticipating enhanced resistance to infection in the absence of CXCL9 and CXCL10 activation. This hypothesis not only proved incorrect (Fig. 2C), but results at week 4 also indicated delayed parasite killing in CXCR3−/− mice. While mRNA expression of CXCL11 (interferon-inducible T-cell alpha chemoattractant), CXCR3's third chemokine ligand, could be detected in B6 mice (e.g., see Table S2), B6 mice do not produce functional CXCL11 protein (22, 23).
(ii) C-C chemokines.
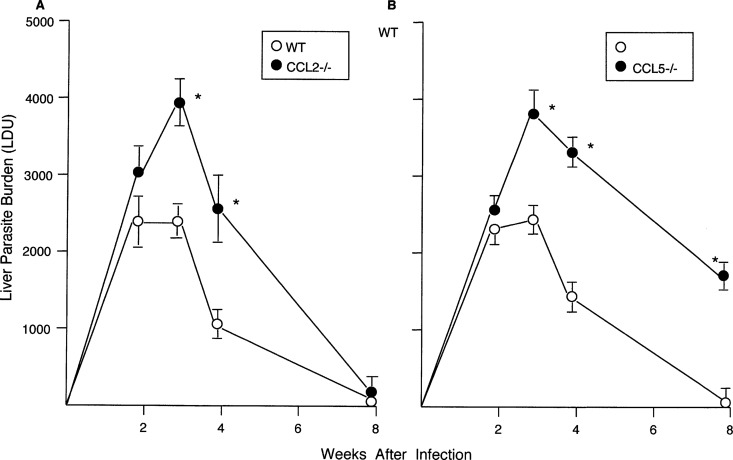
The kinetics and/or outcome of liver infection in mice deficient in CCL2 or CCL5 contrasted with the preceding results since the effects of each were required to properly control early-stage infection and efficiently kill L. donovani (Fig. 3). While CCL2−/− mice proceeded to self-cure by week 8, parasite killing in CCL5−/− mice was incomplete and week 8 liver burdens in CCL5−/− mice were 12-fold higher than those in WT mice. Although spleen parasite burdens were not routinely measured, amastigotes were readily identified in CCL5−/− mouse spleen imprints at week 8, while they were scarce in WT mouse spleens (not shown).
FIG 3.
Course of L. donovani infection in livers of WT, CCL2−/−, and CCL5−/− mice. Results from 2 experiments (A) and 3 experiments (B), indicated as mean ± SEM values, are for 6 to 7 mice (A) and 9 mice (B) at each time point. *, P < 0.05 versus the WT liver parasite burden (LDU).
(iii) Chemokine treatment.
Our results in CXCL10−/− and anti-CXCL10-treated WT mice suggested that endogenous CXCL10 paradoxically promotes infection (Fig. 2 and S3). In contrast, exogenous CXCL10 given prophylactically (on days +1, +3, and +7 after L. donovani challenge) has been reported to induce liver parasite killing (24), an effect also reported in the same model for a single injection of CCL2 on day +7 (25). Because of this discrepancy in CXCL10's apparent action and to test treatment effects in established infection, starting on day +14, WT mice were given 3 alternate-day intraperitoneal (i.p.) injections of 5 or 10 μg/kg of body weight of CXCL10 (24) or a single injection of CCL2 (5 μg/kg) (25). In our hands, however, neither CXCL10 nor CCL2 treatment had an effect on day +21 on either liver parasite burdens or cell recruitment to parasitized tissue foci (2 experiments, 7 to 8 mice per group; data not shown).
Tissue inflammatory responses and granuloma assembly. (i) WT and IFN-γ−/− mice.
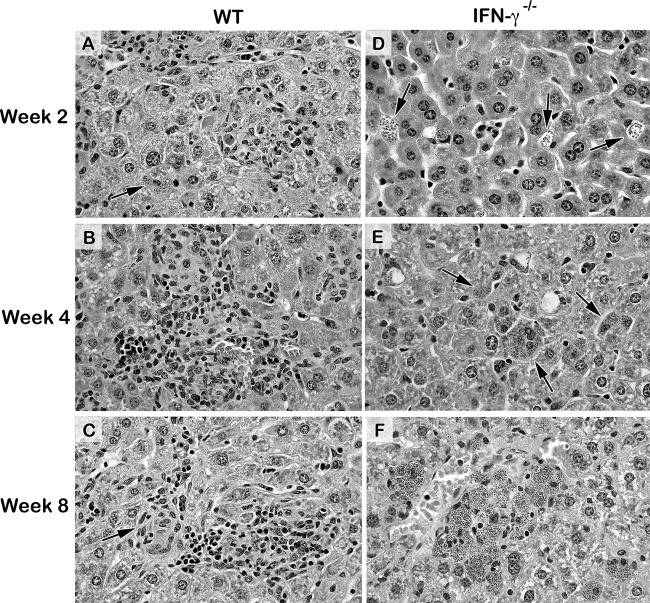
L. donovani parasitizes resident macrophages in the liver, resulting in a range of recruited inflammatory cells directed at initially infected Kupffer cells in both B6 and similarly behaving BALB/c WT mice (3, 5). CD4+ and CD8+ T cells, NK and NKT cells, and blood monocytes are primary influxing cells (1–3, 5, 8, 26, 27); neutrophils are also initially recruited to the liver (1, 5, 7, 8, 28) but are indifferent or not active toward amastigotes (8, 29), limited in granulomas (8), and seldom discernible microscopically by or after week 2 (2, 3, 27). Although the dynamic mononuclear cell response is recognized to be asynchronous (8, 30), in the livers of WT mice it is usually (i) evident at week 2, with early-developing granulomas being ∼50% of parasitized foci, (ii) fully expressed by week 4, with ∼90% of infected foci being surrounded by developing or mature (sometimes parasite-free) granulomas, and (iii) comprised at week 8 of mostly parasite-free, mature, and/or involuting granulomas (3, 30). IFN-γ−/− mice showed none of these effects, with virtually no cells being recruited to progressively more heavily parasitized foci until weeks 8 to 12 (3, 4, 13). Figures 4 and S4 illustrate the histologic responses in WT and IFN-γ−/− mice for comparison to the responses in chemokine-deficient mice.
FIG 4.
Histologic responses to L. donovani infection 2 to 8 weeks after challenge in livers of WT and IFN-γ−/− mice. In WT mice, infected foci show intracellular parasites and inflammatory responses ranging from little or none (arrows) to developing granulomas at week 2 (A), mature granulomas at week 4 (B), and mature and involuting (arrows), largely parasite-free granulomas at week 8 (C). In contrast, in IFN-γ−/− mice at both week 2 (D) and week 4 (E), increasingly heavily infected Kupffer cells (arrows) attracted few if any mononuclear cells, while at week 8 (F), scanty influxing cells (morphologically, lymphocytes) were present at some of the remarkably heavily parasitized foci. Magnifications, ×400. See Fig. S4 for the corresponding low-power photomicrographs.
(ii) Chemokine- and chemokine receptor-deficient mice.
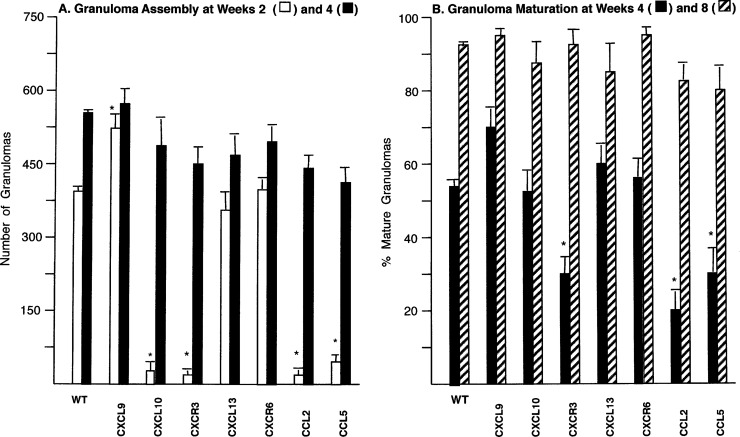
Figure 5 shows the results for granuloma assembly (the number of granulomas at weeks 2 and 4) and maturation (the percentage of parasitized foci scored as mature and/or parasite-free granulomas at weeks 4 and 8) (6, 31). Responses were intact in CXCL13−/− and CXCR6−/− mice. However, the other gene-deficient mice showed altered responses, and histologic correlates of the kinetics of granuloma assembly and maturation are illustrated in Fig. 6, 7, S5, and S6. Together, these results indicated (i) accelerated early granuloma assembly at week 2 in CXCL9−/− mice, (ii) little cell recruitment at week 2 and therefore deficient initial granuloma assembly in CXCL10−/−, CXCR3−/− (as previously reported [21]), CCL2−/−, and CCL5−/− mice, (iii) additionally impaired granuloma maturation at week 4 in CCL2−/− and CCL5−/− mice, and (iv) the presence of delayed-onset, compensatory mechanisms that permitted CXCL10−/−, CXCR3−/− (21), CCL2−/−, as well as CCL5−/− mice to assemble granulomas by week 4 and express intact maturation by week 8. Thus, as judged by the findings in the livers of gene-deficient mice, four of the selected IFN-γ-induced chemokines, CXCL9, CXCL10, CCL2, and CCL5, appear to regulate the provoked granulomatous response, seemingly in a nonredundant fashion. The effects of these individual chemokines were, however, limited to early- and/or midstage infection (weeks 2 to 4), and while CXCL10, CCL2, and CCL5 promoted mononuclear cell recruitment and tissue inflammation, CXCL9 appeared to have a paradoxical restraining effect.
FIG 5.
Granuloma assembly at weeks 2 and 4 (A) and granuloma maturation at weeks 4 and 8 (B) in the livers of WT and chemokine- or chemokine receptor-deficient mice. Results from 2 experiments for each group of deficient mice are shown as mean ± SEM values for 4 mice per group at each time point. WT mice were infected in parallel in each experiment performed in deficient mice; however, for clarity, results are for WT mice in panel A and represent pooled data from 20 WT mice in panel B. *, P < 0.05 versus the corresponding mean result for WT mice infected in the same experiments with the designated deficient mice.
FIG 6.
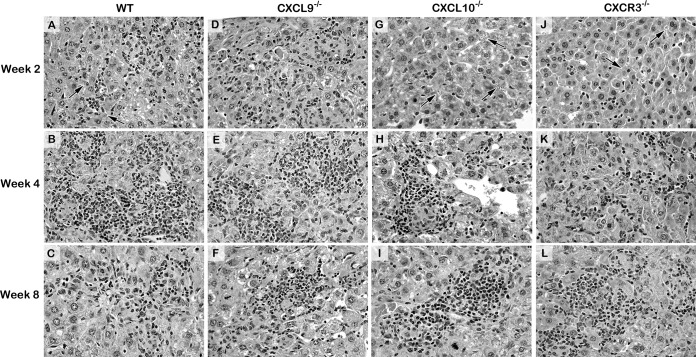
Histologic reaction to L. donovani infection 2 to 8 weeks after challenge in the livers of WT, CXCL9−/−, CXCL10−/−, and CXCR3−/− mice. The photomicrographs shown for WT mice are from mice infected in parallel with CXCR3−/− mice (histologic reactions were similar in WT mice infected in parallel with CXCL9−/− and CXCL10−/− mice). In WT mice, the inflammatory responses at parasitized foci at week 2 range from none (arrows) to developing granulomas (A); at week 4, granulomas are mature and coalescing (B); and at week 8, mature and involuting granulomas are parasite-free (C). (D to F) CXCL9−/− mice show an accelerated response with well-established mononuclear cell recruitment at virtually all infected foci at week 2 (D) and mature, largely parasite-free granulomas at week 4 (E). In contrast, in both CXCL10−/− mice (G to I) and CXCR3−/− mice (J to L), few parasitized Kupffer cells (arrows) have attracted inflammatory cells at week 2 (G, J); however, by week 4 (H) or week 8 (L), granulomatous responses are well established in both groups. Magnifications, ×400. See Fig. S5 for the corresponding low-power photomicrographs.
FIG 7.
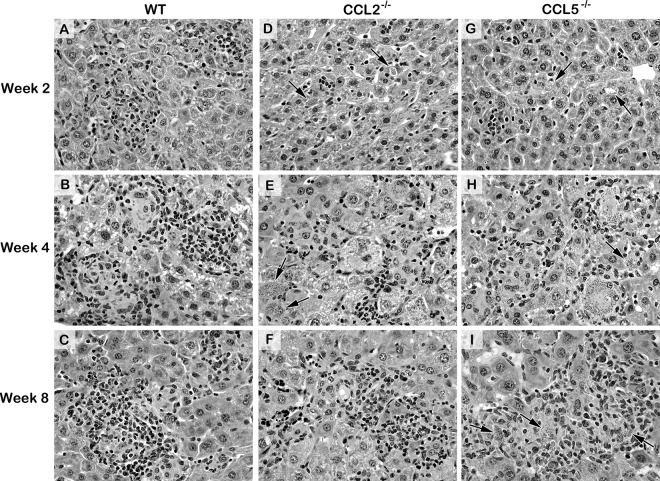
Histologic responses to L. donovani infection at 2 to 8 weeks after challenge in the livers of WT, CCL2−/−, and CCL5−/− mice. The photomicrographs shown for WT mice are from those infected in parallel with CCL5−/− mice (histologic reactions were similar in WT mice infected in parallel with CCL2−/− mice). (A to C) See the legends to Fig. 4 and 5 for descriptions of the reactions in WT mice. In CCL2−/− mice (D to F) and CCL5−/− mice (G to I), (i) few parasitized Kupffer cells (arrows) have attracted inflammatory cells at week 2 (D, G), in contrast to the findings for WT mice (A), and (ii) developing granulomas are present at week 4 at most but not all (arrows) now more heavily infected foci (E, H), and (iii) while mature granulomas are well established in both groups by week 8 and largely parasite-free in CCL2−/− mice (F), the same structures in CCL5−/− mice contain abundant amastigotes (arrows) (I). Magnifications, ×400. See Fig. S6 for the corresponding low-power photomicrographs.
Response to chemotherapy.
In the L. donovani-infected liver, the effects of IFN-γ also convert the efficacy of Sb chemotherapy from being leishmanistatic to being leishmanicidal (12). Thus, we completed the analysis of these IFN-γ-induced chemokines by testing the responses to treatment given at week 2 (day +14). As anticipated (12), Sb-treated WT mice showed >90% parasite killing on day +21 and IFN-γ−/− animals showed none (Table S3). All seven groups of chemokine- or chemokine receptor-deficient mice responded normally to chemotherapy, however, including those (CXCL10−/−, CXCR3−/−, CCL2−/−, and CCL5−/− mice) that expressed little or no tissue inflammation at the time that treatment was administered.
Additional testing in CCL5−/− mice.
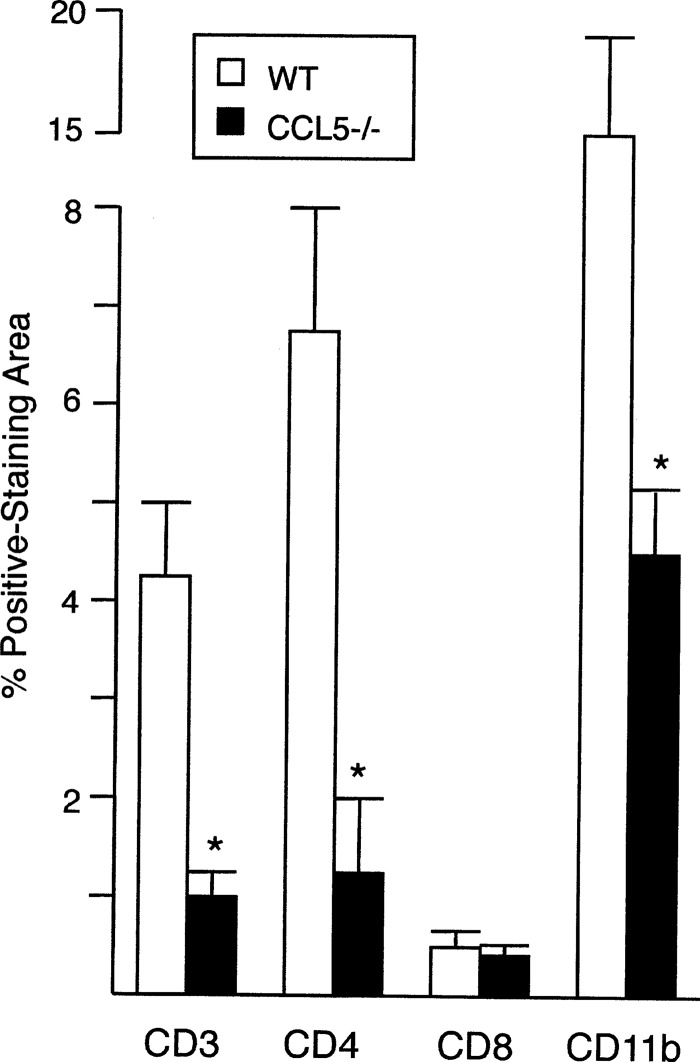
All chemokine-deficient mice eventually generated mature-appearing granulomas and expressed the self-cure phenotype at week 8, with one exception: CCL5−/− mice (Fig. 7). To better understand the action of CCL5 in L. donovani infection, we characterized mononuclear cell recruitment and cytokine secretion in deficient mice. Consistent with CCL5's regulation of T cell and monocyte trafficking (16, 32, 33), the livers of CCL5−/− mice showed reduced expression of CD3, CD4, and CD11b (Fig. 8), used as markers for T cells, CD4+ cells, and presumptive monocytes, respectively, while CD8 expression was intact. (CD11b is also expressed by other types of myeloid cells [5, 27].) To assess cytokine secretion, we focused on IFN-γ and interleukin-10 (IL-10), primary macrophage-activating versus macrophage-deactivating cytokines, respectively, in this model (12, 34). In two experiments, antigen-restimulated spleen cells from CCL5−/− and WT mice that had been infected for 3 weeks (n = 6 per group) secreted similar levels of IFN-γ (2.7 ± 0.2 versus 2.2 ± 0.2 ng/ml) and IL-10 (256 ± 32 versus 164 ± 34 pg/ml) (P > 0.05). IL-10 was not detected in serum from the same infected animals (n = 6); however, IFN-γ activity was appreciably lower in CCL5−/− mice (336 ± 62 pg/ml) than in WT mice (1,565 ± 174 pg/ml) P < 0.05), suggesting a suboptimal Th1 cell-type response.
FIG 8.
Mononuclear cell recruitment, as judged by the immunohistochemical expression of CD3, CD4, CD8, and CD11b, in the livers of WT and CCL5−/− mice 2 weeks after L. donovani infection. Results are from 2 experiments, and mean ± SEM values for 6 mice per group are indicated. *, P < 0.05 versus the corresponding result for WT mice.
DISCUSSION
In inducing the control of intracellular L. donovani infection in the livers of initially susceptible WT mice (e.g., B6 and BALB/c mice), IFN-γ's diverse effects certainly include but also extend beyond direct macrophage activation. One such IFN-γ-dependent effect, granulomatous inflammation, typically accompanies a successful antileishmanial defense in the liver (3, 7, 8, 30).
To better understand mononuclear cell recruitment to sites of parasitized resident macrophages and subsequent assembly into granulomas, we focused on selected IFN-γ-regulated chemokines provoked by L. donovani infection. Liver granulomas in this model in B6 and BALB/c WT mice are complex and dynamic (5, 8, 30), allowing initially parasitized Kupffer cells, recruited mononuclear cells, inflammatory cytokines, and the intracellular mechanisms of the activated macrophage to interdigitate and eventually eradicate L. donovani (3, 14, 35). Administered chemotherapy also interacts with the same factors in the parasitized tissue focus, and for pentavalent antimony (Sb), expression of its intracellular killing action in the liver is also usually associated with tissue inflammation. Sb's leishmanicidal efficacy requires or is optimized by T cells and monocytes (2, 36), IFN-γ and other cytokines (10–12, 37, 38), and IFN-γ-induced antileishmanial macrophage mechanisms (i.e., inducible nitric oxide synthase [iNOS], phagocyte oxidase, Irgm3) likely acting in concert (14, 39).
To gauge the roles of the chemokines designated here to be prominently expressed and IFN-γ regulated, three IFN-γ-dependent responses were assayed in gene-deficient mice: (i) granuloma assembly and maturation, (ii) the kinetics of initial parasite replication and killing and the outcome of infection, and (iii) responsiveness to chemotherapy (3, 12, 13). We used the responses in the livers of deficient mice to infer nonredundant roles for individual chemokines, and we refer below to the six that we studied as “IFN-γ-regulated chemokines.” We understand, however, that IFN-γ induces other chemokines in the L. donovani-infected liver (Table S2), additional chemokines are active in this model (e.g., CCL3 [40] and CCL19/21 [41]), and chemokines and their receptor ligands have pleiotropic effects beyond mononuclear cell recruitment. The latter include T cell stimulation, cytokine secretion, and direct macrophage activation (15–17, 19, 40–42). In addition, we did not analyze chemokine-associated responses in spleen or bone marrow, organs also involved in visceral infection. Nevertheless, our results point to the following conclusions about the six selected chemokines in L. donovani infection.
Initial granuloma assembly.
IFN-γ-regulated chemokines exert diverse effects on mononuclear cell recruitment in early-stage liver infection. Individual effects range from none (CXCL13 and CXCL16 [inferred from CXCR6−/− mice {20)}]) to an unexpected restraining action (CXCL9) and the anticipated promotion of mononuclear cell recruitment (CXCL10, CCL2, CCL5). The requirements for CXCL10, CCL2, or CCL5 in granuloma assembly were not long-lived, however, since compensatory mechanisms restored the granuloma number (Fig. 5A).
Granuloma maturation.
CCL2 and CCL5 optimize the initial conversion of developing granulomas to histologically mature-appearing structures (3, 8) in the infected liver, a process that does not involve IFN-γ-regulated C-X-C chemokines, including CXCL10. Delayed-onset mechanisms for inducing granuloma maturity, however, are similarly in place to compensate for CCL2 and CCL5 (Fig. 5B).
Initial parasite replication.
Individual IFN-γ-regulated chemokines also exert a range of effects in the control of intracellular parasite replication during early-stage infection (weeks 2 to 3). CXCL9, CXCL13, and CXCL16 have no effect, CXCL10 appears to paradoxically promote liver infection, and CCL2 and CCL5 clearly enhance control. At week 3, liver parasite burdens in CCL2−/− and CCL5−/− mice were nearly at the level of those in IFN-γ-deficient mice (Fig. 3 and S1).
Parasite killing.
IFN-γ-regulated chemokines are not involved in the initiation of L. donovani killing in the liver. Killing was under way in all gene-deficient mice by week 4; however, the extent of killing varied considerably. Compared with the results for WT mice infected in parallel, the results for gene-deficient mice suggested that parasite killing was unaffected by CXCL13 and CXCL16, suppressed not only by CXCL10 but also by CXCL9, and enhanced by CCL2 and especially by CCL5 (Table S4).
Outcome of infection.
IFN-γ-regulated chemokines are not required for expression of the self-cure phenotype in L. donovani infection in the liver, with one exception, CCL5. Infection persisted in CCL5−/− mice (Fig. 3), despite the emergence of an intact granulomatous response (Fig. 7). Had observations been extended beyond week 8, it is possible that CCL5−/− mice might have reduced parasite burdens further.
Responsiveness to chemotherapy.
Individual IFN-γ-regulated chemokines are not required for host responses that enable the leishmanicidal activity of Sb chemotherapy in the liver.
Our results for chemokine-deficient mice also serve to reemphasize that in L. donovani infection in the liver, (i) recruited mononuclear cells and granulomas are not necessary to control intracellular infection or respond to Sb chemotherapy and (ii) granuloma assembly, control of infection, and Sb's efficacy are not invariably linked expressions of the same or a similar T cell-dependent, cytokine-mediated, macrophage-activating mechanism (3, 5, 8, 12, 30, 36, 37). In the presence of little or no discernible tissue inflammation, for example, early parasite replication was better controlled in one setting (in CXCL10−/− mice) and Sb's killing action was entirely preserved in others (in CXCL10−/−, CCL2−/−, CCL5−/−, and CXCR3−/− mice).
Additional examples of intact (even enhanced) control of L. donovani in the liver, despite no tissue inflammation or a poorly organized or immature granulomatous response, have also challenged the idea that granulomas are necessary hallmarks of protective antileishmanial immunity (8). Examples of such control can be found in (i) gene-disrupted naive B6 or BALB/c mice deficient in transcription factors (43, 44), cytokines (28, 45, 46), and chemokine receptors (40), (ii) rechallenged immune WT BALB/c mice (3), and (iii) innately resistant WT mice (31). Conversely, a fully established, structurally mature-appearing granulomatous response in the liver also does not necessarily translate into effective control of L. donovani. This dissociation, demonstrated here in CCL5−/− mice, has been observed in the context of sustained macrophage deactivation (IL-10 transgenic mice [34]) and in mice lacking a particular antileishmanial factor, including CD40L, iNOS, and even IFN-γ (13, 35, 47, 48). In IFN-γ−/− mice, granulomas induced by anti-CD40 or anti-IL-10 receptor monoclonal antibody treatment are not effective in controlling L. donovani (47, 48); however, granulomas induced in IFN-γ−/− mice by exogenous IL-12 treatment are associated with inhibition of parasite replication (13).
In addition to our findings in CXCL10−/−, CCL2−/−, and CCL5−/− mice, accumulated evidence also indicates that an intact leishmanicidal response to Sb chemotherapy does not require mononuclear cell recruitment or granuloma assembly. In such a setting, drug efficacy is preserved in early infection in (i) B6 and BALB/c mice deficient in signaling (6, 49), granular proteins (31), cytokine secretion (13, 50), macrophage activation (34), and/or individual intracellular antileishmanial mechanisms (12, 14) and (ii) in innately resistant mice (31). However, even if they are not microscopically in situ at the time that treatment is given, host T cells are nevertheless required to convert Sb from an inactive to leishmanicidal form (36), likely via endogenous IFN-γ, which is required for expression of Sb's intracellular killing action (12).
Both CXCL9 and, especially, CXCL10 ligands that bind to and activate CXCR3 are involved in Th1 cell-type inflammatory responses and are well recognized to regulate activated CD4+ effector cell trafficking into inflamed tissues (22, 23, 51, 52). However, our results in CXCL9−/− and CXCL10−/− mice suggest initially suppressive actions on mononuclear cell recruitment (CXCL9) and control of parasite replication (CXCL10). The latter observation highlights the variable actions reported for CXCL10, since, depending upon the pathogen, model, and organ examined (53), CXCL10 deletion or neutralization can have no effect on (54, 55), reduce (51, 52, 56), or apparently even enhance (57, 58) host resistance to infection. CXCL10 also influences and recruits suppressive CXCR3+ regulatory T cells to the tissues (59, 60), and the absence of CXCR3+ regulatory T cells might enhance the control of L. donovani replication in CXCL10−/− mice. That said, however, it is not clear why early-stage infection was better controlled in CXCL10−/− mice than CXCR3−/− mice, although mice of both backgrounds showed similarly deficient initial mononuclear cell recruitment. Possible explanations might involve CXCL10's CXCR3-independent actions unrelated to chemotaxis (61), CXCR3 effects unrelated to CXCL10 (19, 62), or a predisposition of CXCR3-deficient macrophages toward an alternatively activated (M2) state (63).
CCL5 also has pleiotropic actions and is well recognized in T cell and monocyte recruitment as well as in attraction/trafficking of a range of inflammatory mononuclear cells expressing CCR1, CCR3, or CCR5 (16, 19, 32, 64, 65). CCL5 stood out in our analysis because, in addition to regulating (i) CD4+ cell and presumed monocyte recruitment, (ii) circulating IFN-γ activity, and (iii) resistance to L. donovani in early-stage infection, CCL5−/− mice were the only chemokine-deficient mice that we tested in which liver infection persisted. An antileishmanial role for endogenous CCL5 has already been demonstrated in cutaneous L. major infection (66), and relevant to antileishmanial mechanisms, CCL5 has been reported to upregulate Th1 cell-type responses (66) and, like IFN-γ, to directly activate macrophages to secrete nitric oxide (67). CCL5's reported in vivo effects also extend to protective actions in both early and late chronic stages of other intracellular infections (32, 33, 68).
MATERIALS AND METHODS
Animals and liver infection.
WT female C57BL/6 mice, purchased from The Jackson Laboratory (Bar Harbor, ME), were used as controls in each experiment performed in gene-deficient mice. Breeding pairs of the following mice, all on a C57BL/6 background and backcrossed for at least 10 generations, were obtained and bred at Weill Cornell Medical College: (i) IFN-γ−/−, CCL2 (MCP-1)−/−, and CXCR6−/− mice (The Jackson Laboratory), (ii) CCL5 (RANTES)−/− (69), (iii) CXCL9 (Mig)−/− (originally developed by J. Farber), and CXCL10 (IFN-γ-inducible protein 10 [IP-10])−/− mice (22, 51), and (iv) CXCR3−/− and CXCL13 (B lymphocyte chemoattractant)−/− mice (generously provided, respectively, by A. Satoskar [Ohio State University, Columbus, OH [21]) and J. Cyster [University of California, San Francisco, CA] [70]). A pair of CXCL16−/− mice was generously provided by I. Charo (University of California, San Francisco) but did not breed; in their place, CXCR6−/− mice were used since CXCR6 is the only known receptor ligand for CXCL16 (20).
Mice were female and 7 to 13 weeks old at the time of infection. Groups of 3 to 5 mice were injected via the tail vein with 1.5 × 107 hamster spleen homogenate-derived L. donovani amastigotes (LV9 strain). Infection was assessed microscopically using Giemsa-stained liver imprints in which parasite burdens were measured by blind counting of the number of amastigotes per 500 cell nuclei × organ weight (in milligrams) (Leishman-Donovan units [LDUs]) (6). Parasite killing at week 4 (indicated as the percent reduction in the mean liver burden) was calculated by comparing the number of LDUs at week 4 to the peak number of LDUs at week 3. These studies were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
Gene and mRNA expression in liver tissue.
A high-density oligonucleotide microarray system, the Murine Genome U74A array, version 2, containing 12,488 genes (Affymetrix, Santa Clara, CA), was used to test liver tissue from naive and infected WT and IFN-γ−/− mice. Total RNA was isolated from freshly obtained tissue, and samples from 4 or 5 mice from each group were pooled. The performance of the microarray experiment was determined and the associated data analyses were carried out as previously described (6, 71)
For quantitative real-time RT-PCR testing, total RNA was isolated from liver tissue from individual mice using an RNeasy minikit (Qiagen, Hilden, Germany) and reverse transcribed into cDNA. RT-PCR was performed in an ABI 7400 system using a SYBR green PCR kit. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA expression was used as the control for quantitative analysis. The primers for the detected genes are listed in Table S1 in the supplemental material. PCR cycling conditions were as follows: an initial incubation step of 2 min at 50°C and reverse transcription for 60 min at 60°C and 94°C for 2 min, followed by 40 cycles of 15 s at 95°C for denaturation and 2 min at 62°C for annealing and extension. To calculate relative RNA levels, we used the formula: 2(−ΔCT), where ΔCT = the threshold cycle (CT) number for the specific gene − the CT number for GAPDH (71).
Tissue granuloma responses.
The histologic response to infection was evaluated by microscopic analysis of liver sections stained with hematoxylin and eosin. The number of granulomas (the number of infected Kupffer cells which had attracted ≥5 mononuclear cells) in 100 consecutive ×40-magnification fields was counted, and at 100 parasitized foci, the granulomatous reaction was scored as none, developing or mature, and/or parasite-free (6). Mature granulomas show a core of fused parasitized Kupffer cells, numerous surrounding mononuclear cells, and epithelioid-type changes (3).
Immunohistochemistry.
Liver samples were immediately embedded and frozen in OCT compound (Sakura, Torrance, CA) cooled by dry ice and isopentane. Tissue samples were sectioned at a 5-μm thickness, placed on glass slides, and fixed for 10 min in acetone at 4°C. Sections were stained using primary antibodies for CD3 (clone SP7; Vector Laboratories Inc., Burlingame, CA), CD4 (clone H129.29; BD Pharmingen, San Diego, CA), CD8 (clone 53-6.7; BD Pharmingen), and CD11b (clone M1/70; BD Pharmingen) on a Leica Bond RX automated staining platform (Leica Biosystems, Buffalo Grove, IL). Primary antibodies were applied at a concentration of 1:100, followed by application of a secondary antibody (BA-4001; Vector Laboratories) and a polymer detection system (Novocastra bond polymer refine detection; Leica Biosystems) using diaminobenzidine (DAB) as the chromogen and hematoxylin as the counterstain. Normal mouse spleen, thymus, and liver prepared by the same method were used as positive controls. A negative reagent control was prepared from each experimental tissue specimen by substituting nonspecific immunoglobulins of the same species for the primary antibody. Coverslips were placed on the slides, and images were acquired with an Olympus BX45 microscope using a 40× objective, a DP25 camera, and CellSens Entry (version 1.9) software (Olympus Imaging America, Inc., Center Valley, PA). For each stain in each animal, 6 images measuring 141,790 μm2 were acquired from randomly selected areas. Images were then analyzed using ImageJ (version 1.47) software (National Institutes of Health, Bethesda, MD) with the Color Deconvolution (version 1.5) plug-in to determine the staining area (72). After deconvolution with the H DAB vector, the Threshold tool (in which lower and upper values were set at 0 and 80, respectively) was applied to the DAB component to produce a black-and-white image. The Measure tool was used to determine the percentage of positive (black) pixels (the positively staining area). The mean positively staining area for each stain in sections from each animal's liver was calculated from the values obtained from the 6 images. Results are expressed as the percentage of the positively staining area.
Treatments.
Groups of 3 to 5 infected mice were injected i.p. with 0.2 ml of saline containing (i) pentavalent antimony (Sb; sodium stibogluconate; Pentostam; Wellcome Foundation Ltd., London, UK) once on day +14 at an optimal leishmanicidal dose (500 mg/kg) (6), (ii) recombinant murine CXCL10/IP-10 (R&D Systems, Minneapolis, MN) on days +14, +16, and +18 at 5 or 10 μg/kg per injection (24), or (iii) recombinant murine CCL2/MCP-1 (R&D Systems) once on day +14 at 5 μg/kg (25). The source of CXCL10 and CCL2, the number of injections given, and the doses used were selected from studies in which chemokine treatment reportedly induced antileishmanial effects against L. donovani in the liver (24, 25). The liver parasite burdens in Sb- and chemokine-treated mice were determined on day +21, and parasite killing was calculated as indicated in the Table S3 footnote (6). Additional WT mice were injected i.p., starting 4 h after L. donovani challenge, with 0.2 ml of saline containing 200 μg of hamster anti-mouse CXCL10 (52, 56) or hamster IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Anti-CXCL10 was given on alternate days until day +26, and liver burdens were measured on days +14 and +28.
IFN-γ and IL-10 protein determination.
IFN-γ and IL-10 activities in serum and spleen cell culture supernatants were measured using enzyme-linked immunosorbent assay kits from BD Biosciences (San Jose, CA) and BioLegend (San Diego, CA), respectively (31). Spleen cells (5 × 106 cells/ml) were stimulated for 48 h with 30 μg/ml of soluble L. donovani antigen, generously provided by A. Satoskar (73).
Statistical analysis.
Differences between mean values were analyzed by a two-tailed Student's t test. A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grants 5R01AI083219 (to H.W.M. and X.M.) and CA069212 (to A.D.L.) and a grant from the Sheng Yushou Foundation to Shanghai Jiaotong University (to H.Z.).
We are grateful to Marissa Flack-Mitchell for technical assistance and to Sebastien Monette for the immunohistochemical analysis.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00824-16.
REFERENCES
- 1.McElrath JJ, Murray HW, Cohn ZA. 1988. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med 167:1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray HW. 2000. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect Immun 68:6294–6299. doi: 10.1128/IAI.68.11.6294-6299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray HW. 2001. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol 82:249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray HW. 2005. Prevention of relapse after chemotherapy in a chronic intracellular infection: mechanisms in experimental visceral leishmaniasis. J Immunol 174:4916–4923. doi: 10.4049/jimmunol.174.8.4916. [DOI] [PubMed] [Google Scholar]
- 5.Beattie L, Peltan A, Maroof A, Kirby A, Brown N, Coles M, Smith DF, Kaye PM. 2010. Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog 6:e1000805. doi: 10.1371/journal.ppat.1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray HW, Zhang Y, Zhang Y, Raman VS, Reed SG, Ma X. 2013. Regulatory actions of Toll-like receptor 2 (TLR2) and TLR4 in Leishmania donovani infection in the liver. Infect Immun 81:2318–2326. doi: 10.1128/IAI.01468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faleiro RJ, Kumar R, Hfber LM, Engwerda CR. 2014. Immune regulation during chronic visceral leishmaniasis. PLoS Negl Trop Dis 8:e2914. doi: 10.1371/journal.pntd.0002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye PM, Beattie L. 2016. Lessons from other diseases: granulomatous inflammation in leishmaniasis. Semin Immunopathol 38:249–260. doi: 10.1007/s00281-015-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray HW. 2004. Progress in treatment of a neglected disease: visceral leishmaniasis. Expert Rev Anti Infect Ther 2:279–292. doi: 10.1586/14787210.2.2.279. [DOI] [PubMed] [Google Scholar]
- 10.Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombacher F. 2000. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol 30:2935–2943. doi:. [DOI] [PubMed] [Google Scholar]
- 11.McFarlane E, Carter KC, McKenzie AN, Kaye PM, Brombacher F, Alexander J. 2011. Endogenous IL-13 plays a crucial role in liver granuloma maturation during Leishmania donovani infection, independent of IL-4Rα-responsive macrophages and neutrophils. J Infect Dis 204:36–43. doi: 10.1093/infdis/jir080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray HW, Delph-Etienne S. 2000. Role of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun 68:288–293. doi: 10.1128/IAI.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor A, Murray HW. 1997. Intracellular antimicrobial activity in the absence of interferon-γ: effect of interleukin 12 in experimental visceral leishmaniasis in interferon-γ gene-disrupted mice. J Exp Med 185:1231–1239. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray HW, Mitchell-Flack M, Taylor GA, Ma X. 2015. IFN-γ-induced macrophage antileishmanial mechanisms in mice: a role for immunity-related GTPases, Irgm1 and Irgm3, in Leishmania donovani infection in the liver. Exp Parasitol 157:103–109. doi: 10.1016/j.exppara.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira MJ, Teixeira CR, Andrade BB, Barral-Netto M, Barral A. 2006. Chemokines in host-parasite interactions in leishmaniasis. Trends Parasitol 22:32–40. doi: 10.1016/j.pt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Charo IF, Ransohoff RM. 2006. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 17.Oghumu S, Lezama-Davila CM, Isaac-Marquez AP, Satoskar AR. 2010. Role of chemokines in regulation of immunity against leishmaniasis. Exp Parasitol 126:389–396. doi: 10.1016/j.exppara.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGovern KE, Wilson EH. 2013. Role of chemokines and trafficking of immune cells in parasitic infections. Curr Immunol Rev 9:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz O, Hammerschmidt SI, Moschovakis GL, Forster R. 2016. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol 34:203–242. doi: 10.1146/annurev-immunol-041015-055649. [DOI] [PubMed] [Google Scholar]
- 20.Lee LN, Ronan EO, de Lara C, Franken KLMC, Ottenhoff THM, Tchilian EZ, Beverley PCL. 2011. CXCR6 is a marker for protective antigen-specific cells in the lungs after intranasal immunization against Mycobacterium tuberculosis. Infect Immun 79:3328–3337. doi: 10.1128/IAI.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbi J, Oghumu S, Rosas LE, Carlson T, Lu B, Gerard C, Lezama-Davila CM, Satoskar AR. 2007. Lack of CXCR3 delays the development of hepatic inflammation but does not impair resistance to Leishmania donovani. J Infect Dis 195:1713–1717. doi: 10.1086/516787. [DOI] [PubMed] [Google Scholar]
- 22.Campanella GSV, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. 2008. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A 105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller M, Carter S, Hofer MJ, Campbell IL. 2010. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—a tale of conflict and conundrum. Neuropathol Appl Neurobiol 36:368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta G, Bhattacharjee S, Bhattacharyya S, Bhattacharya P, Adhikari A, Mukerjee A, Majumdar SB, Majumdar S. 2009. CXC chemokine-mediated protection against visceral leishmaniasis: involvement of the proinflammatory response. J Infect Dis 200:1300–1310. doi: 10.1086/605895. [DOI] [PubMed] [Google Scholar]
- 25.Dey R, Majumder N, Majumbar SB, Bhattacharjee S, Banerjee S, Roy S, Majumdar S. 2007. Induction of host protective Th1 immune response by chemokines in Leishmania donovani-infected BALB/c mice. Scand J Immunol 66:671–683. doi: 10.1111/j.1365-3083.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 26.Amprey JL, Im JS, Turco SJ, Murray HW, Porcelli S, Spath GF. 2004. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med 200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervia J, Rosen H, Murray HW. 1993. Effector role of blood monocytes in experimental visceral leishmaniasis. Infect Immun 61:1330–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrazas C, Varikuti S, Kimble J, Moretti E, Boyaka PN, Satoskar AR. 2016. IL-17A promotes susceptibility during experimental visceral leishmaniasis caused by Leishmania donovani. FASEB J 30:1135–1143. doi: 10.1096/fj.15-277202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFarlane E, Perez C, Charmony M, Allenbach C, Carter KC, Alexander J, Tacchini-Cottier F. 2008. Neutrophils contribute to the development of a protective immune response during onset of infection with Leishmania donovani. Infect Immun 76:532–541. doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore JW, Moyo D, Beattie L, Andrews PS, Timmis J, Kaye PM. 2013. Functional complexity of the Leishmania granuloma and the potential of in silico modeling. Front Immunol 4:35. doi: 10.3389/fimmu.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray HW, Mitchell-Flack M, Zheng H, Ma X. 2015. Granzyme-mediated regulation of host defense in the liver in experimental Leishmania donovani infection. Infect Immun 83:702–712. doi: 10.1128/IAI.02418-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford A, Angelosanto JM, Ndwodny KL, Blackburn SD, Wherry EJ. 2011. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog 7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vesosky B, Rottinghaus EK, Stromberg P, Turner J, Beamer G. 2010. CCL5 participates in early protection against Mycobacterium tuberculosis. J Leukoc Biol 87:1153–1165. doi: 10.1189/jlb.1109742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. 2002. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun 70:6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray HW, Nathan CF. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen vs. intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med 189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray HW, Oca MJ, Schreiber RD. 1989. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Invest 83:1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray HW, Montelibano C, Peterson R, Sypek JP. 2000. Interleukin 12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J Infect Dis 182:1497–1502. doi: 10.1086/315890. [DOI] [PubMed] [Google Scholar]
- 38.Murray HW, Jungbluth A, Ritter E, Montelibano C, Marino MW. 2000. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect Immun 68:6289–6293. doi: 10.1128/IAI.68.11.6289-6293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray HW, Xiang Z, Ma X. 2006. Responses to Leishmania donovani in mice deficient in both phagocyte oxidase and inducible nitric oxide synthase. Am J Trop Med Hyg 74:1013–1015. [PubMed] [Google Scholar]
- 40.Sato N, Kuziel WA, Melby PC, Reddick RL, Kostecki V, Zhao W, Maeda N, Ahuja S, Ahuja SS. 1999. Defects in the generation of IFN-γ are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1α-, or CCR2-deficient mice. J Immunol 163:5519–5525. [PubMed] [Google Scholar]
- 41.Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. 2006. Loss of dendritic cell migration and impaired resistance to Leishmania donovani in mice deficient in CCL19 and CCL21. J Immunol 176:5486–5493. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannheimer SB, Hariprashad J, Stoeckle MY, Murray HW. 1996. Induction of macrophage antiprotozoal activity by monocyte chemotactic and activating factor. FEMS Immunol Med Microbiol 14:59–61. doi: 10.1111/j.1574-695X.1996.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 43.Rosas LE, Snider HM, Barbi J, Satoskar AA, Lugo-Villarino G, Keiser T, Papenfuss T, Durbin JE, Radzioch D, Glimcher LH, Satoskar AB. 2006. Cutting edge: STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J Immunol 177:22–25. doi: 10.4049/jimmunol.177.1.22. [DOI] [PubMed] [Google Scholar]
- 44.Khadem F, Mou Z, Liu D, Varikuti S, Satoskar A, Uzonna JE. 2014. Deficiency of p110 isoform of the phosphoinositide 3 kinase leads to enhanced resistance to Leishmania donovani. PLoS Negl Trop Dis 8:e2951. doi: 10.1371/journal.pntd.0002951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stager S, Alexander J, Carter KC, Brombacher F, Kaye PM. 2003. Both interleukin-4 (IL-4) and IL-4 receptor α signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect Immun 71:4804–4807. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray HW, Tsai CW, Liu J, Ma X. 2006. Visceral Leishmania donovani infection in interleukin-13−/− mice. Infect Immun 74:2487–2490. doi: 10.1128/IAI.74.4.2487-2490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray HW, Lu CM, Brooks EB, Fichtl RE, DeVecchio JL, Heinzel FP. 2003. Modulation of T cell costimulation as immuno- or immunochemotherapy in experimental visceral leishmaniasis. Infect Immun 71:6453–6462. doi: 10.1128/IAI.71.11.6453-6462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray HW, Moreira AL, Lu CM, DeVecchio JL, Matsuhashi M, Ma X, Heinzel FP. 2003. Determinants of response to interleukin 10 receptor blockade immunotherapy in experimental visceral leishmaniasis. J Infect Dis 188:458–464. doi: 10.1086/376510. [DOI] [PubMed] [Google Scholar]
- 49.Oghumu S, Gupta G, Snider HM, Varikuti S, Terrazas CA, Papenfuss TL, Kaplan MH, Satoskar AR. 2014. STAT4 is critical for immunity but not for antileishmanial activity of antimonials in experimental visceral leishmaniasis. Eur J Immunol 44:450–459. doi: 10.1002/eji.201343477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray HW, Tsai CW, Liu J, Ma X. 2006. Responses to Leishmania donovani in mice deficient in interleukin-12 (IL-12), IL-12/IL-23, or IL-18. Infect Immun 74:4370–4376. doi: 10.1128/IAI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dufour JH, Dzlejman M, Liu MT, Leung JH, Lane TE, Luster AD. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 52.Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, Luster AD. 2000. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483–494. doi: 10.1016/S1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 53.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, Stiles JK. 2011. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev 22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsunoda I, Lane TE, Blackett J, Fujinami RS. 2004. Distinct roles for IP-10/CXCL10 in three animal models, Theiler's virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Multiple Sclerosis 10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- 55.Molesworth-Kenyon S, Mates A, Yin R, Streiter R, Oakes J, Lausch R. 2005. CXCR3, IP-10 and Mig are required for CD4+ T cell recruitment during the DTH response to HSV-1 yet are independent of the mechanism for viral clearance. Virology 333:1–9. doi: 10.1016/j.virol.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Zeng X, Moore TA, Newstead MW, Deng JC, Kunkel SL, Luster AD, Standiford TJ. 2005. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect Immun 73:8226–8236. doi: 10.1128/IAI.73.12.8226-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valbuena G, Walker DH. 2004. Effect of blocking the CXCL9/10-CXCR3 chemokine system in the outcome of endothelial-target rickettsial infections. Am J Trop Med Hyg 71:393–399. [PubMed] [Google Scholar]
- 58.Nie CQ, Bernard NJ, Norman MU, Amante FH, Lundie RJ, Crabb BS, Heath WR, Engwerda CR, Hickey MJ, Schofield L, Hansen DS. 2009. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog 5:e100039. doi: 10.1371/journal.ppat.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Majumder S, Bhattacharkee A, Paul Chowdhury B, Bhattacharyya Majumdar S, Majumdar S. 2014. Antigen-pulsed CpG-ODN-activated dendritic cells induce host-protective immune response by regulating T regulatory cell functioning in Leishmania donovani-infected mice: critical role of CXCL10. Front Immunol 5:261. doi: 10.3389/fimmu.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lunardi S, Lim SY, Muschel RJ, Brunner TB. 2015. IP-10/CXCL10 attracts regulatory T cells: implication for pancreatic cancer. Oncoimmunology 4:e1027473. doi: 10.1080/21622402X.2015.1027473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer L, Sandborn WJ, Stepanov Y, Geboes K, Hardi R, Yelloin M, Tao X, Xu LA, Salter-Cid L, Gujrathi S, Aranda R, Luo AY. 2014. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomized study. Gut 63:442–450. doi: 10.1136/gutjnl-2012-303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groom JR, Luster AD. 2011. CXCR3 in T cell function. Exp Cell Res 317:620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, Ganju RK, Satoskar AR. 2014. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology 143:109–119. doi: 10.1111/imm.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marques RE, Guabiraba R, Russo RC, Teixeira MM. 2013. Targeting CCL5 in inflammation. Expert Opin Ther Targets 17:1439–1460. doi: 10.1517/14728222.2013.837886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aldinucci D, Colombatti A. 2014. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm 2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santiago HC, Oliveira CF, Santiago L, Ferra FO, de Souza DG, De-Freitas LAR, Afonso LCC, Teixeira MM, Gazzinelli RT, Vieira LQ. 2004. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect Immun 72:4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villalta F, Zhang Y, Bibb KE, Kappes JC, Lima MF. 1998. The cysteine-cysteine family of chemokines RANTES, MIP-1alpha, and MIP-1beta induce trypanocidal activity in human macrophages via nitric oxide. Infect Immun 66:4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Machado FS, Loyama NS, Carregaro V, Ferreira BR, Milanezi CM, Teixeira MM, Rossi MA, Silva JS. 2005. CCR5 plays a critical role in the development of myocarditis and host protection in mice infected with Trypanosoma cruzi. J Infect Dis 191:627–636. doi: 10.1086/427515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. 2004. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol 76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 70.Ansel KM, Harris RB, Cyster JG. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16:67–76. doi: 10.1016/S1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 71.Shi X, Liu L, Xiang Z, Mitsuhashi M, Wu R, Ma X. 2004. Gene expression analysis in interleukin-12-induced suppression of mouse mammary carcinoma. Int J Cancer 110:570–578. doi: 10.1002/ijc.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruifrok AC, Johnston DA. 2001. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23:291–299. [PubMed] [Google Scholar]
- 73.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. 2006. Interleukin-27R (WSX-1/T cell cytokine receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am J Pathol 168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.