ABSTRACT
Lyme disease (LD), the most prevalent tick-borne illness in North America, is caused by Borrelia burgdorferi. The long-term survival of B. burgdorferi spirochetes in the mammalian host is achieved though VlsE-mediated antigenic variation. It is mathematically predicted that a highly variable surface antigen prolongs bacterial infection sufficiently to exhaust the immune response directed toward invariant surface antigens. If the prediction is correct, it is expected that the antibody response to B. burgdorferi invariant antigens will become nonprotective as B. burgdorferi infection progresses. To test this assumption, changes in the protective efficacy of the immune response to B. burgdorferi surface antigens were monitored via a superinfection model over the course of 70 days. B. burgdorferi-infected mice were subjected to secondary challenge by heterologous B. burgdorferi at different time points postinfection (p.i.). When the infected mice were superinfected with a VlsE-deficient clone (ΔVlsE) at day 28 p.i., the active anti-B. burgdorferi immune response did not prevent ΔVlsE-induced spirochetemia. In contrast, most mice blocked culture-detectable spirochetemia induced by wild-type B. burgdorferi (WT), indicating that VlsE was likely the primary target of the antibody response. As the B. burgdorferi infection further progressed, however, reversed outcomes were observed. At day 70 p.i. the host immune response to non-VlsE antigens became sufficiently potent to clear spirochetemia induced by ΔVlsE and yet failed to prevent WT-induced spirochetemia. To test if any significant changes in the anti-B. burgdorferi antibody repertoire accounted for the observed outcomes, global profiles of antibody specificities were determined. However, comparison of mimotopes revealed no major difference between day 28 and day 70 antibody repertoires.
KEYWORDS: Borrelia burgdorferi, Lyme disease, antibody response, suppression, protective efficacy, VlsE, antibody repertoire, mimotopes
INTRODUCTION
Lyme disease (LD), the most prevalent tick-borne illness in North America and Europe, is caused by spirochetes in the genus Borrelia. Borrelia burgdorferi, the principal human pathogen in the United States, is responsible for approximately 300,000 LD cases per year (1). LD is problematic because early diagnosis is easily missed due to flu-like symptoms, which only transiently appear in humans during an early stage of disease (2–5). When missed and therefore left untreated, LD becomes chronic, presenting itself as skin lesions, arthritis, and carditis and occasionally with subsequent nervous system involvement (6, 7). No preventable or therapeutic vaccine for humans is currently available.
The long-term survival of B. burgdorferi spirochetes in the mammalian host is achieved though the B. burgdorferi antigenic variation system (8). This elaborate system, first identified on a 28-kb linear plasmid (lp28-1) of the B. burgdorferi B31 strain, is composed of a vlsE expression site and 15 noncoding silent cassettes. As a result of segmental conversion from the cassettes into the vlsE gene, variants of the VlsE (variable major protein-like sequence expressed) surface lipoprotein are generated (9, 10). The vls-mediated variation of VlsE is absolutely required for persistence in mice as murine antibody clears the vls-deficient B. burgdorferi clone or the B. burgdorferi clone with nonswitchable VlsE (sVlsE, for static VlsE) (11–17). Besides VlsE, however, B. burgdorferi expresses numerous other surface (lipo)proteins that, in contrast to VlsE, are invariant (18). Antibody developed to non-VlsE surface antigens can protect mice from B. burgdorferi infection when variable VlsE is absent (17).
Two potential, not mutually exclusive, mechanisms of vls-mediated avoidance have been proposed (13, 17, 19–21). The first is vls-mediated masking whereby VlsE may physically shield B. burgdorferi surface antigens from antibody. The second is VlsE-mediated immune suppression (17, 22). As an immunodominant surface lipoprotein (23), VlsE may be directly or indirectly involved in suppression of the host antibody response. A mathematical model that considers the interplay between bacterial pathogens with an antigenic variation system, the immune response, and immune exhaustion may support the latter (24). Specifically, the model predicted that antigenic variation of dominant antigens of Trypanosoma or Plasmodium falciparum prolongs infection sufficiently to allow the immune response against invariant antigens to become exhausted. This exhaustion was predicted to occur before the immune response to invariant antigens could control infection. It is therefore plausible that, during B. burgdorferi infection, the antibody response to invariant (non-VlsE) surface antigens is suppressed via direct or indirect involvement of highly variable VlsE proteins. In this case, it is expected that antibody to invariant surface antigens will become inefficient in clearing vls-deficient B. burgdorferi clones. The central hypothesis that this study attempts to test states that the protective efficacy of the antibody response to B. burgdorferi non-VlsE surface antigens declines as B. burgdorferi infection progresses.
A recently developed superinfection model (25) was utilized to assess whether the protective efficacy of the host antibody changes over the course of B. burgdorferi infection. Furthermore, an approach involving random peptide phage display libraries (RPPDL) and next-generation sequencing (NGS) was undertaken to compare specificities of serum antibody developed during the early and late stages of B. burgdorferi infection. Overall, the present data show that the protective efficacy of the antibody response to VlsE and other surface antigens does change as B. burgdorferi infection progresses in the murine host.
RESULTS
Generation and characterization of the ΔVlsE Gentr clone.
In order to test whether the protective efficacy of host antibody to non-VlsE surface antigens declines as the B. burgdorferi infection progresses, a recently developed superinfection model was utilized (25). The experimental design involved wild-type B. burgdorferi 297 strain (26) and B31 A3 clones with antibiotic resistance cassettes for the primary and secondary challenge (superinfection), respectively. The antibiotic resistance allowed us to differentiate between the primary and superinfecting B. burgdorferi clones. Previously generated B31 A3 lp25::kan (WT Kanr) and B31 A3 lp28-1 Δvls (ΔVlsE Kanr) clones were initially chosen for the in vivo assay (16, 25). However, the use of the ΔVlsE Kanr clone in the superinfection model represented a potential caveat. In the prior work, it was noticed that a truncated lp28-1 plasmid was lost by B. burgdorferi spirochetes upon their recovery from infected C3H or SCID mice (17). To overcome this, a vls-deficient mutant that would retain an antibiotic resistance cassette in vivo had to be generated. This was achieved by an insertion, via allelic exchange, of a gentamicin (gent) antibiotic resistance cassette in the bbe02 locus of lp25. The lp25 plasmid is essential for murine infectivity (12, 27), whereas inactivation of bbe02, a putative restriction modification gene, does not result in loss of infectivity in mice (28–30). Thus, B31 A3 lp28-1 Δvls lp25::gent (ΔVlsE Gentr), the clone that possessed both the kanamycin (kan) and gentamicin resistance cassettes on lp28-1 and lp25, respectively, was generated. A total of 20 transformants were chosen for initial PCR analysis to screen for the kan gene. Five clones were further PCR tested for the presence of all parental B. burgdorferi plasmids. Infectivity of a ΔVlsE Gentr clone that retained the full parent plasmid profile was verified. The ΔVlsE Gentr clone demonstrated spirochetemia in 100% of C3SnSmn.CB17-Prkdcscid/J (SCID) and C3H/HeNHsd (C3H) mice (five animals per group) (see Table S1 in the supplemental material). As expected, the ΔVlsE Gentr clone was not able to establish persistent infection in C3H mice due to the lack of the vls locus (16).
Each superinfecting B. burgdorferi clone was used as a host-adapted variant. Host adaptation allowed B. burgdorferi to presumably mimic expression of surface antigens at levels comparable to those found during active infection at the time of challenge. For example, VlsE expression becomes approximately 32-fold higher in mice than that detected under in vitro growth conditions (23). To obtain host-adapted B. burgdorferi clones, the in vitro-grown WT Kanr, ΔVlsE Kanr, or ΔVlsE Gentr clone was subcutaneously injected into SCID mice as previously described (17, 31). Ears from the infected SCID mice were then harvested at day 21 postinfection (p.i.). The infectivity of host-adapted WT Kanr, ΔVlsE Kanr, or ΔVlsE Gentr was every time confirmed in C3H mice at the time of or a few days after each superinfection (three mice per group) (Tables S2, S3, and S4). Blood (∼50 μl) and other murine tissues (bladder, heart, ear, and joint) were harvested at days 7 and 21 postchallenge, respectively, from each control mouse. The murine tissues were incubated in Barbour-Stoenner-Kelly II (BSK-II) medium at 35°C under 2.5% CO2 for up to 4 weeks. The presence or absence of viable spirochetes from tissues was confirmed by dark-field microscopy. Expectedly, blood samples from the mice infected with either host-adapted ΔVlsE Kanr or ΔVlsE Gentr were culture positive, whereas their bladder, heart, ear, and joint tissues were culture negative. All the murine tissues from the control mice infected with host-adapted WT Kanr were culture positive for B. burgdorferi spirochetes. The results demonstrated that host-adapted mutant clones of B. burgdorferi were infectious at each superinfection.
Assessment of anti-B. burgdorferi immune response via a superinfection model.
The experimental design included 90 C3H mice that were initially infected with the B. burgdorferi 297 strain. The infection was confirmed in all animals by culturing murine blood and ear tissues harvested at day 7 p.i. At day 14, 21, 28, 42, 56, or 70 p.i., actively infected mice (five animals per group) were subjected to secondary challenge (superinfection) by host-adapted WT Kanr, ΔVlsE Kanr, or ΔVlsE Gentr. Finally, blood and other tissues (bladder, heart, ear, and joint) were harvested from each mouse at days 7 and 21 postsuperinfection, respectively, and then assessed for the presence of superinfecting spirochetes via culture. Individual tissues were placed in the respective antibiotic-containing BSK-II medium to select for the superinfecting clones. Each mouse had an ongoing B. burgdorferi 297-induced infection, as confirmed by positive culture of ear tissues harvested immediately prior to secondary challenge (data not shown).
At day 14 p.i., spirochetemia was established in at least 60% of the animals superinfected with either VlsE-competent (wild type) or -deficient (ΔVlsE) clones (Table 1). At day 21 p.i., 4 out of 10 mice exhibited spirochetemia induced by ΔVlsE Kanr/ΔVlsE Gentr (ΔVlsE hereafter), whereas 0 out of 5 mice demonstrated WT Kanr spirochetes in their blood. Overall, no statistical difference was observed between the WT Kanr and ΔVlsE groups at either day 14 or 21 p.i. At day 28 p.i. both VlsE-deficient clones had the capacity to establish culture-detectable spirochetemia in 10 out of 10 mice. In contrast, WT Kanr-induced spirochetemia was detected in only one mouse (P = 0.0037). As the primary infection further progressed, the number of mice with ΔVlsE-induced spirochetemia decreased from 50% (day 42 p.i.) to 0% (day 56 p.i.; P = 0.0163), indicating that the immune response became more protective against the VlsE-deficient clones, hence, non-VlsE surface antigens. The rate of WT Kanr-induced spirochetemia remained relatively constant as superinfecting VlsE-competent spirochetes were detected in only 1 and 2 mice at days 42 and 56 p.i., respectively (Table 1). At day 70 p.i., however, 5 out of 5 mice were blood culture positive for WT Kanr as opposed to only 2 out of 10 animals with ΔVlsE-induced spirochetemia (P = 0.0070). This observation demonstrates that the host antibody response against the VlsE-competent WT Kanr clone was no longer protective at this late stage. This, in turn, may suggest that, in contrast to findings at day 28 p.i., VlsE was not the primary target of the immune response. Thus, outcomes of secondary challenge significantly varied between VlsE-competent and -deficient B. burgdorferi clones and were dependent on a stage of primary B. burgdorferi infection.
TABLE 1.
Spirochetemia of superinfecting Borrelia burgdorferi detected in 297-infected C3H mice at day 7 post-secondary challenge
| Day of superinfection post-primary infection | Frequency of superinfecting clones detected in blooda |
|||
|---|---|---|---|---|
| WT Kanr | ΔVlsE Kanr | ΔVlsE Gentr | ΔVlsE Kanr + ΔVlsE Gentr | |
| 14 | 3/5 | 4/5 | 3/5 | 7/10 |
| 21 | 0/5 | 1/5 | 3/5 | 4/10 |
| 28 | 1/5 | 5/5 | 5/5 | 10/10 |
| 42 | 1/5 | 3/5 | 2/5 | 5/10 |
| 56 | 2/5 | 0/5 | 0/5 | 0/10 |
| 70 | 5/5 | 2/5 | 0/5 | 2/10 |
Values listed correspond to numbers of cultures positive/number of cultures tested.
In order to examine whether superinfecting B. burgdorferi clones had the capacity to persist in superinfected mice and whether actively infected mice lacking culture-detectable spirochetemia had completely prevented superinfection, various murine tissues were harvested at day 21 post-secondary challenge (Table 2). As a result, no VlsE-deficient clones were cultured from any of the tissues, suggesting that spirochetemic mice ultimately prevented blood-borne dissemination of superinfecting VlsE-deficient B. burgdorferi clones. This is in contrast to WT Kanr-challenged animals whose harvested tissues were positive for the superinfecting clone. This observation indicates that only the VlsE-competent B. burgdorferi had the capacity to establish long-term superinfection, which is consistent with the previous data (25). Detection of wild-type spirochetes in tissues of mice that lacked culture-detectable spirochetemia suggests that wild-type spirochetes were still present in the mouse blood upon secondary challenge, but at very low numbers.
TABLE 2.
Detection of superinfecting Borrelia burgdorferi in mouse tissues harvested at day 21 post-secondary challenge
| Day of superinfection post-primary infection | Frequency of superinfecting clones detected in murine tissuesa |
||
|---|---|---|---|
| WT Kanr | ΔVlsE Kanr | ΔVlsE Gentr | |
| 14 | 4/5 | 0/5 | 0/5 |
| 21 | 5/5 | 0/5 | 0/5 |
| 28 | 5/5 | 0/5 | 0/5 |
| 42 | 4/5 | 0/5 | 0/5 |
| 56 | 4/5 | 0/5 | 0/5 |
| 70 | 4/5 | 0/5 | 0/5 |
Tissues include ear, heart, bladder, and joint. Values correspond to numbers of cultures positive/number of cultures tested.
Characterization of antibody response via serology.
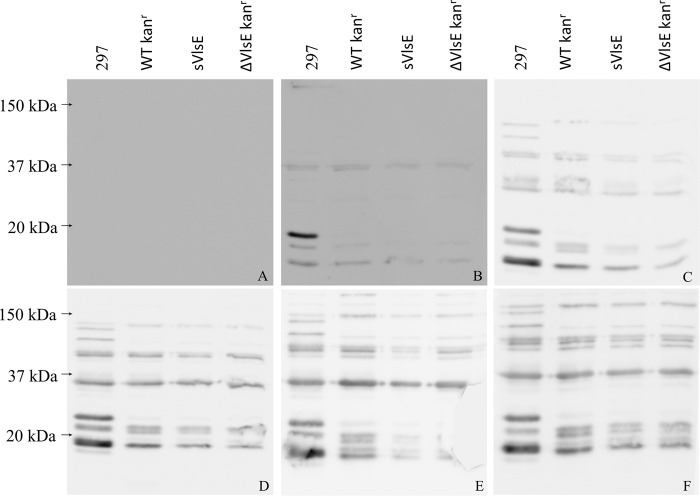
The data showed that the VlsE-competent and -deficient B. burgdorferi clones differ in their abilities to establish culture-detectable spirochetemia in mice actively infected with B. burgdorferi. This difference may be explained by variations in quantity and quality of the anti-B. burgdorferi antibody response over the course of B. burgdorferi infection. Therefore, in order to examine whether the antibody response was changing in strain 297-infected mice during the entire infection period, immune sera collected from animals at days 14, 21, 28, 42, 56, and 70 p.i. were analyzed by Western blotting (Fig. 1). The sera were blotted against whole-cell lysates of the 297, WT Kanr, sVlsE, or ΔVlsE Kanr clone and were reactive to a number of proteins, presumably including surface-localized antigens (32, 33). As expected, the lowest signal was observed in serum harvested at day 14 p.i., the time point at which anti-B. burgdorferi IgG antibody starts to appear in inbred mouse strains (34). The overall antibody response became more pronounced as the B. burgdorferi infection progressed in 297-infected mice. However, no noticeable difference could be noted for day 28 and 70 sera between the two clones (Fig. 1). In contrast to the three B31-derived clones, one prominent band was observed in the strain 297 lane at ∼23 kDa. The 23-kDa band likely corresponds to outer surface protein C (OspC), which generates a strong antibody response (35). Given that there is 78% identity between strain 297 and B31 OspC (297-OspC and B31-OspC, respectively) amino acid sequences, it is possible that antibodies against 297-OspC do not react well with B31-OspC. Similarly, two additional bands in the 70- to 90-kDa range were prominent in the 297 lane but not in the other lanes (Fig. 1).
FIG 1.
Analysis of anti-B. burgdorferi 297 immune sera by Western blotting. The whole-cell lysates of the 297, WT Kanr, sVlsE, and ΔVlsE Kanr (106 cells per lane) clones were treated with preimmune sera collected from uninfected C3H mice (A) and anti-297 immune sera harvested from B. burgdorferi-infected C3H mice at days 14 (B), 28 (C), 42 (D), 56 (E), and 70 (F) postinfection. The blots can be compared to Coomassie blue-stained whole-cell lysates of the 297, WT Kanr, sVlsE, and ΔVlsE Kanr clones shown in Fig. S1 (17).
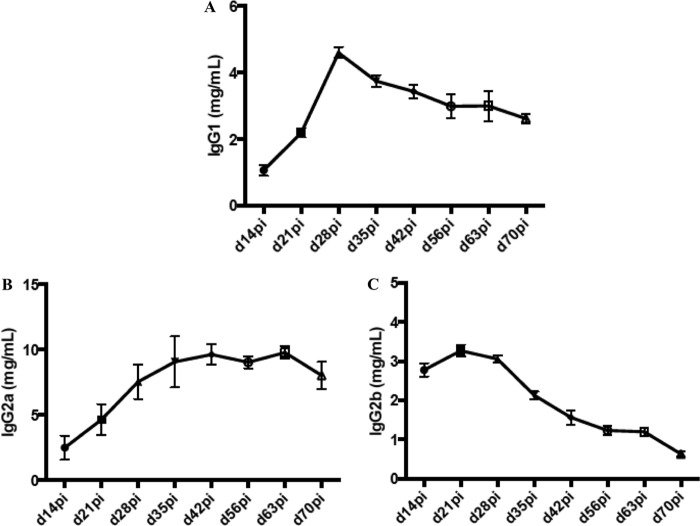
To further characterize the anti-297 antibody response, total serum amounts of different antibody isotypes, IgG1, IgG2a, and IgG2b, were quantified via enzyme-linked immunosorbent assays (ELISAs) (Fig. 2). During the first month of infection, IgG1 and IgG2b, but not IgG2a, were the dominant IgG isotypes. At a later stage of infection, day 70 p.i., the trend was reversed, indicating a shift from a Th2 to Th1 response. This finding is consistent with previous work, which demonstrated alike a Th1/Th2 imbalance in B. burgdorferi-infected C3H mice at week 5 p.i. (36).
FIG 2.
Quantification of total serum antibody isotypes in B. burgdorferi 297-infected C3H mice. Three C3H mice were subcutaneously challenged with B. burgdorferi 297 at 1 × 104 cells per mouse. Individual serum samples were collected weekly and then assessed, as indicated, for total IgG1, IgG2a, and IgG2b isotype-specific responses by ELISAs. d, day; pi, postinfection.
Profiling of the antibody response via random peptide phage display libraries.
Random peptide phage display libraries (RPPDL) were utilized in the present study (37). Although RPPDL have been widely used for mapping epitopes (38–40), the tool was previously applied for epitope discovery of B. burgdorferi proteins by only two studies (41, 42). The necessity to sequence individual phage clones definitely limited RPPDL application in the past (43). The advent of next-generation sequencing (NGS), however, has allowed RPPDL to be widely applied for generating global profiles of antibody specificities (40).
To test whether during persistent B. burgdorferi infection antibody repertoires change over time, the present study involved both RPPDL and NGS. For that, the Ph.D.-7 phage library was utilized to detect any significant difference between antibody repertoires in immune sera collected at days 28 and 70 p.i. from individual 297-infected mice. As a result, approximately 1.4 × 105 distinct peptide sequences were generated from each anti-B. burgdorferi serum sample. The data analyses revealed that only 366 and 14 antibody specificities, respectively, were associated with the day 28 and day 70 serum samples. The difference between the two numbers was statistically significant, with a P value estimated via a permutation test being below 0.25%. However, when a multiple testing correction was applied, no significant difference in antibody repertoires was identified between the two time points of B. burgdorferi infection.
In order to compare anti-VlsE antibody responses between days 28 and 70 p.i., identified peptides were mapped to strain 297 VlsE (297-VlsE) or strain B31 VlsE (B31-VlsE) via blastp analysis (Fig. 3). The epitope mapping against the linear B31-VlsE structure showed no significant difference between the reactivities of day 28 and day 70 antibodies. Predicted cross-reactivity of anti-297-VlsE antibody to linear B31-VlsE is partially consistent with the anti-VlsE antibody reactivity of LD patients (44). Microarray-based epitope mapping demonstrated that IgG antibody of patients with chronic LD were mainly reactive to six peptides of B31-VlsE. The immunodominant epitopes were located within two invariable domains (VlsE residues 21 to 31, 61, 96, and 336 to 343) and one variable domain (VlsE residues 196 and 271 to 291). Consistently, reactivity of anti-297 antibody was predicted to invariant region 6 (IR6). IR6 has been shown to be highly immunogenic in humans, monkeys, and mice (22, 45, 46). In addition to IR6, C3H mice may also develop a strong antibody response to IR2 and IR4 (46). However, reactivity to these conserved regions was not pronounced in the present study (Fig. 3). Interestingly, IR2 and IR4 are not antigenic in humans and monkeys (46). The analysis also predicted strong reactivity of anti-297 antibody to the C-terminal invariable domain (amino acids 372 to 380) within the primary structure of 297-VlsE as opposed to that of B31-VlsE. Murine antibodies were consistently developed against the C-terminal region during the entire infection period as reactivity was detected in all mouse sera taken at day 28 and day 70 p.i. This fully supports the previous findings that the C-terminal invariable domain is highly immunodominant (20) but shows limited antigenic conservation among B. burgdorferi strains (47). It was previously shown that the C-terminal domain sequences of 11 B. burgdorferi strains were not identical to the C-terminal sequence of the B31 strain (47). Likewise, due to a high degree of divergence with 46% identity and 53% similarity between B31-VlsE and 297-VlsE (8), strong antibody reactivity was predicted only to IR1 of 297-VlsE. Finally, no significant difference between the reactivities of day 28 and day 70 antibodies to the primary structures of translated cassettes vls2 to vls16 (vls2-vls16) (9) was identified (Fig. S2).
FIG 3.
Epitope mapping of VlsE. Primary structure of B31-VlsE illustrating two direct repeats (DR1 and DR2; green) that demarcate one variable domain and two invariable domains. Shown are also six invariable (IR; gray) and variable (VR; pink) regions (59) (A). Heat maps were generated from predicted reactivity of anti-297 antibody to the primary structure of B31-VlsE (B) and 297-VlsE (C). Anti-297 sera were harvested from B. burgdorferi persistently infected C3H mice at days 28 and 70 postinfection (five animals per time point). The linear B31-VlsE structure is scaled to the B31-VlsE heat map.
Similar to computational mapping of VlsE epitopes, identified peptides were also mapped to decorin-binding protein A (DbpA), decorin-binding protein (DbpB), and P35. These B. burgdorferi surface proteins were shown to be immunogenic and afforded protection in mice against B. burgdorferi infection (48–50). Consistently, no significant difference in antibody reactivities to contiguous epitopes of these immunogenic proteins was detected between day 28 and day 70 serum samples (Fig. S3).
DISCUSSION
A mathematical model previously developed as differential equations attempted to assess how duration of a bacterial infection may influence immune responses to variable and invariant surface antigens (24). It was calculated that antigenic variation of dominant surface antigen lengthens a bacterial infection to the point at which the immune response to invariant antigens becomes exhausted. In contrast, an immunodominant response to variable antigen does not predictably become exhausted but is, rather, transiently stimulated before the relevant antigenic variant is cleared (24). Therefore, it is possible that, during B. burgdorferi persistence, the antibody response to invariant (non-VlsE) surface epitopes is suppressed via direct or indirect involvement of highly variable and immunodominant VlsE proteins. If the model is correct, it is expected that the antibody response to invariant antigens will become nonprotective when B. burgdorferi infection is protracted in the host. To test the latter, changes in the protective efficacy of the immune response to non-VlsE surface antigens were monitored via the superinfection murine model.
In prior work, the superinfection in vivo assay demonstrated that the presence of the vls system may influence the ability of superinfecting B. burgdorferi clones to establish culture-detectable spirochetemia in persistently B. burgdorferi-infected mice (25). The superinfecting ΔVlsE clone established spirochetemia in three out of five 297-infected C3H mice, whereas wild-type spirochetes were not detected in any of five B. burgdorferi-infected animals (25). To confirm this observation, two independent experiments that involved isogenic VlsE-deficient clones, ΔVlsE Kanr and ΔVlsE Gentr, were reproduced in the present study. As a result, the data demonstrated that the ΔVlsE clones consistently exhibited the capacity to establish culture-detectable spirochetemia in 10 out of 10 animals at day 28 p.i., which statistically validates the earlier observation (25). The lack of culture-detectable spirochetemia by a VlsE-competent B. burgdorferi clone (wild type) consistently observed both in the previous (25) and current work suggests that, at this stage of B. burgdorferi infection, the protective antibody response was mainly targeting B31-VlsE variants and not the other surface antigens.
As the B. burgdorferi infection progressed, however, reversed outcomes were observed (Fig. 4). At day 70 p.i., the host immune response to non-VlsE antigens became sufficiently potent to clear spirochetemia by the ΔVlsE clone and yet failed to prevent WT-induced spirochetemia. At day 70 p.i., superinfecting wild-type B. burgdorferi established spirochetemia in all mice as opposed to a level of only 20% spirochetemia in animals infected with the ΔVlsE clone. This finding indicates that, over time, the infected mice were able to mount a more efficacious immune response against non-VlsE surface antigens. Moreover, at this later stage, the antibody response to B31-VlsE became inefficient, which may be accounted for by greater reactivity of a late antibody response to nonprotective VlsE epitopes via enhanced antigen processing (51). Furthermore, antibody reactivity to the membrane-proximal VlsE epitopes may sharply increase from early to late LD, yet these epitopes are inaccessible on intact spirochetes (51).
FIG 4.
Dynamics of protective antibody responses directed against VlsE and non-VlsE surface antigens. The diagram shows how the protective efficacy of antibody responses changes in C3H mice infected with B. burgdorferi strain 297 over the course of B. burgdorferi infection. At the early stage of B. burgdorferi infection, day 28 postinfection (p.i.), the anti-B. burgdorferi antibody response to invariant (non-VlsE) surface epitopes (light blue) is not protective against the VlsE-deficient B31 A3 clone (ΔVlsE). The ΔVlsE clone is able to consistently establish culture-detectable spirochetemia in strain 297-infected C3H mice at this stage. In contrast, the anti-VlsE immune response (dark red) is sufficiently potent to block culture-detectable spirochetemia by a VlsE-competent B31 A3 clone. As the B. burgdorferi infection progresses, however, the immune response to non-VlsE surface epitopes becomes sufficiently strong (dark blue) to prevent spirochetemia by ΔVlsE (day 70 p.i.). Inversely, the anti-VlsE antibody response is no longer protective (light red) to clear culture-detectable superinfecting wild-type strain in the blood.
These observations taken together indicate that anti-B. burgdorferi antibody responses were quantitatively and qualitatively changing as the infection progressed from day 28 through day 70 p.i. The antibody response became more pronounced and Th2 skewed in 297-infected C3H mice, as demonstrated by Western blotting and ELISA, respectively. These changes may partially account for the reversed outcomes of superinfection for both VlsE-competent and -deficient B. burgdorferi clones between the two time points of infection. It is also possible that, at this early stage, the antibody response to non-VlsE antigens was transiently weakened via temporal suppression of germinal center (GC) B cell responses (52). As early as 24 h p.i., B. burgdorferi spirochetes tend to colonize murine lymph tissues, which results in destruction of T and B cell zones (53, 54). The affected lymph nodes remain persistently infected with B. burgdorferi spirochetes (54, 55). Furthermore, germinal centers become structurally abnormal and fail to generate long-lived plasma and B cell memory cells for months (52). Thus, the present data indirectly support the idea that the LD pathogen requires only temporal suppression of the B cell response for fulfillment of its life cycle (52).
A possibility also exists that the observed dynamics in the protective efficacy of the anti-B. burgdorferi antibody response were due to changes in anti-B. burgdorferi antibody repertoires. For example, anti-B. burgdorferi antibody developed by day 28 p.i. could have been predominantly generated against VlsE epitopes, and, as the B. burgdorferi infection progressed, specificities of the anti-B. burgdorferi antibody were shifted toward non-VlsE surface antigens. To examine this possibility, global mimotope profiles derived from day 28 and 70 serum samples were compared to each other. Since mimotopes are peptides that may mimic both continuous and discontinuous antigens of a different nature (e.g., proteins, polysaccharides, and lipids) (56), the global mimotope comparison considered a variety of B. burgdorferi epitopes, including lipid and carbohydrate epitopes. However, the comparison revealed no major difference in antibody repertoires between the two time points of infection. Similarly, no significant changes were identified between the two antibody repertoires when identified mimotopes were mapped against primary structures of VlsE, DbpA, DbpB, and P35 (Fig. 3; see Fig. S3 in the supplemental material). Given, however, that many antibody epitopes of native proteins are discontinuous (57, 58), the mapping inherently underestimated repertoires of antibody developed to conformational epitopes of these B. burgdorferi surface proteins. Moreover, the BLAST-based analyses of antibody repertoires missed mimotopes that mimicked lipid and carbohydrate epitopes (43).
The present study also confirmed the previous observation that variable VlsE is a requirement for B. burgdorferi to establish persistent superinfection (25). Despite the variable ability of B. burgdorferi mutants to exhibit culture-detectable spirochetemia, only the B. burgdorferi clone with the intact vls system was consistently detected in various murine tissues harvested at day 21 postsuperinfection. In contrast, neither ΔVlsE clone introduced at different time points of B. burgdorferi infection was recovered from any of the tissues tested. Since B. burgdorferi is most likely required to invade the blood to reach various host tissues, the results suggest that a cross-protective anti-VlsE antibody response was not efficient at completely clearing the superinfecting spirochetes during the early stage of the primary infection. This consistent finding reiterates the importance of VlsE-mediated antigenic variation for persistent superinfection (25). Thus, the mathematical model, which predicted that antigenic variation of dominant surface antigen prolongs a bacterial infection to the point at which the immune response to invariant antigens is exhausted (24), may not apply to the LD pathogen. Therefore, based on the outcomes of day 70 superinfection, the tested hypothesis, which states that the protective efficacy of antibody response to B. burgdorferi non-VlsE surface antigens declines as B. burgdorferi infection progresses, can be rejected.
In summary, the study provides insights into a dynamic interplay between the host immune response and Lyme spirochetes in the context of VlsE antigenic variation. The data demonstrated that, during persistent B. burgdorferi infection, the host antibody response to B. burgdorferi invariant surface antigens could be transiently weakened and that, at this stage, variable VlsE might be the primary target of host immunity. Future studies aimed at identifying how and what B. burgdorferi antigens directly or indirectly manipulate host responses are warranted. Acquiring such knowledge may have direct implications in designing efficacious intervention strategies for LD patients.
MATERIALS AND METHODS
Ethics statement.
The animal experimental procedures outlined in this work were performed under a Texas A&M University-approved animal use protocol. The animals were maintained at Texas A&M University in an animal facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Texas A&M University institutional policies and guidelines are in full compliance with the U.S. Public Health Service policy on humane care and use of laboratory animals.
Bacterial strains and culture conditions.
B31 A3 lp25::kan (WT Kanr) and B31 A3 lp28-1 Δvls::vlsE (sVlsE) clones were generated and characterized in prior studies (25) and were generous gifts from Troy Bankhead (Table 3). The B. burgdorferi 297 strain and B31 A3 lp28-1 Δvls (ΔVlsE Kanr) clone, respectively, were kind gifts from Scott Samuels and George Chaconas by way of Troy Bankhead. All B. burgdorferi clones were cultivated in liquid Barbour-Stoenner-Kelly II (BSK-II) medium supplemented with 6% rabbit serum (Gemini Bio-Products, CA) and incubated at 35°C under 2.5% CO2.
TABLE 3.
Borrelia burgdorferi B31 clones used in the study
| B. burgdorferi clone (description) | Genetic profilea |
Reference or source | |
|---|---|---|---|
| vls2-vls16 | vlsE | ||
| B31 A3 lp25::kan (WT Kanr) | + | + | 25 |
| B31 A3 lp28-1Δvls (ΔVlsE Kanr) | − | − | 16 |
| B31 A3 lp28-1Δvls::vlsE (sVlsE) | − | + | 17 |
| B31 A3 lp28-1Δvls lp25::gent (ΔVlsE Gentr) | + | + | This study |
All clones lacked plasmid cp9. vls2-vls16 denotes silent cassettes of the vls locus.
Mutant generation.
To produce B31 A3 lp28-1 Δvls lp25::gent (ΔVlsE Gentr), the previously generated pAR15 plasmid was used to disrupt the bbe02 gene localized on lp25 (NCBI reference sequence NC_001850.1) (25). This pJET1.2-derived plasmid contained the bbe02 target (coordinates 361 to 4130) and the flgBp-driven gentamicin resistance gene (25). B. burgdorferi cells were electroporated according to a previously established protocol (16). In short, a total of 25 μg of DNA was used for electroporation. Borrelia cells were recovered at 35°C for 18 h and then diluted in 100 ml of prewarmed BSK-II medium supplemented with 100 μg ml−1 gentamicin. The transformed cell suspension was aliquoted into 96-well plates and incubated at 35°C for 21 days. Genomic DNA was extracted from positive cultures utilizing a DNeasy blood and tissue kit (Qiagen, MD, USA). The insertion of a flgBp-gent cassette within the bbe02 gene was confirmed by PCR using P168/169 primers (5′-CAGTTGCGCAGCCTGAATGG-3′ and 5′-AGGTGGCGGTACTTGGGTCG-3′) as previously described (25). Finally, the plasmid profile of each PCR-positive clone was screened as described (11).
Murine infection.
Male C3H/HeJ (C3H), C3SnSmn.CB17-Prkdcscid/J (SCID), and BALB/cJ (BALB/c) mice of 4 to 6 weeks of age were obtained from Jackson Laboratories (ME, USA). The primary infection was performed on 4- to 6-week-old animals via subcutaneous inoculation of 1 × 104 total spirochetes in the scapular region. The inoculum of each mutant clone of B. burgdorferi with a recombinant plasmid was first cultured in BSK-II medium containing an antibiotic, followed by dilution (approximately 1:1,000) with antibiotic-free BSK-II medium prior to murine infection. B. burgdorferi clones used for challenge were passaged in vitro no more than two times.
Secondary challenge (superinfection) was performed via subcutaneous transplantation of ear tissue (host-adapted B. burgdorferi) in the lumbar area as previously described (17, 31). In short, ear tissues were harvested from B. burgdorferi-infected SCID mice at day 21 postinfection (p.i.) and stored at −80°C until use. At the time of challenge ear pinnae were excised into small, circular pieces (2 mm in diameter) by a sterile ear punch and subcutaneously inserted via a skin incision in the lumbar region (two pieces per mouse). Given the identical sizes of ear tissues used for mouse challenges, the spirochetal loads of B. burgdorferi mutants were presumably similar. The infectivity of host-adapted B. burgdorferi was tested on naive C3H mice (three male mice per group). Specifically, the control mice were challenged with two ear pieces (2 mm in diameter) derived from two pinnae of each infected SCID mouse (one tissue piece from each ear) either at the time of or a few days after each superinfection (see Tables S2, S3, and S4 in the supplemental material).
Generation of host-adapted B. burgdorferi clones.
Each mouse challenge was confirmed by culturing approximately 50 μl of blood aseptically drawn via maxillary bleed in 3 ml of BSK-II medium that contained a Borrelia antibiotic cocktail (0.02 mg ml−1 phosphomycin, 0.05 mg ml−1 rifampin, and 2.5 mg ml−1 amphotericin B). Infection was monitored by culturing ear, heart, bladder, and tibiotarsal joint tissues aseptically harvested at days 7, 21, and 28 p.i. in BSK-II medium with the antibiotic cocktail. Blood or heart tissues were transferred into 8-ml polystyrene tubes (Becton Dickinson Labware, NJ, USA) containing 3 ml of BSK-II medium. Tissues of bladder, tibiotarsal joint, or ear were cultured in 1.7-ml polypropylene microcentrifuge tubes (Denville Scientific, Inc., MA, USA) with 1.0 ml of BSK-II medium. The tissues were incubated at 35°C under 2.5% CO2 for up to 4 weeks. The presence or absence of viable spirochetes from tissues was confirmed by dark-field microscopy.
Western blot analysis.
B. burgdorferi clones 297, WT Kanr, sVlsE, and ΔVlsE Kanr were grown in BSK-II medium to the late stationary phase. B. burgdorferi cells were counted, pelleted by centrifugation at 6,000 × g for 10 min at 4°C, and then washed twice with ice-cold phosphate-buffered saline (PBS). After PBS was removed, the cells were suspended in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer (100 mM Tris [pH 6.8], 2% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue) and incubated at 95°C for 10 min. Approximately 1 × 106 cells were loaded onto a 15% acrylamide minigel (SDS-PAGE analysis is shown in Fig. S1). Then, resolved proteins were transferred onto polyvinylidene difluoride (PVDF) membrane with a pore size of 0.45 μm (Bio-Rad Laboratories, CA, USA). After the blot was blocked with 5% nonfat dry milk in PBS for 18 h at 4°C, it was then incubated in the same solution supplemented with mouse anti-297 immune or preimmune serum diluted 1:1,000 for 1 h. The immune serum samples were taken from 297-infected C3H mice at days 14, 28, 42, 56, and 70 p.i. At each time point blood was collected, and an equal amount of immune serum derived from five animals per time point was pooled and filter sterilized by passage through a 0.22-μm-pore-size syringe filter. Three naive C3H mice served as a source of preimmune sera. After four washes of 10 min each with PBS plus Tween 20 (PBST), the primary antibodies were detected using goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (Bio-Rad Laboratories, CA, USA) diluted to 1:1,000 in Tris-buffered saline with Tween 20 (TBST) for 30 min. The blot was first washed three times in TBST for 10 min each and then once with nano-pure water. The blots were visualized using enhanced chemiluminescence (ECL) development.
ELISA.
The immune sera were derived from 297-infected C3H mice at days 14, 28, 42, 56, and 70 p.i. as described above. Individual serum samples were tested by ELISAs to quantitate total IgG1, IgG2a, and IgG2b isotypes according to the manufacturer's instructions (eBioscience, Inc., CA, USA). Serum was diluted to 1:10,000 and plated at 4°C overnight. Samples were tested in duplicate, and each assay included preimmune serum as a negative control.
Generating serum antibody repertoire profiles using the Ph.D.-7 random peptide library.
Twenty microliters of mouse serum and 10 μl of the Ph.D.-7 random peptide library (NEB, MA, USA) were diluted in 200 μl of Tris-buffered saline (TBST) buffer containing 0.1% Tween 20 and 1% bovine serum albumin (BSA) and then incubated overnight at room temperature. The phages bound to antibodies were isolated by applying 20 μl of protein G-agarose beads (Santa Cruz Biotechnology, Inc., TX, USA) to the phage-antibody mixture for 1 h. To eliminate unbound phages, the mixture with beads was transferred to a 96-well MultiScreen-Mesh filter plate (EMD Millipore, MA, USA) containing a 20-μm-pore-size nylon mesh on the bottom. Unbound phages were removed by applying a vacuum to the outside of the nylon mesh. The beads were washed four times with 100 μl of TBST buffer per well. Antibody-bound phages were eluted with 100 μl of 100 mM Tris-glycine buffer (pH 2.2). Then the buffer was replaced with 20 μl of 1 M Tris buffer (pH 9.1). The eluted phages were amplified by infecting bacteria according to the manufacturer's instructions. Amplified phages were subjected to two additional rounds of biopanning. Antibody-bound phages were isolated using protein G-agarose beads. DNA was isolated via phenol-chloroform extraction and ethanol precipitation. The 21-nucleotide (nt)-long DNA fragments coding random peptides were then PCR amplified using the following forward and reverse primers, respectively: 5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT(INDEX)TGGTACCTTTCTATTCTCACTCT-3′ and 5′-CAAGCAGAAGAGGGCATACGAGCTCTTCCGATCTAACAGTTTCGGCCGAACCTCCACC-3′. The INDEX in the sequence of the forward primer indicates a 6-nt barcode, which allows sequencing multiple libraries using a single line of the Illumina flow cell. For each mouse serum, a distinct forward primer with unique index sequence was used. The multiplexed PCR-amplified DNA library was then purified on agarose gel and sequenced using an Illumina HiSeq 2500 platform.
Next-generation data analysis.
As a result of sequencing, a total of about 116 million DNA reads were obtained. Reads were demultiplexed based on the barcodes. Each read contained a unique index sequence of 6 nt in length and a 21-nt sequence coding a random peptide: 5′-(INDEX)GTGGTACCTTTCTATTCTCACTCT(21-nt sequence)G-3′. Then the 21-nt sequences were extracted from each read between positions 30 and 50 and translated to 7-mer peptides in the first frame (Text S1). Peptides that contained stop codons were not included in the analysis. The average number of all peptides per serum sample was approximately 1 × 107. The number of distinct peptides identified was approximately 1.4 × 105 per sample. The data were then analyzed via the Python programming language (Python Software Foundation [https://www.python.org]).
The strength of association between a peptide and a serum sample was measured as follows. A peptide, P, was associated with day 28 serum if X(P), the lowest frequency of P among day 28 serum samples, was higher than Y(P), the highest frequency of P among day 70 serum samples. The strength of association was then measured by the size of the gap: X(P) − Y(P). Similarly, a peptide was associated with day 70 serum samples if its smallest frequency among day 70 serum samples was higher than the highest frequency among day 28 serum samples.
Position coverage by peptides of VlsE and other B. burgdorferi proteins.
For each serum sample, all peptides were mapped to VlsE of B. burgdorferi 297 (297-VlsE) (GenBank accession number AB041949.1) or B31 (B31-VlsE) (GenBank accession number AAC45733.1) strains using blastp with an identity threshold of 4 (i.e., only alignments with at least four exact amino acid matches were taken into account). For each VlsE position X, a peptide with the amino acid matched to position X and with K different VlsE matches contributed its frequency divided by K to the coverage of X. Overall, the coverage of X, C(X), was computed as the sum of contributions of all peptides matched to X. Similarly, all peptides were mapped to other B. burgdorferi surface proteins of the two B. burgdorferi strains, decorin-binding proteins A (DbpA), DbpB, and P35.
Statistical analysis.
A one-tailed Fisher's exact test was used for comparison of mouse groups. A P value of <0.05 was considered significantly different. The statistical significance of the difference between the number of peptides associated with the day 28 serum and the number of peptides associated with the day 70 serum was measured using a permutation test. The permutation test was used because of the comparatively small number of samples (five serum samples per time point). For each of the possible permutations, the difference between the numbers of associated peptides was found and compared with the actual difference.
Supplementary Material
ACKNOWLEDGMENTS
We thank Troy Bankhead for providing the WT Kanr, ΔVlsE Kanr, and sVlsE clones. We also thank Anna and Antonia Rogovska for generation of Fig. 4.
The work was supported through the Department of Veterinary Pathobiology, Texas A&M College of Veterinary Medicine and Biomedical Sciences. The work at Georgia State University was partially supported by the NSF grant CCF-16119110 Algorithmic Techniques for Inferring Transmission Networks from Noisy Sequencing Data and a Molecular Basis of Disease Fellowship. The work at Roswell Park Cancer Institute was partially supported by the Philip Hubbell Family Fund.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00890-16.
REFERENCES
- 1.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvikar SL, Steere AC. 2015. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 29:269–280. doi: 10.1016/j.idc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lantos PM. 2015. Chronic Lyme disease. Infect Dis Clin North Am 29:325–340. doi: 10.1016/j.idc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melia MT, Lantos PM, Auwaerter PG. 2015. Laboratory testing for Lyme neuroborreliosis—reply. JAMA Neurol 72:126. doi: 10.1001/jamaneurol.2014.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchers AT, Keen CL, Huntley AC, Gershwin ME. 2015. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun 57:82–115. doi: 10.1016/j.jaut.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Marques AR. 2010. Lyme disease: a review. Curr Allergy Asthma Rep 10:13–20. doi: 10.1007/s11882-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 7.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 8.Norris SJ. 2014. vls antigenic variation systems of Lyme disease Borrelia: eluding host immunity through both random, segmental gene conversion and framework heterogeneity. Microbiol Spectr 2:6. doi: 10.1128/microbiolspec.MDNA3-0038-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JR, Hardham JM, Barbour AG, Norris SJ. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275–285. doi: 10.1016/S0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 10.Norris SJ. 2006. Antigenic variation with a twist—the Borrelia story. Mol Microbiol 60:1319–1322. doi: 10.1111/j.1365-2958.2006.05204.x. [DOI] [PubMed] [Google Scholar]
- 11.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labandeira-Rey M, Skare JT. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun 69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labandeira-Rey M, Seshu J, Skare JT. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun 71:4608–4613. doi: 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer R, Kalu O, Purser J, Norris S, Stevenson B, Schwartz I. 2003. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect Immun 71:3699–3706. doi: 10.1128/IAI.71.7.3699-3706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrenz MB, Wooten RM, Norris SJ. 2004. Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1. Infect Immun 72:6577–6585. doi: 10.1128/IAI.72.11.6577-6585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankhead T, Chaconas G. 2007. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol 65:1547–1558. doi: 10.1111/j.1365-2958.2007.05895.x. [DOI] [PubMed] [Google Scholar]
- 17.Rogovskyy AS, Bankhead T. 2013. Variable VlsE is critical for host reinfection by the Lyme disease spirochete. PLoS One 8:e61226. doi: 10.1371/journal.pone.0061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenedy MR, Lenhart TR, Akins DR. 2012. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol 66:1–19. doi: 10.1111/j.1574-695X.2012.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang FT, Jacobs MB, Bowers LC, Philipp MT. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med 195:415–422. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipp MT, Bowers LC, Fawcett PT, Jacobs MB, Liang FT, Marques AR, Mitchell PD, Purcell JE, Ratterree MS, Straubinger RK. 2001. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J Infect Dis 184:870–878. doi: 10.1086/323392. [DOI] [PubMed] [Google Scholar]
- 21.Palmer GH, Bankhead T, Seifert HS. 2016. Antigenic variation in bacterial pathogens. Microbiol Spectr 4:1. doi: 10.1128/microbiolspec.VMBF-0005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang FT, Alvarez AL, Gu Y, Nowling JM, Ramamoorthy R, Philipp MT. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol 163:5566–5573. [PubMed] [Google Scholar]
- 23.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson PLF, Kochin BF, Ahmed R, Antia R. 2012. How do antigenically varying pathogens avoid cross-reactive responses to invariant antigens? Proc Biol Sci 279:2777–2785. doi: 10.1098/rspb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogovskyy AS, Bankhead T. 2014. Bacterial heterogeneity is a requirement for host superinfection by the Lyme disease spirochete. Infect Immun 82:4542–4552. doi: 10.1128/IAI.01817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N Engl J Med 308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 27.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol 48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawabata H, Norris SJ, Watanabe H. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect Immun 72:7147–7154. doi: 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrenz MB, Kawabata H, Purser JE, Norris SJ. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect Immun 70:4798–4804. doi: 10.1128/IAI.70.9.4798-4804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs MB, Norris SJ, Phillippi-Falkenstein KM, Philipp MT. 2006. Infectivity of the highly transformable BBE02− lp56− mutant of Borrelia burgdorferi, the Lyme disease spirochete, via ticks. Infect Immun 74:3678–3681. doi: 10.1128/IAI.00043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barthold SW. 1993. Antigenic stability of Borrelia burgdorferi during chronic infections of immunocompetent mice. Infect Immun 61:4955–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks CS, Vuppala SR, Jett AM, Akins DR. 2006. Identification of Borrelia burgdorferi outer surface proteins. Infect Immun 74:296–304. doi: 10.1128/IAI.74.1.296-304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes JL, Nolder CL, Nowalk AJ, Clifton DR, Howison RR, Schmit VL, Gilmore RD Jr, Carroll JA. 2008. Borrelia burgdorferi surface-localized proteins expressed during persistent murine infection are conserved among diverse Borrelia spp. Infect Immun 76:2498–2511. doi: 10.1128/IAI.01583-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaible UE, Kramer MD, Wallich R, Tran T, Simon MM. 1991. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur J Immunol 21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 35.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun 61:2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keane-Myers A, Nickell SP. 1995. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J Immunol 155:2020–2028. [PubMed] [Google Scholar]
- 37.Dias-Neto E, Nunes DN, Giordano RJ, Sun J, Botz GH, Yang K, Setubal JC, Pasqualini R, Arap W. 2009. Next-generation phage display: integrating and comparing available molecular tools to enable cost-effective high-throughput analysis. PLoS One 4:e8338. doi: 10.1371/journal.pone.0008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cwirla SE, Peters EA, Barrett RW, Dower WJ. 1990. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A 87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JK, Smith GP. 1990. Searching for peptide ligands with an epitope library. Science 249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 40.Ryvkin A, Ashkenazy H, Smelyanski L, Kaplan G, Penn O, Weiss-Ottolenghi Y, Privman E, Ngam PB, Woodward JE, May GD, Bell C, Pupko T, Gershoni JM. 2012. Deep panning: steps towards probing the IgOme. PLoS One 7:e41469. doi: 10.1371/journal.pone.0041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouzmitcheva GA, Petrenko VA, Smith GP. 2001. Identifying diagnostic peptides for Lyme disease through epitope discovery. Clin Diagn Lab Immunol 8:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamby CV, Llibre M, Utpat S, Wormser GP. 2005. Use of peptide library screening to detect a previously unknown linear diagnostic epitope: proof of principle by use of Lyme disease sera. Clin Vaccine Immunol 12:801–807. doi: 10.1128/CDLI.12.7.801-807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Hu Q, Liu S, Tallo LJ, Sadzewicz L, Schettine CA, Nikiforov M, Klyushnenkova EN, Ionov Y. 2013. Serum antibody repertoire profiling using in silico antigen screen. PLoS One 8:e67181. doi: 10.1371/journal.pone.0067181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandra A, Latov N, Wormser GP, Marques AR, Alaedini A. 2011. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin Immunol 141:103–110. doi: 10.1016/j.clim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang FT, Philipp MT. 2000. Epitope mapping of the immunodominant invariable region of Borrelia burgdorferi VlsE in three host species. Infect Immun 68:2349–2352. doi: 10.1128/IAI.68.4.2349-2352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang FT, Philipp MT. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect Immun 67:6702–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang FT, Bowers LC, Philipp MT. 2001. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect Immun 69:3224–3231. doi: 10.1128/IAI.69.5.3224-3231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fikrig E, Barthold SW, Sun W, Feng W, Telford SR III, Flavell RA. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531–539. doi: 10.1016/S1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 49.Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun 66:2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, Dorward DW, Hook M. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun 66:2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacek E, Tang KS, Komorowski L, Ajamian M, Probst C, Stevenson B, Wormser GP, Marques AR, Alaedini A. 2016. Epitope-specific evolution of human B Cell responses to Borrelia burgdorferi VlsE protein from early to late stages of Lyme disease. J Immunol 196:1036–1043. doi: 10.4049/jimmunol.1501861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsner RA, Hastey CJ, Olsen KJ, Baumgarth N. 2015. Suppression of long-lived humoral Immunity following Borrelia burgdorferi infection. PLoS Pathog 11:e1004976. doi: 10.1371/journal.ppat.1004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hastey CJ, Ochoa J, Olsen KJ, Barthold SW, Baumgarth N. 2014. MyD88- and TRIF-independent induction of type I interferon drives naive B cell accumulation but not loss of lymph node architecture in Lyme disease. Infect Immun 82:1548–1558. doi: 10.1128/IAI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tunev SS, Hastey CJ, Hodzic E, Feng S, Barthold SW, Baumgarth N. 2011. Lymphoadenopathy during Lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS Pathog 7:e1002066. doi: 10.1371/journal.ppat.1002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hastey CJ, Elsner RA, Barthold SW, Baumgarth N. 2012. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J Immunol 188:5612–5622. doi: 10.4049/jimmunol.1103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geysen HM, Rodda SJ, Mason TJ. 1986. A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol Immunol 23:709–715. doi: 10.1016/0161-5890(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 57.Barlow DJ, Edwards MS, Thornton JM. 1986. Continuous and discontinuous protein antigenic determinants. Nature 322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- 58.Benjamin DC. 1995. B-cell epitopes: fact and fiction. Adv Exp Med Biol 386:95–108. doi: 10.1007/978-1-4613-0331-2_8. [DOI] [PubMed] [Google Scholar]
- 59.Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem 277:21691–21696. doi: 10.1074/jbc.M201547200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.